Oral microbiota: Roles and treatment in radiation injury (Review)
- Authors:
- Published online on: August 8, 2025 https://doi.org/10.3892/ol.2025.15218
- Article Number: 472
-
Copyright: © Song et al. This is an open access article distributed under the terms of Creative Commons Attribution License.
Abstract
Introduction
One of the primary therapies for malignant tumors is radiotherapy, the effectiveness of which is closely correlated with the radiation dose. Following radiotherapy for tumors, patients may experience various acute and chronic radiation injuries (RIs) in different sites of the body; a prime example is radiation-induced lung injury (RILI), which encompasses any lung toxicity induced by radiation therapy and manifests acutely as radiation pneumonitis and chronically as radiation pulmonary fibrosis. RI is the main factor that restricts the effectiveness of radiotherapy, as well as affecting the prognosis and quality of life of patients, as its severity increases rapidly with higher radiation doses (1). Nevertheless, currently, our understanding of the mechanisms underlying different RI types remains limited, and there is a lack of adequate clinical approaches for prevention/treatment. Therefore, there is a pressing need for novel and efficient solutions to prevent and treat RI.
Given the ongoing advancements in 16S ribosomal RNA sequencing and quantitative macro-genome detection technology, combined with decreasing costs, the investigation of the human microbiota as the ‘second genome’ has attracted widespread attention from medical researchers both domestically and internationally (2). Comprehensive analysis of the microbiota genome structure in various regions of the human body, such as the intestinal tract, oral cavity, respiratory tract, vagina and skin, has deepened our understanding of the diversity and functional abundance of microbiota that play a vital role in maintaining fundamental physiological functions and contribute to the development of associated diseases (3). The oral microbiota and its dysregulated metabolites have been shown to contribute significantly to the onset, progression, metastasis, diagnosis and prognosis of malignant tumors (4–8). It is worth noting that the oral microbiota is strongly linked to several categories of RIs. Therefore, the oral microbiota has demonstrated significant promise in the prevention and treatment of RI, prompting extensive exploration by researchers worldwide.
Within this review, the processes by which oral microbiota participates in 10 distinct categories of RI are delineated. Additionally, the impact of the oral microbiota on RI located at various sites is discussed. The aim of the present review is to present the most up-to-date medical evidence, paving an efficient path toward the prevention and treatment of RI.
Overview of oral microbiota and RI
The oral microbiota are a diverse group of microorganisms that include viruses, protozoa, fungi, archaea and bacteria. According to the Human Oral Microbiome Database (www.homd.org), the oral cavity harbors 774 microbial species. These species, categorized at the phylum level, primarily consist of Actinobacteria, Bacteroidetes, Firmicutes, Ascomycota, Spirochaetes and Saccharomycetes. These species play a crucial role in defending against external pathogens and preserving oral health. Nevertheless, they can also induce oral disorders, such as dental caries, periodontal disease and oral malignancies (9–11). The most notable pathogens among these are Porphyromonas gingivalis (P. gingivalis) and Fusobacterium nucleatum (F. nucleatum), both gram-negative anaerobic bacteria (8). In addition, there is growing evidence that oral microbiota can affect distal organs through a variety of mechanisms. Specifically, oral microbiota can cause disease through direct translocation to distal organs, such as translocation to the lungs leading to aspiration pneumonia (12), and translocation to the gastrointestinal tract leading to gastrointestinal diseases and malignant tumors (13).
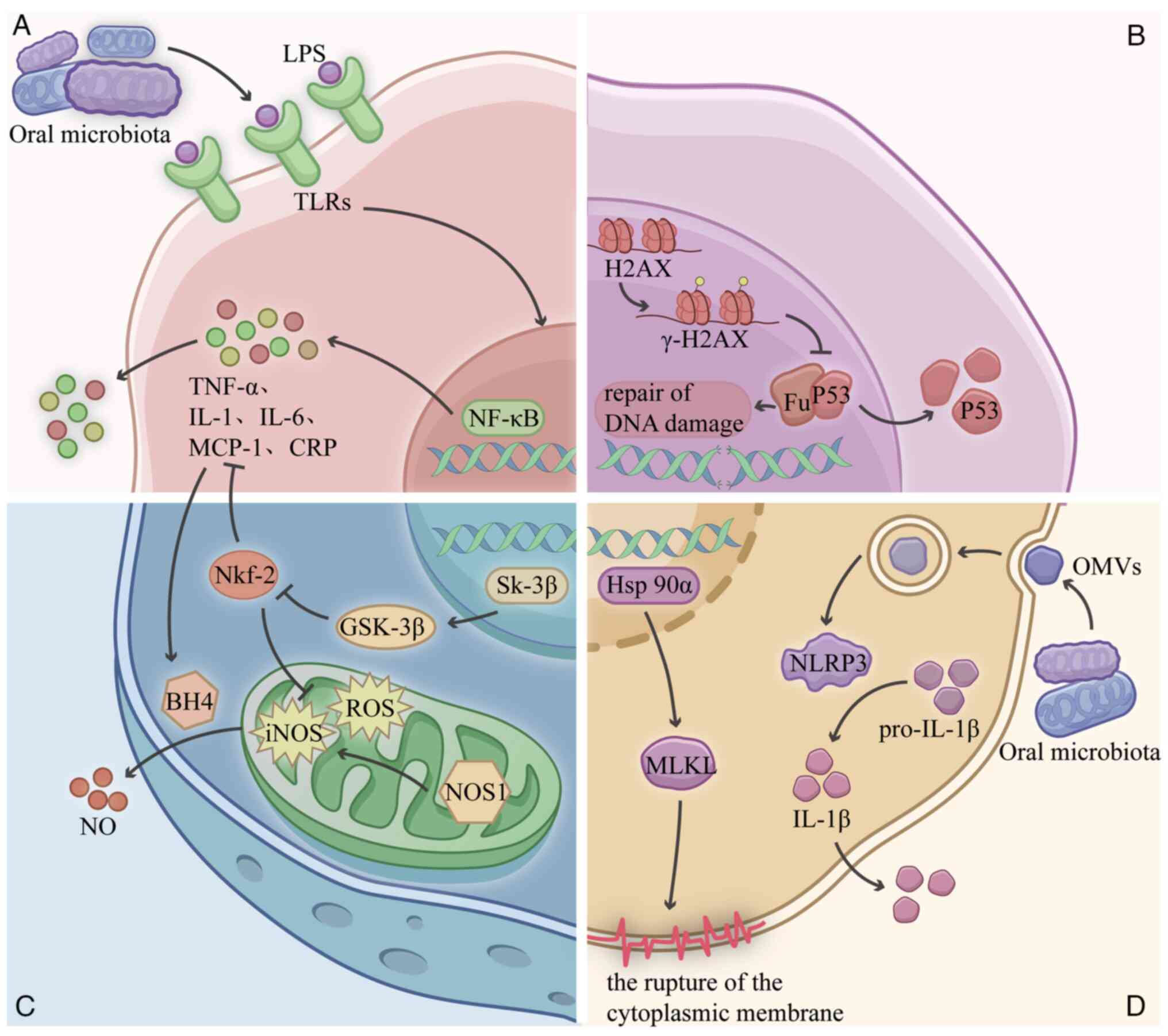
Another mechanism acts indirectly through the body's immune system and inflammatory responses leading to distal diseases, such as exacerbating atherosclerosis by inducing the apoptosis of smooth muscle cells and inhibiting the apoptosis of macrophages (14), and causing inflammation in the nerves resulting in Alzheimer's disease (15). Therefore, during radiation therapy, oral microbiota and their metabolites can reach various organs in the body through multiple pathways (Fig. 1) and participate in the development of RI through multiple mechanisms (Fig. 2) (16–21).
Radiotherapy, a cornerstone of oncological treatment, utilizes precisely targeted high-energy radiation to eradicate malignant tumor cells. This therapeutic intervention inevitably inflicts collateral damage on healthy tissues through three multifactorial mechanisms: Direct cellular injury, the radiation-associated bystander effect (22), and inflammatory cascades induced by activation of both innate and adaptive immune systems (1). These pathological processes collectively lead to RI (Fig. 1). These injuries can manifest as both acute and chronic adverse reactions. Acute adverse effects are prevalent, with the majority of patients experiencing mild to moderate fatigue, skin and mucous membrane damage in the irradiated area, fever, nausea and vomiting, and reduced peripheral blood counts following radiotherapy. These harmful processes substantially compromise long-term survival outcomes and quality of life, thereby acting as a dose-limiting factor that forces clinicians to balance tumor control against toxicity risks and restricts feasible radiation dose escalation, even when higher doses might theoretically improve survival (23).
Investigation of the association between oral microbiota and different categories of RI
Radiation-induced brain injury (RIB)
RIB is a significant adverse reaction following radiotherapy for brain or head and neck malignant tumors. RIB includes damage to the hippocampus and white matter, vascular abnormalities, demyelination, localized neurological deficits and elevated intracranial pressure (24). RIB is primarily characterized by cognitive impairment and neurological inflammation (25). According to statistics, 50 to 90% of brain tumor patients with survival periods >6 months develop RIB (26). Previous research demonstrated that the connections between the oral microbiota and the central nervous system, known as the oral-microbiota-brain axis (27), are linked to cognitive function and neuroinflammation, through which the oral microbiota and their metabolites interact with the immune system. For example, bacteria can secrete immunostimulants such as lipopolysaccharide (LPS) and peptidoglycan into the circulation, enabling them to enter the brain. LPS from Gram-negative bacteria in the oral cavity induces the expression of neuronal inflammatory proteins in SH-SY5Y and HMC3 cells (28). Oral microbiota components or some of their metabolites can even cross the blood-brain barrier directly, altering the inflammatory state of the central nervous system and leading to cognitive dysfunction through a variety of mechanisms (29). For example, Bulgart et al (30) demonstrated that P. gingivalis metabolite gingipain protease disrupts the processing of the transmembrane protein amyloid precursor protein, thereby affecting synaptic stability and neurite growth; and a more recent study showed that P. gingivalis can cause cognitive dysfunction by activating the p38 mitogen-activated protein kinase (MAPK) signaling pathway (31). Prior studies have established that excessive activation of microglia in the central nervous system is crucial for the development and advancement of radiation-induced brain damage (32,33), whereas oral microbiota can affect brain function via intracranial microglia. The oral microbiota can affect brain function via microglial activation. Chuang et al (34) revealed that outer membrane vesicles derived from P. gingivalis cause neurotoxicity and microglial activation.
Radiation-induced oral injury
Ultrafast division of oral mucosal cells makes them susceptible to radiation. Radiation-induced oral mucositis (RIOM) is the predominant adverse effect on the oral mucosa following head and neck radiotherapy. RIOM is characterized by congestion, edema, ulceration and discomfort in the oral mucosa. Statistics indicate that nearly all patients undergoing head and neck radiation experience RIOM, with over half of them developing severe oral mucositis (35,36).
The oral microbiota undergo changes during radiation therapy; for example, a clinical study by Nodit et al (37) systematically explored the dynamics of the oral microbiome during cancer RIOM. At radiation therapy completion, significant microbial shifts were observed, including increased abundance of Fusobacterium and Streptococcus, alongside decreased levels of Rothia and Lautropia. Numerous studies have shown that alterations in the oral microbiota after radiotherapy have the potential to be valuable in predicting the incidence and evaluating the severity of mucositis. Hou et al (38) investigated the characteristics of changes in the oropharyngeal mucosal microbiota of patients with nasopharyngeal carcinoma during radiotherapy, and no significant change was found in the bacterial α-diversity of the mucosal microbiota, whereas the abundance of Prevotella, Clostridium, Mycobacterium, Porphyromonas and Aeromonas showed significant dynamic changes in abundance, with their peaks coinciding with episodes of severe mucositis (38). By contrast, Zhu et al (39) found that patients with severe mucositis had significant microbial α-diversity and higher abundance of Actinobacteria, while Vesty et al (40) demonstrated that the presence of Gram-negative bacilli, Clostridium innocuum and Porphyromonas, correlated with the severity of RIOM. In addition, a retrospective multicenter study of 326 patients by Nishii et al (41) revealed that higher levels of RIOM were significantly associated with increased incidence of oral candidiasis.
These clinical studies suggest that dysregulation of oral microbial ecology may exacerbate RIOM severity. There is also growing evidence that changes in the oral microbiota also influence the development and prognosis of mucositis through a range of mechanisms. Groeger and Meyle (42) found that P. gingivalis could mediate enhanced IL-33 secretion by epithelial cells, which in turn augmented the Th2 cytokine-mediated inflammatory response. Hirahara et al (43) demonstrated that the oral microbiota can cause excessive persistence of inflammation by inducing aberrant polarization of M1/M2 macrophages. Suárez et al (44) similarly found that Toll-like receptors (TLRs) initiate a pro-inflammatory response and upregulate antigen presentation by immune cells bound to LPS, exacerbating the oral inflammatory response. Experiments showed that the oral microbiota interacts with TLRs to regulate the immune system. Specifically, LPS from Gram-negative microbiota activate TLRs, subsequently activating the NF-κB pathway, driving the release of TNF-α and IL-6, and triggering an inflammatory cascade (17). Therefore, modulation of the oral microbiota is a potential target for combating (45). Researchers have identified several protective agents within the microbiome against RIOM: Zhao et al (46) developed fullerenol, an antioxidant that inhibits apoptosis, maintains microbial homeostasis and protects against RIOM by scavenging radiation-induced excess reactive oxygen species and upregulating intrinsic enzyme-activated antioxidants. Topical Omega-3 nanoemulgel demonstrated a beneficial effect in preventing RIOM, mediated by its ability to regulate oral microbial dysbiosis; specifically, Morsy et al (47) found it significantly reduced the Firmicutes/Bacteroidetes ratio in patients with head and neck cancer undergoing radiotherapy.
Radiation-induced esophageal injury
As a common inflammatory reaction to thoracic radiotherapy, radiation-induced esophagitis (RE) is most often observed following radiotherapy for esophageal cancer, lung cancer, breast cancer, mediastinal malignancy, lymphoma and other thoracic cancers (48). Following deglutition, the oral microbiota translocate to the esophagus via the saliva, forming an esophageal microbiota that is compositionally analogous to its oral counterpart (49). Thus, in addition to acting indirectly through the immune system and inflammatory response, oral microbiota may be directly involved in the progression of radiation esophagitis by migrating into the esophagus. Research has demonstrated that Gram-negative microbiota exhibit predominance in the esophageal microbiota of individuals with esophagitis (50), mirroring the alterations in the oral microbiota observed in people with oral mucositis. Furthermore, it has been established that LPS present in Gram-negative microbiota stimulate TLRs to exacerbate inflammatory reactions. Notably, LPS induces nitric oxide synthase (NOS)1 upregulation and increases the production of inducible NOS, resulting in the relaxation of the lower esophageal sphincter (51). In addition, LPS impairs the rate of stomach emptying via COX-2 stimulation, consequently increasing gastric pressure (50) and thereby elevating esophagitis risk. Although no specific research has revealed a direct link between the oral microbiota and the onset of radiation esophagitis, the aforementioned evidence suggests a possible association between the two.
RILI
RILI is a severe complication from radiation to the lung tissue, characterized by early radiation pneumonitis (RP) and late radiation pulmonary fibrosis, reported in 25% of treated cases (52). Clinically, RILI not only impacts the health and treatment outcomes of cancer patients, but may lead to life-threatening complications in severe cases. The oral cavity and the lungs are anatomically contiguous, allowing microbial translocation via salivary aspiration and subsequent inhalation into the lungs. Furthermore, the organization of the microbiota in both the oral cavity and the lungs is similar (53). Multiple studies have demonstrated that the oral cavity and the lungs exert mutual influence (54,55). Thus, researchers proposed the oral-lung axis (56), with the oral microbiota significantly contributing to the pathogenesis of pulmonary inflammatory conditions.
The animal model described in the study by Benedyk et al (16) showed a significant elevation in the peripheral blood concentrations of TNF-α, IL-6, monocyte chemotactic protein-1 and C-reactive protein following intratracheal administration of P. gingivalis. These inflammatory mediators promote airway inflammation by mediating the recruitment and activation of key immune cells, including neutrophils and monocytes, within the respiratory tract (16). Other researchers found that P. gingivalis and F. nucleatum, whose pathogenic mechanism is similar to that of P. gingivalis, can induce the expression of IL-6 and IL-8 in the bronchial epithelial cells, triggering inflammatory cascades, with IL-8 stimulating the production of the extracellular matrix by lung fibroblasts, a critical mechanism in fibrogenesis (57,58). Mechanistically, Feng et al (20) proved that P. gingivalis stimulates mixed lineage kinase domain-like pseudokinase-mediated necroptosis by increasing the expression of Hsp90α, resulting in plasma membrane disruption. This directly triggers and indirectly spreads inflammatory responses. Concurrently, F. nucleatum can activate MAPKs and NF-κB, which in turn stimulate matrix metalloproteinases that play a role in airway reconstruction (59). Beyond direct cellular effects, oral pathogens can synergistically interact with pulmonary microbiota to enhance pulmonary inflammatory responses. P. gingivalis or its virulence factors can potentiate the pathogenicity with Streptococcus pneumoniae and Pseudomonas aeruginosa to intensify the inflammatory disease response (57,60). Clinical corroboration comes from a clinical study by O'Dwyer et al (61), which found that oral microbial diversity predicted disease severity and death in patients with pulmonary fibrosis. Despite these compelling associations linking oral microbiota with pulmonary inflammation and pulmonary fibrosis, their involvement in RILI remains unclear.
Radiation-induced gastrointestinal injury (RIGI)
Primary malignant tumors in the abdomen and pelvis mostly cause radiation-induced gastrointestinal damage. This damage includes radiation-induced enteritis (REn), gastric injury, duodenitis and proctitis. Among these complications, REn is the most prevalent and can be life-threatening for patients in severe cases (23,62). The mouth is the beginning of the digestive tract, and although these two loci are physiologically distinct, they are directly connected and can interact in a number of ways. Therefore, the microbiota in the mouth and intestines can mutually interact to establish an oral-intestinal axis (63,64). Yamazaki (65) established that the oral microbiota induced intestinal dysbiosis, including various inflammatory disorders, by inducing dysbiosis of the intestinal microbiota. Emerging evidence further implicates the oral microbiota in RIGI progression. For example, oral microbiota can lead to reduced expression of tight junction protein genes in intestinal tissues and disrupt intestinal barrier function (65). Atarashi et al (66) found that Klebsiella spp. isolated from the oral cavity could colonize the mouse intestine and trigger Th1-mediated inflammatory responses. Dong et al (67) found that oral microbial transplantation (OMT) impaired the therapeutic efficacy of radiotherapy in colorectal malignant tumors. Furthermore, notably, it was found experimentally that colorectal cancer mice treated with radiation therapy in combination with metronidazole had reduced overall tumor load and REn (49). This suggests the potential of metronidazole in counteracting oral microbiota-mediated radiotherapy complications.
Radiation-induced heart injury
Due to the anatomical proximity of the heart, radiotherapy to the chest can result in radiation-induced heart disease (RIHD). This may include damage to coronary arteries or valves, obstruction of the conduction system, pericardial injury, myocardial injury, myocardial fibrosis and other related conditions (68,69). RIHD represents the primary cause of non-cancer-related mortality in thoracic radiotherapy recipients (70). Emerging evidence implicates periodontal pathogens in cardiovascular pathophysiology through multiple mechanisms.
In experimental models, Peron et al (71) demonstrated that P. gingivalis causes elevated numbers of DNA breaks, increased malondialdehyde levels, increased oxidized protein levels and enhanced macrophage infiltration in the myocardium, leading to adverse effects. Bijla et al (72) revealed that gingival protease released by P. gingivalis impairs autophagy by preventing the fusion of autophagosomes and lysosomes in cardiomyocytes, resulting in reduced cardiomyocyte viability and promoting apoptosis.
Radiation-induced skin injury (RISI)
RISI manifests universally in radiotherapy recipients due to the high mitotic index of epidermal keratinocytes. RISI occurs to varying degrees after radiotherapy and can be categorized into acute and chronic injuries. Acute RISI includes dry and wet desquamation, skin necrosis, ulceration and hemorrhage, while chronic RISI comprises chronic ulceration, actinic keratosis, capillary dilatation, fibrosis and skin malignant tumors (73). Several studies have demonstrated microbiota alterations as a potential pathogenetic mechanism for radiation dermatitis (74,75). There is also a link between the oral microbiota and skin inflammation. Li and Yosipovitch (76) found that patients with atopic dermatitis had significantly reduced oral microbiota diversity, characterized by a decreased abundance of pro-inflammatory-associated microbiota compared with that in healthy individuals, with functional antagonism between these microbial communities, suggesting that skin inflammation can be controlled based on the modulation of the oral microbiota. Notably, as aforementioned, the oral microbiota can promote pro-inflammatory cytokines such as IL-1 to initiate an inflammatory cascade response. Janko et al (77) found that IL-1 or IL-1 receptor-deficient mice exhibited reduced inflammation and attenuated radiation dermatitis severity. Collectively, microbial-mediated therapy may be a viable option for RISI.
Radiation-induced bone injury (RIBI)
Considering the characteristically high calcium concentration in bone tissue, it can absorb up to 30 to 40% more radiation than the surrounding tissues when exposed. This elevated absorption makes bone particularly vulnerable to RI at equivalent exposure doses (78). RIBI includes several conditions, such as osteoporosis, osteomyelitis, pathological fracture, osteonecrosis and growth restriction (57). Recent studies have revealed that radiation-induced osteonecrosis of the jaw, which is caused by radiotherapy for head and neck malignant tumors, is associated with notable alterations in the oral microbiota (58). These changes interfere with bone regeneration by regulating particular metabolic pathways that enhance osteoclast activity, thereby exacerbating radiation-induced osteonecrosis (79). However, the precise mechanisms underlying how oral microbiota influence osteogenesis have not yet been fully elucidated. Evidence suggests that the oral microbiota imparts osteoimmunomodulatory effects and modulates bone resorption (80). For instance, Xu et al (81) demonstrated that P. gingivalis and F. nucleatum promote bone resorption by activating bone remodeling cells through pro-inflammatory cytokines and modulation of the key axis, the receptor activator of nuclear factor-κB ligand-receptor activator of nuclear factor-κB-osteoprotegerin axis. In an experimental model, P. gingivalis LPS activated various cell types, including monocytes, endothelial cells and epithelial cells, resulting in the release of pro-inflammatory mediators that initiate an immune-inflammatory response in host tissues. Kovtonyuk et al (82) proposed that microbiota drive inflammation in hematopoietic stem cells via the microbiota/IL-1/IL-1R1 pathway. These findings collectively suggest the mechanistic involvement of the oral microbiota in RIBI progression.
Radiation-induced urinary system injury
The kidney is highly susceptible to radiotherapy, and radiation-induced kidney injury (RIKI) may result from abdominal or paraspinal malignancies, or systemic radiotherapy. The acute phase of RIKI is marked by malaise, edema, headache, hypertensive crisis and cardiorenal syndrome, whereas the chronic phase is defined by hypertension, anemia and progressive renal atrophy (83). The bladder is a critical organ susceptible to pelvic tumor radiotherapy. Radiation-induced bladder injury manifests as hemorrhagic cystitis, dysuria and overactive bladder syndrome (84). Yuan et al (85) introduced the oral-genitourinary axis derived from epidemiological and clinical research, identifying oral microbiota such as P. gingivalis, Streptococcus oralis and Capnocytophaga ochracea, and periodontal pathogens as contributors to elevated instances of prostatic hyperplasia, prostatitis, bladder cancer and other bladder diseases, via inflammation and the Akt signaling pathway (86–88). An increasing number of researchers have undertaken comprehensive investigations into the oral microbiota involved in the advancement of kidney disorders. Tooth brushing and mastication facilitate the entry of oral microbiota into the bloodstream through ulcerated periodontal pockets, resulting in bacteremia. Proinflammatory mediators from microbiota in the systemic circulation exacerbate the inflammatory burden on renal tissues (89). Kajiwara et al (90) revealed that the LPS of P. gingivalis stimulates Mac-1/podoplanin-positive macrophages through the overexpression of vascular cell adhesion protein 1 and E-selectin in glomerular infiltration, leading to renal inflammation characterized by glomerulosclerosis and tubulitis. Consequently, the oral microbiota may have a role in the etiology of RIKI.
Radiation-induced liver injury
Radiation-induced liver injury (RILI) is a common complication of radiotherapy for thoracic and abdominal neoplasms, particularly hepatocellular carcinoma (91). Clinical data reveal that ~60% of patients with liver cancer receive radiotherapy to varying degrees, and RILI significantly limits the clinical utility of this treatment (91). Researchers have proposed the oral-gut-liver axis following an in-depth examination of the association between the liver and oral microbiota (92). The connection between the liver and the oral cavity may occur via the gut, where impaired intestinal permeability allows the direct translocation of oral microbiota metabolites and inflammatory mediators from the oral cavity into the systemic circulation or to the liver. Upon entering the liver, the oral microbiota may promote the progression of liver disease via multiple mechanisms. For instance, vesicles from P. gingivalis can be released into the cytoplasm of hepatocytes to diminish phospho-glycogen synthase kinase 3 activation and inhibit gluconeogenesis via the insulin/insulin receptor substrate-1 receptor signaling pathway (68). The LPS of P. gingivalis prompts the release of galectin-3 from hepatocytes and macrophage-2 antigen-positive Kupffer cells, subsequently activating the TGF-β1/TGFβR2 cascade, which upregulates α-smooth muscle actin transcription and facilitates the differentiation of hepatic stellate cells into myofibroblasts (93). Research has demonstrated that the oral microbiota provoke hepatic dysfunction and fibrosis by enhancing immune cell infiltration and inflammatory gene expression in the liver, thereby activating the pro-inflammatory NF-κB pathway (94). However, to date, the comprehensive understanding of the causal association between the oral microbiota and RILI remains incomplete. This causal association between the oral microbiota and hepatic RI requires further elucidation.
Oral microbiota-based therapeutic approaches for RI
Oral microbiota-based approaches may be a useful method for the prevention and treatment of RI (Fig. 3). The homeostatic balance of oral microbiota contributes to systemic equilibrium. Healthy lifestyle choices, low-carbohydrate diets and good oral hygiene practices all help to maintain dynamic oral microbiota equilibrium and reduce the oral microbiota burden (95). By introducing beneficial bacterial species and substrates, probiotics, prebiotics and synbiotics help to reverse microbial dysbiosis (96). As aforementioned, Streptococcus salivarius K12, a probiotic, has been shown to restore the oral microbiota and cure radiographic oral mucositis (97). By reducing oral microbiota's load and obstructing pathogenic pathways, antibiotics and inhibitors that target harmful microbiota and their derivatives can successfully mitigate the pathogenic consequences of pathogens. Protease inhibitors, for instance, can reduce the inflammatory response and the P. gingivalis microbiota load (13). Notably, OMT has the ability to directly alter the microbiota ecosystem of the recipient, which makes it a potentially useful therapeutic strategy for RI.
Probiotics, antibiotics and inhibitors
Oral microbial dysbiosis can affect RI, and so probiotics, as a relatively predictable and safe measure to regulate microecology, are of interest to researchers.
First, despite the clinical trial by De Sanctis et al (98) demonstrating that Lactobacillus brevis CD2 lozenges failed to prevent RIOM in patients with head and neck cancer, strategic modulation of salivary microbiota homeostasis remains a compelling therapeutic avenue. Subsequently, a clinical trial by Peng et al (99) demonstrated that the probiotic Streptococcus salivarius K12 alleviated RIOM through suppression of opportunistic pathogens and reconstruction of murine oral flora. These findings are consistent with animal experiments conducted by Wang et al (97), which revealed that Streptococcus salivarius K12 ameliorate RIOM by suppressing oral anaerobic bacterial overgrowth. A clinical trial further showed that topical application of Streptococcus salivarius K12 lozenges effectively decrease RIOM incidence, delay symptom onset and shorten disease duration (45). Moreover, nisin (a lantibiotic) was found to modify the oral microbiota composition in healthy rats and dogs (100,101). Animal studies by Gao et al (102) additionally demonstrated that nisin-producing Lactococcus lactis probiotics significantly lowered periodontal pathogen levels, attenuated alveolar bone loss and modulated host responses to oral/systemic inflammatory diseases, offering therapeutic implications for RI. In the context of RIB, Zhao et al (103) reported that nisin treatment significantly suppressed radiation-induced upregulation of pro-inflammatory cytokines (IL-1β, IL-6 and TNF-α) in brain tissue affected by periodontal infections, suggesting therapeutic potential for RIB.
Furthermore, strategies targeting pathogenic bacteria through antibiotics or virulence factor inhibitors may mitigate pathogenicity via microbial load reduction and pathogenic pathway blockade. For instance, protease inhibitors have been shown to decrease P. gingivalis colonization and inflammatory responses (13). Notably, as previously mentioned, Dong et al (67) experimentally demonstrated that metronidazole combined with radiotherapy in mice with colorectal cancer resulted in reduced tumor burden and RE severity, implying therapeutic potential through modulation of oral microbiota-mediated interference with radiotherapeutic efficacy.
OMT
OMT, defined as the deliberate transfer of beneficial oral microbiota from healthy donors to patients, aims to establish microbial communities capable of modulating inflammatory responses (104). Accumulating evidence has demonstrated significant associations between oral microbiota dysbiosis and RI, prompting exploration of OMT as a therapeutic intervention for RI. In a murine model of radiation-induced head and neck injury, Xiao et al (105) administered OMT from healthy mice to irradiated mice with head and neck lesions, with mitigated oral mucositis demonstrating its potential as a novel treatment for RIOM. Clinically, Goloshchapov et al (106) recently reported successful OMT application in preventing chemotherapeutic oral mucositis. In this clinical trial, researchers showed that OMT effectively prevented oral mucositis following chemotherapy. This was achieved by administering repeated daily injections of donor saliva from healthy donors to patients for three chemotherapy cycles and before autologous hematopoietic cell transplantation (106). OMT can also be used to treat RP. AbdelMassih et al (107) hypothesized the therapeutic potential of OMT for low-grade inflammatory illnesses based on established links between oral ecological imbalance and pneumonia development. However, in investigations on radiotherapy for colorectal malignant tumors, scientists have discovered that OMT may have an adverse effect. Dong et al (67) discovered that OMT reduced the effectiveness of radiotherapy for colorectal malignant tumors.et al Verification of the use of this antibiotic for pretreatment of clinical colorectal malignant tumors radiation requires further investigation through clinical studies. Clinical translation warrants robust validation. Although OMT research has advanced, its clinical application remains experimental, mandating methodologically rigorous trials to establish therapeutic efficacy and safety.
Clinical translation program for treatment strategies
For current prevention and treatment strategies, future studies should prioritize expanding cohort sizes and diversifying research populations to validate the reliability and generalizability of current findings.
However, the mechanistic interplay between the oral microbiota and RI remains exploratory. Future investigations should focus on the following: i) Elucidating specific microbiota-radiation interaction mechanisms through establishment of oral microbiota-organ axis animal models; ii) deciphering molecular pathways mediating microbial effects on irradiated tissues across body regions; and iii) characterizing microbiota-immune system crosstalk during radiation pathogenesis. This systematic approach will advance a comprehensive understanding of host-microbe dynamics to inform novel RI prevention and therapeutic strategies.
In addition, based on the existing research results, precise intervention strategies can be developed. For example, for targeted bacterial inhibition technologies, exploration is required of the development of effective intervention strategies against oral microbial pathogens, such as P. gingivalis and F. nucleatum, including precision-targeted inhibitors, antibiotics and oral cleansers. These strategies aim to reduce the number or pathogenicity of pathogenic bacteria in the oral cavity, thereby reducing the risk of RI. For immunomodulatory strategies, oral mucosa-targeted vaccines can be developed to induce specific IgA responses, and phage therapy can be explored to selectively remove drug-resistant pathogenic bacteria. Microecological remodeling techniques, such as OMT to rebuild healthy flora, are able to construct pH-responsive slow-release systems to maintain oral microenvironmental homeostasis.
Notably, research on the oral microbiota and RI across anatomical sites involves multiple disciplines, including dentistry, oncology, radiation oncology, immunology and systems-level expertise. Consequently, enhanced interdisciplinary collaboration is required to accelerate research progress in this domain. Through integration of cross-disciplinary resources and technical capabilities, researchers can more comprehensively elucidate the intrinsic mechanisms underlying this association and develop more effective preventive and therapeutic strategies.
In the future, a multimodal diagnosis and treatment system can also be established, integrating a rapid saliva flora detection chip, an artificial intelligence-assisted diagnostic system and a personalized flora regulation program to establish a closed-loop management system of early warning of the risk of RI, early intervention and efficacy assessment.
Patient management strategies for radiotherapy
The management of patients with malignant tumors throughout the course of their illness will be an indispensable medical treatment in the future. The radiotherapy-phase management system has sparked considerable deliberation among healthcare professionals, prompting the emergence of a multitude of management modalities to mitigate the incidence and severity of RI (83–85). Zhao et al (108) demonstrated that the bound healthcare cohort management model can effectively prevent RILI and improve the quality of life of patients. The core of the bound medical and nursing cohort management model lies in the establishment of a medical and nursing bound care team, with members performing their respective roles to provide health education, basic care, psychological intervention and continuity of care for patients. Wang et al (109) found that the application of the 4F (all-weather, all-process, all-system and all-service) nursing management model in patients who underwent nasopharyngeal carcinoma radiotherapy had a very good effect, helping to enhance treatment engagement, improve self-care ability, promote quality of life and reduce the incidence of radiotherapy-related adverse reactions. The retrospective cohort study by Xiao et al (110) illustrated that the 4R (reduction, readiness, response and recovery) crisis management theory could significantly reduce the incidence and severity of RE in patients during radiotherapy for breast malignant tumors. These evidence-based management strategies provide actionable insights for RI patient care, effectively enhancing patient engagement and self-management capacity.
Summary and outlook
With marked advances in radiotherapy technology, its clinical applications in oncology have become increasingly widespread. However, there are still substantial challenges in the clinical management of RI, particularly regarding accurate diagnosis and evidence-based prevention and therapeutic strategies. The purpose of this review is to establish a systematic and effective microbiota-based intervention through the in-depth study of oral microbiota and different categories of RI. The present review describes the association between the two, and the potential prevention and treatment strategies. Nevertheless, critical questions persist regarding the precise mechanisms through which the oral microbiota modulate radiotherapy outcomes, while these proposed prevention and treatment strategies require rigorous clinical validation to assess their clinical feasibility and safety profiles.
Acknowledgements
Not applicable.
Funding
This study was supported by the National Natural Science Foundation of China (grant no. 82304072, 2024.01-2026.12) and the Chongqing Graduate Student Research Innovation Project (grant no. CYS240301, 2024.07.01-2026.06.30).
Availability of data and materials
Not applicable.
Authors' contributions
JS drafted the manuscript and contributed to writing the content. LX drafted the manuscript and revised the content. Both authors have read and approved the final manuscript. Data authentication is not applicable.
Ethics statement and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
|
De Ruysscher D, Niedermann G, Burnet NG, Siva S, Lee AWM and Hegi-Johnson F: Radiotherapy toxicity. Nat Rev Dis Primers. 5:132019. View Article : Google Scholar : PubMed/NCBI | |
|
Zhao Q, Chen Y, Huang W, Zhou H and Zhang W: Drug-microbiota interactions: An emerging priority for precision medicine. Signal Transduct Target Ther. 8:3862023. View Article : Google Scholar : PubMed/NCBI | |
|
Durrant MG and Bhatt AS: Microbiome genome structure drives function. Nat Microbiol. 4:912–913. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Spencer CN, McQuade JL, Gopalakrishnan V, McCulloch JA, Vetizou M, Cogdill AP, Khan MAW, Zhang X, White MG, Peterson CB, et al: Dietary fiber and probiotics influence the gut microbiome and melanoma immunotherapy response. Science. 374:1632–1640. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Jin C, Lagoudas GK, Zhao C, Bullman S, Bhutkar A, Hu B, Ameh S, Sandel D, Liang XS, Mazzilli S, et al: Commensal microbiota promote lung cancer development via γδ T cells. Cell. 176:998–1013.e16. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Pernigoni N, Zagato E, Calcinotto A, Troiani M, Mestre RP, Calì B, Attanasio G, Troisi J, Minini M, Mosole S, et al: Commensal bacteria promote endocrine resistance in prostate cancer through androgen biosynthesis. Science. 374:216–224. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Sivan A, Corrales L, Hubert N, Williams JB, Aquino-Michaels K, Earley ZM, Benyamin FW, Lei YM, Jabri B, Alegre ML, et al: Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science. 350:1084–1089. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Vétizou M, Pitt JM, Daillère R, Lepage P, Waldschmitt N, Flament C, Rusakiewicz S, Routy B, Roberti MP, Duong CP, et al: Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science. 350:1079–1084. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Baker JL, Morton JT, Dinis M, Alverez R, Tran NC, Knight R and Edlund A: Deep metagenomics examines the oral microbiome during dental caries, revealing novel taxa and co-occurrences with host molecules. Genome Res. 31:64–74. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Di Stefano M, Polizzi A, Santonocito S, Romano A, Lombardi T and Isola G: Impact of oral microbiome in periodontal health and periodontitis: A critical review on prevention and treatment. Int J Mol Sci. 23:51422022. View Article : Google Scholar : PubMed/NCBI | |
|
Irfan M, Delgado RZR and Frias-Lopez J: The oral microbiome and cancer. Front Immunol. 11:5910882020. View Article : Google Scholar : PubMed/NCBI | |
|
Scannapieco FA: Poor oral health in the etiology and prevention of aspiration pneumonia. Clin Geriatr Med. 39:257–271. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Park SY, Hwang BO, Lim M, Ok SH, Lee SK, Chun KS, Park KK, Hu Y, Chung WY and Song NY: Oral-gut microbiome axis in gastrointestinal disease and cancer. Cancers (Basel). 13:21242021. View Article : Google Scholar : PubMed/NCBI | |
|
Bruno JS, Al-Qadami GH, Laheij AMGA, Bossi P, Fregnani ER and Wardill HR: From pathogenesis to intervention: The importance of the microbiome in oral mucositis. Int J Mol Sci. 24:82742023. View Article : Google Scholar : PubMed/NCBI | |
|
Dominy SS, Lynch C, Ermini F, Benedyk M, Marczyk A, Konradi A, Nguyen M, Haditsch U, Raha D, Griffin C, et al: Porphyromonas gingivalis in Alzheimer's disease brains: Evidence for disease causation and treatment with small-molecule inhibitors. Sci Adv. 5:eaau33332019. View Article : Google Scholar : PubMed/NCBI | |
|
Benedyk M, Mydel PM, Delaleu N, Płaza K, Gawron K, Milewska A, Maresz K, Koziel J, Pyrc K and Potempa J: Gingipains: Critical factors in the development of aspiration pneumonia caused by Porphyromonas gingivalis. J Innate Immun. 8:185–198. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Vasconcelos RM, Sanfilippo N, Paster BJ, Kerr AR, Li Y, Ramalho L, Queiroz EL, Smith B, Sonis ST and Corby PM: Host-microbiome cross-talk in oral mucositis. J Dent Res. 95:725–733. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Geng F, Zhang Y, Lu Z, Zhang S and Pan Y: Fusobacterium nucleatum caused DNA damage and promoted cell proliferation by the Ku70/p53 Pathway in oral cancer cells. DNA Cell Biol. 39:144–151. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Sampath C, Okoro EU, Gipson MJ, Chukkapalli SS, Farmer-Dixon CM and Gangula PR: Porphyromonas gingivalis infection alters Nrf2-phase II enzymes and nitric oxide in primary human aortic endothelial cells. J Periodontol. 92:54–65. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Feng N, Han X, Peng D, Geng F, Li Q, Pan C, Wang H, Pan Y and Tan L: P. gingivalis alters lung microbiota and aggravates disease severity of COPD rats by up-regulating Hsp90α/MLKL. J Oral Microbiol. 16:23345882024. View Article : Google Scholar : PubMed/NCBI | |
|
Gong T, Chen Q, Mao H, Zhang Y, Ren H, Xu M, Chen H and Yang D: Outer membrane vesicles of Porphyromonas gingivalis trigger NLRP3 inflammasome and induce neuroinflammation, tau phosphorylation, and memory dysfunction in mice. Front Cell Infect Microbiol. 12:9254352022. View Article : Google Scholar : PubMed/NCBI | |
|
Mladenov E, Li F, Zhang L, Klammer H and Iliakis G: Intercellular communication of DNA damage and oxidative status underpin bystander effects. Int J Radiat Biol. 94:719–726. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Wang K and Tepper JE: Radiation therapy-associated toxicity: Etiology, management, and prevention. CA Cancer J Clin. 71:437–454. 2021.PubMed/NCBI | |
|
Turnquist C, Harris BT and Harris CC: Radiation-induced brain injury: Current concepts and therapeutic strategies targeting neuroinflammation. Neurooncol Adv. 2:vdaa0572020.PubMed/NCBI | |
|
Xu L, Huang H, Liu T, Yang T and Yi X: Exposure to X-rays causes depression-like behaviors in mice via HMGB1-mediated pyroptosis. Neuroscience. 481:99–110. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Makale MT, McDonald CR, Hattangadi-Gluth JA and Kesari S: Mechanisms of radiotherapy-associated cognitive disability in patients with brain tumours. Nat Rev Neurol. 13:52–64. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Bowland GB and Weyrich LS: The oral-microbiome-brain axis and neuropsychiatric disorders: An anthropological perspective. Front Psychiatry. 13:8100082022. View Article : Google Scholar : PubMed/NCBI | |
|
Verma A, Azhar G, Patyal P, Zhang W, Zhang X and Wei JY: Proteomic analysis of P. gingivalis-lipopolysaccharide induced neuroinflammation in SH-SY5Y and HMC3 cells. Geroscience. 46:4315–4332. 2024. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang J, Yu C, Zhang X, Chen H, Dong J, Lu W, Song Z and Zhou W: Porphyromonas gingivalis lipopolysaccharide induces cognitive dysfunction, mediated by neuronal inflammation via activation of the TLR4 signaling pathway in C57BL/6 mice. J Neuroinflammation. 15:372018. View Article : Google Scholar : PubMed/NCBI | |
|
Bulgart HR, Neczypor EW, Wold LE and Mackos AR: Microbial involvement in Alzheimer disease development and progression. Mol Neurodegener. 15:422020. View Article : Google Scholar : PubMed/NCBI | |
|
Jin R, Ning X, Liu X, Zhao Y and Ye G: Porphyromonas gingivalis-induced periodontitis could contribute to cognitive impairment in Sprague-Dawley rats via the P38 MAPK signaling pathway. Front Cell Neurosci. 17:11413392023. View Article : Google Scholar : PubMed/NCBI | |
|
Liu Q, Huang Y, Duan M, Yang Q, Ren B and Tang F: Microglia as therapeutic target for radiation-induced brain injury. Int J Mol Sci. 23:82862022. View Article : Google Scholar : PubMed/NCBI | |
|
Wang Y, Tian J, Liu D, Li T, Mao Y and Zhu C: Microglia in radiation-induced brain injury: Cellular and molecular mechanisms and therapeutic potential. CNS Neurosci Ther. 30:e147942024. View Article : Google Scholar : PubMed/NCBI | |
|
Chuang WC, Yang CN, Wang HW, Lin SK, Yu CC, Syu JH, Chiang CP, Shiao YJ and Chen YW: The mechanisms of Porphyromonas gingivalis-derived outer membrane vesicles-induced neurotoxicity and microglia activation. J Dent Sci. 19:1434–1442. 2024. View Article : Google Scholar : PubMed/NCBI | |
|
Maria OM, Eliopoulos N and Muanza T: Radiation-Induced oral mucositis. Front Oncol. 7:892017. View Article : Google Scholar : PubMed/NCBI | |
|
Li J, Zhu C, Zhang Y, Guan C, Wang Q, Ding Y and Hu X: Incidence and risk factors for radiotherapy-induced oral mucositis among patients with nasopharyngeal carcinoma: A meta-analysis. Asian Nurs Res (Korean Soc Nurs Sci). 17:70–82. 2023.PubMed/NCBI | |
|
Nodit L, Kelley JR, Panella TJ, Bruckbauer A, Nodit PG, Shope GA, Peyton K, Klingeman DM, Zaretzki R, Carrell A and Podar M: Oral microbiome and mycobiome dynamics in cancer therapy-induced oral mucositis. Sci Data. 12:4632025. View Article : Google Scholar : PubMed/NCBI | |
|
Hou J, Zheng H, Li P, Liu H, Zhou H and Yang X: Distinct shifts in the oral microbiota are associated with the progression and aggravation of mucositis during radiotherapy. Radiother Oncol. 129:44–51. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Zhu XX, Yang XJ, Chao YL, Zheng HM, Sheng HF, Liu HY, He Y and Zhou HW: The potential effect of oral microbiota in the prediction of mucositis during radiotherapy for nasopharyngeal carcinoma. EBioMedicine. 18:23–31. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Vesty A, Gear K, Biswas K, Mackenzie BW, Taylor MW and Douglas RG: Oral microbial influences on oral mucositis during radiotherapy treatment of head and neck cancer. Support Care Cancer. 28:2683–2691. 2020.PubMed/NCBI | |
|
Nishii M, Soutome S, Kawakita A, Yutori H, Iwata E, Akashi M, Hasegawa T, Kojima Y, Funahara M, Umeda M and Komori T: Factors associated with severe oral mucositis and candidiasis in patients undergoing radiotherapy for oral and oropharyngeal carcinomas: A retrospective multicenter study of 326 patients. Support Care Cancer. 28:1069–1075. 2020.PubMed/NCBI | |
|
Groeger S and Meyle J: Oral mucosal epithelial cells. Front Immunol. 10:2082019. View Article : Google Scholar : PubMed/NCBI | |
|
Hirahara L, Takase-Minegishi K, Kirino Y, Iizuka-Iribe Y, Soejima Y, Yoshimi R and Nakajima H: The roles of monocytes and macrophages in Behçet's disease with focus on M1 and M2 polarization. Front Immunol. 13:8522972022. View Article : Google Scholar : PubMed/NCBI | |
|
Suárez LJ, Arboleda S, Angelov N and Arce RM: Oral versus gastrointestinal mucosal immune niches in homeostasis and allostasis. Front Immunol. 12:7052062021. View Article : Google Scholar : PubMed/NCBI | |
|
Li Y, Li Z, Zheng S and Xu X: Probiotics in the management of radiation-induced oral mucositis. Front Cell Infect Microbiol. 14:14771432024. View Article : Google Scholar : PubMed/NCBI | |
|
Zhao M, Wang C, Ji C, Liu R, Xie J, Wang Y and Gu Z: Ascidian-inspired temperature-switchable hydrogels with antioxidant fullerenols for protecting radiation-induced oral mucositis and maintaining the homeostasis of oral microbiota. Small. 19:e22065982023. View Article : Google Scholar : PubMed/NCBI | |
|
Morsy BM, El Domiaty S, Meheissen MAM, Heikal LA, Meheissen MA and Aly NM: Omega-3 nanoemulgel in prevention of radiation-induced oral mucositis and its associated effect on microbiome: A randomized clinical trial. BMC Oral Health. 23:6122023. View Article : Google Scholar : PubMed/NCBI | |
|
Zou XY, Xiang SX and Pan Y: Research advances in traditional Chinese and Western medicine treatments for radiation-induced esophagitis. J Esophagus Dis. 7:129–134. 2025. | |
|
Liu S, Wang S, Zhang N and Li P: The oral microbiome and oral and upper gastrointestinal diseases. J Oral Microbiol. 16:23558232024. View Article : Google Scholar : PubMed/NCBI | |
|
Zou Q, Feng L, Cai X, Qian Y and Xu L: Esophageal microflora in esophageal diseases. Front Cell Infect Microbiol. 13:11457912023. View Article : Google Scholar : PubMed/NCBI | |
|
Yang L, Francois F and Pei Z: Molecular pathways: Pathogenesis and clinical implications of microbiome alteration in esophagitis and Barrett esophagus. Clin Cancer Res. 18:2138–2144. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Hanania AN, Mainwaring W, Ghebre YT, Hanania NA and Ludwig M: Radiation-induced lung injury: Assessment and management. Chest. 156:150–162. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Li R, Li J and Zhou X: Lung microbiome: New insights into the pathogenesis of respiratory diseases. Signal Transduct Target Ther. 9:192024. View Article : Google Scholar : PubMed/NCBI | |
|
Pathak JL, Yan Y, Zhang Q, Wang L and Ge L: The role of oral microbiome in respiratory health and diseases. Respir Med. 185:1064752021. View Article : Google Scholar : PubMed/NCBI | |
|
Dong J, Li W, Wang Q, Chen J, Zu Y, Zhou X and Guo Q: Relationships between oral microecosystem and respiratory diseases. Front Mol Biosci. 8:7182222022. View Article : Google Scholar : PubMed/NCBI | |
|
Gaeckle NT, Pragman AA, Pendleton KM, Baldomero AK and Criner GJ: The oral-lung axis: The impact of oral health on lung health. Respir Care. 65:1211–1220. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Li Q, Wang H, Tan L, Zhang S, Lin L, Tang X and Pan Y: Oral pathogen Fusobacterium nucleatum coaggregates with Pseudomonas aeruginosa to modulate the inflammatory cytotoxicity of pulmonary epithelial cells. Front Cell Infect Microbiol. 11:6439132021. View Article : Google Scholar : PubMed/NCBI | |
|
Takahashi Y, Watanabe N, Kamio N, Yokoe S, Suzuki R, Sato S, Iinuma T and Imai K: Expression of the SARS-CoV-2 Receptor ACE2 and proinflammatory cytokines induced by the periodontopathic bacterium Fusobacterium nucleatum in human respiratory epithelial cells. Int J Mol Sci. 22:13522021. View Article : Google Scholar : PubMed/NCBI | |
|
Suzuki R, Kamio N, Sugimoto K, Maruoka S, Gon Y, Kaneko T, Yonehara Y and Imai K: Periodontopathic bacterium Fusobacterium nucleatum affects matrix metalloproteinase-9 expression in human alveolar epithelial cells and mouse lung. In Vivo. 36:649–656. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Okabe T, Kamiya Y, Kikuchi T, Goto H, Umemura M, Suzuki Y, Sugita Y, Naiki Y, Hasegawa Y, Hayashi JI, et al: Porphyromonas gingivalis components/secretions synergistically enhance pneumonia caused by Streptococcus pneumoniae in mice. Int J Mol Sci. 22:127042021. View Article : Google Scholar : PubMed/NCBI | |
|
O'Dwyer DN, Kim JS, Ma SF, Ranjan P, Das P, Lipinski JH, Metcalf JD, Falkowski NR, Yow E, Anstrom K, et al: Commensal oral microbiota, disease severity, and mortality in fibrotic lung disease. Am J Respir Crit Care Med. 209:1101–1110. 2024. View Article : Google Scholar : PubMed/NCBI | |
|
Wang Q, Xu G, Yan O, Wang S and Wang X: Radiation-induced injury and the gut microbiota: Insights from a microbial perspective. Therap Adv Gastroenterol. 18:175628482513473472025. View Article : Google Scholar : PubMed/NCBI | |
|
Yamazaki K and Kamada N: Exploring the oral-gut linkage: Interrelationship between oral and systemic diseases. Mucosal Immunol. 17:147–153. 2024. View Article : Google Scholar : PubMed/NCBI | |
|
Wang A, Zhai Z, Ding Y, Wei J, Wei Z and Cao H: The oral-gut microbiome axis in inflammatory bowel disease: From inside to insight. Front Immunol. 15:14300012024. View Article : Google Scholar : PubMed/NCBI | |
|
Yamazaki K: Oral-gut axis as a novel biological mechanism linking periodontal disease and systemic diseases: A review. Jpn Dent Sci Rev. 59:273–280. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Atarashi K, Suda W, Luo C, Kawaguchi T, Motoo I, Narushima S, Kiguchi Y, Yasuma K, Watanabe E, Tanoue T, et al: Ectopic colonization of oral bacteria in the intestine drives TH1 cell induction and inflammation. Science. 358:359–365. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Dong J, Li Y, Xiao H, Zhang S, Wang B, Wang H, Li Y, Fan S and Cui M: Oral microbiota affects the efficacy and prognosis of radiotherapy for colorectal cancer in mouse models. Cell Rep. 37:1098862021. View Article : Google Scholar : PubMed/NCBI | |
|
Zou B, Schuster JP, Niu K, Huang Q, Rühle A and Huber PE: Radiotherapy-induced heart disease: A review of the literature. Precis Clin Med. 2:270–282. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Podlesnikar T, Berlot B, Dolenc J, Goričar K and Marinko T: Radiotherapy-induced cardiotoxicity: The role of multimodality cardiovascular imaging. Front Cardiovasc Med. 9:8877052022. View Article : Google Scholar : PubMed/NCBI | |
|
Liu QH and Liu ZY: Research advances in radiation-induced heart injury. J Microcirc. 34:92–97. 2024. | |
|
Peron D, Prates RA, Antonio EL, Teixeira ILA, de Oliveira HA, Mansano BSDM, Bergamo A, Almeida DR, Dariolli R, Tucci PJF and Serra AJ: A common oral pathogen Porphyromonas gingivalis induces myocarditis in rats. J Clin Periodontol. 49:506–517. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Bijla M, Saini SK, Pathak AK, Bharadwaj KP, Sukhavasi K, Patil A, Saini D, Yadav R, Singh S, Leeuwenburgh C and Kumar P: Microbiome interactions with different risk factors in development of myocardial infarction. Exp Gerontol. 189:1124092024. View Article : Google Scholar : PubMed/NCBI | |
|
Yang HJ, Zhang Y, Peng O and Zou BW: Radiation-induced heart disease: Current status and challenges. Sichuan Da Xue Xue Bao Yi Xue Ban. 53:1127–1134. 2022.(In Chinese). PubMed/NCBI | |
|
Miyamae N, Ogai K, Kunimitsu M, Fujiwara M, Nagai M, Okamoto S, Okuwa M and Oe M: Relationship between severe radiodermatitis and skin barrier functions in patients with head and neck cancer: A prospective observational study. Asia Pac J Oncol Nurs. 12:1006252024. View Article : Google Scholar : PubMed/NCBI | |
|
Hülpüsch C, Neumann AU, Reiger M, Fischer JC, de Tomassi A, Hammel G, Gülzow C, Fleming M, Dapper H, Mayinger M, et al: Association of skin microbiome dynamics with radiodermatitis in patients with breast cancer. JAMA Oncol. 10:516–521. 2024. View Article : Google Scholar : PubMed/NCBI | |
|
Li W and Yosipovitch G: The role of the microbiome and microbiome-derived metabolites in atopic dermatitis and non-histaminergic itch. Am J Clin Dermatol. 21 (Suppl 1):S44–S50. 2020. View Article : Google Scholar | |
|
Janko M, Ontiveros F, Fitzgerald TJ, Deng A, DeCicco M and Rock KL: IL-1 generated subsequent to radiation-induced tissue injury contributes to the pathogenesis of radiodermatitis. Radiat Res. 178:166–172. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Curi MM, Cardoso CL, de Lima HG, Kowalski LP and Martins MD: Histopathologic and histomorphometric analysis of irradiation injury in bone and the surrounding soft tissues of the jaws. J Oral Maxillofac Surg. 74:190–199. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Li Z, Fu R, Huang X, Wen X and Zhang L: Oral microbiota may affect osteoradionecrosis following radiotherapy for head and neck cancer. J Transl Med. 21:3912023. View Article : Google Scholar : PubMed/NCBI | |
|
Hathaway-Schrader JD, Aartun JD, Poulides NA, Kuhn MB, McCormick BE, Chew ME, Huang E, Darveau RP, Westwater C and Novince CM: Commensal oral microbiota induces osteoimmunomodulatory effects separate from systemic microbiome in mice. JCI Insight. 7:e1407382022. View Article : Google Scholar : PubMed/NCBI | |
|
Xu J, Yu L, Ye S, Ye Z, Yang L and Xu X: Oral microbiota-host interaction: The chief culprit of alveolar bone resorption. Front Immunol. 15:12545162024. View Article : Google Scholar : PubMed/NCBI | |
|
Kovtonyuk LV, Caiado F, Garcia-Martin S, Manz EM, Helbling P, Takizawa H, Boettcher S, Al-Shahrour F, Nombela-Arrieta C, Slack E and Manz MG: IL-1 mediates microbiome-induced inflammaging of hematopoietic stem cells in mice. Blood. 139:44–58. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Klaus R, Niyazi M and Lange-Sperandio B: Radiation-induced kidney toxicity: Molecular and cellular pathogenesis. Radiat Oncol. 16:432021. View Article : Google Scholar : PubMed/NCBI | |
|
Wang WP, Zhang FQ and Yuan SH: Clinical practice guidelines for the prevention and treatment of radiation-induced bladder injury. Chin J Cancer Prev Treat. 30:187–193. 2023. | |
|
Yuan S, Fang C, Leng WD, Wu L, Li BH, Wang XH, Hu H and Zeng XT: Oral microbiota in the oral-genitourinary axis: Identifying periodontitis as a potential risk of genitourinary cancers. Mil Med Res. 8:542021.PubMed/NCBI | |
|
Porter CM, Shrestha E, Peiffer LB and Sfanos KS: The microbiome in prostate inflammation and prostate cancer. Prostate Cancer Prostatic Dis. 21:345–354. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Prakash P, Verma S and Gupta S: Influence of microbiome in intraprostatic inflammation and prostate cancer. Prostate. 84:1179–1188. 2024. View Article : Google Scholar : PubMed/NCBI | |
|
Wang SY, Cai Y, Hu X, Li F, Qian XH, Xia LY, Gao B, Wu L, Xie WZ, Gu JM, et al: P. gingivalis in oral-prostate axis exacerbates benign prostatic hyperplasia via IL-6/IL-6R pathway. Mil Med Res. 11:302024.PubMed/NCBI | |
|
Liu ZH, Zhou XD and Zhang LL: Research progress in the correlation between oral microbiota and chronic kidney disease. Sichuan Da Xue Xue Bao Yi Xue Ban. 54:66–70. 2023.(In Chinese). PubMed/NCBI | |
|
Kajiwara K, Sawa Y, Fujita T and Tamaoki S: Immunohistochemical study for the expression of leukocyte adhesion molecules, and FGF23 and ACE2 in P. gingivalis LPS-induced diabetic nephropathy. BMC Nephrol. 22:32021. View Article : Google Scholar : PubMed/NCBI | |
|
Zhu W, Zhang X, Yu M, Lin B and Yu C: Radiation-induced liver injury and hepatocyte senescence. Cell Death Discov. 7:2442021. View Article : Google Scholar : PubMed/NCBI | |
|
Acharya C, Sahingur SE and Bajaj JS: Microbiota, cirrhosis, and the emerging oral-gut-liver axis. JCI Insight. 2:e94416–94416. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Albuquerque-Souza E and Sahingur SE: Periodontitis, chronic liver diseases, and the emerging oral-gut-liver axis. Periodontol 2000. 89:125–141. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Bai L, Wang YL, Chen YL, Li HX, Zhu SW, Liu Y, Song ZC and Duan SZ: The combination of experimental periodontitis and oral microbiota from periodontitis patients aggravates liver fibrosis in mice. J Clin Periodontol. 49:1067–1078. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Jia G, Zhi A, Lai PFH, Wang G, Xia Y, Xiong Z, Zhang H, Che N and Ai L: The oral microbiota-a mechanistic role for systemic diseases. Br Dent J. 224:447–455. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Huang X, Huang X, Huang Y, Zheng J, Lu Y, Mai Z, Zhao X, Cui L and Huang S: The oral microbiome in autoimmune diseases: Friend or foe? J Transl Med. 21:2112023. View Article : Google Scholar : PubMed/NCBI | |
|
Wang Y, Li J, Zhang H, Zheng X, Wang J, Jia X, Peng X, Xie Q, Zou J, Zheng L, et al: Probiotic Streptococcus salivarius K12 alleviates radiation-induced oral mucositis in mice. Front Immunol. 12:6848242021. View Article : Google Scholar : PubMed/NCBI | |
|
De Sanctis V, Belgioia L, Cante D, LA Porta MR, Caspiani O, Guarnaccia R, Argenone A, Muto P, Musio D, DE Felice F, et al: Lactobacillus brevis CD2 for prevention of oral mucositis in patients with head and neck tumors: A multicentric randomized study. Anticancer Res. 39:1935–1942. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Peng X, Li Z, Pei Y, Zheng S, Liu J, Wang J, Li R and Xu X: Streptococcus salivarius K12 alleviates oral mucositis in patients undergoing radiotherapy for malignant head and neck tumors: A randomized controlled trial. J Clin Oncol. 42:1426–1435. 2024. View Article : Google Scholar : PubMed/NCBI | |
|
Cunha E, Valente S, Nascimento M, Pereira M, Tavares L, Dias R and Oliveira M: Influence of the dental topical application of a nisin-biogel in the oral microbiome of dogs: A pilot study. PeerJ. 9:e116262021. View Article : Google Scholar : PubMed/NCBI | |
|
Wu L, Li F, Ran L, Gao Y, Xie P, Yang J, Ke F, Liu L, Wang Q and Gao X: Insight into the effects of nisin and cecropin on the oral microbial community of rats by high-throughput sequencing. Front Microbiol. 11:10822020. View Article : Google Scholar : PubMed/NCBI | |
|
Gao L, Kuraji R, Zhang MJ, Martinez A, Radaic A, Kamarajan P, Le C, Zhan L, Ye C, Rangé H, et al: Nisin probiotic prevents inflammatory bone loss while promoting reparative proliferation and a healthy microbiome. NPJ Biofilms Microbiomes. 8:452022. View Article : Google Scholar : PubMed/NCBI | |
|
Zhao C, Kuraji R, Ye C, Gao L, Radaic A, Kamarajan P, Taketani Y and Kapila YL: Nisin a probiotic bacteriocin mitigates brain microbiome dysbiosis and Alzheimer's disease-like neuroinflammation triggered by periodontal disease. J Neuroinflammation. 20:2282023. View Article : Google Scholar : PubMed/NCBI | |
|
Nascimento MM: Oral microbiota transplant: A potential new therapy for oral diseases. J Calif Dent Assoc. 45:565–568. 2017.PubMed/NCBI | |
|
Xiao H, Fan Y, Li Y, Dong J, Zhang S, Wang B, Liu J, Liu X, Fan S, Guan J and Cui M: Oral microbiota transplantation fights against head and neck radiotherapy-induced oral mucositis in mice. Comput Struct Biotechnol J. 19:5898–5910. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Goloshchapov OV, Chukhlovin AB, Bug DS, Polev DE, Kosarev OV, Klementeva RV, Izmailova EA, Kazantsev IV, Khalipskaia MS, Goloshchapova МО, et al: Safety, feasibility, and advantages of oral microbiota transplantation: The first clinical case. J Pediatr Hematol Oncol. 46:287–296. 2024. View Article : Google Scholar : PubMed/NCBI | |
|
AbdelMassih A, Gadalla M, Hussein E, Elahmady M, Zahra N, Eid MA, Hussein M, Hassan AA, Abou-Zeid AS, Hassan A, et al: The forgotten oral microbial transplantation for improving the outcomes of COVID-19. New Microbes New Infect. 43:1009232021. View Article : Google Scholar : PubMed/NCBI | |
|
Zhao F, Huang D and Cao M: Application of bonded medical care peer management model in patients with radiotherapy-induced radiation lung injury for lung cancer. Qilu J Nurs China. 27:19–22. 2021.(In Chinese). View Article : Google Scholar | |
|
Wang N, Wang J and Fang F: Application of 4F nursing management model in patients undergoing radiotherapy for nasopharyngeal carcinoma. Qilu J Nurs China. 29:135–138. 2023.(In Chinese). | |
|
Xiao B, Zou T and Liu Y: Application of 4R crisis management theory in the management of radiation dermatitis in patients with breast cancer during radiotherapy. Mod Med Health. 40:2238–2241. 2024. |