Function of SP1 in tumors and focused treatment approaches for immune evasion (Review)
- Authors:
- Published online on: August 14, 2025 https://doi.org/10.3892/ol.2025.15230
- Article Number: 483
-
Copyright: © Wang et al. This is an open access article distributed under the terms of Creative Commons Attribution License.
Abstract
Introduction
Ovarian cancer has one of the highest mortality rates among gynecological malignancies, largely because early-stage disease lacks reliable diagnostic markers. Research has indicated that overexpression of specificity protein 1 (SP1) is strongly linked to ovarian cancer. Previous studies have shown that SP1 interacts with GC-rich promoters to control gene expression (1). SP1-encoded proteins participate in several biological processes, including cell differentiation, apoptosis, immunological responses, chromatin remodeling and DNA damage response. Post-translational changes (such as acetylation, phosphorylation, glycosylation and protein hydrolysis) impact the biological function of this protein (1). Overexpression of SP1 can drive cancer progression by increasing the expression of genes that enhance cell proliferation, invasion, metastasis and chemotherapy (2). It has been identified that both the increase and decrease in SP1 levels control oncogenes, thereby influencing cancer spread and tumor development (3,4). SP1 accelerates tumor formation by stimulating blood vessel growth and preventing cell death in cancer cells. High levels of SP1 expression enhance the autophagic flux, which promotes tumorigenesis (5). In addition, the levels of SP1 in tumor-derived exosomes increase, which favors the secretion of interleukin (IL)-1β by neutrophils through the activation of the Toll-like receptor 4 (TLR4)-nuclear factor-κB (NF-κB) pathway, ultimately aggravating lung metastasis of breast cancer (6). It is expected that research in this area will greatly benefit antitumor treatment.
Structure and polymorphism of SP1
Structural features of SP1
The structural features of SP1 were initially identified by Dynan and Tjian in 1983; the protein was recognized as a promoter-specific binding factor necessary for transcribing the crucial immediate early gene of the simian vacuolating virus 40 polyomavirus (7). SP1 belongs to a group of transcription factor-specific proteins that also include C2H2-type zinc fingers. Members of the SP family exhibit multiple similarities with the Krüppel-like family (KLF) (8). SP family transcription factors bind to the GC-frame of the promoter region of the target gene, while KLF has a preference for the CACCC-frame of the promoter region of the target gene (9). SP family members are categorized into two main groups, namely SP1-4 and SP5-9. SP1-4 share structural similarities, whereas SP5-9 are more akin to KLF (10).
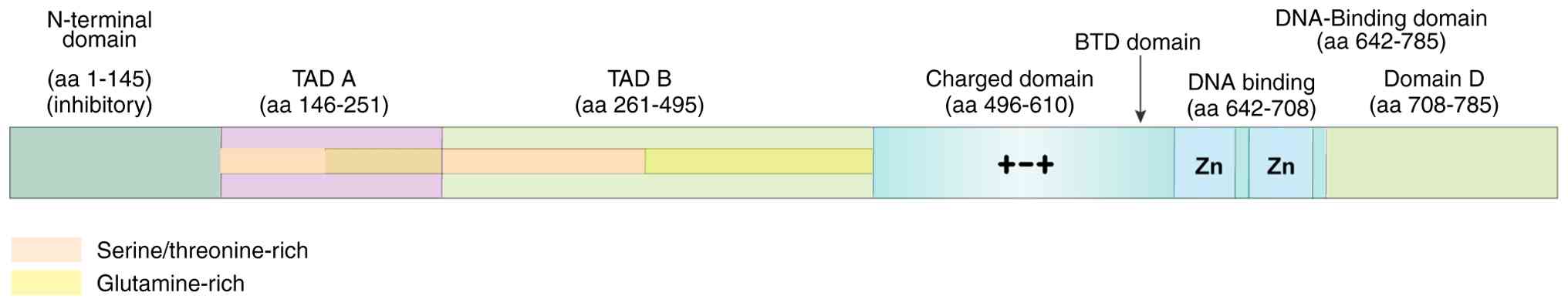
SP1 is organized into four structural domains: i) A double-stranded DNA-binding domain; ii) the SP1 transcriptional activity domain; iii) a buttonhead (Btd) domain; and iv) the SP domain (10). The C-terminus of SP1 has three standard Cys2His2 zinc finger structures. Two of these structures are located near the N-terminus, and each bind to one of the four bases of double-stranded DNA; the other two bases of DNA are attached to the zinc finger structure close to the C-terminus. This unique configuration enhances the capability of SP1 to bind to the target gene promoter.
The transcriptionally active part of SP1 consists of two glutamine-rich transactivation domains [tight adherence (TAD)A and TADB], namely the charged domain and C-terminal domain. These components interact to create a tetramer. The SP1 tetramer is formed by coordinating TADA, TADB and the D region, causing the DNA in the promoter region of the target gene to bend into a ring. This allows the SP1 protein to bind to the promoter and initiate target gene expression (11). The Btd and SP domains may influence SP1 transcriptional activity, which is associated with the hydrolysis of the SP1 protein (Fig. 1) (12). It has been reported that the transcription factor SP1 is abundantly present in mammalian cells and controls the expression of several genes and the biological functions of cells. Previous research has classified it as a housekeeping gene (13). SP1 is also implicated in the regulation of biological processes in colorectal, gastric, breast, ovarian and lung cancer cells. A previous study has demonstrated that SP1 regulates the expression of genes linked to tissue development (14). Examination of chromosomal gene sequences has revealed that the human genome includes 12,000 binding sites for SP1, which control the majority of cellular functions (15). In addition, SP1 interacts with several proteins and factors in the cell, such as other transcription factors, components of the transcription initiation complex and epigenetic factors (16).
Association of SP1 polymorphisms with its function and tumor process
SP1 function is affected by single nucleotide polymorphisms and structural domain variants, which in turn affect tumorigenesis, metastasis and drug resistance (17). It has been shown that SP1 interacts with interferon-inducible protein 16, activates heme oxygenase 1 transcription, inhibits iron death and leads to radiotherapy resistance. Therefore, patients with gliomas showing high clinical expression of SP1 tend to be resistant to temozolomide (TMZ) and have a poorer prognosis (18).
Mutations in the zinc finger (ZNF)3 structural domain of SP1, which is essential for liquid-liquid phase separation (LLPS), can affect oncogenic activity. For example, in lung adenocarcinoma, SP1 promotes metastasis by forming nuclear condensates through phase separation, recruiting coactivators [such as p300 and histone deacetylases (HDACs)], and activating the expression of regulator of G protein signaling factor 20 (RGS20) (19).
Transmembrane structural domain variants also influence the oncogenic effects of SP1. It has been shown that, in low-grade fibromucinous sarcoma, FUS-cAMP responsive element binding protein 3 like 2 fusion proteins remodel the endoplasmic reticulum through phase separation, leading to aberrant protein hydrolysis, oncogenic fragmentation into the nucleus and activation of oncogenes (20).
SP1 polymorphisms cause TMZ resistance in glioblastoma. SP1 inhibits TMZ-induced DNA damage and apoptosis by upregulating cytochrome P450 17A1 family 17 subfamily A member 1, which promotes dehydroepiandrosterone production (21). Betulinic acid inhibits SP1 and activates the PKR-like ER kinase/CCAAT-enhancer-binding protein homologous protein apoptotic pathway, thereby enhancing TMZ efficacy (22).
SP1 promotes immune escape by regulating programmed cell death-ligand 1 (PD-L1) and metabolic reprogramming. Circular RNA-encoded protein downstream of SP1 (circPETH-147aa) enhances pyruvate kinase M2 (PKM2) activity through m6A modification and inhibits CD8+ T cell function. However, the natural compound norathyriol blocks circPETH-147aa and restores the efficacy of anti-programmed cell death protein-1 (anti-PD-1) (23). Polymorphisms in SP1 play a key role in tumorigenesis, metastasis and therapeutic resistance by affecting its transcriptional activity, phase-separation ability and downstream signaling pathways. Future studies should focus on developing precise therapeutic strategies targeting different SP1 variants.
Mechanism of action of SP1
Molecular mechanism of SP1
SP1 primarily functions by recruiting the underlying transcriptional complex and interacting with components of the transcription factor II D (TFIID) complex. The TFIID complex comprises the TATA-binding protein (TBP) and other TBP-associated factor proteins. These proteins attach to the gene promoter, initiating the creation and assembly of the transcription start complex. The interaction mechanism of SP1 can be modulated by various transcriptional activators (24). Previous research indicates that the state of chromosomes can influence the transcriptional activity of SP1. SP1 can directly interact with histone acetylase P300, thereby altering the chromosomal structure to a more relaxed state and increasing the DNA-binding capacity of SP1 (25). A previous study has demonstrated that SP1 and P300 can interact in the promoter area of P21 in neural precursor cells to activate P21 expression, which inhibits cell proliferation and induces cell cycle arrest (26). Previous research identified that the activation of reporter genes in the Drosophila SE2 cell line was moderate when SP1 expression vectors were created along with reporter genes and only one SP1-binding site was present. However, the presence of two SP1-binding sites induced a 78-fold increase in the level of transcription (27). Electron microscopy findings indicated that the cooperative activation of proximal and distal binding sites was facilitated by the bending of the intermediate DNA double strand, allowing SP1 proteins situated at both ends to engage with each other (28). SP1 initially assembles into a tetramer in the promoter region of a target gene during transcription initiation. This observation indicates that transcriptional synergy of SP1 occurs through the formation of a tetramer by multiple SP1 monomers in the promoter region of the gene (29).
Previous gel blocking experiments have demonstrated that the first SP1 monomer binds to the promoter of each gene during interactions with the target genes. As the concentration of SP1 protein increases, a second SP1 protein molecule sequentially appears on the gene promoter (16). These findings indicate that the synergistic transcriptional activation by SP1 is not attributable to enhanced DNA binding affinity, but rather to a cooperative enhancement of transcriptional output that surpasses the additive effects of SP1 and DNA transcription alone (16). SP1 is active in all cell types and circumstances; however, its activity is tightly regulated, leading to varied expression results for several tumor suppressor and oncogene genes (16). One of those processes involves interaction with other proteins. For example, octamer transcription factor 1 (Oct1) interacts with structural domain B and the serine/threonine-rich area near SP1. This enhances its DNA-binding affinity by binding to distant regulatory genes, thereby enhancing transcription (30). O-GlcNAc in a serine/threonine-rich region of SP1 prevents the interaction between SP1 and Oct1, inhibiting the activation of U2 small nuclear RNA genes by these proteins (31). Estrogen can bind to SP1 to activate transcription. For example, when the endoplasmic reticulum binds to SP1, it increases the SP1-DNA binding to estrogen response elements even in the absence of estrogen. However, the transcriptional activation of the gene is only enhanced in the presence of estrogen. This indicates that the regulation of SP1 influences the transcriptional outcome differently (32).
Regulatory mechanism of SP1
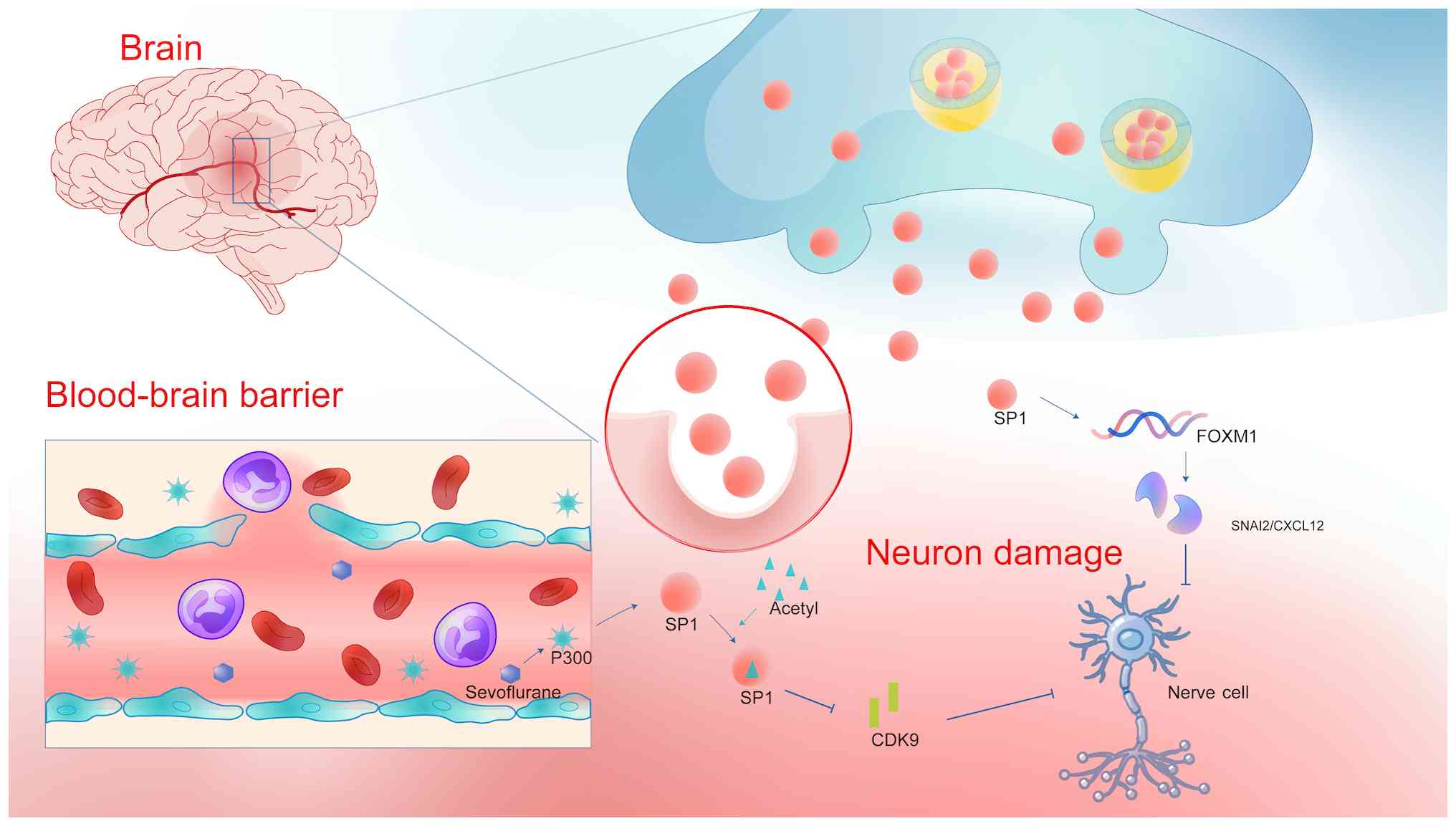
Jin et al (33) reported that myeloma cells with increased expression of the IQ motif containing GTPase activating protein 1 (IQGAP1) gene stimulate the Ras/Raf/MEK/extracellular signal-regulated kinase (Ras/Raf/MEK/ERK) pathway. A previous bioinformatics study identified SP1 as an upstream regulator of IQGAP1. SP1 binding to the IQGAP1 promoter region was confirmed by chromatin immunoprecipitation analysis. The study found that blocking SP1 or P300 reduced the levels of ERK1/2 and IQGAP1, whereas increasing SP1 or P300 levels exerted the opposite effect. Increased expression of SP1 or P300 substantially enhanced the activity of the IQGAP1 gene promoter, with the SP1/P300 complex controlling IQGAP1 gene expression in myeloma cells. Zhou et al (34) reported that sevoflurane-induced P300 suppressed SP1 activity by enhancing SP1 acetylation, reduced cyclin-dependent kinase (CDK)9 expression and stimulated neuronal death. Dong and Gao (35) observed high expression levels of SP1, forkhead box M1 (FOXM1), Snail family transcriptional repressor 2 (SNAI2) and C-X-C motif chemokine ligand 12 (CXCL12) in mice and MN9D cells damaged by rotenone. FOXM1 suppression delayed rotenone-induced damage to dopaminergic neurons in vitro. Experimental evidence demonstrated that SP1 contributed to dopaminergic neuronal damage by activating the FOXM1/SNAI2/CXCL12 pathway in living organisms. SP1 silencing provides a neuroprotective impact on dopaminergic neurons, indicating that dopaminergic neurons rely on the inactivated FOXM1/SNAI2/CXCL12 axis (Fig. 2) (35).
Furthermore, the expression of SP1 varies over time and under different circumstances within the same malignancy. SP1 expression is elevated in cancer cells and tissues compared with normal cells and tissues. However, SP1 is closely regulated during both the initial and advanced phases of tumor development, thus impacting the evolution of cancer. Its expression levels are significantly increased in lung cancer cells exhibiting low invasiveness and in individuals diagnosed with stage I lung adenocarcinoma. SP1 expression was decreased in lung cancer cells exhibiting high invasiveness and in stage IV lung adenocarcinoma. Furthermore, SP1 has an inverse regulatory effect on the migration, invasion and metastasis of lung cancer cells in living organisms. The expression levels of SP1 were reduced in highly invasive lung cancer cells because the SP1 protein became unstable. Enhanced SP1 expression in highly invasive lung adenocarcinoma cells upregulated E-calmodulin, a metastasis inhibitor, and attenuated the translocation of β-catenin into the cell nucleus that leads to tumor malignancy (36). Previous studies on lung cancer cohorts have demonstrated that SP1 is elevated and stimulates cancer progression in the majority of patients with early-stage lung cancer. In advanced lung cancer, low SP1 levels are linked to a negative prognosis. E2 raises RING finger protein 4 (RNF4) to decrease SP1 levels, which enhances CD44 expression by reducing microRNAs (miRNAs or miRs), thereby resulting in a poor prognosis for young women with lung cancer (37). Therefore, treatment approaches aimed at suppressing SP1 may not be appropriate for all patients with lung cancer, regardless of their stage of disease.
Molecular mechanisms of SP1 in migration and invasion
SP1 is a widely expressed transcription factor. A previous study has revealed that SP1 plays a key role in the migration and invasion of numerous cancer types; its main molecular mechanisms include transcriptional regulation of pro-metastatic genes, modulation of the WNT signaling pathway to promote metastasis, enhancement of cancer-promoting transcription by LLPS, metabolic reprogramming to promote invasion and immune microenvironmental regulation (38). SP1 promotes cancer cell migration and invasion through direct activation of several genes associated with epithelial-mesenchymal transition (EMT) and extracellular matrix remodeling. For example, the promoter of vimentin binds to SP1 to promote expression and enhance cancer cell migration (38). In addition, SP1 inhibits E-cadherin, and promotes cancer cell dedifferentiation and invasion by upregulating Snail/Twist (39). Regarding the regulation of the WNT signaling pathway, SP1 drives the activation of WNT/β-catenin signaling, and promotes the dynamic communication between cancer cells and the microenvironment, which enhances the invasiveness of cancer cells. In addition, WNT signaling can further activate c-Myc and cyclin D1, thus promoting cancer cell proliferation and metastasis (40).
SP1 enhances pro-oncogenic transcription through LLPS. A previous study has shown that the demethylase inhibitor GSK-J4 disrupts SP1 phase separation and inhibits its pro-metastatic activity (19), suggesting that targeting SP1-LLPS may be a new anti-metastatic strategy. SP1 regulates key enzymes of glycolysis, such as PKM2 and lactate dehydrogenase A to promote the Warburg effect, enhance cancer cell energy supply and support migration (41).
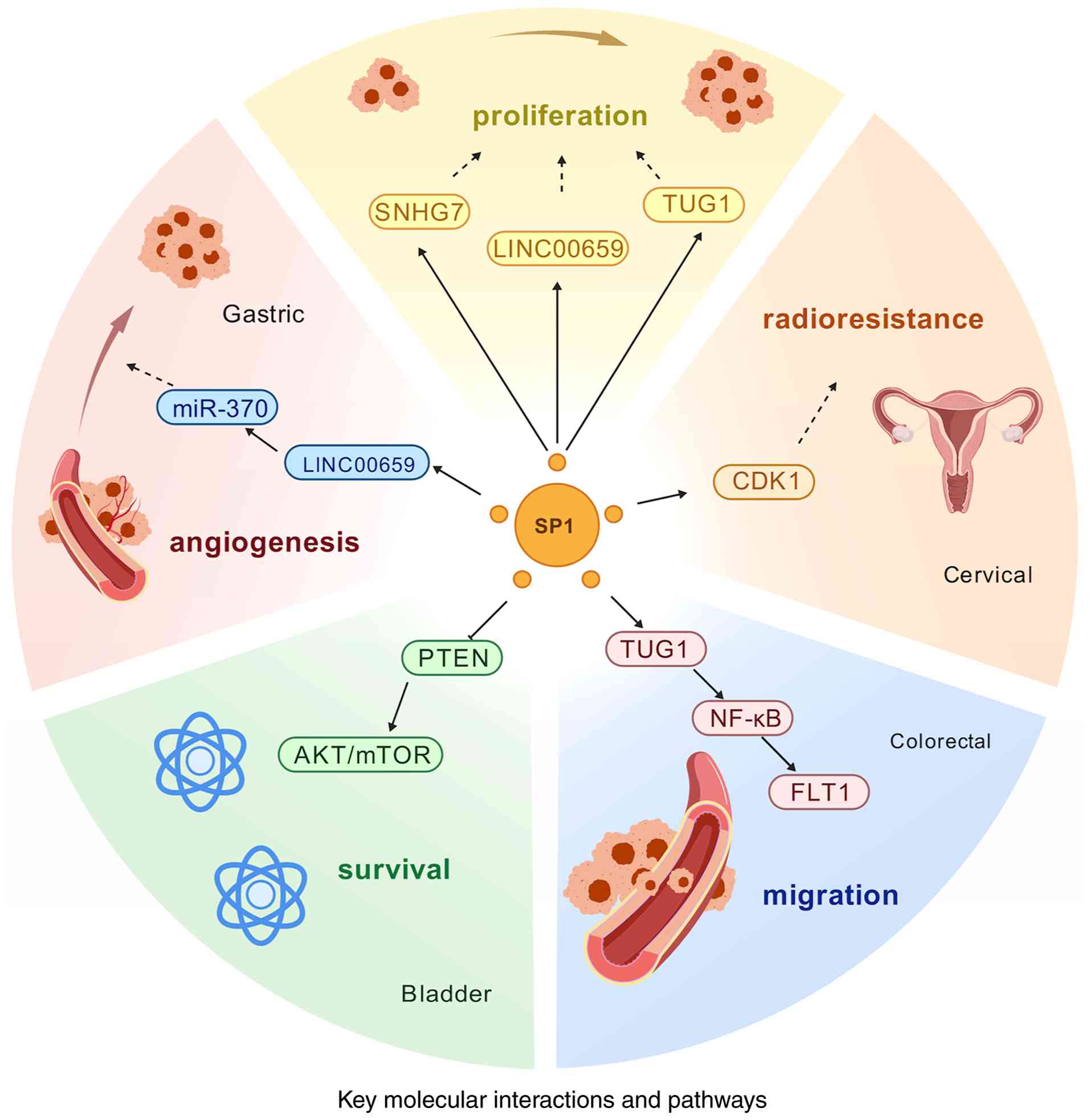
In conclusion, SP1 promotes cancer cell migration and invasion through transcriptional regulation, phase separation and other mechanisms. Targeting SP1 or its downstream effector molecules, such as WNT and PKM2, may become a new direction for anti-metastatic therapy. However, challenges such as off-target effects and drug resistance remain to be addressed (Fig. 3).
SP1 is associated with cardiomyopathy
SP1 is important in controlling tumor growth and is a major factor in the development of common illnesses. Ström et al (30) observed that mice with reduced SP1 levels developed hypertrophic cardiomyopathy, which was characterized by significant cardiac hypertrophy, interstitial fibrosis and disorganized myofilament fibers. Furthermore, inhibition of SP1 led to a significant increase in the cell area of the human induced pluripotent stem cell-derived cardiomyocytes and caused intracellular myofibrillar disorganization similar to that of hypertrophic cardiomyocytes in hypertrophic cardiomyopathy. Tuftelin 1 was identified as an essential target gene for SP1. SP1 overexpression inhibited the progression of hypertrophic cardiomyopathy in myosin heavy chain R404Q/+ mutant mice and corrected the hypertrophic characteristics in human induced pluripotent stem cell-derived cardiomyocytes with hypertrophic cardiomyopathy. SP1 may be a promising target for the treatment of hypertrophic cardiomyopathy.
SP1 and regulation of the tumor microenvironment (TME)
Involvement of SP1 in the formation of an immunosuppressive microenvironment
Upregulated miR-21 in tumor-associated mesenchymal stem cells diminishes the DNA methylation level of the miR-21 promoter region via the SP1/DNA methyltransferase 1 pathway, thus facilitating the elevated expression of miR-21 and augmenting the immunosuppressive capacity of myeloid-derived suppressor cells (42).
Immunosuppressive function of tumor-associated macrophages (TAMs)
TAMs suppress T-cell function both directly and indirectly through multiple effects, including the expression of immunological checkpoints such as PD-L1, the production of inhibitory cytokines (such as IL-10 and transforming growth factor-β) and modifications in metabolic activity. TAMs restrict T-cell infiltration by modulating the vascular architecture and extracellular matrix, thereby preventing T cells from accessing intratumoral regions. Exosomal miR-21-5p in hepatocellular carcinoma (HCC) cells influences HCC cell progression via modulating SP1/X-box binding protein 1 (XBP1) and facilitating the M2 polarization of TAMs, consequently impacting the unfavorable prognostic outcomes of patients with HCC (43).
Regulation of neutrophil function
Chronic stress leads to increased SP1 levels in tumor-derived exosomes, which are internalized by lung neutrophils, thereby stimulating the release of IL-1β via the TLR4-NFκβ-κB pathway. This results in an immunosuppressive milieu that aggravates lung metastasis of breast cancer (6).
Influence on macrophage polarity
The polarity of macrophages, as indicated by CXCL9 and SPP1, is a crucial characteristic of the TME (44). The CXCL9:SPP1 ratio can delineate the prevalence of antitumor immune cells within a tumor, the gene expression profiles of each tumor-infiltrating cell type, and can quantify tumor control or progression, the modulation of communication networks and the response to immunotherapy (45).
Influence on immune cell infiltration
In HCC, SPP1+ macrophages and cancer-associated fibroblasts co-localize in the tumor periphery, creating a tumor immune barrier that obstructs T-cell infiltration into the tumor core, resulting in suboptimal immunotherapy outcomes. Conversely, in a mouse model with SPP1 knockdown, the therapeutic efficacy improved following anti-PD-1 treatment, indicating that SPP1 may modulate the immunological milieu by affecting immune cell infiltration (46).
SP1 in immune escape
Role in gastric cancer
Chemotherapeutic drugs enhance chromatin accessibility and facilitate self-activation of the SP1 gene promoter region in gastric cancer cells. Activated SP1 enhances the expression of solute carrier family 6 member 6 (SLC6A6), leading gastric cancer cells to absorb increased quantities of taurine from the microenvironment to mitigate the effects of chemotherapeutic agents. Concurrently, this results in taurine depletion within the microenvironment, which diminishes the expression and functionality of immune checkpoints in CD8+ T cells, ultimately yielding a suboptimal response to immune checkpoint inhibitors in patients. The SP1-SLC6A6 regulatory axis is a crucial element connecting chemotherapy and immunotherapy resistance. It offers a clear molecular rationale for the clinical observation of diminished effectiveness of immune checkpoint inhibitors following treatment with numerous chemotherapy regimens (47).
Role in colorectal cancer
Histone demethylase Jumonji domain containing 2D (JMJD2D) functions as a transcriptional coactivator for the factors SP1, signal transducer and activator of transcription 3 (STAT3) and interferon regulatory factor transcription factor (IRF)-1. The interaction between JMJD2D and the DNA-binding domain of SP1 results in increased production of the interferon-γ receptor 1 gene. STAT3 and IRF1 collaborate with the coactivator JMJD2D to increase PD-L1 gene expression, resulting in immune evasion in colorectal cancer (48).
Role in cellular pyroptosis
The transcription factor SP1 binds to the promoter region of gasdermin-E (GSDME), thereby enhancing the expression of GSDME, a crucial execution protein in tumor cells experiencing pyroptosis. This process is initiated by the cleavage of the upstream caspase-3 (CASP3) protein, leading to the formation of a detrimental pore-like structure in the cell membrane, resulting in cell death, rupture and release of inflammatory mediators. This procedure is critical for the survival of tumor cells and their method of cell death. Inhibition of this system increases tumor cell resistance to chemotherapeutic agents, exhibiting a synergistic interaction with the STAT3 transcriptional regulatory pathway and an antagonistic association with DNA methylation. Silencing of SP1 or pharmacological inhibition diminishes GSDME expression, thereby decreasing the N-terminal levels of GSDME, which is cytotoxic during pyroptosis. This effect eventually reduces cell death and the release of cellular contents (49).
Role of SP1 in different tumors
SP1 is linked to a diverse array of malignancies (≥20 types), most notably colorectal, breast, pancreatic, lung and liver cancer.
SP1 in colorectal cancer
Multiple studies have indicated that SP1 serves as a connector between crucial oncogenic and metastatic signaling pathways in colorectal cancer. It is involved in tumor cell movement, invasion, EMT and response to treatment. Huang et al (50) demonstrated that SP1 interacted with the taurine upregulated 1 (TUG1) promoter to control its expression, leading to the upregulation of TUG1. This upregulation contributes to the oncogenic characteristics of colorectal cancer.
Previous studies employed immunoblotting to confirm that the colon cancer 1 (MACC1)/MET signaling pathway associated with oncogenic metastasis is deactivated due to miR-320a-induced SP1 downregulation, which enhances MACC1 transcription (51). Inhibition of miR-320a by SP1 leads to proliferation and invasiveness of colorectal cancer cells (51). Based on cell function studies, Li et al (52) found that miR-1224-5P suppressed colorectal cancer cell motility, invasiveness and EMT by targeting SP1. Furthermore, SP1 stimulated the phosphorylation of P65, thereby promoting the advancement of EMT in colorectal cancer cells. In summary, SP1 facilitates the NF-κB signaling pathway to enhance metastasis and EMT in colorectal cancer (52). Xu et al (53) demonstrated that miR-375 limits the growth of colorectal cancer cells via targeting SP1. Yu et al (54) showed that SP1 increased the levels of long noncoding RNA (lncRNA) terminal differentiation-induced noncoding RNA (TINCR), leading to the advancement of colorectal cancer by acting as a sponge for miR-7-5P. Chen et al (55) showed that SP1 stimulated ZNFX1 antisense RNA 1 to increase vascular endothelial growth factor A (VEGFA) levels via binding to miR-150-5P, thereby enhancing the development of colorectal cancer (55). Sun et al (56) reported that SP1 played a role in the suppressive impact of miRNA-382 on cell proliferation and movement in colorectal cancer. Furthermore, SP1 plays a vital role in colorectal cancer migration and invasiveness through several pathways, including the miR-150-5P/VEGFA axis (55), the death receptor 4/neurofibromin 1 switch axis (57) and the WNT/β-collagen pathway (58). It was established that SP1-dependent promoter progenitors stimulate FOXO3A gene transcription in colorectal cancer cells, and upregulation of the FOXO3A gene by SP1 is necessary for the development of colorectal cancer (59). The aforementioned experimental investigations concerning SP1 offer a foundation for prospective targeted treatment in patients with colorectal cancer.
SP1 in gastric cancer
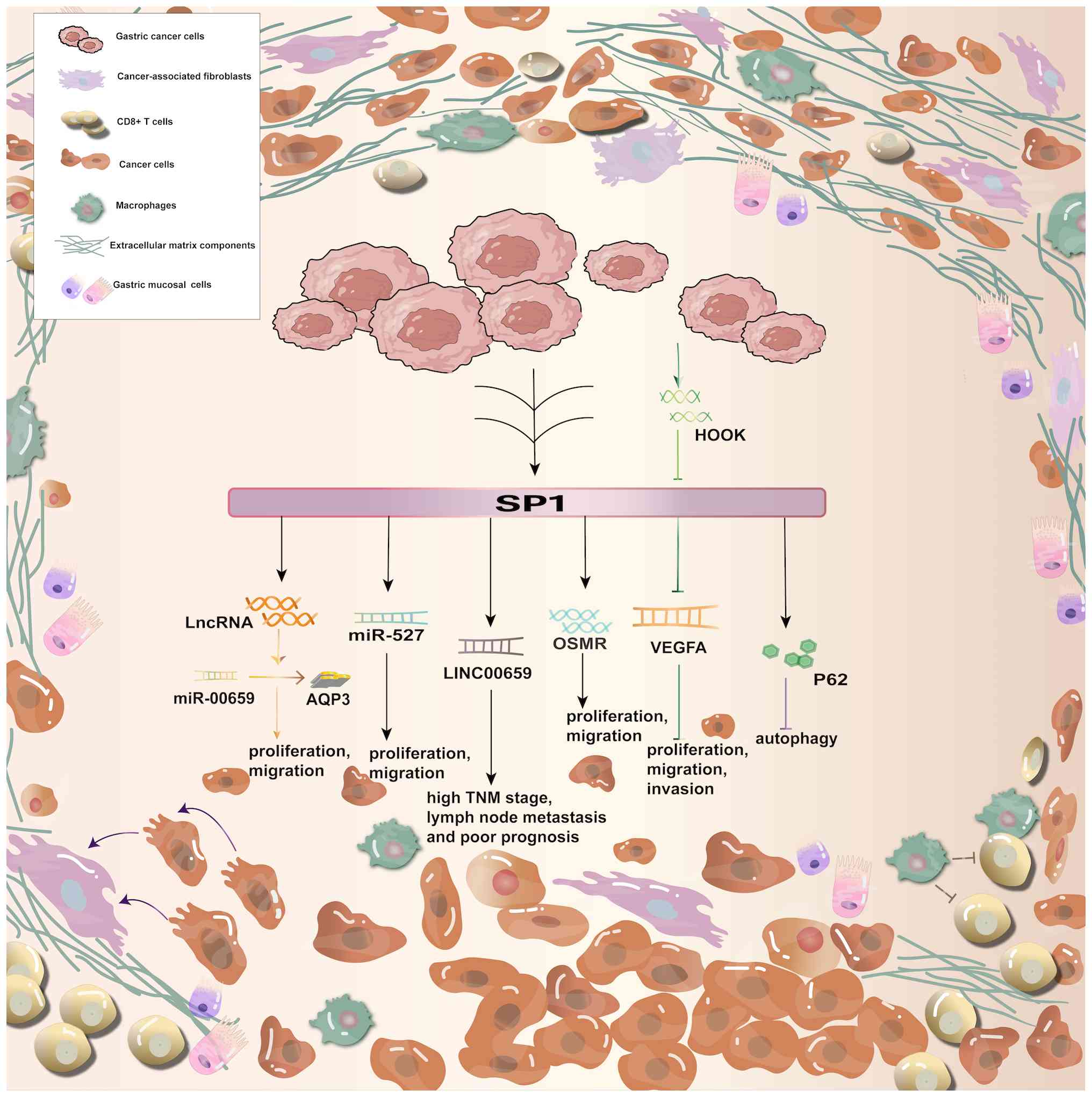
Shi and Zhang (60) determined that SP1 expression was positively correlated with the degree of tumor infiltration, tumor-node-metastasis (TNM) stage, lymph node metastasis and Lauren stage; however, it was not correlated with tumor differentiation. Kaplan-Meier analysis demonstrated that SP1 mRNA expression was inversely related to the overall and progression-free survival of patients with gastric cancer. Additionally, SP1 protein expression was elevated in gastric cancer tissues compared with normal tissues, and was linked to the depth of infiltration and TNM stage of gastric cancer. The inverse association between tripartite motif containing 25 (TRIM25) and SP1 protein levels in human gastric cancer tissues confirms that elevated SP1 levels and decreased TRIM25 levels are associated with a worse outcome for patients with gastric cancer (61). Xu et al (5) revealed that SP1 activated P62, subsequently reducing the autophagic flux of the cells. Furthermore, the absence of SP1 led to an elevated autophagy rate in gastric cancer cells. That study presented evidence of a new method for controlling autophagy in gastric cancer cells. Wang et al (62) validated that SP1 could increase the expression of long intergenic non-protein coding RNA 659 (LINC00659) in gastric cancer. Clinical analyses revealed a correlation between elevated LINC00659 levels and TNM stage, lymph node metastasis and a worse prognosis. The authors also experimentally verified that SP1 triggered the increase of lncRNAs, which controlled the miR-00659/ aquaporin 3 axis, consequently fostering the progression of gastric cancer. Zhang et al (63) demonstrated that SP1 is important in advancing gastric cancer, and its participation in the miR-527/SP1 axis enhances the proliferation and spread of gastric cancer cells. Yu et al showed that SP1 interacts with the promoter region (−255 to −246 region) of the human oncostatin M receptor (OSMR) gene, as well as with OSMR that is overexpressed in gastric cancer cells. Consequently, SP1 has a positive regulatory function in promoting the development and metastasis of gastric cancer cells through this gene (64).
Pan et al (49) showed that SP1 promotes the spread of gastric cancer through fatty acids. Previous research verified that miR-149 reduced the expression of zinc finger and BTB domain containing 2 and SP1, thus suppressing the cancer-promoting activity of gastric cancer associated transcript 1 (53). SP1 can increase urothelial cancer associated 1 expression in gastric cancer, and enhance gastric cancer cell proliferation by engaging enhancer of zeste 2 polycomb repressive complex 2 subunit (EZH2) and activating the protein kinase B (AKT) pathway specific to gastric cancer (57). Recent research indicates that hook microtubule tethering protein 3 (HOOK3) controls VEGFA expression in gastric cancer cells by suppressing SP1, and restrains the proliferation, movement and infiltration of gastric cancer cells. The mechanism via which HOOK3 controls SP1 remains unidentified and requires further investigation (65). SP1 is essential for the development and spread of gastric cancer. Further studies are warranted to fully understand the mechanisms of SP1 and its role in regulation (Fig. 4).
SP1 in breast cancer
Previous studies have shown that SP1 increases the expression of TINCR, leading to enhanced cell proliferation, growth anchoring and decreased apoptosis in breast cancer cells (66). Monteleone et al (67) reported that STAT3 and SP1 may act together to promote increased Ras homolog family member U levels and enhance the migration of breast cancer cells. Li et al (68) observed that miR-212-3p suppressed VEGFA expression through SP1, leading to decreased angiogenesis in breast cancer. SP1 is responsible for epigenetic dysregulation, and is involved in determining the responsiveness of human epidermal growth factor receptor 2-overexpressing breast cancer to treatment with HDAC inhibitors (69). It was reported that miR-539 functioned as a tumor suppressor by targeting SP1 (70). Zhang et al (71) noticed the involvement of TIMELESS circadian regulator and SP1 in sphingolipid metabolism, with SP1 playing a role in promoting the development of breast cancer. SP1 is implicated in several signaling pathways in breast cancer, including the miR-2110/SP1 axis (72) and ERK/SP1 signaling network (73). Previous research has shown that miR-506 decreases the methylation of maternally expressed 3 through a SP1/SP3-dependent process, leading to a decrease in breast cancer cell motility and invasiveness (74). SP1 is essential in several signaling pathways related to breast cancer, including the miR-539/SP1, miR-212-3p/SP1 and miR-2110/SP1 axes, and the ERK/SP1 signaling pathway.
SP1 in ovarian cancer
Bai et al (75) found that the SP1 molecule triggered the activation of lncRNA small nucleolar RNA host gene (SNHG)7 and interacted with EZH2 to enhance the development of ovarian cancer. Cui et al (76) demonstrated that SP1 stimulated differentiation antagonizing non-protein coding RNA to facilitate ovarian cancer development. Previous studies have demonstrated a strong positive correlation between the expression levels of exosomal CUB domain containing protein 1 (CDCP1) and SP1 in patients with ovarian cancer. Both proteins are highly expressed in ovarian cancer cells. Furthermore, SP1-regulated matrix metalloproteinase (MMP)2 and MMP9 proteins are positively correlated with CDCP1, suggesting a synergistic association between CDCP1 and SP1. However, the exact mechanism underlying this association remains unknown (77). Previous research suggests that exosomal miR-21-5p in HCC cells can affect HCC cell development by controlling SP1/XBP1 and encouraging the M2 polarization of TAMs, ultimately influencing responses associated with poor prognosis in patients with HCC (43).
Wang et al (78) demonstrated that ferritin-1 hindered cisplatin-induced ovarian damage and granulosa cell death both in vivo and in vitro, thus expanding on the connection between SP1 and ovarian illness. The expression levels of acyl-CoA synthetase long chain family member 4 (ACSL4) and glutathione peroxidase 4 were considerably and simultaneously altered. The ACSL4 inhibitor rosiglitazone reduced ovarian damage in mice treated with chemotherapy. Cisplatin enhanced the expression of SP1, which in turn bound to the promoter of ACSL4 to boost transcription (78). SP1 is crucial in the development of ovarian cancer, as well as in ovarian damage and granulosa cell death. However, additional studies are required to identify the precise mechanism involved in these processes.
SP1 in lung cancer
SP1 is closely controlled at various stages of lung cancer, influencing cancer cell advancement in diverse ways at different points in time. Young et al (37) found that decreased SP1 expression in premenopausal patients with advanced lung cancer was associated with a poor prognosis. The study verified that estradiol suppressed SP1 levels, resulting in decreased miR-3194-5P expression and increased CD44 expression, eventually promoting cancer advancement. Cancer stem cell-related proteins can enhance SP1 activity. In non-small cell lung cancer, resistance to pemetrexed was closely linked to SP1 activity (79). Hu et al (80) showed that the decaprenyl diphosphate synthase subunit 2 (PDSS2) promoter harbored binding sites for SP1 and GATA binding protein 1. SP1 and PDSS2 expression are negatively regulated, and increased SP1 expression and decreased PDSS2 expression are strongly linked to a poor prognosis in lung cancer. In lung cancer cells, the suppression of PDSS2 transcription by SP1 leads to pathogenicity. Previous research has shown that SP1 is involved in the AKT and ERK1/2 signaling pathways in non-small-cell lung cancer, promoting cancer cell proliferation and migration (81). In addition, the miR-326/SP1/KLF3 axis plays a role in the development of lung cancer (82). These findings demonstrate the important contribution of SP1 to lung cancer advancement.
SP1 in prostate cancer
Wang et al (83) demonstrated that SP1 increased SNHG4 expression in prostate cancer. The elevated SNHG4 levels were strongly associated with lymph node metastases, tumor stage and worse overall survival in patients with prostate cancer. Experimental evidence revealed that SP1 enhanced the progression of prostate cancer by increasing the expression of SNHG4. Previous research indicated that SP1 triggered the WNT/β-cyclin signaling pathway and facilitated EMT, leading to increased proliferation and invasiveness of prostate cancer cells. Furthermore, overexpression of glypican 5 (GPC5) counteracted this effect, suggesting that GPC5 exerts a cancer-suppressing effect by inhibiting SP1. In summary, GPC5 can have a cancer-suppressive impact by blocking SP1, and SP1 affects lymph node metastasis and tumor stage in prostate cancer by increasing SNHG4 expression. Additionally, SP1 triggers the WNT/β-linker protein signaling pathway, leading to EMT. This process enhances the proliferation and invasiveness of prostate cancer cells, and reduces overall survival in patients.
SP1 in cervical cancer
Previous research has demonstrated that the activation of lung cancer associated transcript 1 by SP1 contributes to the development of cancer in the cervix by enhancing the proliferation, migration and invasiveness of cervical cancer cells (84). Deng et al (85) reported a notable correlation between high SP1 expression and advanced International Federation of Gynecology and Obstetrics stage, lymph node metastases, and lymph node interstitial infiltration in cervical cancer. Expression of SP1 in cervical cancer cell lines increased in a dose-dependent manner at both the mRNA and protein levels. SP1 impacted the longevity of cervical cancer cells following radiotherapy. Suppression of SP1 caused cell cycle arrest at the G2/M phase in cervical cancer cells, which led to a significant improvement in cell response to radiotherapy. Increased SP1 expression decreased G2/M cell cycle arrest in cervical cancer cells, which was associated with increased expression of CDK1. SP1 may hinder G2/M phase arrest and enhance the effectiveness of radiotherapy for cervical cancer by affecting CDK1 (85). Previous research indicated that magnolin (MGL), a chemical derived from the magnolia plant, exhibited inhibitory effects on tumor cell invasiveness and proliferation. MGL hindered cellular metastasis by affecting IL-10/IL-10 receptor B expression, which reduced the JUN N-terminal kinase/SP1-mediated production of MMP15, thereby influencing the cervical cancer microenvironment (86). SP1 plays an oncogenic function in cervical cancer by enhancing the proliferation, migration and invasiveness of cervical cancer cells. It also impacts the survival of these cells following radiotherapy, which is detrimental to patients. The introduction of MGL significantly reduced the negative effects of SP1 on the cervical cancer microenvironment. Further investigation should uncover the precise mechanism involved in this process, offering a theoretical foundation for the treatment of cervical cancer.
SP1 in osteosarcoma
Signal regulatory protein α (SIRPA) is increased in osteosarcoma tissues, particularly in metastatic tissues, and is linked to a worse prognosis. Reducing SIRPA levels results in decreased stability of SP1 and arginine absorption, which impacts the migration of osteosarcoma cells. SIRPA activates ERK to phosphorylate SP1, preventing its degradation by the proteasome. SP1 boosts solute carrier family 7 member 3 (SLC7A3) expression by attaching to the SLC7A3 promoter, which leads to increased arginine absorption and encourages the migration of osteosarcoma cells. Arginine improves the stability of SP1, creating the ‘SP1 stabilization loop’. Previous research demonstrated that increased SIRPA expression enhanced osteosarcoma migration through the ‘SP1 stability circle’ and SLC7A3-mediated arginine absorption (87). Lysine demethylase 3A (KDM3A) is abundantly present in osteosarcoma tissues and cells. KDM3A boosts SP1 gene expression by removing methyl groups from its promoter region. SP1 interacts with the 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 4 (PFKFB4) promoter to stimulate its transcription and enhance its expression. Overexpression of PFKFB4 markedly enhanced the proliferation and metastasis of osteosarcoma cells, and increased the glycolytic activity in these cells. Activation of the KDM3A-SP1 axis increased the expression of PFKFB4, which led to improved aerobic glycolysis in osteosarcoma and the promotion of tumor growth (88). Mi et al (89) showed that the SP1 gene was upregulated in osteosarcoma, facilitating osteosarcoma progression by increasing LINC00514 expression through competitive binding to miR-708. Hu et al (90) showed that the transcription factor SP1 functioned as a tumor promoter in osteosarcoma by increasing the interleukin enhancer binding factor 3-antisense 1 levels through the control of the miR-212-SRY-box transcription factor 5 pathway. Another study on osteosarcoma revealed that trans-chalcone impacted the expression of SP1 and P53 in transcription and proteasome regulation, respectively, thereby influencing the development of this cancer type (91).
SP1 in pancreatic cancer
LIM domain-containing protein Ajuba is a recently identified transcriptional co-regulator that is involved in the development of various types of cancer. Elevated protein and mRNA expression levels of Ajuba and SP1 in pancreatic cancer tissues are positively connected, and are associated with a worse prognosis in patients. Ajuba binds to the C-terminus of SP1 and functions as a coactivator to increase SP1 gene expression and stimulate cell proliferation. The Ajuba promoter includes active SP1 response elements, and Ajuba is a target gene of SP1. The Ajuba/SP1 complex can create a feed-forward loop that stimulates the transcription of SP1 target genes and enhances cell proliferation in pancreatic cancer. Ajuba and SP1 may serve as diagnostic markers and potential targets for the treatment of pancreatic cancer (92). The nuclear factor of activated T cells (NFAT) transcription factor plays a major role in the oncogenicity of pancreatic cancer. Tumor necrosis factor α (TNFα) is a target gene for the interaction between NFATc2 and SP1. It has been demonstrated that small interfering RNAs (siRNAs) decrease cell proliferation by blocking NFATc2, SP1 and TNFα (93). Through survival analysis, Xu et al (53) showed that adenylate kinase 4 pseudogene 1 (AK4P1) was a significant predictor of poor prognosis in patients with pancreatic cancer. Subcellular localization studies revealed that AK4P1 was mostly found in the cytoplasm, and may compete for binding with miR-375 in pancreatic cancer cells. SP1 has been identified as a possible gene that is regulated by miR-375 in pancreatic cancer. Previous expression analyses confirmed that SP1 could enhance AK4P1 levels in pancreatic cancer. The AK4P1/miR-375/SP1 pathway is crucial for the progression of pancreatic cancer (94). Previous research showed that protein phosphatase 3 catalytic subunit β inhibited pancreatic cancer progression by promoting atonal bHLH transcription factor 8 translocation and transcriptionally regulating SP1 (95). Yang et al (96) analyzed the transcription of Yes-associated protein 1 (YAP1) in pancreatic cancer cells, and found that the transcription factor SP1 was upregulated by the atypical protein kinase C isoform t and subsequently bound to multiple sites in the YAP1 promoter to drive the transactivation of YAP1 in pancreatic cancer cells carrying mutant KRAS (Kirsten rat sarcoma viral oncogene homolog (KRAS). Gao et al (97) suggested that propofol represses pathological biological behaviors associated with pancreatic cancer cells through the suppression of SP1.
SP1 in endometrial cancer
Shao et al (74) reported that SP1 was a direct target of miR-490. Knocking down SP1 could counteract the impact of miR-490 inhibition on the aggressive behavior of cancer cells. Knockdown of deleted in lymphocytic leukemia 1 (DLEU1) suppressed the phosphoinositide-3-kinase/AKT/glycogen synthase kinase 3β (PI3K/AKT/GSK3β) pathway induced by miR-490 suppression, while SP1 knockdown may counteract this pathway. These findings indicate that DLEU1 facilitates the development of endometrial cancer by controlling SP1 expression (98). Liu et al (99) identified natural killer cell-related genes involved in cytotoxicity, including CASP3. Further analysis indicated that CASP3 reduced the expression of poly (ADP-ribose) polymerase 1 (PARP1). In addition, analysis of data from the Transcriptional Regulatory Relationships Unraveled by Sentence-based Text database indicated that SP1 regulated CASP3. By conducting an in-depth investigation on uterine natural killer cell-related genes, it was suggested that the SP1-CASP3-PARP1 axis leads to recurrent miscarriage. Previous research has demonstrated that elevated SP1 levels promote the development of endometrial fibrosis (100), which is an important characteristic in individuals experiencing recurrent miscarriage (101). Elevated levels of SP1 were detected in ovarian endometriosis, and it was shown that miR-25-3p directly interacted with SP1. This evidence indicates a new miRNA/SP1 pathway in the development of endometriosis, although further investigation is warranted to determine the precise underlying mechanism (102).
SP1 in bladder cancer
Bladder cancer cells overexpress SP1. Suppression of SP1 hindered bladder cancer cell proliferation, migration and invasion, while enhancing programmed cell death. Phosphatase and tensin homolog (PTEN) enhanced tumor cell survival, migration and invasion, while decreasing cell death; these effects were reversed by SP1 depletion. Reducing SP1 levels lessened the activation of the AKT/mechanistic target of rapamycin kinase (AKT/mTOR) pathway induced by PTEN reduction. Previous in vivo experiments showed that reducing SP1 expression suppressed tumor development, elevated PTEN levels and reduced the expression of proteins linked to the AKT/mTOR pathway. SP1 enhanced the progression of bladder cancer by blocking the PTEN-mediated AKT/mTOR pathway (103). Elevated SP1 mRNA expression was observed in urothelial bladder cancer tissues compared with normal bladder tissues using reverse transcription-quantitative PCR. Immunohistochemistry revealed a strong correlation between elevated SP1 expression and histological grade, tumor stage, vascular invasion, lymph node metastasis and distant metastasis (P<0.05). According to the results of log-rank test, elevated SP1 expression in cancer tissues was associated with worse overall survival and disease-free survival compared with low SP1 expression (P<0.05). High levels of SP1 expression in bladder urothelial carcinoma may serve as a marker for identifying patients with a poor prognosis and aggressive disease (104). Yan et al (105) found that miR-300 suppressed the migration of bladder cancer cells via controlling the SP1/MMP9 pathway. The study revealed that miR-300 directly targeted SP1 and suppressed its expression by selectively binding to its 3′-non-coding region. Reducing MMP9 led to the migration of bladder cancer cells. SP1 promoted bladder cancer progression by inhibiting the PTEN-mediated AKT/mTOR pathway. Overall, the aforementioned findings indicate that SP1 has a significant impact on bladder cancer (Table I).
SP1 and clinical treatment
As a widely expressed transcription factor, SP1 regulates several pro-oncogenes, such as survivin and VEGF. However, direct inhibition of SP1 may affect the physiological functions of normal cells and lead to toxicity. In addition, due to the lack of highly selective SP1 inhibitors, existing drugs such as mithramycin A have limited clinical application due to side effects; thus, there is a certain degree of complexity in targeting SP1 inhibition (106).
SP1 promotes chemoresistance by upregulating anti-apoptotic proteins such as B-cell lymphoma 2 (Bcl-2) and metabolic reprogramming such as enhanced glycolysis (107). In addition, SP1 can synergize with KRAS signaling, thereby exacerbating immunosuppression in the TME and decreasing immunotherapeutic response, making resistance challenging (108). When the TME is impaired, the dense fibrotic mesenchyme of pancreatic cancer can hinder drug delivery (109), and SP1-driven EMT further promotes invasion and metastasis (110). EMT and metastasis can be reduced by developing SP1 inhibitors such as mithramycin A, which has inhibited SP1-DNA binding in preclinical models; however, further optimization is needed to reduce toxic effects such as myelosuppression. Additionally, efficacy can be enhanced by screening natural compounds such as curcumin derivatives, possibly by blocking SP1 phosphorylation, including in the AKT/ERK pathway (111).
Combination targeted therapy could be a promising approach. For example, SP1 combined with KRAS inhibition may be an option. SP1 is dependent on KRAS signaling, and co-inhibition can overcome drug resistance (112). Previous research has shown that DNA nanocarriers such as tubular DNA origami structures (~70 nm) can selectively deliver SP1 siRNA or chemotherapeutic agents to KRAS-mutant tumors to reduce off-target effects. Therefore, the development of nanodelivery technologies could be a promising intervention (113). In terms of immune microenvironment regulation, SP1 knockdown enhanced CD8+ T-cell infiltration; if combined with PD-1 inhibitors, it may reverse immune escape (114).
In the future, SP1 subtype- or structural domain-specific inhibitors, such as zinc finger domain blockers, could be developed to precisely target therapy and reduce systemic toxicity. In addition, the dynamic regulatory network of SP1 in tumor heterogeneity may be resolved by combining single cell sequencing and chromatin immunoprecipitation sequencing. Phase 1 clinical trials can also be advanced by optimizing the pharmacokinetics of SP1 inhibitors. Furthermore, SP1 inhibitors plus epigenetic drugs, such as HDAC inhibitors or lysoviruses, may be utilized to enhance tumor cell killing (115).
SP1 and clinical treatment
New strategies for immunotherapy
Regarding the role of circular RNA derived from PIAS1 (circPIAS1) in melanoma, a previous study revealed that the 108 amino acid peptide encoded by circPIAS1 markedly inhibited immunogenic iron death triggered by immune checkpoint inhibitor therapies by modulating the equilibrium between the SUMOylation and phosphorylation of STAT1, thus facilitating immune evasion. This finding offers an innovative approach to enhance the effectiveness of immune checkpoint inhibitor therapy (116). PD-1/PD-L1 immune checkpoint inhibitors represent an important advancement in cancer treatment; they work by blocking the interaction between PD-1 and PD-L1, thereby releasing T cells from inhibition and restoring their ability to attack cancer cells (76).
SP1 inhibitors and clinical therapy
In recent years, SP1 inhibitors, such as plicamycin/plicamycin/mithramycin A and IMB-S7, have been investigated for antitumor therapy. SP1 inhibitors inhibit the transcription of the oncogene RGS20 by blocking SP1-DNA binding. Furthermore, they inhibit super-enhancer-driven oncogene expression by disrupting the LLPS of SP1 (such as GSK-J4), in addition to enhancing the sensitivity of chemotherapy or immunotherapy by modulating the downstream PI3K/AKT signaling pathway (19).
Plicamycin inhibits the binding of SP1 to DNA by directly binding to its zinc finger structural domain. In lung adenocarcinoma, plicamycin inhibits the SP1-RGS20 axis and reduces tumor metastasis (19). In HCC, procamycin decreases GSDME expression, reduces chemotherapy-induced focal death and enhances drug resistance (49). Due to myelosuppression and hepatotoxicity, the clinical application of plicamycin is limited, and this drug is mainly used for adjuvant treatment of osteosarcoma and testicular cancer. Low-dose combination chemotherapy is currently being explored to minimize side effects.
A novel SP1 small molecule inhibitor called IMB-S7 inhibits the transcriptional activity of SP1 without affecting other GC-box binding proteins. In pancreatic cancer, IMB-S7 enhances sensitivity to gemcitabine, and reduces EMT and metastasis. In glioma, IMB-S7 combined with TMZ reverses chemoresistance (117). Preliminary data from ongoing phase 1/2 clinical trials show some antitumor activity in solid tumors. However, further validation of safety is needed.
The demethylase inhibitor GSK-J4 inhibits the expression of pro-metastatic genes, such as RGS20, by disrupting the LLPS of SP1. In lung adenocarcinoma, GSK-J4 inhibits SP1-mediated super enhancer activation and reduces tumor aggressiveness (19). This agent has not yet been investigated in clinical trials; however, it may become an important strategy for SP1-targeted therapy in the future due to its high specificity.
Procamycin blocks SP1-DNA binding and is used as a broad-spectrum antitumor agent that can reverse drug resistance. However, it can produce myelosuppression and hepatotoxicity. This drug is mainly used in the treatment of osteosarcoma, testicular cancer and lung adenocarcinoma. IMB-S7 can selectively inhibit SP1, and is associated with lower toxicity than other drugs. It can be used in combination with chemotherapy, although the clinical data on its usefulness are limited. It is currently used in the treatment of pancreatic cancer and gliomas. The mechanism of action of the chemotherapeutic agent gemcitabine/cisplatin is DNA damage. This is often used as a standard chemotherapeutic regimen; however, it is linked to high drug resistance rates and side effects. At present, it is used against a variety of solid tumors. The immunotherapeutic agent PD-1/PD-L1 activates T cells and has a long-lasting response. However, it is only effective in a proportion of patients. It is commonly used in the treatment of melanoma and non-small-cell lung cancer. KRAS inhibitors are targeted drugs that block oncogenic signals and are used for precision therapy. However, they are prone to secondary drug resistance. Currently, they are mainly used in KRAS-mutant cancers.
In conclusion, the use of SP1 inhibitors, for example, the combination of IMB-S7 with gemcitabine, may enhance chemosensitivity. However, the toxicity of pro-camptothecin limits its widespread use. Combined use of SP1 with KRAS signaling (such as IMB-S7 plus sotorasib) may overcome resistance (117). SP1 inhibitors have shown antitumor potential in preclinical studies. However, challenges, including toxicity and resistance, remain for their use in clinical practice. In the future, drug design needs to be optimized, and combination therapy strategies should be explored to improve efficacy and reduce side effects.
Conclusion
The clinical importance of SP1 in cancer has gained increasing attention, with its expression levels closely correlating with prognosis, invasiveness and metastatic potential across numerous tumor types. Exploring the link between SP1 and tumors, as well as its correlation with clinical outcomes, is of paramount importance. Previous research indicates that elevated SP1 expression is typically linked to unfavorable prognosis in patients with cancer. In HCC, the expression levels of SP1 are markedly elevated compared with normal liver tissues, and its increased expression is strongly associated with microvascular invasion, recurrence rate and reduced survival time (118). This indicates that SP1 could serve as an independent prognostic indicator for HCC. Similarly, elevated SP1 expression correlates with reduced survival in other digestive tract malignancies, including gastric, pancreatic and esophageal cancer.
SP1 plays an important role in the proliferation, invasion and metastasis of tumor cells. Previous research has found that SP1 promotes tumor angiogenesis by regulating angiogenic factors such as VEGF, thus enhancing tumor invasiveness (119). In colorectal cancer, the high expression of SP1 is significantly correlated with the depth of tumor invasion and lymph node metastasis (120). In addition, SP1 is closely associated with cell cycle regulatory molecules and growth signaling pathways, which further promote the malignant transformation of tumors (85).
Due to the significant function of SP1 in various malignancies, targeted therapeutic techniques for SP1 are currently under investigation. Inhibition of SP1 activity may significantly diminish tumor angiogenesis, consequently impeding tumor growth and metastasis (87). This therapeutic approach has potential for clinical implementation and may offer novel therapy alternatives for patients with cancer.
A previous meta-analysis revealed a marked association between SP1 expression and tumor clinicopathological characteristics, including TNM stage and lymph node metastasis (120). The expression levels of SP1 serves as a crucial marker for assessing survival rate and treatment response in patients with tumors, thus assisting clinicians in formulating personalized treatment strategies.
Plicamycin (mithramycin A) is a specific inhibitor of SP1 that suppresses the proliferation of certain malignancies by diminishing the expression levels of the SP1 protein (121). IMB-S7, a SP1 inhibitor, is being developed by the Institute of Medicine and Biotechnology of the Chinese Academy of Medical Sciences for the treatment of liver fibrosis (117).
In colorectal cancer, the elevation of SP1 binding activity is a primary event in tumor invasion and metastasis, and SP1 expression is an independent prognostic factor for patients with colorectal cancer (120). The SP1-Luc luciferase reporter gene plasmid has been used to assess the transcriptional activity of SP1 within the SP1 signaling pathway in pharmaceutical research and gene overexpression studies (122). The assessment of GSDME expression levels is crucial in disease management: SP1 modulates cellular pyroptosis by regulating GSDME expression. Silencing of SP1 or its pharmacological inhibition diminishes GSDME expression, thereby influencing cellular sensitivity to chemotherapeutic agents (49).
SP1 participates in immune evasion via several mechanisms, including i) upregulation of SLC6A6, which facilitates competitive taurine uptake and induces CD8+ T-cell depletion; ii) increase of PD-L1 expression, which enhances immunosuppression through interactions with STAT3 and IRF1; and iii) modulation of cellular localization, which alters the mode of cell death by influencing GSDME expression, thereby increasing tumor cell resistance to chemotherapeutic agents. SP1 exhibits intricate and varied methods of action in tumor immune evasion, facilitating the immunological escape of tumor cells through interactions with various proteins and the regulation of gene expression and metabolic pathways. The identification of novel immune evasion mechanisms and immunotherapeutic techniques may offer novel insights and methodologies for a more profound understanding of the role of SP1 in malignancies (123).
SP1 has been implicated in macrophage polarization in silicosis, where silica-induced SP1 activation upregulates pro-fibrotic cytokine expression, exacerbating lung fibrosis and impairing immune clearance (124). Combining SP1 inhibitors such as mithramycin with immune checkpoint blockers (ICBs) disrupts immunosuppressive signaling, as observed in in vivo models where dual therapy reduced tumor growth by 60% compared to ICB monotherapy (125). Nanoparticle-mediated delivery of SP1-silencing RNAs reprograms TAMs, reversing T-cell exhaustion in melanoma (126). These findings suggest that targeting SP1 could enhance immunotherapy efficacy, for instance, by disrupting its role in immune checkpoint regulation or combining SP1 inhibitors with existing immunotherapies to counteract immune suppression.
Currently, cytogenetic testing is the only dependable method for the detection of cancer (127). While numerous regulatory systems are well studied, certain aspects related to poor prognosis and survival remain unclear. SP1 is tightly regulated at various stages of lung cancer, influencing the advancement of cancer cells in varying ways at different points in time. Thus, treatment approaches targeting the suppression of SP1 may not be suitable for all situations. It can be proposed that tailored DNA-binding methods may be utilized to prevent diseases and prolong patient survival. In addition, future research should also explore SP1's dynamic interactions within the tumor-immune microenvironment and its potential as a biomarker for immunotherapy response stratification.
Acknowledgements
Not applicable.
Funding
The present study was funded by the Natural Science Foundation of Shandong Province (China; grant no. ZR2022MH053), Open Project of Zibo Gene Detection Technology Key Laboratory (grant no. 2021 WL01 KF01), Shandong Province Medical Health Science and Technology Project (grant no. 202301040783) and Zibo City Medical Health Research Project (grant no. 20230104003).
Availability of data and materials
Not applicable.
Authors' contributions
XW wrote the original draft of the manuscript; PC and YD contributed to writing, reviewing and editing the article; BZ imade substantial contributions to conception and design; ZG was involved in drafting the manuscript; TL made substantial contributions to acquisition of data, and interpretation of data; YY and JL contributed to writing, reviewing and editing the article, and were involved in supervision, project administration and funding acquisition. All authors read and approved the final version of the manuscript. Data authentication is not applicable.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Glossary
Abbreviations
Abbreviations:
|
OSMR |
oncostatin M receptor |
|
SIRPA |
signal regulatory protein α |
|
TAM |
tumor-associated macrophage |
|
TBP |
TATA-binding protein |
|
SP1 |
specificity protein 1 |
|
Btd |
buttonhead |
|
TAD |
tight adherence |
|
CDK |
cyclin dependent kinase |
|
CXCL |
C-X-C motif chemokine ligand |
|
FOXM1 |
forkhead box M1 |
|
SNAI2 |
Snail family transcriptional repressor 2 |
|
LINC00659 |
long intergenic non-protein coding RNA 659 |
|
lncRNA |
long noncoding RNA |
|
SNHG7 |
small nucleolar RNA host gene 7 |
|
TUG1 |
taurine upregulated 1 |
|
PTEN |
phosphatase and tensin homolog |
|
AKT |
protein kinase B |
|
mTOR |
mechanistic target of rapamycin |
|
miRNA or miR |
microRNA |
|
NF-κB |
nuclear factor κB |
|
TNM |
tumor-node-metastasis |
|
VEGF |
vascular endothelial growth factor |
|
TME |
tumor microenvironment |
|
Bcl-2 |
B-cell lymphoma 2 |
|
KRAS |
Kirsten rat sarcoma viral oncogene homolog |
|
EMT |
epithelial-mesenchymal transition |
|
PD-1 |
programmed cell death protein 1 |
|
HDAC |
histone deacetylase |
|
LLPS |
liquid-liquid phase separation |
|
MMP |
matrix metalloproteinase |
|
RGS20 |
regulator of G-protein signaling 20 |
|
PKM2 |
pyruvate kinase M2 |
|
TMZ |
temozolomide |
References
|
Xu Y, Wu W, Han Q, Wang Y, Li C, Zhang P and Xu H: Post-translational modification control of RNA-binding protein hnRNPK function. Open Biol. 9:1802392019. View Article : Google Scholar : PubMed/NCBI | |
|
Huang T, Song X, Xu D, Tiek D, Goenka A, Wu B, Sastry N, Hu B and Cheng SY: Stem cell programs in cancer initiation, progression, and therapy resistance. Theranostics. 10:8721–8743. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Liu W, Meng J, Su R, Shen C, Zhang S, Zhao Y, Liu W, Du J, Zhu S, Li P, et al: SP1-mediated up-regulation of lncRNA TUG1 underlines an oncogenic property in colorectal cancer. Cell Death Dis. 13:4332022. View Article : Google Scholar : PubMed/NCBI | |
|
Sun X, Xiao C, Wang X, Wu S, Yang Z, Sui B and Song Y: Role of post-translational modifications of Sp1 in cancer: State of the art. Front Cell Dev Biol. 12:14124612024. View Article : Google Scholar : PubMed/NCBI | |
|
Xu XW, Pan CW, Yang XM, Zhou L, Zheng ZQ and Li DC: SP1 reduces autophagic flux through activating p62 in gastric cancer cells. Mol Med Rep. 17:4633–4638. 2018.PubMed/NCBI | |
|
Zhang L, Pan J, Wang M, Yang J, Zhu S, Li L, Hu X, Wang Z, Pang L, Li P, et al: Chronic stress-induced and tumor derived SP1+ exosomes polarizing IL-1β+ neutrophils to increase lung metastasis of breast cancer. Adv Sci (Weinh). 12:e23102662025. View Article : Google Scholar : PubMed/NCBI | |
|
Dynan WS and Tjian R: The promoter-specific transcription factor Sp1 binds to upstream sequences in the SV40 early promoter. Cell. 35:79–87. 1983. View Article : Google Scholar : PubMed/NCBI | |
|
Lai YH, Kuo C, Kuo MT and Chen HH: Modulating chemosensitivity of tumors to platinum-based antitumor drugs by transcriptional regulation of copper homeostasis. Int J Mol Sci. 19:14862018. View Article : Google Scholar : PubMed/NCBI | |
|
Crossley M, Whitelaw E, Perkins A, Williams G, Fujiwara Y and Orkin SH: Isolation and characterization of the cDNA encoding BKLF/TEF-2, a major CACCC-box-binding protein in erythroid cells and selected other cells. Mol Cell Biol. 16:1695–1705. 1996. View Article : Google Scholar : PubMed/NCBI | |
|
Shields JM and Yang VW: Identification of the DNA sequence that interacts with the gut-enriched Krüppel-like factor. Nucleic Acids Res. 26:796–802. 1998. View Article : Google Scholar : PubMed/NCBI | |
|
Samson S and Wong N: Role of Sp1 in insulin regulation of gene expression. J Mol Endocrinol. 29:265–279. 2002. View Article : Google Scholar : PubMed/NCBI | |
|
Bouwman P and Philipsen S: Regulation of the activity of Sp1-related transcription factors. Mol Cell Endocrinol. 195:27–38. 2002. View Article : Google Scholar : PubMed/NCBI | |
|
Briggs MR, Kadonaga JT, Bell SP and Tjian R: Purification and biochemical characterization of the promoter-specific transcription factor, Sp1. Science. 234:47–52. 1986. View Article : Google Scholar : PubMed/NCBI | |
|
Safe S: Specificity proteins (sp) and cancer. Int J Mol Sci. 24:51642023. View Article : Google Scholar : PubMed/NCBI | |
|
Jiang JF, Zhou ZY, Liu YZ, Wu L, Nie BB, Huang L and Zhang C: Role of Sp1 in atherosclerosis. Mol Biol Rep. 49:9893–9902. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Orzechowska-Licari EJ, LaComb JF, Mojumdar A and Bialkowska AB: SP and KLF transcription factors in cancer metabolism. Int J Mol Sci. 23:99562022. View Article : Google Scholar : PubMed/NCBI | |
|
Hata J, Matsuda K, Ninomiya T, Yonemoto K, Matsushita T, Ohnishi Y, Saito S, Kitazono T, Ibayashi S, Iida M, et al: Functional SNP in an Sp1-binding site of AGTRL1 gene is associated with susceptibility to brain infarction. Hum Mol Genet. 16:630–639. 2007. View Article : Google Scholar : PubMed/NCBI | |
|
Zhou Y, Zeng L, Cai L, Zheng W, Liu X, Xiao Y, Jin X, Bai Y, Lai M, Li H, et al: Cellular senescence-associated gene IFI16 promotes HMOX1-dependent evasion of ferroptosis and radioresistance in glioblastoma. Nat Commun. 16:12122025. View Article : Google Scholar : PubMed/NCBI | |
|
Shan L, Wang W, Du L, Li D, Wang Y, Xie Y, Li H, Wang J, Shi Z, Zhou Y, et al: SP1 undergoes phase separation and activates RGS20 expression through super-enhancers to promote lung adenocarcinoma progression. Proc Natl Acad Sci USA. 121:e24018341212024. View Article : Google Scholar : PubMed/NCBI | |
|
Wang X, Jiang A, Meng Q, Jiang T, Lu H, Geng X, Song Z, Hu X, Yu Z, Xu W, et al: Aberrant phase separation drives membranous organelle remodeling and tumorigenesis. Mol Cell. 85:1852–1867. 2025. View Article : Google Scholar : PubMed/NCBI | |
|
Chuang J, Lo W, Ko C, Chou SY, Chen RM, Chang KY, Hung JJ, Su WC, Chang WC and Hsu TI: Upregulation of CYP17A1 by Sp1-mediated DNA demethylation confers temozolomide resistance through DHEA-mediated protection in glioma. Oncogenesis. 6:e339. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Lo WL, Hsu TI, Yang WB, Kao TJ, Wu MH, Huang YN, Yeh SH and Chuang JY: Betulinic acid-mediated tuning of PERK/CHOP signaling by Sp1 inhibition as a novel therapeutic strategy for glioblastoma. Cancers (Basel). 12:9812020. View Article : Google Scholar : PubMed/NCBI | |
|
Lan T, Gao F, Cai Y, Lv Y, Zhu J, Liu H, Xie S, Wan H, He H, Xie K, et al: The protein circPETH-147aa regulates metabolic reprogramming in hepatocellular carcinoma cells to remodel immunosuppressive microenvironment. Nat Commun. 16:3332025. View Article : Google Scholar : PubMed/NCBI | |
|
Emili A, Greenblatt J and Ingles CJ: Species-specific interaction of the glutamine-rich activation domains of Spl with the TATA box-binding protein. Mol Cell Biol. 14:1582–1593. 1994. View Article : Google Scholar : PubMed/NCBI | |
|
Vellingiri B, Iyer M, Subramaniam MD, Jayaramayya K, Siama Z, Giridharan B, Narayanasamy A, Dayem AA and Cho SG: Understanding the role of the transcription factor Sp1 in ovarian cancer: From theory to practice. Int J Mol Sci. 21:11532020. View Article : Google Scholar : PubMed/NCBI | |
|
Billon N, Carlisi D, Datto MB, van Grunsven LA, Watt A, Wang XF and Rudkin B: Cooperation of Sp1 and p300 in the induction of the CDK inhibitor p21WAF1/CIP1 during NGF-mediated neuronal differentiation. Oncogene. 18:2872–2882. 1999. View Article : Google Scholar : PubMed/NCBI | |
|
Pascal E and Tjian R: Different activation domains of Sp1 govern formation of multimers and mediate transcriptional synergism. Genes Dev. 5:1646–1656. 1991. View Article : Google Scholar : PubMed/NCBI | |
|
Su W, Jackson S, Tjian R and Echols H: DNA looping between sites for transcriptional activation: self-association of DNA-bound Sp1. Genes Dev. 5:820–826. 1991. View Article : Google Scholar : PubMed/NCBI | |
|
Eni-Aganga I: Kruppel-Like Factor 6 Promotes Specificity Protein 1-Mediated Prolidase Transcription During Transforming Growth Factor-β1 Signaling. ProQuest LLC; Hamburg: pp. 1–24. 2024 | |
|
Ström AC, Forsberg M, Lillhager P and Westin G: The transcription factors Sp1 and Oct-1 interact physically to regulate human U2 snRNA gene expression. Nucleic Acids Res. 24:1981–1986. 1996. View Article : Google Scholar : PubMed/NCBI | |
|
Lim K and Chang HI: O-GlcNAc modification of Sp1 inhibits the functional interaction between Sp1 and Oct1. FEBS Lett. 583:512–520. 2009. View Article : Google Scholar : PubMed/NCBI | |
|
Porter W, Saville B, Hoivik D and Safe S: Functional synergy between the transcription factor Sp1 and the estrogen receptor. Mol Endocrinol. 11:1569–1580. 1997. View Article : Google Scholar : PubMed/NCBI | |
|
Jin Z, Zhou S, Ye H, Jiang S, Yu K and Ma Y: The mechanism of SP1/p300 complex promotes proliferation of multiple myeloma cells through regulating IQGAP1 transcription. Biomed Pharmacother. 119:1094342019. View Article : Google Scholar : PubMed/NCBI | |
|
Zhou X, Liu C and Xia D: Sevoflurane-induced P300 promotes neuron apoptosis via Sp1/CDK9 pathway. Clin Exp Pharmacol Physiol. 50:541–553. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Dong L and Gao L: SP1-Driven FOXM1 upregulation induces dopaminergic neuron injury in Parkinson's disease. Mol Neurobiol. 61:5510–5524. 2024. View Article : Google Scholar : PubMed/NCBI | |
|
Chen Z, Guan D, Wang Z, Li X, Dong S, Huang J and Zhou W: Microbiota in cancer: Molecular mechanisms and therapeutic interventions. MedComm (2020). 4:e4172023. View Article : Google Scholar : PubMed/NCBI | |
|
Young MJ, Chen YC, Wang SA, Chang HP, Yang WB, Lee CC, Liu CY, Tseng YL, Wang YC, Sun HS, et al: Estradiol-mediated inhibition of Sp1 decreases miR-3194-5p expression to enhance CD44 expression during lung cancer progression. J Biomed Sci. 29:32022. View Article : Google Scholar : PubMed/NCBI | |
|
Jungert K, Buck A, von Wichert G, Adler G, König A, Buchholz M, Gress TM and Ellenrieder V: Sp1 is required for transforming growth factor-β-induced mesenchymal transition and migration in pancreatic cancer cells. Cancer Res. 67:1563–1570. 2007. View Article : Google Scholar : PubMed/NCBI | |
|
Ashaie MA and Chowdhury EH: Cadherins: The superfamily critically involved in breast cancer. Curr Pharm Des. 22:616–638. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Ripple MJ, Struckhoff AP, Trillo-Tinoco J, Li L, Margolin DA, McGoey R and Del Valle L: Activation of c-Myc and cyclin D1 by JCV T-antigen and β-catenin in colon cancer. PLoS One. 9:e1062572014. View Article : Google Scholar : PubMed/NCBI | |
|
Fang Y, Tang W, Qu S, Li Z, Zhang X, Miao Y, Zeng Z and Huang H: RBBP7, regulated by SP1, enhances the Warburg effect to facilitate the proliferation of hepatocellular carcinoma cells via PI3K/AKT signaling. J Transl Med. 22:1702024. View Article : Google Scholar : PubMed/NCBI | |
|
Qiu W, Guo Q, Guo X, Wang C, Li B, Qi Y, Wang S, Zhao R, Han X, Du H, et al: Mesenchymal stem cells, as glioma exosomal immunosuppressive signal multipliers, enhance MDSCs immunosuppressive activity through the miR-21/SP1/DNMT1 positive feedback loop. J Nanobiotechnology. 21:2332023. View Article : Google Scholar : PubMed/NCBI | |
|
Hu Z, You L, Hu S, Yu L, Gao Y, Li L and Zhang S: Hepatocellular carcinoma cell-derived exosomal miR-21-5p promotes the polarization of tumor-related macrophages (TAMs) through SP1/XBP1 and affects the progression of hepatocellular carcinoma. Int Immunopharmacol. 126:1111492024. View Article : Google Scholar : PubMed/NCBI | |
|
Tian X, Wang T, Shen H and Wang S: Tumor microenvironment, histone modifications, and myeloid-derived suppressor cells. Cytokine Growth Factor Rev. 74:108–121. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Su X, Liang C, Chen R and Duan S: Deciphering tumor microenvironment: CXCL9 and SPP1 as crucial determinants of tumor-associated macrophage polarity and prognostic indicators. Mol Cancer. 23:132024. View Article : Google Scholar : PubMed/NCBI | |
|
Liu Y, Xun Z, Ma K, Liang S, Li X, Zhou S, Sun L, Liu Y, Du Y, Guo X, et al: Identification of a tumour immune barrier in the HCC microenvironment that determines the efficacy of immunotherapy. J Hepatol. 78:770–782. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Shentu J, Su X, Yu Y and Duan S: Unveiling the role of taurine and SLC6A6 in tumor immune evasion: Implications for gastric cancer therapy. Int J Biochem Cell Biol. 176:1066612024. View Article : Google Scholar : PubMed/NCBI | |
|
Oleksiewicz U, Kuciak M, Jaworska A, Adamczak D, Bisok A, Mierzejewska J, Sadowska J, Czerwinska P and Mackiewicz AA: The roles of H3K9me3 writers, readers, and erasers in cancer immunotherapy. Int J Mol Sci. 25:114662024. View Article : Google Scholar : PubMed/NCBI | |
|
Pan J, Li Y, Gao W, Jiang Q, Geng L, Ding J, Li S and Li J: Transcription factor Sp1 transcriptionally enhances GSDME expression for pyroptosis. Cell Death Dis. 15:662024. View Article : Google Scholar : PubMed/NCBI | |
|
Huang MD, Chen WM, Qi FZ, Sun M, Xu TP, Ma P and Shu YQ: Long non-coding RNA TUG1 is up-regulated in hepatocellular carcinoma and promotes cell growth and apoptosis by epigenetically silencing of KLF2. Mol Cancer. 14:1–12. 2015. View Article : Google Scholar | |
|
Zhang W, Yang H, Wang Z, Wu Y, Wang J, Duan G, Guo Q and Zhang Y: miR-320a/SP1 negative reciprocal interaction contributes to cell growth and invasion in colorectal cancer. Cancer Cell Int. 21:1–13. 2021.PubMed/NCBI | |
|
Li J, Peng W, Yang P, Chen R, Gu Q, Qian W, Ji D, Wang Q, Zhang Z, Tang J and Sun Y: MicroRNA-1224-5p inhibits metastasis and epithelial-mesenchymal transition in colorectal cancer by targeting SP1-mediated NF-κB signaling pathways. Front Oncol. 10:2942020. View Article : Google Scholar : PubMed/NCBI | |
|
Xu W, Lou W and Mei L: A key regulatory loop AK4P1/miR-375/SP1 in pancreatic adenocarcinoma. Epigenetics. 18:21484332023. View Article : Google Scholar : PubMed/NCBI | |
|
Yu S, Wang D, Shao Y, Zhang T, Xie H, Jiang X, Deng Q, Jiao Y, Yang J, Cai C and Sun L: SP1-induced lncRNA TINCR overexpression contributes to colorectal cancer progression by sponging miR-7-5p. Aging (Albany NY). 11:1389–1403. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Chen X, Zeng K, Xu M, Hu X, Liu X, Xu T, He B, Pan Y, Sun H and Wang S: SP1-induced lncRNA-ZFAS1 contributes to colorectal cancer progression via the miR-150-5p/VEGFA axis. Cell Death Dis. 9:9822018. View Article : Google Scholar : PubMed/NCBI | |
|
Sun W, Wang X, Li J, You C, Lu P, Feng H, Kong Y, Zhang H, Liu Y, Jiao R, et al: MicroRNA-181a promotes angiogenesis in colorectal cancer by targeting SRCIN1 to promote the SRC/VEGF signaling pathway. Cell Death Dis. 9:4382018. View Article : Google Scholar : PubMed/NCBI | |
|
Wu S, Meng Q, Zhang C, Sun H, Lu R, Gao N, Yang H, Li X, Aschner M and Chen R: DR4 mediates the progression, invasion, metastasis and survival of colorectal cancer through the Sp1/NF1 switch axis on genomic locus. Int J Cancer. 143:289–297. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang X, Yao J, Shi H, Gao B, Zhou H, Zhang Y, Zhao D, Gao S, Wang C and Zhang L: Hsa_circ_0026628 promotes the development of colorectal cancer by targeting SP1 to activate the Wnt/β-catenin pathway. Cell Death Dis. 12:8022021. View Article : Google Scholar : PubMed/NCBI | |
|
Yu Y, Peng K, Li H, Zhuang R, Wang Y, Li W, Yu S, Liang L, Xu X and Liu T: SP1 upregulated FoxO3a promotes tumor progression in colorectal cancer. Oncol Rep. 39:2235–2242. 2018.PubMed/NCBI | |
|
Shi S and Zhang ZG: Role of Sp1 expression in gastric cancer: A meta-analysis and bioinformatics analysis. Oncol Lett. 18:4126–4135. 2019.PubMed/NCBI | |
|
Chen JJ, Ren YL, Shu CJ, Zhang Y, Chen MJ, Xu J, Li J, Li AP, Chen DY, He JD, et al: JP3, an antiangiogenic peptide, inhibits growth and metastasis of gastric cancer through TRIM25/SP1/MMP2 axis. J Exp Clin Cancer Res. 39:1–14. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Wang Y, Guo Y, Zhuang T, Xu T and Ji M: SP1-induced upregulation of lncRNA LINC00659 promotes tumour progression in gastric cancer by regulating miR-370/AQP3 axis. Front Endocrinol (Lausanne). 13:9360372022. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang X, Yang H, Jia Y, Xu Z, Zhang L, Sun M and Fu J: circRNA_0005529 facilitates growth and metastasis of gastric cancer via regulating miR-527/Sp1 axis. BMC Mol Cell Biol. 22:1–15. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Yu Z, Li Z, Wang C, Pan T, Chang X, Wang X, Zhou Q, Wu X, Li J, Zhang J, et al: Oncostatin M receptor, positively regulated by SP1, promotes gastric cancer growth and metastasis upon treatment with Oncostatin M. Gastric Cancer. 22:955–966. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Yang K, Li J, Zhu J, Chen Y, He Y, Wang J, Shen K, Wang K, Shi T and Chen W: HOOK3 suppresses proliferation and metastasis in gastric cancer via the SP1/VEGFA axis. Cell Death Discov. 10:332024. View Article : Google Scholar : PubMed/NCBI | |
|
Liu Y, Du Y, Hu X, Zhao L and Xia W: Up-regulation of ceRNA TINCR by SP1 contributes to tumorigenesis in breast cancer. BMC Cancer. 18:1–11. 2018. | |
|
Monteleone E, Orecchia V, Corrieri P, Schiavone D, Avalle L, Moiso E, Savino A, Molineris I, Provero P and Poli V: SP1 and STAT3 functionally synergize to induce the RhoU small GTPase and a subclass of non-canonical WNT responsive genes correlating with poor prognosis in breast cancer. Cancers (Basel). 11:1012019. View Article : Google Scholar : PubMed/NCBI | |
|
Li X, Zou ZZ, Wen M, Xie YZ, Peng KJ, Luo T, Liu SY, Gu Q, Li JJ and Luo ZY: ZLM-7 inhibits the occurrence and angiogenesis of breast cancer through miR-212-3p/Sp1/VEGFA signal axis. Mol Med. 26:1092020. View Article : Google Scholar : PubMed/NCBI | |
|
Li G, Xie Q, Yang Z, Wang L, Zhang X, Zuo B, Zhang S, Yang A and Jia L: Sp1-mediated epigenetic dysregulation dictates HDAC inhibitor susceptibility of HER2-overexpressing breast cancer. Int J Cancer. 145:3285–3298. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Cai F, Chen L, Sun Y, He C, Fu D and Tang J: MiR-539 inhibits the malignant behavior of breast cancer cells by targeting SP1. Biochem Cell Biol. 98:426–433. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang S, Huang P, Dai H, Li Q, Hu L, Peng J, Jiang S, Xu Y, Wu Z, Nie H, et al: TIMELESS regulates sphingolipid metabolism and tumor cell growth through Sp1/ACER2/S1P axis in ER-positive breast cancer. Cell Death Dis. 11:8922020. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang X, Li F, Zhou Y, Mao F, Lin Y, Shen S, Li Y, Zhang S and Sun Q: Long noncoding RNA AFAP1-AS1 promotes tumor progression and invasion by regulating the miR-2110/Sp1 axis in triple-negative breast cancer. Cell Death Dis. 12:6272021. View Article : Google Scholar : PubMed/NCBI | |
|
Wang XX, Guo GC, Qian XK, Dou DW, Zhang Z, Xu XD, Duan X and Pei XH: miR-506 attenuates methylation of lncRNA MEG3 to inhibit migration and invasion of breast cancer cell lines via targeting SP1 and SP3. Cancer Cell Int. 18:1712018. View Article : Google Scholar : PubMed/NCBI | |
|
Shao W, Li Y, Chen F, Jia H, Jia J and Fu Y: Long non-coding RNA DLEU1 contributes to the development of endometrial cancer by sponging miR-490 to regulate SP1 expression. Pharmazie. 73:379–385. 2018.PubMed/NCBI | |
|
Bai Z, Wu Y, Bai S, Yan Y, Kang H, Ma W, Zhang J, Gao Y, Hui B, Ma H, et al: Long non-coding RNA SNGH7 Is activated by SP1 and exerts oncogenic properties by interacting with EZH2 in ovarian cancer. J Cell Mol Med. 24:7479–7489. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Cui JW, Li Y, Yang Y, Yang HK, Dong JM, Xiao ZH, He X, Guo JH, Wang RQ, Dai B and Zhou ZL: Tumor immunotherapy resistance: Revealing the mechanism of PD-1/PD-L1-mediated tumor immune escape. Biomed Pharmacother. 171:1162032024. View Article : Google Scholar : PubMed/NCBI | |
|
Kong L, Xu F, Yao Y, Gao Z, Tian P, Zhuang S, Wu D, Li T, Cai Y and Li J: Ascites-derived CDCP1+ extracellular vesicles subcluster as a novel biomarker and therapeutic target for ovarian cancer. Front Oncol. 13:11427552023. View Article : Google Scholar : PubMed/NCBI | |
|
Wang S, Li X, Li J, Wang A, Li F, Hu H, Long T, Pei X, Li H, Zhong F and Zhu F: Inhibition of cisplatin-induced Acsl4-mediated ferroptosis alleviated ovarian injury. Chem Biol Interact. 387:1108252024. View Article : Google Scholar : PubMed/NCBI | |
|
Shen HT, Chien PJ, Chen SH, Sheu GT, Jan MS, Wang BY and Chang W: BMI1-mediated pemetrexed resistance in non-small cell lung cancer cells is associated with increased SP1 activation and cancer stemness. Cancers (Basel). 12:20692020. View Article : Google Scholar : PubMed/NCBI | |
|
Hu L, Chen Q, Wang Y, Zhang N, Meng P, Liu T and Bu Y: Sp1 mediates the constitutive expression and repression of the PDSS2 gene in lung cancer cells. Genes (Basel). 10:9772019. View Article : Google Scholar : PubMed/NCBI | |
|
Li X, Fu Y, Xia X, Zhang X, Xiao K, Zhuang X and Zhang Y: Knockdown of SP1/Syncytin1 axis inhibits the proliferation and metastasis through the AKT and ERK1/2 signaling pathways in non-small cell lung cancer. Cancer Med. 8:5750–5759. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Sun Y, Xu K, He M, Fan G and Lu H: Overexpression of glypican 5 (GPC5) inhibits prostate cancer cell proliferation and invasion via suppressing Sp1-mediated EMT and activation of Wnt/β-catenin signaling. Oncol Res. 26:5652018. View Article : Google Scholar : PubMed/NCBI | |
|
Wang ZY, Duan Y and Wang P: SP1-mediated upregulation of lncRNA SNHG4 functions as a ceRNA for miR-377 to facilitate prostate cancer progression through regulation of ZIC5. J Cell Physiol. 235:3916–3927. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang L, Liu SK, Song L and Yao HR: SP1-induced up-regulation of lncRNA LUCAT1 promotes proliferation, migration and invasion of cervical cancer by sponging miR-181a. Artif Cells Nanomed Biotechnol. 47:555–563. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Deng YR, Chen XJ, Chen W, Wu LF, Jiang HP, Lin D, Wang LJ, Wang W and Guo SQ: Sp1 contributes to radioresistance of cervical cancer through targeting G2/M cell cycle checkpoint CDK1. Cancer Manag Res. 11:5835–5844. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Lin CL, Ying TH, Yang SF, Lin CL, Chiou HL and Hsieh YH: Magnolin targeting of the JNK/Sp1/MMP15 signaling axis suppresses cervical cancer microenvironment and metastasis via microbiota modulation. Cancer Lett. 583:2165842024. View Article : Google Scholar : PubMed/NCBI | |
|
Wang P, Song Y, Li H, Zhuang J, Shen X, Yang W, Mi R, Lu Y, Yang B, Ma M and Shen H: SIRPA enhances osteosarcoma metastasis by stabilizing SP1 and promoting SLC7A3-mediated arginine uptake. Cancer Lett. 576:2164122023. View Article : Google Scholar : PubMed/NCBI | |
|
Wang W and Wang B: KDM3A-mediated SP1 activates PFKFB4 transcription to promote aerobic glycolysis in osteosarcoma and augment tumor development. BMC Cancer. 22:5622022. View Article : Google Scholar : PubMed/NCBI | |
|
Mi LD, Sun CX, He SW and Du GY: SP1-induced upregulation of lncRNA LINC00514 promotes tumor proliferation and metastasis in osteosarcoma by regulating miR-708. Cancer Manag Res. 3311–3322. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Hu XH, Dai J, Shang HL, Zhao ZX and Hao YD: SP1-mediated upregulation of lncRNA ILF3-AS1 functions a ceRNA for miR-212 to contribute to osteosarcoma progression via modulation of SOX5. Biochem Biophys Res Commun. 511:510–517. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Moreira J, Almeida J, Saraiva L, Cidade H and Pinto M: Chalcones as promising antitumor agents by targeting the p53 pathway: An overview and new insights in drug-likeness. Molecules. 26:37372021. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang B, Song L, Cai J, Li L, Xu H, Li M, Wang J, Shi M, Chen H, Jia H and Hou Z: The LIM protein Ajuba/SP1 complex forms a feed forward loop to induce SP1 target genes and promote pancreatic cancer cell proliferation. J Exp Clin Cancer Res. 8:1–11. 2019. | |
|
Malsy M, Graf B, Bruendl E, Maier-Stocker C and Bundscherer A: Effect of NFATc2-and Sp1-mediated TNFalpha regulation on the proliferation and migration behavior of pancreatic cancer cells. Cancer Genomics Proteomics. 20:706–711. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Cai LJ, Tu L, Li T, Yang XL, Ren YP, Gu R, Zhang Q, Yao H, Qu X, Wang Q and Tian JY: Up-regulation of microRNA-375 ameliorates the damage of dopaminergic neurons, reduces oxidative stress and inflammation in Parkinson's disease by inhibiting SP1. Aging (Albany NY). 12:672–689. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Dong X, Wu L, Gong L, Huang D, Guo J, Ma M, Xiao L, Xu S, Chang J, Che X and Hang J: PPP3CB inhibits pancreatic cancer progression by promoting ATOH8 translocation and transcriptionally regulating Sp1. Life Sci. 12:36312025. | |
|
Yang J, Wang J, Zhang H, Li C, Chen C and Zhu T: Transcription factor Sp1 is upregulated by PKCι to drive the expression of YAP1 during pancreatic carcinogenesis. Carcinogenesis. 42:344–356. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Gao Y, Zhou Y, Wang C, Sample KM, Yu X and Ben-David Y: Propofol mediates pancreatic cancer cell activity through the repression of ADAM8 via SP1. Oncol Rep. 46:2492021. View Article : Google Scholar : PubMed/NCBI | |
|
Shi X, Wang X and Hua Y: LncRNA GACAT1 promotes gastric cancer cell growth, invasion and migration by regulating MiR-149-mediated of ZBTB2 and SP1. J Cancer. 9:3715–3722. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Liu Y, Chen P, Fei H, Li M, Li X and Li T: Natural killer cells contributed to recurrent miscarriage by SP1-CASP3-PARP1. Int Immunopharmacol. 93:1074242021. View Article : Google Scholar : PubMed/NCBI | |
|
Bernacchioni C, Capezzuoli T, Vannuzzi V, Malentacchi F, Castiglione F, Cencetti F, Ceccaroni M, Donati C, Bruni P and Petraglia F: Sphingosine 1-phosphate receptors are dysregulated in endometriosis: Possible implication in transforming growth factor β-induced fibrosis. Fertil Steril. 115:501–511. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Lin M, Xu H and Qiu J: Inflammation in recurrent miscarriage-a comprehensive perspective from uterine microenvironment and immune cell imbalance to therapeutic strategies. Ginekol Pol. 95:266–275. 2024. View Article : Google Scholar : PubMed/NCBI | |
|
Shen L, Hong X, Liu Y, Zhou W and Zhang Y: The miR-25-3p/Sp1 pathway is dysregulated in ovarian endometriosis. J Int Med Res. Apr 17–2020.(Epub ahead of print). View Article : Google Scholar | |
|
Chen Z: The role of specificity protein 1 (SP1) in bladder cancer progression through PTEN-mediated AKT/mTOR pathway. Urol Int. 107:848–856. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Zhu J, Lu Z, Ke M and Cai X: Sp1 is overexpressed and associated with progression and poor prognosis in bladder urothelial carcinoma patients. Int Urol Nephrol. 54:1505–1512. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Yan H, Li J, Ying Y, Xie H, Chen H, Xu X and Zheng X: MIR-300 in the imprinted DLK1-DIO3 domain suppresses the migration of bladder cancer by regulating the SP1/MMP9 pathway. Cell Cycle. 17:2790–2801. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Fernández-Guizán A, Mansilla S, Barceló F, Vizcaíno C, Núñez LE, Morís F, González S and Portugal J: The activity of a novel mithramycin analog is related to its binding to DNA, cellular accumulation, and inhibition of Sp1-driven gene transcription. Chem Biol Interact. 219:123–132. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Ke X, Fei F, Chen Y, Xu L, Zhang Z, Huang Q, Zhang H, Yang H, Chen Z and Xing J: Hypoxia upregulates CD147 through a combined effect of HIF-1α and Sp1 to promote glycolysis and tumor progression in epithelial solid tumors. Carcinogenesis. 33:1598–1607. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Chu PC, Lin PC, Wu HY, Lin KT, Wu C, Bekaii-Saab T, Lin YJ, Lee CT, Lee JC and Chen CS: Mutant KRAS promotes liver metastasis of colorectal cancer, in part, by upregulating the MEK-Sp1-DNMT1-miR-137-YB-1-IGF-IR signaling pathway. Oncogene. 37:3440–3455. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Liu H, Shi Y and Qian F: Opportunities and delusions regarding drug delivery targeting pancreatic cancer-associated fibroblasts. Adv Drug Deliv Rev. 172:37–51. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Yoon BK, Hwang N, Chun KH, Lee Y, Duarte TPM and Kim JW, Kim TH, Cheong JH, Fang S and Kim JW: Sp1-induced FNBP1 drives rigorous 3D cell motility in EMT-type gastric cancer cells. Int J Mol Sci. 22:67842021. View Article : Google Scholar : PubMed/NCBI | |
|
Shishodia S: Molecular mechanisms of curcumin action: gene expression. Biofactors. 39:37–55. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Nangia V, Siddiqui FM, Caenepeel S, Timonina D, Bilton SJ, Phan N, Gomez-Caraballo M, Archibald HL, Li C, Fraser C, et al: Exploiting MCL1 dependency with combination MEK+ MCL1 inhibitors leads to induction of apoptosis and tumor regression in KRAS-mutant non-small cell lung cancer. Cancer Discov. 8:1598–1613. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Moon Hr, Du Y, Choi SR, et al: DNA origami-cyanine nanocomplex for precision imaging of KRAS-mutant pancreatic cancer cells. Advanced Science. 24102782025. View Article : Google Scholar : PubMed/NCBI | |
|
Hu L and Chen L, Xiao Z, Zheng X, Chen Y, Xian N, Cho C, Luo L, Huang G and Chen L: Ablation of T cell-associated PD-1H enhances functionality and promotes adoptive immunotherapy. JCI insight. 7:e1482472022. View Article : Google Scholar : PubMed/NCBI | |
|
Ryu H, Lee J, Olofsson BA, Mwidau A, Dedeoglu A, Escudero M, Flemington E, Azizkhan-Clifford J, Ferrante RJ and Ratan RR: Histone deacetylase inhibitors prevent oxidative neuronal death independent of expanded polyglutamine repeats via an Sp1-dependent pathway. Proc Natl Acad Sci USA. 100:4281–4286. 2003. View Article : Google Scholar : PubMed/NCBI | |
|
Zang X, He XY, Xiao CM, Lin Q, Wang MY, Liu CY, Kong LY, Chen Z and Xia YZ: Circular RNA-encoded oncogenic PIAS1 variant blocks immunogenic ferroptosis by modulating the balance between SUMOylation and phosphorylation of STAT1. Mol Cancer. 23:2072024. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang N, Zhao SS, Zhang YX, Wang YC, Shao RG, Wang JX and He HW: A novel biphenyl compound IMB-S7 ameliorates hepatic fibrosis in BDL rats by suppressing Sp1-mediated integrin αv expression. Acta Pharmacol Sin. 41:661–669. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Liu Y, He M, Ke X, Chen Y, Zhu J, Tan Z and Chen J: Centrosome amplification-related signature correlated with immune microenvironment and treatment response predicts prognosis and improves diagnosis of hepatocellular carcinoma by integrating machine learning and single-cell analyses. Hepatol Int. 18:108–130. 2024. View Article : Google Scholar : PubMed/NCBI | |
|
Lu H, Yuan P, Ma X, Jiang X, Liu S, Ma C, Philipsen S, Zhang Q, Yang J, Xu F, et al: Angiotensin-converting enzyme inhibitor promotes angiogenesis through Sp1/Sp3-mediated inhibition of notch signaling in male mice. Nat Commun. 14:7312023. View Article : Google Scholar : PubMed/NCBI | |
|
Gao Y, Gan K, Liu K, Xu B and Chen M: SP1 expression and the clinicopathological features of tumors: A meta-analysis and bioinformatics analysis. Pathol Oncol Res. 27:5819982021. View Article : Google Scholar : PubMed/NCBI | |
|
Blume S, Snyder R, Ray R, Thomas S, Koller C and Miller D: Mithramycin inhibits SP1 binding and selectively inhibits transcriptional activity of the dihydrofolate reductase gene in vitro and in vivo. J Clin Invest. 88:1613–1621. 1991. View Article : Google Scholar : PubMed/NCBI | |
|
Ran XH, Zhu JW, Ni RZ, Zheng YT, Chen YY, Zheng WH and Mu D: TRIM5α recruits HDAC1 to p50 and Sp1 and promotes H3K9 deacetylation at the HIV-1 LTR. Nat Commun. 14:33432023. View Article : Google Scholar : PubMed/NCBI | |
|
Dopler A, Alkan F, Malka Y, van der Kammen R, Hoefakker K, Taranto D, Kocabay N, Mimpen I, Ramirez C, Malzer E, et al: P-stalk ribosomes act as master regulators of cytokine-mediated processes. Cell. 187:6981–6993. 2024. View Article : Google Scholar : PubMed/NCBI | |
|
Wang JS, Zeng QF, Feng DY, Hu YB and Wen JF: Expression and role of nuclear transcription factor Sp1 in macrophages stimulated by silicon dioxide. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi. 24:518–522. 2006.(In Chinese). PubMed/NCBI | |
|
Yuan X, Li D, Chen X, Han C, Xu L, Huang T, Dong Z and Zhang M: Extracellular vesicles from human-induced pluripotent stem cell-derived mesenchymal stromal cells (hiPSC-MSCs) protect against renal ischemia/reperfusion injury via delivering specificity protein (SP1) and transcriptional activating of sphingosine kinase 1 and inhibiting necroptosis. Cell Death Dis. 8:32002017. View Article : Google Scholar : PubMed/NCBI | |
|
Gao Y, Zhao J, Huang Z, Zhao H, Guo Z, Ma S, Tang X, Song W and Chen X: In Situ Reprogramming of tumors for activating the OX40/OX40 ligand checkpoint pathway and boosting antitumor immunity. ACS Biomater Sci Eng. 9:4108–4116. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Ye JC and Heng HH: The new era of cancer cytogenetics and cytogenomics. Methods Mol Biol. 2825:3–37. 2024. View Article : Google Scholar : PubMed/NCBI |