Multidisciplinary strategies including local treatment to achieve drug‑free status after atezolizumab plus bevacizumab treatment in hepatocellular carcinoma
- Authors:
- Published online on: July 31, 2025 https://doi.org/10.3892/ol.2025.15212
- Article Number: 466
Abstract
Introduction
Hepatocellular carcinoma (HCC) is one of the most common malignant tumors worldwide, ranking as the sixth most commonly diagnosed cancer and the third leading cause of cancer-related death (1,2). Treatment strategies for advanced HCC have diversified in recent years owing to the introduction of several regimens after sorafenib [regorafenib, lenvatinib, ramucirumab, atezolizumab plus bevacizumab (AteBev), cabozantinib and tremelimumab plus durvalumab] (3). In addition, there have been a growing effort to optimize survival outcomes by stratifying treatment regimens according to variations in therapeutic response and biomarker profiles (4). Thus, the treatment outcomes for advanced HCC are expected to continue improving.
In the IMbrave150 trial, AteBev treatment, a combination of anti-programmed cell death-ligand 1 inhibitor and anti-vascular endothelial growth factor inhibitor, demonstrated superior survival outcomes and treatment response to sorafenib. In addition, it is accepted as the first-line treatment worldwide for advanced-stage HCC, according to the Barcelona Clinic Liver Cancer (BCLC) classification (5,6). Owing to its high response rate, the concept of conversion therapy, combining AteBev and sequential local treatments, which could prolong overall survival (OS) and progression-free survival (PFS), has attracted considerable attention in recent years (7,8). However, the definition of conversion therapy varies among institutions, and there are no standardized strategies regarding the timing and modality of local treatment, or whether systemic chemotherapy should be continued after local treatment. Kudo et al (9) proposed the ‘clinical complete response (CR)’ and ‘drug-off’ criteria, and patients meeting these criteria with or without curative conversion achieved good clinical outcomes. However, the study cohort was limited to patients with BCLC stage B, and most of the local treatment modalities were transarterial chemoembolization (TACE) and radiofrequency ablation (RFA). In addition, the proposed ‘clinical CR’ and ‘drug-off’ criteria are clinically inflexible for adapting to all cases.
Therefore, the present study aimed to assess the outcomes of AteBev treatment for advanced HCC and elucidate the optimal timing for introducing local treatment. The present study also aimed to evaluate the prognostic value of discontinuing systemic chemotherapy and switching to careful observation in patients who achieved a CR based on imaging according to the modified Response Evaluation Criteria in Solid Tumors (RECIST) (10), regardless of tumor marker normalization.
Patients and methods
Study population
The present retrospective study analyzed 123 consecutive patients with HCC treated with AteBev at Kobe University Hospital (Kobe, Japan) and Kobe Minimally invasive Cancer Center (Kobe, Japan) between October 2020 and January 2024. All procedures were performed following the ethical standards of the institutions and the National Research Committee, in accordance with the Declaration of Helsinki. The present study was approved by the ethics committees of Kobe University Hospital and Kobe Minimally invasive Cancer Center (approval no. B240024). An opt-out method was employed in accordance with institutional guidelines. Information about the study was publicly disclosed on the institutional websites of Kobe University Hospital and Kobe Minimally invasive Cancer Center, allowing patients the opportunity to decline participation.
Data collection
The following patient and laboratory data were collected: Sex, age, performance status, serum total bilirubin, albumin, α-fetoprotein (AFP) level, des-γ-carboxy prothrombin level, prothrombin time, neutrophil-to-lymphocyte ratio (NLR) (11) and viral serology. Liver function was evaluated using the Child-Pugh classification (12) and modified albumin-bilirubin (mALBI) grade (13). Performance status was assessed using the Eastern Cooperative Oncology Group (ECOG) Performance Status guidelines (14). HCC was diagnosed histologically and/or radiologically based on contrast-enhanced (CE)-computed tomography (CT) and/or gadolinium-ethoxybenzyl-diethylenetriamine pentaacetic acid (EOB)-enhanced magnetic resonance imaging (MRI) findings according to the practice guidelines proposed by the American Association for the Study of Liver Disease in 2018 (15). The following pretreatment imaging findings were obtained: Tumor number, tumor size, presence of macrovascular invasion, presence of extrahepatic lesions and BCLC staging (6).
Definition of the ‘drug-free’ criteria
The ‘clinical CR’ criteria proposed by Kudo et al (9) were defined as follows: i) CR on CT or MRI according to the modified RECIST; and ii) normalization of the levels of three tumor markers for ≥6 weeks when any of these three markers is elevated. The ‘drug-off’ status was defined in patients who could be curatively resected or in those who received curative locoregional therapy and fulfilled the following criteria: i) Achievement of CR according to the modified RECIST by super-selective TACE with curative intent or RFA/microwave ablation; ii) maintenance of three normalized tumor markers for ≥24 weeks; and iii) complete disappearance of intratumoral arterial flow on CE ultrasound (9). Furthermore, the following definition for the ‘drug-free’ cohort was used, with patients followed up who fulfilled the criteria: i) Achievement of one of the following conditions: CR using AteBev treatment alone based on the modified RECIST or sequential local treatment following AteBev treatment; ii) no apparent residual lesions detected using CE-CT or EOB-MRI; and iii) non-essential normalization of tumor markers.
In the present study, patients with no apparent residual lesions after sequential local treatment or AteBev treatment alone were followed up without any systemic chemotherapy. The modality and timing of the sequential local treatment were decided by the attending physician. After sequential local treatment, observation without AteBev treatment was performed regardless of tumor marker normalization. Another local treatment was administered if residual lesions could be controlled. Patients who achieved CR with AteBev treatment alone were followed up without AteBev treatment.
The present study assessed the survival outcomes between the ‘drug-free’ cohort and patients achieving ‘clinical CR’ or ‘drug-off’ criteria, and the validity of the strategy aiming at a drug-free status.
Study design
The inclusion criteria for patients receiving AteBev treatment were as follows: ECOG performance status of 0-2; clinical diagnosis of advanced HCC; and Child-Pugh classes A and B. Moreover, the exclusion criteria were as follows: Serious complications; ascites refractory or minimally responsive to therapy; uncontrolled gastroesophageal varices; severe autoimmune diseases; and missing follow-up or short follow-up time (≤30 days). A flow diagram of the patient selection process is presented in Fig. S1.
Atezolizumab (1,200 mg) and bevacizumab (15 mg/kg) were administered intravenously every 3 weeks. Laboratory data were collected at the time of administration. Treatment responses were evaluated every 3–12 weeks using CE-CT and/or EOB-MRI according to the RECIST (version 1.1) and modified RECIST (10,16). The changes in tumor size of all patients during AteBev treatment were evaluated using spider plots based on the RECIST (version 1.1). Treatment discontinuation and withdrawal were determined in accordance with the protocol of the IMbrave150 trial (5). To assess the duration of the effectiveness of AteBev treatment, the transition of serum AFP levels was evaluated. Patients with serum AFP levels ≥10 ng/ml (the upper limit of normal), which decreased at the first evaluation, were included in the analysis.
Statistical analyses
OS was defined as the time from the initiation of AteBev treatment to death from any cause. PFS was defined as the time from the initiation of AteBev treatment to disease progression or death from any cause.
All statistical analyses were performed using JMP Pro, version 17 (SAS Institute, Inc.). OS and PFS were estimated using the Kaplan-Meier method and compared using the log-rank test. Changes in tumor size based on RECIST (version 1.1) were expressed using Spider plots. The serum AFP levels transition were expressed using box plots. Clinical factors were analyzed using the χ2 test or Fisher's exact test, as appropriate. Univariate and multivariate analyses of the pretreatment clinical factors associated with poor OS were performed using the Cox proportional hazards model. Factors previously reported to be associated with poor OS among the pretreatment factors (mALBI grade, NLR, macrovascular invasion, BCLC classification and treatment line) were included in the multivariate analysis. P<0.05 was considered to indicate a statistically significant difference.
Results
Baseline patient characteristics
A total of 123 consecutive patients were enrolled in the present study, and their baseline characteristics are listed in Table I. The cohort included 98 men and 25 women, with a median age of 73 years. A total of ~50% of the patients had a non-viral etiology (n=62). Furthermore, 109 patients (88.6%) were classified as Child-Pugh A, and 74 (60.2%) as mALBI grade 1 + 2a. A total of 72 patients (58.5%) were classified as BCLC stage C. Additionally, 82 patients (66.7%) were administered AteBev as a first-line treatment.
Treatment efficacy and survival outcomes
At the data cutoff date (January 31, 2024), the median follow-up duration in all patients was 13.0 months [95% confidence interval (CI), 12.6–15.0] Treatment responses are presented in Table SI. The objective response rate (ORR) and disease control rate (DCR) based on the RECIST (version 1.1) were 29.3 and 78.9%, respectively, whilst the ORR and DCR based on the modified RECIST were 38.2 and 78.9%, respectively. The median survival time (MST) was 21.7 months (95% CI, 15.9–25.4), and the median PFS was 7.4 months (95% CI, 5.0–10.2) (Fig. 1A and B). The OS and PFS were well stratified by the RECIST (version 1.1): CR, not reached (95% CI, NA-NA) and not reached (95% CI, NA-NA); partial response, 31.1 months (95% CI, 23.3-NA) and 16.9 months (95% CI, 9.7–21.0); stable disease, 15.9 months (95% CI, 12.1–19.4) and 7.1 months (95% CI, 4.4–9.7); and progressive disease, 11.6 months (95% CI, 7.0-NA) and 2.2 months (95% CI, 1.6–2.5) (Fig. 1C and D). The OS and PFS were markedly longer in patients who achieved objective response than in those who achieved non-objective response: MST not reached [95% CI, 24.5-not applicable (NA)] compared with 15.0 months (95% CI, 11.9–18.9) (P<0.01); and median PFS of 20.5 months (95% CI, 14.1-NA) compared with 4.14 months (95% CI, 3.1–5.5; P<0.01) (Fig. 1E and F).
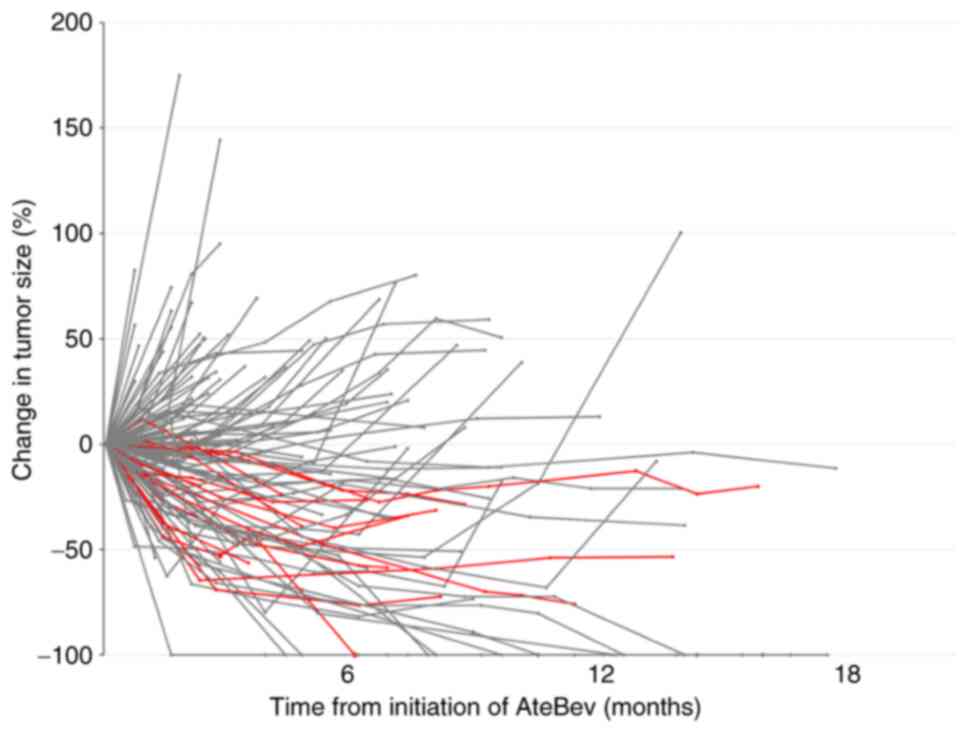
Transition in tumor size and serum AFP level
Spider plots of the changes in tumor size based on the RECIST (version 1.1) are presented in Fig. 2. Almost all patients with sequential local treatment reached a plateau at ~6 months. Moreover, the serum AFP level transition in patients with serum AFP levels of ≥10 ng/ml, which decreased at the first evaluation, is presented in Fig. 3. Of all patients, 55 were included in the analysis. Although the serum AFP ratio [AFP (X month)/AFP at baseline (before AteBev initiation)] decreased until 3 months after AteBev initiation, it increased at ~4 months (Fig. 3A). Furthermore, the serum AFP ratio [AFP (X month)/AFP (X-1 month)] was close to 1 at ~3 months, indicating a plateau in AFP decline, after which the serum AFP level continued to increase (Fig. 3B).
Impact of sequential local treatment on survival outcomes
Out of all the patients, 14 (11.4%) underwent sequential local treatment following AteBev treatment, and eight achieved CR using AteBev treatment alone and were followed up without systemic chemotherapy (‘drug-free’ cohort; Fig. S2). The median duration from the AteBev initiation to local treatment was 7.2 months (95% CI, 6.3–11.1). Sequential local treatment comprised nine patients who underwent particle radiotherapy (PRT), one patient who underwent surgery and four patients who underwent TACE. The survival outcomes were significantly improved in the ‘drug-free’ cohort compared to those not meeting the ‘drug-free’ criteria: MST not reached (95% CI, 25.4-NA) compared with 16.3 months (95% CI, 13.0–21.8; P<0.0001); and median PFS not reached (95% CI, 18.4-NA) compared with 5.0 months (95% CI, 3.7–7.1; P<0.0001) (Fig. 4A and B). In the drug-free cohort, five patients experienced recurrences during the follow-up term. Of the five patients, AteBev was re-administered to two patients, one underwent salvage surgery and two underwent TACE, resulting in a ‘drug-free’ status. The MST (95% CI, NA-NA) and median PFS (95% CI, 18.4-NA) of patients meeting the ‘clinical CR’ criteria were not reached, whereas the MST and median PFS of those who did not meet the ‘clinical CR’ criteria were 16.9 months (95% CI, 13.6–23.0) and 5.5 months (95% CI, 4.1–8.8), respectively (Fig. 4C and D). Moreover, the MST (95% CI, NA-NA) and median PFS (95% CI, NA-NA) of patients meeting the ‘drug-off’ criteria after sequential local treatment or AteBev treatment alone were not reached, whereas the MST and median PFS of those who did not meet the ‘drug-off’ criteria were 18.9 months (95% CI, 14.1–23.0) and 6.0 months (95% CI, 4.1–8.9), respectively (Fig. 4E and F). No patients meeting the ‘clinical CR’ and ‘drug-off’ criteria died during the follow-up term. A total of two patients meeting the ‘clinical CR’ criteria and no patients meeting the ‘drug-off’ criteria experienced recurrences during the follow-up term.
Factors related to OS after AteBev treatment
The associations between achieving a ‘drug-free’ status and clinical parameters are presented in Table II. Among these factors, a significantly higher number of patients with ‘drug-free’ status was observed for patients with greater liver function, as indicated by mALBI grade 1 + 2a, compared with those with grade 2b + 3 (P=0.022). In addition, patients who achieved ‘drug-free’ status tended to have ≤1 intrahepatic lesion (none or single; P=0.053), macrovascular invasion (P=0.053) and administration of AteBev as the first-line treatment (P=0.096); however no significant differences were demonstrated.
To further assess prognostic factors, univariate and multivariate Cox proportional analyses were performed to evaluate baseline clinical characteristics associated with prolonged OS (Table III). Among the pretreatment clinical factors, mALBI grade 1 + 2a was independently associated with an improved OS [hazard ratio (HR), 0.465; 95% CI, 0.263–0.823; P=0.009]. Other clinical variables, such as NLR (HR, 1.173; 95% CI, 0.655–2.102; P=0.591), macrovascular invasion (HR, 1.228; 95% CI, 0.538–2.804; P=0.625), BCLC classification (HR, 1.119; 95% CI, 0.574–2.182; P=0.741) and treatment line (HR, 0.660; 95% CI, 0.363–1.202; P=0.175) were not significantly associated with OS.
Discussion
In the present study, the MST and median PFS were slightly improved compared with those in the IMbrave150 trial and real-world data from Japan (17,18). Recently, Kudo et al (9) reported that achieving ‘clinical CR’ after AteBev treatment followed by curative conversion had a marked survival benefit; however, it is difficult to implement the proposed ‘clinical CR’ and ‘drug-off’ criteria in all cases in clinical practice due to the necessity for long-term normalization of three tumor markers and performing CE ultrasonography. Moreover, the decision to continue or discontinue systemic chemotherapy is often a comprehensive issue. In the present study, patients with no apparent residual lesions after sequential local treatment were followed up without any systemic chemotherapy (‘drug-free’ cohort) despite not meeting the proposed criteria. The OS and PFS of the ‘drug-free’ cohort were comparable with those who achieved ‘clinical CR’ or ‘drug-off’ criteria. The prognosis of patients who meet the ‘clinical CR’ or ‘drug-off’ criteria was favorable; thus, continuing the systemic chemotherapy until meeting these criteria may be considered the optimal treatment option. In addition, according to the report by Aoki et al (19), cautions should be exercised when evaluating complete tumor control solely by modified RECIST-based CR and normalization of serum AFP levels (≥6 weeks) after curative conversion following AteBev treatment. However, the results of the present study, with equally favorable outcomes of the ‘drug-free’ cohort, may be an alternative clinical indicator, especially for those with difficulty continuing systemic chemotherapy. Further advantages of the strategy in the present study include the prevention of liver function exacerbation, maintenance of patients’ quality-of-life, reduction of adverse events and preservation of late-line treatment options. Although further studies are necessary, multidisciplinary strategies aimed at achieving a drug-free status may be a valid option for AteBev treatment.
Concerning the timing of sequential local treatment, serum AFP levels reached a plateau at ~3 months and increased thereafter. Tumor shrinkage by radiological findings reached a plateau at ~6 months, necessitating consideration for the introduction of sequential local treatment 3–6 months after AteBev initiation. The present study also demonstrated that cases of sustained tumor shrinkage beyond 6 months are quite rare. The discrepancy of the transition between serum AFP levels and radiological findings suggested that serum AFP levels may be a more sensitive indicator of early tumor biological activity than radiological findings, and several studies have reported that early AFP decrease predicted improved outcomes in patients with HCC treated with AteBev (20,21). It is important to follow up with careful monitoring of the upward trend in serum AFP levels and shift to sequential local treatment within 6 months of AteBev initiation. Drug sequence changes after AteBev treatment should be considered in the absence of indications for sequential local treatment. These results have important implications regarding the timing of bevacizumab withdrawal when considering sequential hepatectomies. According to a recent questionnaire survey in Japan regarding the duration of treatment with AteBev prior to conversion surgery, the most frequent answers were 4 months (40%) and 6 months (27%) (22). However, there is currently a lack of evidence-based studies regarding the optimal timing for local treatment introduction after AteBev treatment. To the best of our knowledge, the present study is the first to assess the timing of the introduction of local treatment based on changes in AFP levels and radiological findings after AteBev treatment.
The concept of conversion after systemic chemotherapy is a current topic. Several studies have reported a conversion rate of 5.3–19% after AteBev treatment (7,23,24), which is similar to the introduction rate of sequential local treatment in the present study (11.4%). Although conversion therapy generally refers to local treatment after technically or oncologically converting unresectable tumors into resectable HCC (25), the definition of ‘unresectable’ tumors varies among physicians and institutions. The concept of conversion has not yet been unified. However, there are a growing number of reports regarding combination therapy with systemic chemotherapy followed by sequential local treatment (7–9), which contributes to prolonged OS and PFS in patients with advanced HCC. In addition, the introduction of sequential local treatment after AteBev treatment is key to prolonged prognosis (26).
According to previous reports regarding conversion therapy, the predictive factors for transition to conversion therapy were NLR of <3, mALBI grade 1 + 2a and BCLC stage A/B (7,8,27). In the present study, mALBI grade 1 + 2a was associated with both a ‘drug-free’ status and improved OS. Therefore, maintaining normal liver function through multidisciplinary treatment is important. By contrast, macrovascular invasion, categorized as BCLC stage C (6), was also associated with ‘drug-free’ status in the present study. One reason for this is that AteBev may be effective in treating oncologically aggressive tumors (28). Our previous research reported the efficacy of PRT as a curative local treatment for locally advanced HCC (29–31), and it also served an important role in the ‘drug-free’ cohort. Unlike BCLC stage B HCC, there have been very few reports on conversion therapy for BCLC stage C (32). By using several local treatment modalities, including PRT, favorable local control and prolonged survival outcomes may be achieved, even for HCC with macrovascular invasion.
The treatment modalities for sequential local treatment include surgery, RFA and TACE (9). However, there are currently no well-established guidelines regarding the optimal choice of sequential local treatments following systemic chemotherapy. Surgery is generally accepted as the most common curative treatment modality; however, it is controversial whether TACE and radiotherapy are curative local therapies after systemic chemotherapy owing to their lower efficacy (1,33). Nevertheless, previous studies have reported the improved efficacy of local control using TACE after systemic chemotherapy (34–36). As the indications for surgery are often restricted due to liver functional, technical and oncological issues (37), TACE is expected to serve an important role in achieving a ‘drug-free’ status for patients who are unsuitable for surgery. PRT is also a promising and less toxic modality for the local control of HCC due to its high dose concentration at the Bragg peak (38–40) and is recognized as a curative local treatment (41,42). In the present study, the treatment impacts were not directly compared among the local treatment modalities due to the low introduction rate of sequential local treatment. Further analyses with larger sample sizes and long-term observations are required to determine the precise choice of local treatments following systemic chemotherapy.
The present study had several limitations. First, it was performed retrospectively at only two institutions. Although, the number of patients was sufficient for demonstrating the prolonged survival outcomes of the ‘drug-free’ cohort, the number of patients who received local treatment following AteBev was too small and direct comparisons between treatment modalities within the cohort were difficult. Second, the decision for sequential local treatment depended on the physician, and fixed criteria were not defined. Indeed, it is difficult to establish standardized criteria for shifting to local treatment due to liver function, as well as oncological and technical factors. The same applies to the decision to withdraw AteBev treatment when CR is achieved after AteBev alone. Third, AFP-negative (serum AFP levels of <10 ng/ml) patients were not eligible for consideration of the optimal timing of sequential local treatment based on the transition of serum AFP levels. Meticulous observation using radiological imaging is required for AFP-negative patients.
In conclusion, patients with advanced HCC who achieved sequential local treatment or CR after AteBev treatment had improved survival outcomes compared with those who did not achieve treatment. When considering sequential local treatment, the optimal timing is 3–6 months after AteBev initiation. Follow-up with a ‘drug-free’ status after local treatment or achievement of CR may be an option. Moreover, PRT may be a preferred alternative to local treatment after AteBev treatment. However, further evidence on the continuation of systemic chemotherapy after conversion therapy is required.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be requested from the corresponding author.
Authors' contributions
NI, SK, and TF conceptualized the study design. NI, SK, YY, YF and JI collected raw data from electronic medical records. NI wrote the manuscript and performed statistical analyses. SK, YY, YF, JI, MK, HG, KF, TU, HY, HT, YK, and TF interpreted the data, critically revised the manuscript and provided valuable feedback. YK and TF supervised the study. NI and SK confirm the authenticity of all the raw data. All authors read and approved the final manuscript.
Ethics approval and consent to participate
All procedures in the present study were performed following the ethical standards of Kobe University Hospital, Kobe Minimally invasive Cancer Center and the National Research Committee, in accordance with the Declaration of Helsinki. The present study was approved by the ethics committees of Kobe University Hospital and the Kobe Minimally invasive Cancer Center (approval no. B240024). An opt-out method for participants was employed in accordance with institutional guidelines. Information about the study was publicly disclosed on the institutional websites of Kobe University Hospital and Kobe Minimally invasive Cancer Center, allowing patients the opportunity to decline participation.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Glossary
Abbreviations
Abbreviations:
|
HCC |
hepatocellular carcinoma |
|
AteBev |
atezolizumab plus bevacizumab |
|
BCLC |
Barcelona Clinic Liver Cancer |
|
OS |
overall survival |
|
PFS |
progression-free survival |
|
CR |
complete response |
|
TACE |
transarterial chemoembolization |
|
RFA |
radiofrequency ablation |
|
AFP |
α-fetoprotein |
|
NLR |
neutrophil-to-lymphocyte ratio |
|
mALBI |
modified albumin-bilirubin |
|
ECOG |
Eastern Cooperative Oncology Group |
|
CE-CT |
contrast-enhanced computed tomography |
|
EOB-MRI |
gadolinium-ethoxybenzyl-diethylenetriamine pentaacetic acid-enhanced magnetic resonance imaging |
|
RECIST |
Response Evaluation Criteria in Solid Tumors |
|
CI |
confidence interval |
|
ORR |
objective response rate |
|
DCR |
disease control rate |
|
MST |
median survival time |
|
NA |
not applicable |
|
PRT |
particle radiotherapy |
References
|
Llovet JM, Kelley RK, Villanueva A, Singal AG, Pikarsky E, Roayaie S, Lencioni R, Koike K, Zucman-Rossi J and Finn RS: Hepatocellular carcinoma. Nat Rev Dis Primers. 7:62021. View Article : Google Scholar : PubMed/NCBI | |
|
Rumgay H, Arnold M, Ferlay J, Lesi O, Cabasag CJ, Vignat J, Laversanne M, McGlynn KA and Soerjomataram I: Global burden of primary liver cancer in 2020 and predictions to 2040. J Hepatol. 77:1598–1606. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Rimassa L, Finn RS and Sangro B: Combination immunotherapy for hepatocellular carcinoma. J Hepatol. 79:506–515. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Kudo M: Treatment decision-making in unresectable hepatocellular carcinoma: Importance of understanding the different response patterns between IO plus Anti-VEGF and IO plus IO Regimens. Liver Cancer. 14:119–126. 2025.PubMed/NCBI | |
|
Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, Kudo M, Breder V, Merle P, Kaseb AO, et al: Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 382:1894–1905. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Reig M, Forner A, Rimola J, Ferrer-Fàbrega J, Burrel M, Garcia-Criado Á, Kelley RK, Galle PR, Mazzaferro V, Salem R, et al: BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J Hepatol. 76:681–693. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Tomonari T, Tani J, Sato Y, Tanaka H, Tanaka T, Taniguchi T, Kawano Y, Morishita A, Okamoto K, Sogabe M, et al: Clinical features and outcomes of conversion therapy in patients with unresectable hepatocellular carcinoma. Cancers (Basel). 15:52212023. View Article : Google Scholar : PubMed/NCBI | |
|
Kikuchi T, Takeuchi Y, Nouso K, Kariyama K, Kuwaki K, Toshimori J, Iwado S, Moriya A, Hagihara H, Takabatake H, et al: Predictive factors for transition to conversion therapy in hepatocellular carcinoma using atezolizumab plus bevacizumab. Liver Int. 44:1456–1463. 2024. View Article : Google Scholar : PubMed/NCBI | |
|
Kudo M, Aoki T, Ueshima K, Tsuchiya K, Morita M, Chishina H, Takita M, Hagiwara S, Minami Y, Ida H, et al: Achievement of complete response and drug-free status by atezolizumab plus bevacizumab combined with or without curative conversion in patients with transarterial chemoembolization-unsuitable, intermediate-stage hepatocellular carcinoma: A multicenter proof-of-concept study. Liver Cancer. 12:321–338. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Lencioni R and Llovet JM: Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 30:52–60. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Ochi H, Kurosaki M, Joko K, Mashiba T, Tamaki N, Tsuchiya K, Marusawa H, Tada T, Nakamura S, Narita R, et al: Usefulness of neutrophil-to-lymphocyte ratio in predicting progression and survival outcomes after atezolizumab-bevacizumab treatment for hepatocellular carcinoma. Hepatol Res. 53:61–71. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Cholongitas E, Papatheodoridis GV, Vangeli M, Terreni N, Patch D and Burroughs AK: Systematic review: The model for end-stage liver disease-should it replace Child-Pugh's classification for assessing prognosis in cirrhosis? Aliment Pharmacol Ther. 22:1079–1089. 2005. View Article : Google Scholar : PubMed/NCBI | |
|
Hiraoka A, Michitaka K, Kumada T, Izumi N, Kadoya M, Kokudo N, Kubo S, Matsuyama Y, Nakashima O, Sakamoto M, et al: Validation and potential of albumin-bilirubin grade and prognostication in a nationwide survey of 46,681 hepatocellular carcinoma patients in Japan: The need for a more detailed evaluation of hepatic function. Liver Cancer. 6:325–336. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET and Carbone PP: Toxicity and response criteria of the Eastern cooperative oncology group. Am J Clin Oncol. 5:649–655. 1982. View Article : Google Scholar : PubMed/NCBI | |
|
Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, Roberts LR and Heimbach JK: Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American association for the study of liver diseases. Hepatology. 68:723–750. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, et al: New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247. 2009. View Article : Google Scholar : PubMed/NCBI | |
|
Cheng AL, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, Lim HY, Kudo M, Breder V, Merle P, et al: Updated efficacy and safety data from IMbrave150: Atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J Hepatol. 76:862–873. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Nakagawa M, Inoue M, Ogasawara S, Maruta S, Okubo T, Itokawa N, Iino Y, Obu M, Haga Y, Seki A, et al: Clinical effects and emerging issues of atezolizumab plus bevacizumab in patients with advanced hepatocellular carcinoma from Japanese real-world practice. Cancer. 129:590–599. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Aoki T, Kudo M, Nishida N, Ueshima K, Tsuchiya K, Tada T, Morita M, Chishina H, Takita M, Hagiwara S, et al: Proposal of discontinuation criteria of atezolizumab plus bevacizumab after curative conversion therapy for unresectable early-to-intermediate-stage hepatocellular carcinoma: A multicenter proof-of-concept study. J Gastroenterol. 60:738–753. 2025. View Article : Google Scholar : PubMed/NCBI | |
|
Campani C, Bamba-Funck J, Campion B, Sidali S, Blaise L, Ganne-Carrié N, Demory A, Sutter O, Larrey E, Evain M, et al: Baseline ALBI score and early variation of serum AFP predicts outcomes in patients with HCC treated by atezolizumab-bevacizumab. Liver Int. 43:708–717. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Kuzuya T, Kawabe N, Muto H, Tachi Y, Ukai T, Wada Y, Komura G, Nakano T, Tanaka H, Nakaoka K, et al: Characteristics and prognosis of patients with advanced hepatocellular carcinoma treated with atezolizumab/bevacizumab combination therapy who achieved complete response. Curr Oncol. 31:6218–6231. 2024. View Article : Google Scholar : PubMed/NCBI | |
|
Shindoh J, Matsumura M, Okubo S, Okada T, Hashimoto M, Nakamura M and Ohtsuka M: A questionnaire survey to explore the current treatment policies adopted for patients with advanced hepatocellular carcinoma at board-certified HPB training institutions in Japan: A JSHBPS project study 2023, Part 1. J Hepatobiliary Pancreat Sci. 32:360–373. 2025. View Article : Google Scholar : PubMed/NCBI | |
|
Niizeki T, Tokunaga T, Takami Y, Wada Y, Harada M, Shibata M, Nakao K, Sasaki R, Hirai F, Shakado S, et al: Comparison of efficacy and safety of atezolizumab plus bevacizumab and lenvatinib as first-line therapy for unresectable hepatocellular carcinoma: A propensity score matching analysis. Target Oncol. 17:643–653. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Kudo M: A novel treatment strategy for patients with intermediate-stage HCC who are not suitable for TACE: Upfront systemic therapy followed by curative conversion. Liver Cancer. 10:539–544. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Wang MD, Xu XJ, Wang KC, Diao YK, Xu JH, Gu LH, Yao LQ, Li C, Lv GY and Yang T: Conversion therapy for advanced hepatocellular carcinoma in the era of precision medicine: Current status, challenges and opportunities. Cancer Sci. 115:2159–2169. 2024. View Article : Google Scholar : PubMed/NCBI | |
|
Kudo M: Sequential therapy for hepatocellular carcinoma after failure of atezolizumab plus bevacizumab combination therapy. Liver Cancer. 10:85–93. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Shimose S, Iwamoto H, Shirono T, Tanaka M, Niizeki T, Kajiwara M, Itano S, Yano Y, Matsugaki S, Moriyama E, et al: The impact of curative conversion therapy aimed at a cancer-free state in patients with hepatocellular carcinoma treated with atezolizumab plus bevacizumab. Cancer Med. 12:12325–12335. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Ishihara N, Komatsu S, Sofue K, Ueshima E, Yano Y, Fujishima Y, Ishida J, Kido M, Gon H, Fukushima K, et al: Association between tumor morphology and efficacy of atezolizumab plus bevacizumab for advanced hepatocellular carcinoma. Hepatol Res. 54:773–780. 2024. View Article : Google Scholar : PubMed/NCBI | |
|
Komatsu S, Fukumoto T, Demizu Y, Miyawaki D, Terashima K, Niwa Y, Mima M, Fujii O, Sasaki R, Yamada I, et al: The effectiveness of particle radiotherapy for hepatocellular carcinoma associated with inferior vena cava tumor thrombus. J Gastroenterol. 46:913–920. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
Komatsu S, Fukumoto T, Demizu Y, Miyawaki D, Terashima K, Sasaki R, Hori Y, Hishikawa Y, Ku Y and Murakami M: Clinical results and risk factors of proton and carbon ion therapy for hepatocellular carcinoma. Cancer. 117:4890–4904. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
Komatsu S, Terashima K, Ishihara N, Matsuo Y, Kido M, Yanagimoto H, Toyama H, Tokumaru S, Okimoto T and Fukumoto T: Novel concept of ‘sequential particle radiotherapy’ with atezolizumab plus bevacizumab for hepatocellular carcinoma with portal vein tumor thrombus. Surg Today. 54:972–976. 2024. View Article : Google Scholar : PubMed/NCBI | |
|
Takakusagi S, Tanaka H, Naganuma A, Kakizaki S, Shibuya K, Ohno T, Takagi H and Uraoka T: Two cases of hepatocellular carcinoma successfully treated by carbon ion radiotherapy after atezolizumab plus bevacizumab treatment. Clin J Gastroenterol. 16:407–415. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
European Association for the Study of the Liver, . EASL clinical practice guidelines: Management of hepatocellular carcinoma. J Hepatol. 69:182–236. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Wang K, Zhu H, Yu H, Cheng Y, Xiang Y, Cheng Z, Li Y, Li T, Wang D, Zhu Z and Cheng S: Early Experience of TACE combined with atezolizumab plus bevacizumab for patients with intermediate-stage hepatocellular carcinoma beyond up-to-seven criteria: A multicenter, single-arm study. J Oncol. 2023:63530472023. View Article : Google Scholar : PubMed/NCBI | |
|
Zhao C, Xiang Z, Li M, Wang H, Liu H, Yan H and Huang M: Transarterial chemoembolization combined with atezolizumab plus bevacizumab or lenvatinib for unresectable hepatocellular carcinoma: A propensity score matched study. J Hepatocell Carcinoma. 10:1195–1206. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Kudo M, Ueshima K, Saeki I, Ishikawa T, Inaba Y, Morimoto N, Aikata H, Tanabe N, Wada Y, Kondo Y, et al: A phase 2, prospective, multicenter, single-arm trial of transarterial chemoembolization therapy in combination strategy with lenvatinib in patients with unresectable intermediate-stage hepatocellular carcinoma: TACTICS-L trial. Liver Cancer. 13:99–112. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Allaire M, Goumard C, Lim C, Le Cleach A, Wagner M and Scatton O: New frontiers in liver resection for hepatocellular carcinoma. JHEP Rep. 2:1001342020. View Article : Google Scholar : PubMed/NCBI | |
|
Schulz-Ertner D and Tsujii H: Particle radiation therapy using proton and heavier ion beams. J Clin Oncol. 25:953–964. 2007. View Article : Google Scholar : PubMed/NCBI | |
|
Katsuya G, Kato H, Yasuda S, Tsuji H, Yamada S, Haruyama Y, Kobashi G, Ebner DK, Okada NN, Makishima H, et al: Progressive hypofractionated carbon-ion radiotherapy for hepatocellular carcinoma: Combined analyses of 2 prospective trials. Cancer. 123:3955–3965. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Kim TH, Koh YH, Kim BH, Kim MJ, Lee JH, Park B and Park JW: Proton beam radiotherapy vs. radiofrequency ablation for recurrent hepatocellular carcinoma: A randomized phase III trial. J Hepatol. 74:603–612. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Yoo GS, Yu JI and Park HC: Proton therapy for hepatocellular carcinoma: Current knowledges and future perspectives. World J Gastroenterol. 24:3090–3100. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Lee SU and Kim TH: Current evidence and the potential role of proton beam therapy for hepatocellular carcinoma. Clin Mol Hepatol. 29:958–968. 2023. View Article : Google Scholar : PubMed/NCBI |