Efficacy of combined immunotherapy and chemotherapy in recurrent non‑small cell lung cancer during durvalumab maintenance therapy: A case report
- Authors:
- Published online on: September 22, 2025 https://doi.org/10.3892/ol.2025.15288
- Article Number: 542
-
Copyright: © Maze et al. This is an open access article distributed under the terms of Creative Commons Attribution License.
Abstract
Introduction
Non-small cell lung cancer (NSCLC) is the most common type of lung cancer, and treatment strategies vary depending on the stage of disease and patient performance status. For patients with locally advanced NSCLC who have a good performance status, concurrent chemoradiotherapy (CCRT) is the standard treatment, combining systemic chemotherapy with thoracic radiotherapy to enhance local control and improve survival outcomes (1,2). Following definitive CCRT, consolidation with durvalumab, an anti-PD-L1 antibody, has been shown in the phase III PACIFIC trial to significantly improve progression-free and overall survival, and is now widely regarded as the standard post-CCRT treatment (3,4). However, a substantial proportion of patients relapse even after CCRT and durvalumab consolidation therapy, and no consensus exists on how to manage recurrence in this setting. The present case report describes a patient with stage IIIB lung adenocarcinoma who experienced recurrence during durvalumab consolidation and was subsequently treated with a combination of durvalumab, tremelimumab and chemotherapy.
Case report
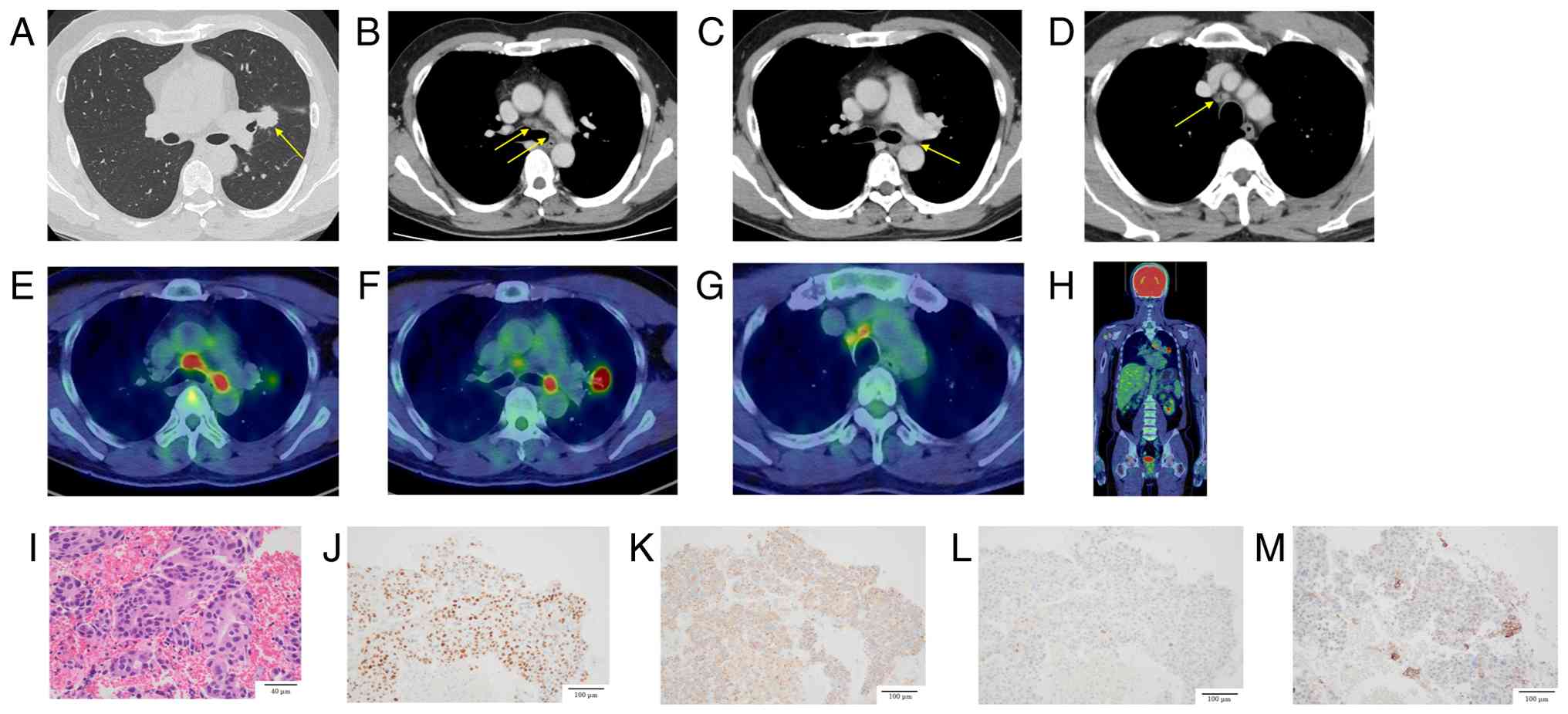
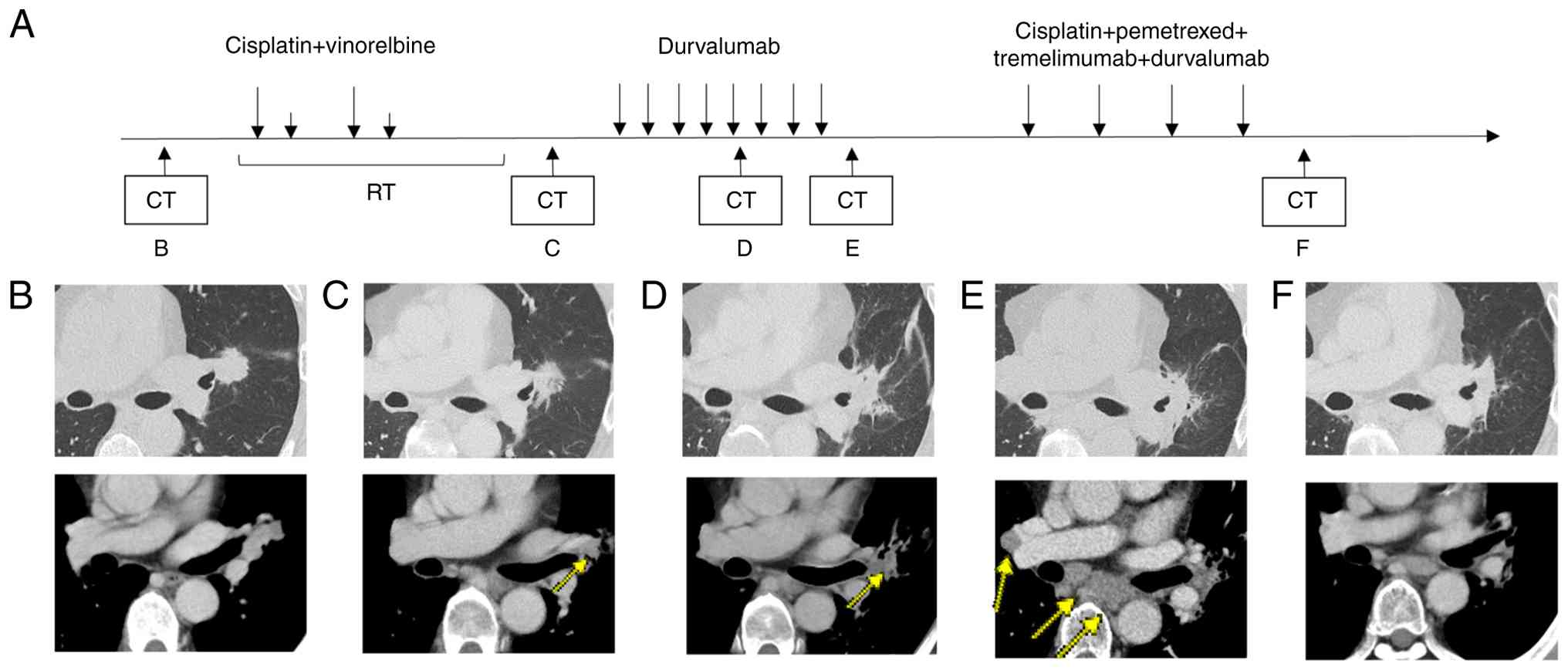
A 52-year-old man with a 20-pack-year smoking history presented to Toranomon Hospital (Tokyo, Japan) in April 2023 with an elevated carcinoembryonic antigen level of 17.8 ng/ml (day 0). Chest computed tomography revealed a 23 mm irregular mass in the left upper lobe hilum, with lymphadenopathy in the left hilar and mediastinal regions (Fig. 1A-D). 18F-fluorodeoxyglucose (18F-FDG) positron emission tomography/computed tomography (PET/CT) revealed FDG uptake in the left upper lobe mass, left hilar lymph nodes, and bilateral paratracheal lymph nodes. Relevant 18F-FDG PET/CT images are presented in Fig. 1E-G. No findings suggestive of distant metastases were observed elsewhere (Fig. 1H). On day 7, bronchoscopy with transbronchial biopsy of the left B3a bronchus and endobronchial ultrasound-guided transbronchial needle aspiration of the 4R lymph node confirmed the presence of an adenocarcinoma (Fig. 1I). Immunohistochemical analysis was negative for cytokeratin 5/6 and p40, positive for thyroid transcription factor 1 and napsin A, and focally positive for hepatocyte nuclear factor 4 alpha. Staining of programmed death-ligand 1 (PD-L1) using the 22C3 assay revealed a tumor proportion score of less than 1% (Fig. 1J-M). Oncomine Dx testing was negative for B-Raf proto-oncogene V600E, epidermal growth factor receptor, and Kirsten rat sarcoma viral oncogene homolog G12C mutations. Mutations in other driver gene could not be detected because of insufficient sample quantity. Based on these findings, stage IIIB primary lung adenocarcinoma (cT1cN3M0) was diagnosed according to the 8th edition of the American Joint Committee on Cancer staging system. On day 30, after 2 cycles of chemotherapy (cisplatin (CDDP) 80 mg/m2, vinorelbine (VNR) 20 mg/m2) and 60 Gy radiation therapy, a reduction in the size of the primary tumor and the left hilar lymph nodes was observed, resulting in a partial response (PR) (Fig. 2C). This was followed by durvalumab (10 mg/kg) consolidation therapy. Radiotherapy was delivered as intensity-modulated radiation therapy (IMRT) using 6 MV photons, at a total dose of 60 Gy in 30 fractions over 6 weeks. In concurrent chemoradiotherapy (CCRT) for stage III non-small cell lung cancer (NSCLC), carboplatin plus paclitaxel (CBDCA plus PTX) is considered one of the standard treatment options (5,6). and CDDP plus etoposide or CDDP plus pemetrexed (PEM) regimens have also been comparatively evaluated. In contrast, CDDP plus VNR has been well studied in Japanese patients, with sufficient data supporting its efficacy and safety (5,7–9). It is also covered by insurance and widely used in clinical practice in Japan. Therefore, in this case, CDDP plus VNR were selected.
During the initial concurrent chemoradiotherapy, no significant adverse events were observed. On day 92, maintenance therapy with durvalumab (10 mg/kg, every 2 weeks) was started. After 5 cycles of durvalumab maintenance therapy, the follow-up chest CT revealed mild radiation pneumonitis around the lesions, which did not require treatment. In addition, a reduction in the size of the primary tumor and left hilar lymph node metastases was observed (Fig. 2D), and the therapeutic response was assessed as partial response (PR). However, after 8 cycles of durvalumab, on day 196, the patient's carcinoembryonic antigen levels increased to 27.8 ng/ml. 18F-FDG PET/CT revealed additional FDG uptake in the right hilar, mediastinal, and bilateral supraclavicular lymph nodes, indicating disease progression (Fig. 2E). Therefore, durvalumab was discontinued. On day 210, tissue specimens were obtained via repeat bronchoscopy. However, the sample collected during the procedure was insufficient for comprehensive analysis; thus, next-generation sequencing could not be performed.
On day 217, the treatment regimen was adjusted to include durvalumab (1,500 mg), tremelimumab (75 mg, every 3 weeks), CDDP (75 mg/m2, every 3 weeks), plus PEM (500 mg/m2, every 3 weeks). Although disease progress occurred, only 2 cycles of CDDP plus VNR were administered during the concurrent chemoradiotherapy, and there is no evidence that the effect of CDDP was insufficient. In the POSEIDON trial, either CDDP or CBDCA could be selected. We chose CDDP plus PEM, which has more extensive data compared to CBDCA plus PEM (10). Following the initial combination phase, the patient transitioned to maintenance therapy with durvalumab (1,500 mg every 4 weeks) plus PEM (500 mg/m2 every 4 weeks), following the dosing protocol established in the phase II and III POSEIDON trial, which led to its approval by the U.S. Food and Drug Administration and subsequent approval in Japan (6). Thyroid function monitoring resulted in the continuation of thyroid hormone replacement therapy because of hypothyroidism as an immune-related adverse event. During the first cycle of this combination therapy, the patient experienced Grade 1 appetite loss, which was resolved without intervention. Subsequently, during the maintenance phase with durvalumab plus PEM, Grade 1 hepatotoxicity was observed. This was managed with ursodeoxycholic acid and did not require treatment interruption. After 4 cycles of combination therapy, the primary tumor and right hilar lymph node metastases showed less than 30% reduction in size, consistent with stable disease (Fig. 2F).
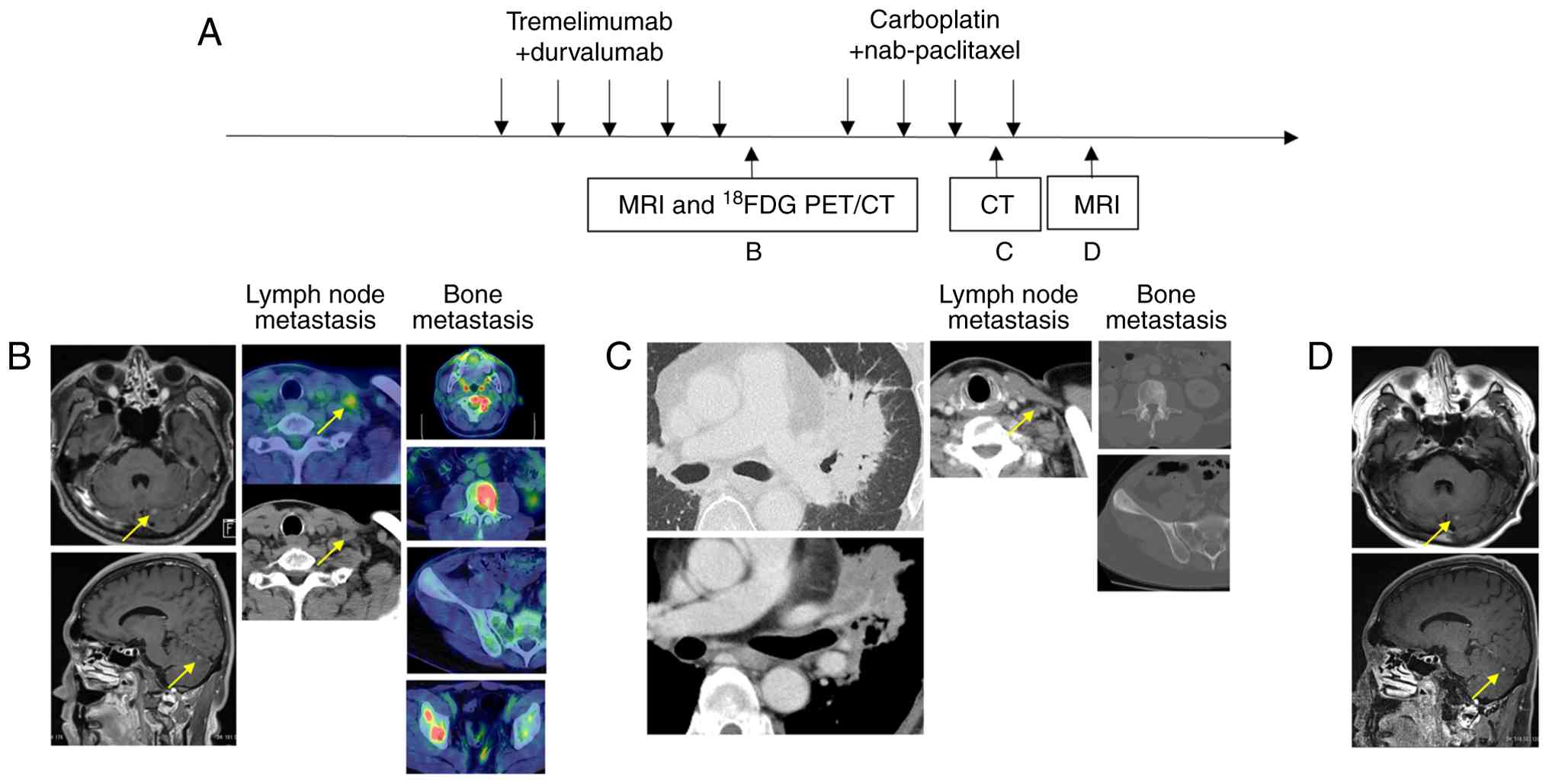
Maintenance therapy was administered for 5 cycles. On day 477, a 6-mm brain metastasis developed in the left cerebellum (Fig. 3B), and on day 509, 18F-FDG PET/CT revealed multiple bone metastases and supraclavicular lymph node metastases (Fig. 3B). Therefore, disease progression (PD) was determined, and maintenance therapy with durvalumab plus PEM was discontinued. Radiotherapy to the cervical spine (C2) was administered at a dose of 7 Gy per fraction for 5 fractions, and Gamma Knife radiosurgery was performed at NTT Medical Center Tokyo for brain metastasis in the left cerebellum.
From day 561, the patient received combination therapy with CBDCA (AUC 6, every 3 weeks) plus nab-PTX (100 mg/m2 on days 1, 8, and 15 of each cycle). After 3 cycles of CBDCA plus nab-PTX, CT showed expansion of sclerosis in the bone metastases of the L3 vertebral body and the right iliac wing, as well as enlargement of the primary lesion and infiltrative shadow around the right hilar lymph nodes, likely due to a radiation recall phenomenon (Fig. 3C). These bone and lung findings were considered treatment-related changes, as the primary and mediastinal lymph nodes maintained their size reduction, and the left subclavian lymph node also showed shrinkage (Fig. 3C), indicating that CBDCA plus nab-PTX was effective. Since the effectiveness of CBDCA plus nab-PTX was observed, a fourth course was also administered. Brain magnetic Resonance Imaging (MRI) after 4 cycles of CBDCA plus nab-PTX confirmed a reduction of the lesion to 4 mm (Fig. 3D). The purpose of Gamma Knife treatment is local tumor control (11,12). In this case, the metastatic brain tumor shrank, and local control was successfully achieved. CBDCA plus nab-PTX treatment is planned to be continued.
Discussion
This case demonstrates the potential of combining durvalumab, tremelimumab, and chemotherapy to treat NSCLC recurrence during durvalumab consolidation therapy after CCRT. A five-year follow-up study from the PACIFIC trial reported a median overall survival (OS) of 47.5 months for patients receiving durvalumab after CCRT compared to 29.1 months in the placebo group (13). In the PACIFIC trial, post-durvalumab therapies consisted predominantly of chemotherapy, often followed by immunotherapy, most commonly with immune checkpoint inhibitors such as nivolumab or pembrolizumab. The current approach for treating recurrent NSCLC without driver mutations involves immunotherapy, with or without chemotherapy. Therefore, despite the absence after durvalumab and CCRT, the use of immunotherapy alone or in combination with chemotherapy remains a viable strategy.
A retrospective analysis of 15 Japanese institutions examined patients with locally advanced or unresectable NSCLC who experienced disease progression after CCRT and durvalumab consolidation therapy. Patients were categorized into 3 groups: early discontinuation (progression within 6 months of durvalumab initiation), late discontinuation (progression between 7 and 12 months after durvalumab initiation), and accomplishment (progression after 12 months of durvalumab initiation) (14). Among the 127 patients analyzed, 50 (39.4%) were in the early discontinuation group. Subsequent treatments included platinum-based chemotherapy combined with immune checkpoint inhibitors in 4 patients (8.0%) in the early discontinuation group. In this group, the median OS of the 4 patients treated with platinum-based chemotherapy and immune checkpoint inhibitors was 10.3 months; however, none of these patients received both durvalumab and tremelimumab.
Previous research showed that patients with metastatic melanoma who progressed after PD-L1 therapy benefited more from a combination of PD-L1 and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) treatments than from CTLA-4 monotherapy, showing an objectively higher response rate (31% vs. 13%; P<0.0001) and longer median OS (20.4 vs. 8.8 months; hazard ratio 0.50; P<0.0001) (15). Similarly, in patients with metastatic NSCLC who experienced disease progression after PD-L1 inhibitor treatment, addition of CTLA-4 antibodies demonstrated efficacy in 10% of patients in phase 2 trials (16).
The phase III POSEIDON trial evaluated first-line treatment for advanced NSCLC by comparing a regimen of durvalumab, tremelimumab, and chemotherapy with chemotherapy alone. This combination significantly improved the progression-free survival and OS of patients (6). Furthermore, addition of durvalumab to chemotherapy significantly increased progression-free survival. However, as the patients had not previously received durvalumab, the effectiveness of the regimen for recurrence during post-CCRT durvalumab therapy remains unverified. In contrast, the CheckMate 9LA trial evaluated a dual-checkpoint strategy using nivolumab and ipilimumab with only 2 chemotherapy cycles while continuing CTLA-4 blockade in the maintenance phase (17). Both trials supported the combination of anti-CTLA-4 and anti-PD-L1 antibodies with chemotherapy for treatment-naive NSCLC. Our patient, who was diagnosed with lung adenocarcinoma without actionable driver mutations (EGFR or ALK) and with negative PD-L1 expression at initial diagnosis, shares key pathological features with the populations enrolled in both trials. The POSEIDON trial reported approximately 70% of enrolled patients with nonsquamous histology and 38% with PD-L1 expression <1%, while the CheckMate 9LA trial included patients with both squamous and nonsquamous histologies and a broad range of PD-L1 expression levels. Thus, from a pathological standpoint, our case is consistent with these study populations.
However, none of these studies included patients with recurrence after concurrent chemoradiotherapy or durvalumab consolidation. Our case differs in this regard, as the patient experienced disease progression during durvalumab consolidation following CCRT, a clinical context not evaluated in either study. Although informative, these findings may not be directly applicable to post-CCRT recurrence. Furthermore, although anti-vascular endothelial growth factor therapy such as bevacizumab is an established option for advanced NSCLC, its administration following stereotactic ablative radiotherapy (SABR) is associated with a markedly increased risk of pulmonary hemorrhage (18). While SABR was not employed in the present case, the potential for hemorrhagic complications associated with prior thoracic irradiation was taken into consideration, and a treatment regimen incorporating dual immune checkpoint inhibitors was selected.
In our patient, the combination of immunotherapy and chemotherapy, including durvalumab, was beneficial for treating NSCLC recurrence during durvalumab maintenance after CCRT. Given the absence of driver mutations and negative PD-L1 expression at the initial diagnosis, this combination was selected to enhance efficacy by targeting different immune checkpoints with durvalumab and tremelimumab. Further accumulation of cases is recommended to strengthen these findings, and additional clinical experience is essential to validate the effectiveness of this treatment strategy in a broader patient population.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be requested from the corresponding author.
Authors' contributions
YM drafted the first version of the manuscript. YM, TM, HN and MT confirmed the authenticity of all the raw data. HU and TF contributed to the pathological evaluation. YM, TM, HN, TK, YT, YN, SH, AM, HU, TF and MT made substantial contributions to the conception and design of the study, including determination of the treatment strategy, acquisition of clinical and pathological data, and analysis and interpretation of data. All authors were involved in drafting the manuscript or revising it critically for important intellectual content. Furthermore, all authors agreed to be accountable for all aspects of the work, ensuring that questions related to the accuracy or integrity of any part of the study are appropriately investigated and resolved. Each author participated sufficiently to take public responsibility for appropriate portions of the content, and all authors have confidence in the accuracy and integrity of the contributions of their co-authors. All authors have read and approved the final version of the manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
The patient provided written informed consent for the case report to be published under anonymity.
Competing interests
SH receives lecture fees from AstraZeneca. The other authors declare that they have no competing interests.
Glossary
Abbreviations
Abbreviations:
|
CCRT |
concurrent chemoradiotherapy |
|
CTLA-4 |
cytotoxic T-lymphocyte-associated protein 4 |
|
NSCLC |
non-small cell lung cancer |
|
OS |
overall survival |
|
PD-L1 |
programmed death-ligand 1 |
References
|
Pritchard RS and Anthony SP: Chemotherapy plus radiotherapy compared with radiotherapy alone in the treatment of locally advanced, unresectable, non-small-cell lung cancer. A mata-anaysis. Ann Intern Med. 125:723–729. 1996. View Article : Google Scholar : PubMed/NCBI | |
|
Marino P, Preatoni A and Cantoni A: Randomized trials of radiotherapy alone versus combined chemotherapy and radiotherapy in stages IIIa and IIIb nonsmall cell lung cancer. A meta-analysis. Cancer. 76:593–601. 1995. View Article : Google Scholar : PubMed/NCBI | |
|
Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, Yokoi T, Chiappori A, Lee KH, de Wit M, et al: Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med. 377:1919–1929. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, Kurata T, Chiappori A, Lee KH, de Wit M, et al: Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N Engl J Med. 379:2342–2350. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Naito Y, Kubota K, Nihei K, Fujii T, Yoh K, Niho S, Goto K, Ohmatsu H, Saijo N and Nishiwaki Y: Concurrent chemoradiotherapy with cisplatin and vinorelbine for stage III Non-small cell lung cancer. J Thorac Oncol. 3:617–622. 2008. View Article : Google Scholar : PubMed/NCBI | |
|
Johnson ML, Cho BC, Luft A, Alatorre-Alexander J, Geater SL, Laktionov K, Kim SW, Ursol G, Hussein M, Lim FL, et al: Durvalumab with or without tremelimumab in combination with chemotherapy as first-line therapy for metastatic non-small-cell lung cancer: The phase III POSEIDON study. J Clin Oncol. 41:1213–1227. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Yamamoto N, Nakagawa K, Nishimura Y, Tsujino K, Satouchi M, Kudo S, Hida T, Kawahara M, Takeda K, Katakami N, et al: Phase III study comparing second- and third-generation regimens with concurrent thoracic radiotherapy in patients with unresectable stage III non-small-cell lung cancer: West Japan Thoracic Oncology Group WJTOG0105. J Clin Oncol. 28:3739–3745. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Sasaki T, Seto T, Yamanaka T, Kunitake N, Shimizu J, Kodaira T, Nishio M, Kozuka T, Takahashi T, Harada H, et al: A randomised phase II trial of S-1 plus cisplatin versus vinorelbine plus cisplatin with concurrent thoracic radiotherapy for unresectable, locally advanced non-small cell lung cancer: WJOG5008L. Br J Cancer. 119:675–682. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Shukuya T, Takahashi T, Harada H, Akamatsu H, Sakaguchi C, Imai H, Ono A, Nakamura Y, Tsuya A, Kenmotsu H, et al: Comparison of vinorelbine plus cisplatin and s-1 plus cisplatin in concurrent chemoradiotherapeutic regimens for unresectable stage III Non-small Cell lung cancer. Anticancer Res. 32:675–680. 2012.PubMed/NCBI | |
|
Scagliotti GV, Parikh P, Von Pawel J, Biesma B, Vansteenkiste J, Manegold C, Serwatowski P, Gatzemeier U, Digumarti R, Zukin M, et al: Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol. 26:3543–3551. 2008. View Article : Google Scholar : PubMed/NCBI | |
|
Rogers SJ, Lomax N, Alonso S, Lazeroms T and Riesterer O: Radiosurgery for five to fifteen brain metastases: A single centre experience and a review of the literature. Front Oncol. 12:8665422022. View Article : Google Scholar : PubMed/NCBI | |
|
Dewan MC, Rattani A, Fieggen G, Arraez MA, Servadei F, Boop FA, Johnson WD, Warf BC and Park KB: Global neurosurgery: The current capacity and deficit in the provision of essential neurosurgical care. Executive summary of the global neurosurgery initiative at the program in global surgery and social change. J Neurosurg. 130:1055–1064. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Spigel DR, Faivre-Finn C, Gray JE, Vicente D, Planchard D, Paz-Ares L, Vansteenkiste JF, Garassino MC, Hui R, Quantin X, et al: Five-year survival outcomes from the PACIFIC trial: Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. J Clin Oncol. 40:1301–1311. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Hasegawa T, Ariyasu R, Tanaka H, Saito R, Kawashima Y, Horiike A, Sakatani T, Tozuka T, Shiihara J, Saiki M, et al: Subsequent treatment for locally advanced non-small-cell lung cancer that progressed after definitive chemoradiotherapy and consolidation therapy with durvalumab: A multicenter retrospective analysis (TOPGAN 2021-02). Cancer Chemother Pharmacol. 92:29–37. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Pires da Silva I, Ahmed T, Reijers ILM, Weppler AM, Betof Warner A, Patrinely JR, Serra-Bellver P, Allayous C, Mangana J, Nguyen K, et al: Ipilimumab alone or ipilimumab plus anti-PD-1 therapy in patients with metastatic melanoma resistant to anti-PD-(L)1 monotherapy: A multicentre, retrospective, cohort study. Lancet Oncol. 22:836–847. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Schoenfeld JD, Giobbie-Hurder A, Ranasinghe S, Kao KZ, Lako A, Tsuji J, Liu Y, Brennick RC, Gentzler RD, Lee C, et al: Durvalumab plus tremelimumab alone or in combination with low-dose or hypofractionated radiotherapy in metastatic non-small-cell lung cancer refractory to previous PD(L)-1 therapy: An open-label, multicentre, randomised, phase 2 trial. Lancet Oncol. 23:279–291. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Paz-Ares L, Ciuleanu TE, Cobo M, Schenker M, Zurawski B, Menezes J, Richardet E, Bennouna J, Felip E, Juan-Vidal O, et al: First-line nivolumab plus ipilimumab combined with two cycles of chemotherapy in patients with non-small-cell lung cancer (CheckMate 9LA): An international, randomised, open-label, phase 3 trial. Lancet Oncol. 22:198–211. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Lau B, No HJ, Wu YF, Ko RB, Devine M, Das M, Neal JW, Wakelee HA, Ramchandran KJ, Wakelee HA, et al: Pulmonary hemorrhage in patients treated with thoracic stereotactic ablative radiotherapy and anti-angiogenic agents. Int J Radiat Oncol Biol Phys. 111:e4232021. View Article : Google Scholar |