Association between FGF10 expression and gland‑forming differentiation pattern in gastric adenocarcinoma: An immunohistochemical analysis
- Authors:
- Published online on: August 12, 2025 https://doi.org/10.3892/ol.2025.15224
- Article Number: 479
-
Copyright: © Fujibayashi et al. This is an open access article distributed under the terms of Creative Commons Attribution License.
Abstract
Introduction
Gastric adenocarcinoma constitutes a significant global health burden, ranking fifth in terms of both incidence and mortality among malignancies worldwide (1). Despite established treatment modalities, advanced gastric cancer maintains dismal prognostic outcomes (2), necessitating personalized therapeutic strategies based on molecular characterization.
The molecular landscape of gastric cancer includes established biomarkers, such as programmed death-ligand 1, human epidermal growth factor receptor 2 (HER2), and claudin 18.2, which are now utilized in clinical practice (3–6). Emerging markers, including mesenchymal-epithelial transition factor amplification, Dickkopf-related protein 1, and fibroblast growth factor (FGF) receptor 2 (FGFR2) amplification, can also expand the available therapeutic targets (7–9). Liquid biopsy via circulating tumor DNA and tumor-infiltrating T lymphocytes offer diagnostic and prognostic insights, supporting personalized treatment strategies (10,11).
Histopathologically, gastric adenocarcinoma is dichotomized into differentiated and undifferentiated subtypes with established prognostic implications (12–14). Differentiated adenocarcinomas encompass papillary adenocarcinoma (pap) and tubular adenocarcinoma (tub), and the latter is further subclassified into well-differentiated (tub1) and moderately differentiated (tub2) variants (15). Tub1 exhibits regular glandular structures with a favorable prognosis (14,15), whereas pap demonstrates papillary architecture with occasionally unfavorable outcomes (16,17).
Undifferentiated adenocarcinomas comprise poorly differentiated adenocarcinoma (por), signet-ring cell carcinoma (sig), and mucinous adenocarcinoma (muc) (15). Por is subdivided into solid (por1) and non-solid (por2) types, with por2 showing diffuse infiltration and a poorer prognosis compared with por1 (15,18). Sig exhibits characteristic intracytoplasmic mucin with peripheral nuclear displacement, whereas muc demonstrates abundant extracellular mucin production (15). The gland-forming differentiation pattern is generally associated with prognosis (14), although it can be modulated by invasion depth and metastatic parameters (19,20).
FGFR2 has emerged as a significant prognostic biomarker for gastric adenocarcinoma (9,21–24), with established therapeutic relevance (25–28). FGFR2 exists in two predominant isoforms, IIIb and IIIc, with FGFR2-IIIb reported to be more frequently overexpressed than FGFR2-IIIc in gastric adenocarcinoma (24). These isoforms have differential binding affinities for various FGFs, with FGF10 selectively activating FGFR2-IIIb (29–31).
FGF10 is a secreted FGF that mediates tissue homeostasis, repair, and morphogenesis throughout development and adult physiology (31). Notably, FGF10 contributes to gastric development and morphogenesis (32). In murine models, Fgf10 expression was localized to mesenchymal compartments subjacent to gastric glands, participating in mesenchymal-epithelial signaling (33). In addition, adult transgenic mice overexpressing Fgf10 demonstrated epithelial proliferation, mucus-secreting cell expansion, and parietal/chief cell reduction, suggesting that FGF10 may influence gastric epithelial differentiation beyond the embryonic stages (33). Notably, FGF10 amplification has been reported in human gastric cancer (34), and its expression level has been associated with a poor prognosis (35).
These findings suggest that the FGF10-FGFR2 axis may be involved in the differentiation of gastric adenocarcinoma, although its clinicopathological significance remains unclear. This study aimed to elucidate the association between FGF10-FGFR2 axis activation and the gland-forming differentiation pattern in human gastric adenocarcinoma. We analyzed FGF10 and FGFR2 expression in surgically resected specimens using immunohistochemistry (IHC), and evaluated the associations with clinicopathological parameters to identify the potential therapeutic and prognostic implications.
Materials and methods
Human samples
This retrospective, single-institution study utilized surgically resected gastric tumor specimens collected at Gifu University Hospital (Gifu, Japan) between June 1, 2000, and December 31, 2008. Clinical records were reviewed to collect information on patient background, treatment, and postoperative course. Cases with histological diagnoses other than adenocarcinoma (e.g., small cell carcinoma, undifferentiated carcinoma, or neuroendocrine tumor) were excluded from the analysis. Patients were also excluded if they were under 20 years old, had incomplete clinical data, exhibited inadequate immunohistochemical staining, or were deemed unsuitable for the study by the attending physician. Histological classification was primarily performed according to the 15th edition of the Japanese Classification of Gastric Carcinoma (15). In addition, tumors were further categorized into intestinal/indeterminate and diffuse types based on the WHO Classification of Digestive System Tumours, 5th edition (36), to facilitate international comparison. Tumor staging was based on the 8th edition of the American Joint Committee on Cancer/Union for International Cancer Control Tumor-Node-Metastasis classification of malignant tumors (37). This study was approved by the Institutional Review Board of Gifu University (approval no. 2024-253; approved on December 4, 2024) and conducted in accordance with the Declaration of Helsinki (2013 version). The study was publicly disclosed on the institution's website, and patients were given the opportunity to opt out of participation. The study design, data collection, analysis, and reporting were performed in accordance with the Reporting Recommendations for Tumor Marker Prognostic Studies guidelines (38).
Immunohistochemistry
All paraffin-embedded tissues were cut into 4-µm-thick serial sections, deparaffinized, and stained with hematoxylin and eosin or used for IHC. For IHC, the sections were placed in citrate buffer (10 mmol/l, pH 6.0) and autoclaved in a pressure chamber at 121°C for 20 min to retrieve the antigens, followed by rinsing and blocking in 3% hydrogen peroxide in methanol for 10 min at room temperature to remove endogenous peroxidase. Non-specific binding sites were blocked in 0.01 M phosphate-buffered saline (pH 7.4) containing 2% bovine serum albumin (Wako Pure Chemical, Osaka, Japan) for 40 min at room temperature. The sections were then incubated overnight (16 h) at 4°C in a humidified chamber with the following primary antibodies: FGF10 (rabbit polyclonal IgG, 1:500; Abcam, Cambridge, UK, #ab80064), FGFR2 (rabbit polyclonal IgG, 1:500; Abcam #ab10648), HER2 (rabbit monoclonal IgG, 1:400, Clone 4B5; Roche Diagnostics, Basel, Switzerland, #790-2991), epidermal growth factor receptor (EGFR) (rabbit monoclonal IgG, 1:200, Clone D38B1; Cell Signaling Technology, Danvers, MA, USA; #4267), CD44 (mouse monoclonal IgG, 1:50, Clone G44-26; BD Pharmingen, San Diego, CA, USA; #550392), and Ki67 (mouse monoclonal IgG, 1:100, Clone MIB-1; DAKO, Agilent Technologies, Santa Clara, CA, USA; #M7240). The next day, the sections were exposed to biotinylated goat anti-rabbit IgG (1:200; Vectastain Elite ABC kit; Vector Laboratories, Newark, CA, USA) for 60 min at room temperature, followed by incubation with avidin-biotin peroxidase complex (Vectastain Elite ABC kit; Vector Laboratories) to activate immunoreactivity. The bound complex was visualized using 3,3′-diaminobenzidine (Sigma, St. Louis, MO, USA) for 3–5 min under a microscope, to prevent overstaining. Finally, the sections were counterstained with hematoxylin for 2 min and dehydrated through graded alcohols. The specificity of the antibodies for IHC was validated using positive and negative controls. FGF10 expression was confirmed in plasma cells and FGFR2 expression in the glandular epithelium in normal intestinal tissue (positive control), and negative controls showed no notable immunoreactivity (Fig. S1). The normal intestinal tissue used for positive and negative controls was obtained from the archival tissue bank of Gifu University Hospital. These samples were non-neoplastic tissues sourced from surgical resections and were processed and paraffin-embedded in the same manner as the study specimens.
Histological assessment of tumor and stromal cells
The distinction between tumor and stromal cells was performed by two board-certified pathologists (K.K. and H.T.) based on standard histopathological assessment of cellular morphology in hematoxylin and eosin-stained sections. Tumor cells were identified as malignant epithelial cells characterized by features such as nuclear pleomorphism and the formation of abnormal glandular structures. Stromal cells were identified as the surrounding non-neoplastic connective tissue components, including fibroblasts, inflammatory cells, and vascular elements.
Immunohistochemical evaluation
The four-tier score was determined based on the visual assessment of staining intensity by two independent pathologists. The categories were defined as follows: 0 (negative) for no discernible immunoreactivity; 1+ (weakly positive) for faint, hard-to-see staining; 2+ (moderately positive) for clear, intermediate-intensity staining; and 3+ (strongly positive) for intense staining. Representative images illustrating these four tiers of staining intensity for FGF10 and FGFR2 are provided in Fig. 1.
For example, expression levels of FGF10 and FGFR2 in tumor cells and FGF10 in the stroma were scored using a four-tier scale: 0 (negative), 1+ (weakly positive), 2+ (moderately positive), and 3+ (strongly positive) (Fig. 1). HER2 expression was classified as negative (0 or 1+) or positive (2+ or 3+), EGFR was classified as negative (0) or positive (1+ to 3+), and CD44 was classified as negative (0), weakly positive (1+), or strongly positive (2+). Immunohistochemical evaluations were independently performed by two board-certified pathologists (K.K. and H.T.) who were blinded to clinical outcomes and patient characteristics. Inter-observer agreement was assessed using weighted Cohen's κ coefficients, demonstrating substantial agreement for FGF10 tumor expression (κ=0.78), FGF10 stromal expression (κ=0.74), and FGFR2 tumor expression (κ=0.82). Discrepancies (6.8%) were resolved through collaborative microscopic review and consensus.
Statistical analysis
Patient characteristics were summarized using medians and ranges for continuous variables and frequencies and percentages for categorical variables. Baseline characteristics were compared between groups defined by FGF10 expression using the Mann-Whitney U test for continuous variables and Fisher's exact test for categorical variables.
The association between FGF10 expression (in tumor cells and stroma separately) or FGFR2 expression and differentiation status was evaluated using multivariable ordinal logistic regression models adjusting for patient age, HER2 status, and EGFR status, with differentiation status as an ordinal outcome (pap, tub1, tub2, por1, por2, and sig). Age is a fundamental demographic factor known to influence tumor biology and clinical outcomes, and HER2 and EGFR were included owing to their known roles as molecular markers in gastric cancer and their potential associations with histological differentiation (5,39). These variables were selected a priori based on biological plausibility and their relevance in previous gastric cancer studies. For the binary classification of differentiation (pap, tub1, tub2, and por1 as intestinal/indeterminate type; and por2 and sig as diffuse type), multivariable binary logistic regression models were employed with the same adjustment factors, and predicted probabilities from the multivariable models were plotted.
Overall survival (OS) was defined as the time from the date of surgery to the date of death from any cause, with data for surviving patients censored at the date of their last follow-up. OS curves were generated using the Kaplan-Meier method, and differences between groups were compared using the log-rank test. Multivariable Cox proportional hazards regression models were used to assess the prognostic impacts of FGF10 and FGFR2 expression on OS, adjusting for patient age, sex, and tumor stage. Covariates included in the multivariable Cox proportional hazards regression models were selected based on their established clinical relevance and biological plausibility in relation to survival outcomes. FGF10 and FGFR2 were treated as continuous variables in all analyses.
Multicollinearity among covariates in all multivariable models was assessed using variance inflation factors (VIF). All statistical analyses were performed using R software version 4.3.2. A two-sided P-value <0.05 was considered to indicate a statistically significant difference for all analyses. A post hoc power analysis indicated that the sample size in this study provided 73% power to detect a medium effect size (Cohen's d=0.5) with a significance level of α=0.05.
In the predicted probability plots, solid lines represent the point estimate of the predicted probability and the gray shaded areas indicate the 95% confidence interval (CI), both derived from a logistic regression model. The estimates were calculated using maximum likelihood estimation. For ordinal logistic regression models, maximum likelihood estimation was used to estimate the variance of the regression coefficients under the proportional odds assumption, and the CIs for the adjusted ORs were constructed using the estimated variance. In the Cox proportional hazards model, the variance of the regression coefficients was estimated using the partial likelihood method, and the CIs for the hazard ratios were similarly constructed using the estimated variance. The CIs for Kaplan-Meier survival probabilities were calculated using Greenwood's formula.
Results
Patient characteristics and IHC analysis
A total of 120 surgically resected gastric tumor specimens were initially collected, of which 117 gastric adenocarcinoma specimens were included in the final analysis, after excluding three non-adenocarcinoma tumors (1 small cell carcinoma, 1 undifferentiated carcinoma, and 1 neuroendocrine tumor). FGF10 and FGFR2 expression levels in these 117 gastric adenocarcinoma tissues were evaluated by IHC (Fig. S2). The clinicopathological characteristics of the 117 patients are shown in Table I. The median age was 68 years (range: 31–92 years), with a male predominance (65.0%). Regarding tumor progression, most cases were T4 (66.7%) and N3 (41.0%). There were no cases of Stage I disease and Stage III was the most common (63.2%), followed by Stage II (23.1%) and Stage IV (13.7%). Notably, lymphatic invasion was present in 95.7% of cases and vascular invasion was observed in 82.1% of cases.
Table I.Clinicopathological characteristics of patients with gastric cancer included in the immunohistochemical analysis. |
The expression patterns of FGF10 and FGFR2 in gastric adenocarcinoma tissues are shown in Fig. 1. FGF10 expression was observed in tumor cells in 72 of 117 cases (61.5%) and in the stroma in 75 cases (64.1%). Among the FGF10-positive cases in tumor cells, 44 cases (37.6%) showed weak expression (1+) and 28 cases (23.9%) showed high expression (≥2+; 13 cases 2+, 15 cases 3+). In the stromal component, 43 cases (36.8%) showed weak expression (1+) and 32 cases (27.4%) demonstrated high expression (≥2+; 25 cases 2+, 7 cases with 3+). FGF10 expression in the stromal compartment was comparable to its expression in tumor cells, suggesting the importance of paracrine signaling within the tumor microenvironment. FGFR2 expression in tumor cells was observed in 114 of 117 cases (97.4%), with 29 cases (24.8%) showing weak expression (1+) and 85 cases showing high expression (≥2+) (72.6%; 49 cases 2+, 36 cases 3+). This extremely high expression frequency suggested an important role of FGFR2 in gastric adenocarcinoma.
When patients were divided into two groups based on the presence or absence of FGF10 expression in tumor cells (negative: 0, positive: 1+ to 3+), significant differences were observed in histological subtype (P=0.041, Fisher's exact test) and MIB-1 index (P=0.012, Mann-Whitney U test). A detailed analysis of histological subtypes revealed that moderately differentiated tubular adenocarcinoma (tub2) had a higher tendency for FGF10-positivity (30/37 cases, 81.1%), whereas poorly differentiated adenocarcinoma (por2) had a more balanced distribution (22/43 cases, 51.2%). The MIB-1 index was significantly higher in the FGF10-positive group compared with the FGF10-negative group (median: 45.7 vs. 37.1, respectively), indicating that FGF10 expression was associated with increased tumor cell proliferation.
FGF10 and FGFR2 expression in gastric adenocarcinoma and its association with gland-forming differentiation pattern and survival
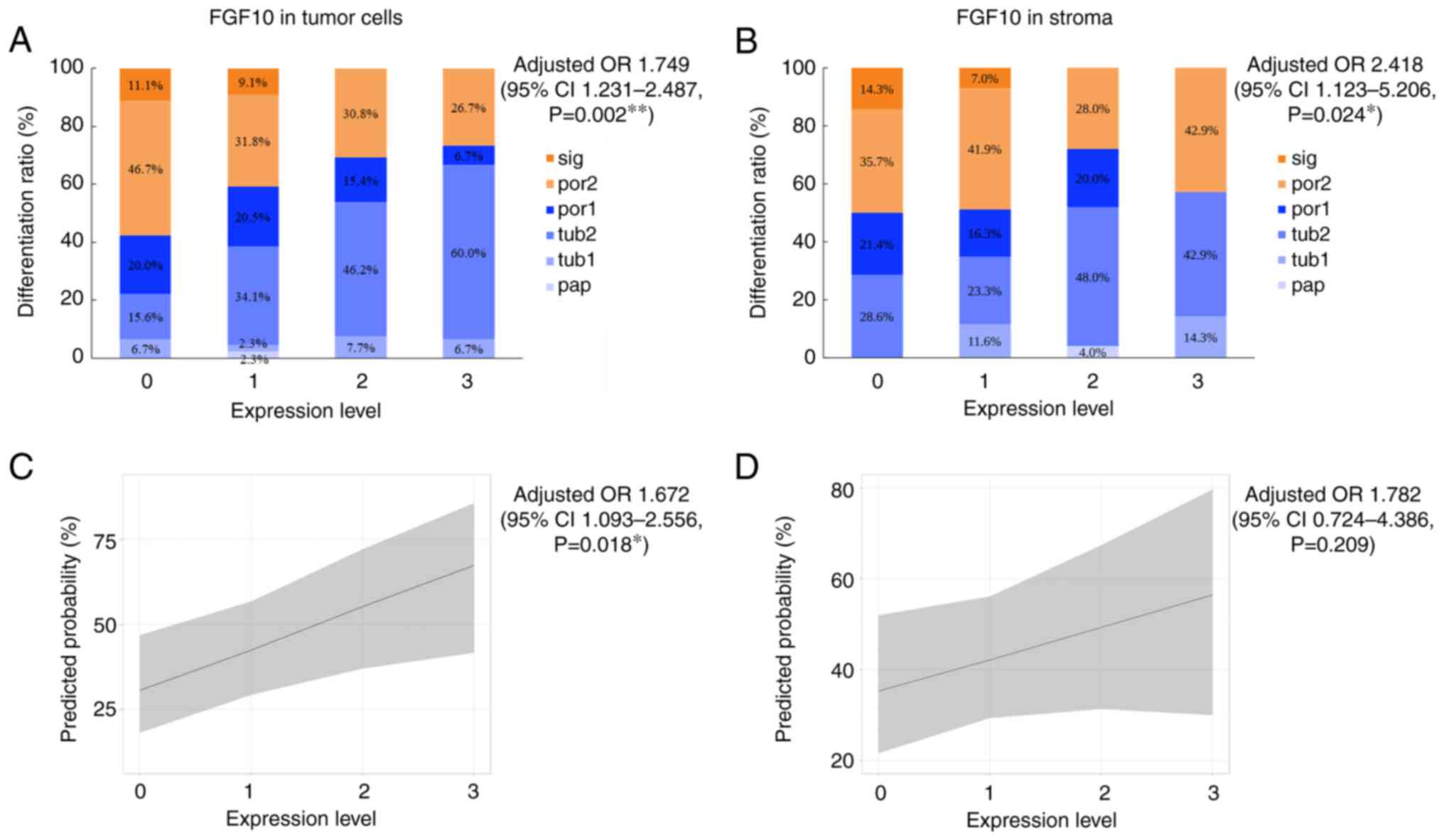
We clarified the relationship between FGF10 and FGFR2 expression levels and the histopathological gland-forming differentiation pattern in gastric adenocarcinoma using a multivariable ordinal logistic regression model. Expression levels of FGF10 showed significant positive associations with histological classification in tumor cells and stromal tissue (Fig. 2). Multivariable ordinal logistic regression analysis, adjusted for age, HER2 status, and EGFR expression, revealed adjusted ORs of 1.749 (95% CI 1.231–2.487, P=0.002) for FGF10 expression in tumor cells (Fig. 2A) and 2.418 (95% CI 1.123–5.206, P=0.024) for stromal FGF10 expression (Fig. 2B). These findings indicate that increased FGF10 expression was associated with a higher probability of well-differentiated histological subtypes. This association was consistently observed in tumor cells and stromal tissue, with stromal FGF10 expression demonstrating a stronger association (higher adjusted OR). Subsequent analysis of FGFR2 found no significant association between FGFR2 expression levels and differentiation (P=0.788) (Fig. 3A).
A predicted probability plot is shown for the classification of pap, tub1, tub2, and por1 as intestinal/indeterminate type and por2 and sig as diffuse type. FGF10 expression in tumor cells had an adjusted OR in multivariable binary logistic regression analysis of 1.672 (95% CI 1.093–2.556, P=0.018), demonstrating a significant association with histological classification (Fig. 2C); however, FGF10 expression in the stroma (adjusted OR=1.782, 95% CI 0.724–4.386, P=0.209) (Fig. 2D) and FGFR2 expression in tumor cells (adjusted OR=0.831, 95% CI 0.31–2.227, P=0.713) showed no significant associations with histological classification (Fig. 3B). These results suggest that higher FGF10 expression levels were more strongly associated with the intestinal/indeterminate type. Furthermore, the association between FGF10 expression and histological classification was stronger in tumor cells than in the stroma.
Survival analysis revealed no significant associations between OS and the expression levels of FGF10 (in tumor cells and stroma) and FGFR2. In addition, Kaplan-Meier survival curve analysis (Fig. 4) showed no significant differences in survival rates in relation to the expression levels of each marker (log-rank test: FGF10 in tumor cells, P=0.596; FGF10 in stroma, P=0.806; FGFR2 in tumor cells, P=0.594). Multivariable Cox proportional hazards regression analysis, adjusted for age, sex, and disease stage, demonstrated no significant associations for FGF10 expression in tumor cells [hazard ratio (HR) 0.823, 95% CI 0.640–1.057, P=0.127], stromal FGF10 expression (HR 0.675, 95% CI 0.385–1.183, P=0.170), or FGFR2 expression in tumor cells (HR 1.080, 95% CI 0.559–2.085, P=0.819).
Assessment of multicollinearity indicated that all VIF values were close to 1.0 across all multivariable regression models, indicating no evidence of multicollinearity among the included covariates.
Discussion
FGF10 expression levels in tumor cells and the stroma were significantly associated with gland-forming differentiation patterns, with higher FGF10 expression levels associated with a higher degree of differentiation. In contrast, FGFR2 expression levels were not associated with differentiation, suggesting that FGF10 may play a specific role in gastric adenocarcinoma differentiation.
The mechanism by which the FGF10-FGFR2 signaling pathway contributes to the gland-forming differentiation pattern remains unclear. FGF10 has a critical role in organogenesis during embryonic development, and the findings of this study suggest that its function during development may be partially recapitulated in adult gastric cancer tissues. Notably, FGF10 may promote glandular formation and contribute to the maintenance of differentiation via epithelial-mesenchymal interactions (31). FGF10 has been reported to promote the maintenance of gastric epithelial progenitor cells and gland-forming differentiation pattern by binding to FGFR2b through epithelial-mesenchymal interactions and activating signaling pathways such as MAPK/ERK and PI3K/AKT (32,40). Moreover, FGF10 cooperates with the Notch and Wnt pathways to determine region-specific differentiation patterns through transcriptional regulation of factors such as Sox2 and GATA4 (32,41). FGF10 stimulation also induces downregulation of E-cadherin expression and induction of epithelial-mesenchymal transition-related molecules, thereby participating in cell adhesion and morphogenesis (42). FGF10 is considered to influence glandular formation and tumor differentiation grade via these molecular mechanisms (32,43). Conversely, excessive activation of the FGF10-FGFR2b pathway has been suggested to enhance tumor cell proliferation and invasive capacity, highlighting its importance as a therapeutic target (43,44). FGF10 is also used to induce the differentiation of gastric epithelial cells in organoid culture systems, with potential for applications in regenerative medicine (43). Further elucidation of the molecular networks centered on FGF10 is expected to contribute to the development of diagnosis and personalized treatment for gastric adenocarcinoma (40,43).
It is noteworthy that, although FGF10 expression was associated with tumor differentiation, FGFR2 expression showed no such association. This finding suggests that FGF10 may act through receptors other than FGFR2, or that the activation state of FGFR2 is more critical than its expression level. Speer et al (33) reported that FGF10-FGFR2b signaling was dispensable for maintaining epithelial proliferation and differentiation in gastric tissues from adult mice under homeostatic conditions (33). The FGF10-FGFR2 signaling pathway, however, has been shown to play a major role in tumor formation in several other cancer types (44). A more detailed analysis of this pathway in gastric cancer is therefore warranted.
This study found no significant association between FGF10 or FGFR2 expression and OS, suggesting that these molecules may not serve as direct prognostic factors. Sun et al reported that FGF10 expression was associated with prognosis in patients with gastric adenocarcinoma; they found no association between FGF10 expression and tumor differentiation, but did identify significant associations with lymph node metastasis and distant metastasis (35). Several methodological differences may account for this apparent discordance. First, our cohort comprised predominantly patients with advanced-stage disease (Stage III–IV: 76.9%) compared with Sun et al's study, which had a higher proportion of early-stage cases (Stage I–II: 45.3%), potentially masking subtle molecular prognostic influences in advanced disease states. Second, we employed a polyclonal antibody (Abcam ab80064) targeting amino acids 50–150 of human FGF10, whereas Sun et al are presumed to have utilized a monoclonal antibody against the C-terminus, which could result in differential detection of FGF10 isoforms or post-translational modifications. Finally, our multivariable Cox regression analysis incorporated comprehensive adjustment for established prognostic factors (age, sex, tumor stage), which may attenuate apparent associations observed in less rigorously adjusted analyses and provide a more precise estimate of independent prognostic impact.
Emerging evidence demonstrates that FGF10-FGFR2 signaling exhibits distinct, tissue-specific oncogenic functions across different cancer types. The FGF10-FGFR2 axis drives context-dependent oncogenic programs: it induces epithelial-mesenchymal transition and invasion in pancreatic ductal adenocarcinoma (45), while promoting invasion and a poor prognosis in gastric adenocarcinoma (35). Paracrine FGF10 enhances androgen receptor signaling and multifocal tumor formation in prostate adenocarcinoma, primarily via FGFR1, contrasting with gastric signaling, which operates independently of hormonal cues (46). Colorectal cancer cells co-express FGF10 and epithelial FGFR2-IIIb, enabling autocrine proliferation distinct from gastric mesenchymal-epithelial paracrine interactions (47). These organ-specific functions reflect unique embryological origins and FGFR2 isoform expression patterns, highlighting the need for tissue-adapted FGF10-FGFR2 targeting strategies (31).
This study had several limitations. The relatively small sample size of 117 patients and potentially limited observation period, evidenced by wide CIs in the survival analyses, may have affected the generalizability of the results. In addition, the study was a retrospective study conducted at a single institution, and the study population was thus limited to patients from Gifu University Hospital, and careful consideration is therefore needed when generalizing the results. Although the sample size of 117 patients was sufficient for certain analyses, a large-scale, multicenter collaborative study is needed to provide more reliable and generalizable findings. IHC scoring was based on visual assessment of staining intensity by two pathologists using a four-tier scale. While discrepancies were resolved by consensus and inter-observer agreement was substantial, the inherent subjectivity of this method may have introduced variability. Furthermore, although this study demonstrated an association between FGF10 and FGFR2 expression and gland-forming differentiation patterns, we did not conduct molecular biological analyses to elucidate the underlying mechanisms. Additionally, this study did not evaluate the relationship between FGF10-FGFR2 signaling and other key molecular markers or genetic mutations involved in gastric cancer progression, such as p53 and E-cadherin. As a result, the relative significance of the FGF10-FGFR2 signaling pathway in gastric cancer remains incompletely understood. Considering these limitations, the findings of this study can be expanded further and refined.
In conclusion, FGF10 expression is significantly associated with gland-forming differentiation patterns in gastric adenocarcinoma, contributing to molecular subtype characterization, despite a lack of prognostic significance. Future investigations into FGF10-FGFR2 signaling mechanisms may reveal novel therapeutic targets.
Supplementary Material
Supporting Data
Acknowledgements
The authors would like to thank Ms. Kyoko Takahashi, Ms. Ayako Suga, Ms. Reiko Kitazumi and Ms. Izumi Toshima (Department of Tumor Pathology, Gifu University Graduate School of Medicine), for their experimental support. The authors would also like to thank Dr Takuma Ishihara (Innovative and Clinical Research Promotion Center, Gifu University Hospital), for their assistance with the statistical analysis, and Dr J. Ludovic Croxford for editing a draft of this manuscript.
Funding
The present study received financial support for the research, authorship and/or publication of this article from JSPS KAKENHI (grant no. JP20K07587 to HT).
Availability of data and materials
The data generated in the present study may be requested from the corresponding author.
Authors' contributions
HT conceptualized the study. SF, DW and HT developed the methodology. DW performed the formal analysis. SF, ME, RY, KM, HH, MK, KK and HT conducted the investigation and data acquisition. KK and HT provided technical assistance. SF, HT, MF, IY, YS, RA, JYT, AH and NM contributed to data collection, organization and interpretation. SF, KK and HT confirm the authenticity of all the raw data. SF and HT wrote the manuscript and performed the subsequent review and editing. KK, DW, ME, RY, KM, HH, MK, MF, IY, YS, RA, JYT, AH and NM contributed to the critical review and revision of the manuscript. AH and NM supervised the study. AH provided research resources and HT acquired the funding. All authors read and approved the final version of the manuscript.
Ethics approval and consent to participate
The present study was approved by the Ethics Committee of Gifu University (approval no. 2024-253, approved on December 4, 2024) and conducted in accordance with The Declaration of Helsinki. The need for informed consent was waived due to the retrospective design, and the study was disclosed on the institutional website with an opt-out option.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Glossary
Abbreviations
Abbreviations:
|
FGF10 |
fibroblast growth factor 10 |
|
FGFR2 |
fibroblast growth factor receptor 2 |
|
CI |
confidence interval |
|
HER2 |
human epidermal growth factor receptor 2 |
|
pap |
papillary adenocarcinoma |
|
tub |
tubular adenocarcinoma |
|
tub1 |
well-differentiated tubular adenocarcinoma |
|
tub2 |
moderately differentiated tubular adenocarcinoma |
|
por |
poorly differentiated adenocarcinoma |
|
sig |
signet-ring cell carcinoma |
|
muc |
mucinous adenocarcinoma |
|
por1 |
solid-type poorly differentiated adenocarcinoma |
|
por2 |
non-solid-type poorly differentiated adenocarcinoma |
|
IHC |
immunohistochemistry |
|
EGFR |
epidermal growth factor receptor |
|
OS |
overall survival |
|
VIF |
variance inflation factors |
|
OR |
odds ratio |
|
HR |
hazard ratio |
References
|
Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I and Jemal A: Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 74:229–263. 2024.PubMed/NCBI | |
|
Kakeji Y, Ishikawa T, Suzuki S, Akazawa K, Irino T, Miyashiro I, Ono H, Suzuki H, Tanabe S, Kadowaki S, et al: A retrospective 5-year survival analysis of surgically resected gastric cancer cases from the Japanese gastric cancer association nationwide registry (2001–2013). Gastric Cancer. 25:1082–1093. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Cancer Genome Atlas Research Network, . Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 513:202–209. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Janjigian YY, Shitara K, Moehler M, Garrido M, Salman P, Shen L, Wyrwicz L, Yamaguchi K, Skoczylas T, Campos Bragagnoli A, et al: First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): A randomised, open-label, phase 3 trial. Lancet. 398:27–40. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Sato Y, Okamoto K, Kawano Y, Kasai A, Kawaguchi T, Sagawa T, Sogabe M, Miyamoto H and Takayama T: Novel biomarkers of gastric cancer: Current research and future perspectives. J Clin Med. 12:46462023. View Article : Google Scholar : PubMed/NCBI | |
|
Shitara K, Lordick F, Bang YJ, Enzinger P, Ilson D, Shah MA, Van Cutsem E, Xu RH, Aprile G, Xu J, et al: Zolbetuximab plus mFOLFOX6 in patients with CLDN18.2-positive, HER2-negative, untreated, locally advanced unresectable or metastatic gastric or gastro-oesophageal junction adenocarcinoma (SPOTLIGHT): A multicentre, randomised, double-blind, phase 3 trial. Lancet. 401:1655–1668. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Kawakami H and Okamoto I: MET-targeted therapy for gastric cancer: The importance of a biomarker-based strategy. Gastric Cancer. 19:687–695. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Shi T, Zhang Y, Wang Y, Song X, Wang H, Zhou X, Liang K, Luo Y, Che K, Wang X, et al: DKK1 promotes tumor immune evasion and impedes anti-PD-1 treatment by inducing immunosuppressive macrophages in gastric cancer. Cancer Immunol Res. 10:1506–1524. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Kim HS, Kim JH and Jang HJ: Pathologic and prognostic impacts of FGFR2 amplification in gastric cancer: A meta-analysis and systemic review. J Cancer. 10:2560–2567. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Gao Y, Zhang K, Xi H, Cai A, Wu X, Cui J, Li J, Qiao Z, Wei B and Chen L: Diagnostic and prognostic value of circulating tumor DNA in gastric cancer: A meta-analysis. Oncotarget. 8:6330–6340. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Lee JS, Won HS, Sun S, Hong JH and Ko YH: Prognostic role of tumor-infiltrating lymphocytes in gastric cancer: A systematic review and meta-analysis. Medicine (Baltimore). 97:e117692018. View Article : Google Scholar : PubMed/NCBI | |
|
Nakamura K, Sugano H and Takagi K: Carcinoma of the stomach in incipient phase: Its histogenesis and histological appearances. Gan. 59:251–258. 1968.PubMed/NCBI | |
|
Feng F, Liu J, Wang F, Zheng G, Wang Q, Liu S, Xu G, Guo M, Lian X and Zhang H: Prognostic value of differentiation status in gastric cancer. BMC Cancer. 18:8652018. View Article : Google Scholar : PubMed/NCBI | |
|
Zu H, Wang H, Li C and Xue Y: Clinicopathologic characteristics and prognostic value of various histological types in advanced gastric cancer. Int J Clin Exp Pathol. 7:5692–5700. 2014.PubMed/NCBI | |
|
Association JGC: Japanese Classification of Gastric Carcinoma 2017. 15th edition. Kanehara; Tokyo: 2017 | |
|
Wang X, Deng J and Liang H: Well differentiated carcinoma with a poor prognosis: A retrospective analysis of papillary gastric adenocarcinoma. Surg Today. 51:1387–1396. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Li HZ, Zhu HT, Chen YY, Zheng RS, Jin GF, Du LB and Cheng XD: Five-year survival analysis of gastric cancer from population-based cancer registration data in Zhejiang province, China. Zhonghua Zhong Liu Za Zhi. 46:862–870. 2024.(In Chinese). PubMed/NCBI | |
|
Kunisaki C, Akiyama H, Nomura M, Matsuda G, Otsuka Y, Ono HA, Nagahori Y, Takahashi M, Kito F and Shimada H: Clinicopathological properties of poorly-differentiated adenocarcinoma of the stomach: Comparison of solid- and non-solid-types. Anticancer Res. 26:639–646. 2006.PubMed/NCBI | |
|
Yasuda K, Adachi Y, Shiraishi N, Maeo S and Kitano S: Papillary adenocarcinoma of the stomach. Gastric Cancer. 3:33–38. 2000. View Article : Google Scholar : PubMed/NCBI | |
|
Shin HD, Bang KB, Kang SH, Moon HS, Sung JK, Jeong HY, Lee DK, Kim KB, Kim SM, Lee SW, et al: Clinical outcome of endoscopic submucosal dissection for papillary type early gastric cancer: A multicenter study. Gut Liver. 18:426–433. 2024. View Article : Google Scholar : PubMed/NCBI | |
|
Kim HS, Kim JH, Jang HJ, Han B and Zang DY: Pathological and prognostic impacts of FGFR2 overexpression in gastric cancer: A meta-analysis. J Cancer. 10:20–27. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Hur JY, Chao J, Kim K, Kim ST, Kim KM, Klempner SJ and Lee J: High-level FGFR2 amplification is associated with poor prognosis and Lower response to chemotherapy in gastric cancers. Pathol Res Pract. 216:1528782020. View Article : Google Scholar : PubMed/NCBI | |
|
Ooki A and Yamaguchi K: The beginning of the era of precision medicine for gastric cancer with fibroblast growth factor receptor 2 aberration. Gastric Cancer. 24:1169–1183. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Yashiro M, Kuroda K, Masuda G, Okuno T, Miki Y, Yamamoto Y, Sera T, Sugimoto A, Kushiyama S, Nishimura S, et al: Clinical difference between fibroblast growth factor receptor 2 subclass, type IIIb and type IIIc, in gastric cancer. Sci Rep. 11:46982021. View Article : Google Scholar : PubMed/NCBI | |
|
Kunii K, Davis L, Gorenstein J, Hatch H, Yashiro M, Di Bacco A, Elbi C and Lutterbach B: FGFR2-amplified gastric cancer cell lines require FGFR2 and Erbb3 signaling for growth and survival. Cancer Res. 68:2340–2348. 2008. View Article : Google Scholar : PubMed/NCBI | |
|
Wainberg ZA, Enzinger PC, Kang YK, Qin S, Yamaguchi K, Kim IH, Saeed A, Oh SC, Li J, Turk HM, et al: Bemarituzumab in patients with FGFR2b-selected gastric or gastro-oesophageal junction adenocarcinoma (FIGHT): A randomised, double-blind, placebo-controlled, phase 2 study. Lancet Oncol. 23:1430–1440. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Kang YK, Qin S, Lee KW, Oh SC, Kim IH, Kim JG, Li Y, Yan Z, Li J, Bai LY, et al: Bemarituzumab plus mFOLFOX6 as first-line treatment in East Asian patients with FGFR2b-overexpressing locally advanced or metastatic gastric/gastroesophageal junction cancer: Subgroup of FIGHT final analysis. Gastric Cancer. 27:1046–1057. 2024. View Article : Google Scholar : PubMed/NCBI | |
|
Wainberg ZA, Kang YK, Lee KW, Qin S, Yamaguchi K, Kim IH, Saeed A, Oh SC, Li J, Turk HM, et al: Bemarituzumab as first-line treatment for locally advanced or metastatic gastric/gastroesophageal junction adenocarcinoma: Final analysis of the randomized phase 2 FIGHT trial. Gastric Cancer. 27:558–570. 2024. View Article : Google Scholar : PubMed/NCBI | |
|
Ornitz DM, Xu J, Colvin JS, McEwen DG, MacArthur CA, Coulier F, Gao G and Goldfarb M: Receptor specificity of the fibroblast growth factor family. J Biol Chem. 271:15292–15297. 1996. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang X, Ibrahimi OA, Olsen SK, Umemori H, Mohammadi M and Ornitz DM: Receptor specificity of the fibroblast growth factor family. The complete mammalian FGF family. J Biol Chem. 281:15694–15700. 2006. View Article : Google Scholar : PubMed/NCBI | |
|
Ornitz DM and Itoh N: The fibroblast growth factor signaling pathway. Wiley Interdiscip Rev Dev Biol. 4:215–266. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Nyeng P, Norgaard GA, Kobberup S and Jensen J: FGF10 signaling controls stomach morphogenesis. Dev Biol. 303:295–310. 2007. View Article : Google Scholar : PubMed/NCBI | |
|
Speer AL, Al Alam D, Sala FG, Ford HR, Bellusci S and Grikscheit TC: Fibroblast growth factor 10-fibroblast growth factor receptor 2b mediated signaling is not required for adult glandular stomach homeostasis. PLoS One. 7:e491272012. View Article : Google Scholar : PubMed/NCBI | |
|
Ooi A, Oyama T, Nakamura R, Tajiri R, Ikeda H, Fushida S, Nakamura H and Dobashi Y: Semi-comprehensive analysis of gene amplification in gastric cancers using multiplex ligation-dependent probe amplification and fluorescence in situ hybridization. Mod Pathol. 28:861–871. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Sun Q, Lin P, Zhang J, Li X, Yang L, Huang J, Zhou Z, Liu P and Liu N: Expression of fibroblast growth factor 10 is correlated with poor prognosis in gastric adenocarcinoma. Tohoku J Exp Med. 236:311–318. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Board WCoTE: Digestive System Tumours. International Agency for Research on Cancer; Lyon: 2019 | |
|
Brierley JD, Gospodarowicz MK and Wittekind C: TNM Classification of Malignant Tumours. 8th edition. Wiley-Blackwell; Hoboken: pp. 63–69. 2017 | |
|
Altman DG, McShane LM, Sauerbrei W and Taube SE: Reporting recommendations for tumor marker prognostic studies (REMARK): Explanation and elaboration. PLoS Med. 9:e10012162012. View Article : Google Scholar : PubMed/NCBI | |
|
Röcken C: Predictive biomarkers in gastric cancer. J Cancer Res Clin Oncol. 149:467–481. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Watson J and Francavilla C: Regulation of FGF10 signaling in development and disease. Front Genet. 9:5002018. View Article : Google Scholar : PubMed/NCBI | |
|
Sankoda N, Tanabe W, Tanaka A, Shibata H, Woltjen K, Chiba T, Haga H, Sakai Y, Mandai M, Yamamoto T, et al: Epithelial expression of Gata4 and Sox2 regulates specification of the squamous-columnar junction via MAPK/ERK signaling in mice. Nat Commun. 12:5602021. View Article : Google Scholar : PubMed/NCBI | |
|
Abolhassani A, Riazi GH, Azizi E, Amanpour S, Muhammadnejad S, Haddadi M, Zekri A and Shirkoohi R: FGF10: Type III epithelial mesenchymal transition and invasion in breast cancer cell lines. J Cancer. 5:537–547. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Lv YQ, Wu J, Li XK, Zhang JS and Bellusci S: Role of FGF10/FGFR2b signaling in mouse digestive tract development, repair and regeneration following injury. Front Cell Dev Biol. 7:3262019. View Article : Google Scholar : PubMed/NCBI | |
|
Clayton NS and Grose RP: Emerging roles of fibroblast growth factor 10 in cancer. Front Genet. 9:4992018. View Article : Google Scholar : PubMed/NCBI | |
|
Ndlovu R, Deng LC, Wu J, Li XK and Zhang JS: Fibroblast growth factor 10 in pancreas development and pancreatic cancer. Front Genet. 9:4822018. View Article : Google Scholar : PubMed/NCBI | |
|
Memarzadeh S, Xin L, Mulholland DJ, Mansukhani A, Wu H, Teitell MA and Witte ON: Enhanced paracrine FGF10 expression promotes formation of multifocal prostate adenocarcinoma and an increase in epithelial androgen receptor. Cancer Cell. 12:572–585. 2007. View Article : Google Scholar : PubMed/NCBI | |
|
Matsuike A, Ishiwata T, Watanabe M and Asano G: Expression of fibroblast growth factor (FGF)-10 in human colorectal adenocarcinoma cells. J Nippon Med Sch. 68:397–404. 2001. View Article : Google Scholar : PubMed/NCBI |