Effect of medroxyprogesterone injection on liver function and histological features of the liver in albino rats
- Authors:
- Published online on: April 2, 2025 https://doi.org/10.3892/wasj.2025.341
- Article Number: 53
-
Copyright : © Alassaf et al. This is an open access article distributed under the terms of Creative Commons Attribution License [CC BY 4.0].
Abstract
Introduction
The well-known injectable hormonal contraceptive, medroxyprogesterone (MdP), is a long-acting progestin. A progesterone-based contraceptive injection is a highly effective birth control method (1). The contraceptive efficacy of MdP involves the inhibition of the secretion of gonadotropin-releasing hormone, preventing the maturation of ovarian follicles and ovulation that leads to a thin endometrial lining. This renders the endometrium unsuitable for implantation (2). The most commonly associated side-effects of MdP are irregular bleeding and weight gain (3). Depression, mood swings, decreased sexual interest and a slight increase in the risk of developing breast cancer have also been reported. However, there is evidence of a protective role of MdP against cancer, such as decreasing the risk of developing ovarian cancer (4).
MdP is commonly used as a family planning method due to its availability, effectiveness with a low failure rate and easy administration as a single intramuscular injection every 12 weeks (5). However, the careful use of MdP is required in patients with diabetes mellitus, as insulin sensitivity and glucose tolerance are affected. Additionally, patients with renal impairment may exhibit elevated levels of urea and creatine with the deterioration of the oxidative stress status. Moreover, the parenteral use of MdP is contraindicated in patients who are at a risk of developing cardiovascular diseases, such as stroke (6,7). The use of MdP with estrogen in menopausal women is associated with an increased risk of cardiovascular disorders and thromboembolic complications (8,9). It is considered that the underlying complications of cardiovascular disorders in women on injectable progestin-only contraceptives are caused by the abnormal lipid status (10-12).
MdP is exclusively cleared by the metabolism of the liver, and this may involve hepatobiliary complications (13-15). Over the years, conflicting data have been reported concerning the use of MdP in women with liver dysfunction. In 2009, The World Health Organization recommended the use of injectable progestogen-only contraception in women with liver disease (14) and it was found that MdP does not affect liver function (16,17). However, MdP was then used in women with liver disease and was classified as category 3 (risks outweigh benefits) (18,19). In 2022, Sathe and Gerriets (20) considered a contraindication of MdP usage in females with hepatic disorders and suggested discontinuing the MdP in case of liver function disturbances. Accordingly, the present study aimed to evaluate the effects of MdP on liver function and histological changes in the livers of female albino rats. The present study aimed to provide clinical insight into the liver-associated risks in users of MdP.
Materials and methods
Study protocol and animals
The study protocol was approved by the Medical Ethical Committee at the University of Mosul, Mosul, Iraq with code number CCMRE-phA-22-13 on 18/10/2022. A total of 20 adult female Wistar albino rats (age range, 3-4 months; weight range, 200-315 g) were obtained from the Animal House at the College of Veterinary at the University of Mosul. A sample size of 20 female albino rats was based on previous studies investigating the effect of MdP on different organs (6,21,22). These rats were healthy and exposed to a light cycle of 12 h light/12 h dark with ~50% humidity at 22±2˚C. The housed animals were hosted in cages for a 14-day adaptation period, which were enriched with bedding, nesting material and environmental supplements to promote natural behaviors and reduce stress. In addition, the rats were caged in small groups to allow social interaction, and were provide with access to water ad libitum and were fed a standard rodent laboratory diet. Animal handling was minimized, and all procedures were performed by trained personnel to reduce stress. To ensure compliance with ethical standards by minimizing pain and distress without compromising the scientific integrity of the study, meloxicam was used subcutaneously (1 mg/kg) on need. The animals were monitored, on a daily basis, for body weight, food and water intake, and for any signs of distress. The behavior of animals was recorded twice daily. The specific criteria for predefined humane endpoints used to determine when animals should be euthanized included either the end of the experiment, so that the liver could be examined for microscopic studies, or if any animal exhibited severe weight loss, inability to drink and eat, or severe irreversible distress signs. The 20 rats were randomly assigned to receive MdP (10 rats) or to serve as a control group (10 rats). All rats were euthanized at the end of the study, and none died during the experiment. The 10 rats in group 1 (G1) represent those prior to MdP administration, while those in group 2 (G2) represent the same rats as those in group 1, but after receiving an intramuscular injection of MdP (3.5 mg/rat/week for 8 weeks). The rats in group 3 (G3) represent the untreated control group at the baseline (starting point of the experiments), which included 10 female Wistar albino rats. The rats in group 4 (G4) are the same rats as those in G3, but at the end of the experiments (after 8 weeks). . The animals were weighed and blood samples were drawn from the lateral tail vein at two time points: At the start of the experiments and at 8 weeks following the MdP administration. Blood samples were allowed to clot and centrifuged for 5 min (1500 x g at room temperature). The supernatant of the blood samples was then collected and stored at -20˚C until tested at the same time in triplicate. Liver function tests were performed using the serum by enzyme-linked immunosorbent assay (ELISA) were purchased from CORMAY (Poland) and included alanine transaminase (ALT) (cat. no. 7-216), aspartate transaminase (AST) (cat. no. 7-214) and alkaline phosphatase (ALP) (cat. no. 7-212) in addition to total bilirubin (cat. no. 7-254). At the endpoint of the 8-week MdP treatment period, the animals were euthanized by an intraperitoneal injection of phenobarbital sodium (150 mg/kg) according to the American Veterinary Medical Association (AVMA) guidelines. The death of the rats was verified by the absence of a heartbeat, corneal reflux and respiration. The total period of the study lasted 10 weeks, including 2 weeks of adaptation and 8 weeks of treatment.
Histopathological examination
The livers of the rats were immediately collected through surgery, washed with cold saline, preserved in 10% neutral buffered formalin and fixed for ~48 h at room temperature (25˚C). The fixed tissues then washed with running water for 6-8 h. The samples were then hydrated through a graded alcohol series, and cleared with xylene, were embedded in paraffin, after which 5-µm-thick sections were prepared. The specimens were deparaffinized and stained with hematoxylin and eosin (H&E; Bio Optica staining kit) as outlined in the instructions provided with the kit, which involved three clearing stations of 3 min each at room temperature (25˚C). The slides were analyzed using a light microscope (Olympus BX43; Olympus Corporation), and images were captured using a digital camera (Olympus XC30; Olympus C Corporation).
Statistical analysis
Statistical analysis was performed using GraphPad Prism 8.0 software (Dotmatics). All data are expressed as the mean ± standard deviation (SD). The Student's t-test was used for two paired variables (pre-and post-administration of MdP), while one-way ANOVA followed by Tukey's post hoc test were used to calculate a significant difference of the means between different examined groups. A P-value ≤0.05 was considered to indicate a statistically significant difference.
Results
Effects of MdP on serum liver biomarkers
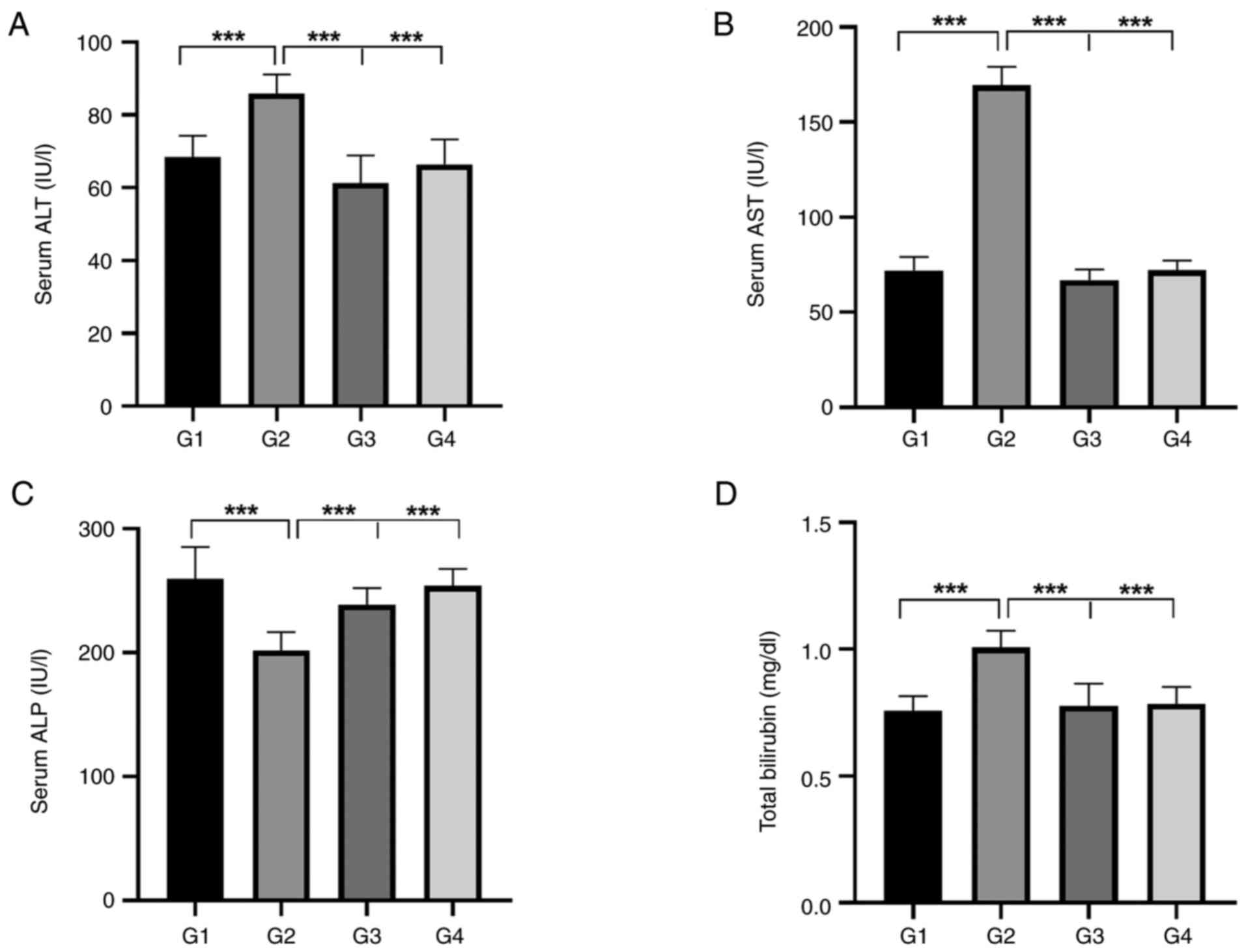
As demonstrated in Table I, a considerable increase was observed in the serum levels of ALT, AST and total bilirubin at 8 weeks following the administration of a weekly dose of MdP, with a significant decrease in the levels of ALP (P<0.001) in G2 compared to the serum levels prior to the administration of MdP (G1). On the other hand, no changes in the liver function tests were noted in the control rats before the start (G3) and after the end of the study (G4), as demonstrated in Table II.
When comparing the examined liver function parameters of the four groups, the results revealed that the serum ALT, AST and total bilirubin levels significantly increased following the administration of 3.5 mg of MdP (G2) in comparison to G1, G3 and G4, respectively (P<0.001), as shown in Fig. 1A, B and D. On the other hand, there was a substantial decrease in the serum levels of ALP in G2 following treatment compared to the other groups (G1, G3 and G4; P<0.001, as shown in Fig. 1C.
Immunohistochemistry of hepatic tissues in rats
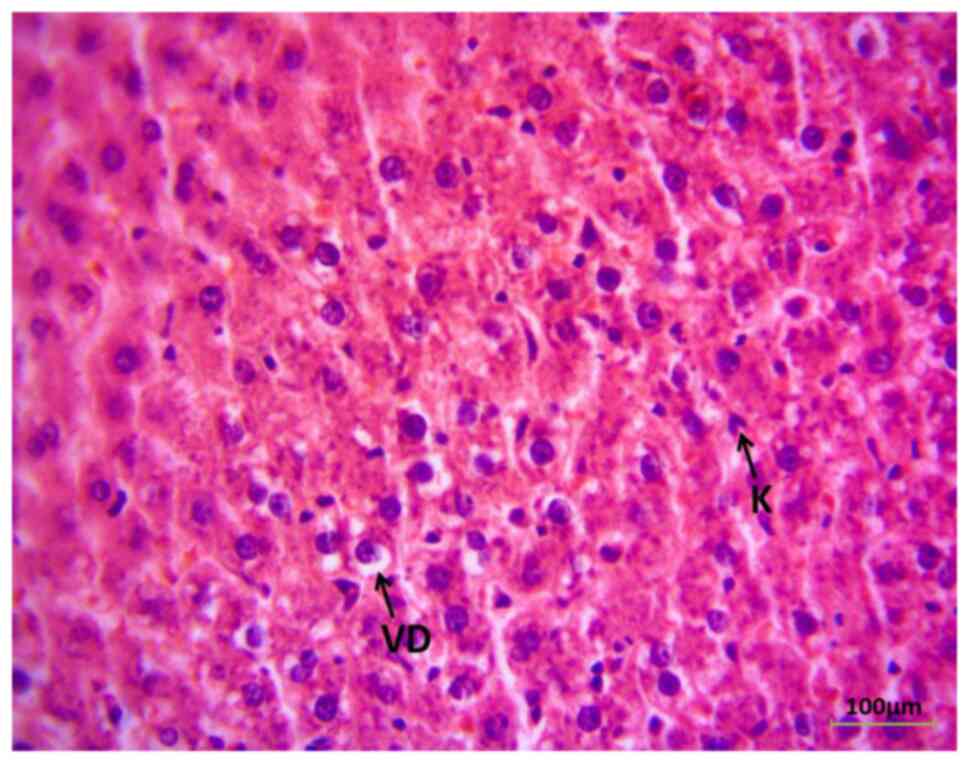
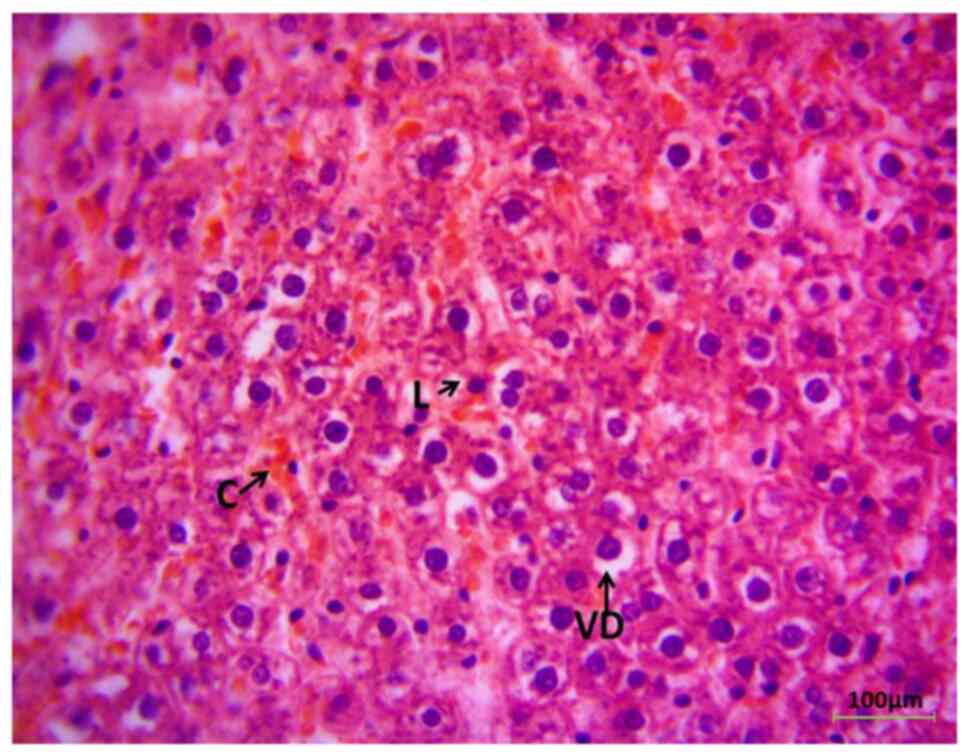
Immunohistochemistry of the liver (G1) revealed the classical histological observation of the liver section in the rats prior to MdP, apart from mild vacuolar degeneration of hepatocytes (Fig. 2). However, the liver histological sections exhibited a pathological appearance following the administration of MdP for 8 weeks, as demonstrated in Fig. 3. Some areas exhibited the congestion of sinusoids with hepatic tissue infiltration of inflammatory cells (lymphocytes).
Discussion
In the present study, the 8-week use of MdP was found to be linked to an increase in AST, ALT and total bilirubin levels, aligning with findings of previous research (23-25). As regards ALP, the present study demonstrated a decreased level in the MdP group compared to the animals not administered MdP. Furthermore, MdP was found to cause the vacuolar degeneration of hepatocytes, the congestion of sinusoids and the infiltration of hepatic tissues with inflammatory cells.
The liver is a crucial organ for several metabolic processes, including the detoxification of medications. AST, ALT, ALP and total bilirubin are among certain biochemical tests that can be used to monitor the overall function of the liver (26). The abnormal levels of any of these markers may be a sign of liver damage, and such tests are required for the determination of the location of liver injury and differential diagnosis (27).
The elevations in the levels of AST and ALT observed in the present study indicate possible MdP-induced hepatocellular damage. Hepatic cell injury results in the release of AST and ALT enzymes in the circulation. This observation is in line with the findings of previous studies that attributed the rise of such liver enzymes to the effect of ethinylestradiol (26-28). Hepatic, cardiac, renal, skeletal muscles, white blood and red blood cells in addition to the brain and lungs contain AST as cytosolic and mitochondrial enzymes. An increase in AST levels may be due to non-hepatic sources and it is not as specific or sensitive as ALT for the liver (31). Conversely, ALT is a cytosolic hepatic enzyme that is found in high concentrations in the liver. An increase in ALT levels is highly specific to liver injury (32).
The significant reduction in the levels of ALP in the present study is contradictory to the results of other studies (21,31) that demonstrated an elevation in the levels of ALP. It is known that hormonal contraception containing progestin is metabolized by the liver, potentially affecting the activity of liver enzymes (34), leading to a decrease in the production or release of ALP or even lowered levels due to damage to the biliary system.
The total bilirubin levels were substantially higher in the present study following the administration of MdP compared with the controls, which is a potential indicator of hepatobiliary damage or issues with the excretory roles of the liver (35). This outcome is consistent with the findings of previous studies demonstrating liver complications with the use of progesterone (21,23,34).
The outcomes of the present study collectively revealed that the use of MdP was associated with increased levels of ALT, AST and total bilirubin, suggesting that the use of MdP is associated with integrity and functionality alterations of the liver. It is a consequence of how medications can alter the metabolic activity of the liver and how injectable contraception leads to elevated levels of hepatic enzymes (37).
The present study also found histopathological lesions in the livers of female rats following hormonal therapy. Hepatocyte vacuolar degeneration, sinusoidal congestion and lymphocyte infiltration by lymphocytes were observed. Previous research has explained vacuolar degeneration, stating that an increase in endoplasmic reticulum, mitochondrial swelling and cellular granularity is caused by the metabolism of female sex hormones in hepatocytes (38); however, mild vacuolar degeneration does not essentially indicate severe liver damage. Additionally, the activation of the inflammatory immune system is reported by the infiltration of immune cells that are potentially responsible for the acceleration of hepatic damage (39).
MdP induces hepatic toxicity via several mechanisms, including an increase in oxidant molecules with a reduction in antioxidant enzymes that may be associated with hepatocellular injury (6). The levels of inflammatory markers increase with the administration of MdP, resulting in inflammation. The alteration of bile acid metabolism associated with MdP indicates a possibility of cholestatic damage (40). Elevated levels of inflammatory cytokines, and the interruption of mitochondrial oxidation further exacerbate hepatocellular injury through cellular homeostasis disruption and provoke apoptosis (41).
MdP is commonly used in hormonal therapies, including birth control and hormone replacement therapy. The present study confirmed the hepatotoxic effects of MdP, suggesting comparable adverse effects in humans. These data may help clinicians monitor liver function in patients with MdP or may provide a safer therapy or an adjuvant treatment for the likely development of hepatotoxicity.
The present study demonstrates the hepatotoxic effects of MdP through liver function tests and histological damage; however, the present study was an observational study. Further studies at the molecular levels, examining inflammatory cytokines, mitochondrial chain reaction, gene expression and oxidative stress markers are recommended to determine the mechanisms through which MdP induces liver damage. Despite this limitation however, the results of the present study highlight the negative effects of MdP on hepatic cells with the need for future research in this area.
In conclusion, the present study confirmed that MdP induces liver injury through mechanisms that are not yet entirely clear. Hepatotoxicity can be caused through induced metabolism, as MdP is metabolized by the liver, during which a reactive metabolite could be formed and cause further liver damage. The activation of the immune response through medication can result in inflammation and injury to hepatocytes, or can cause liver injury via direct MdP toxicity that more likely depends on the dose and duration of the treatment. However, the results of the present study highlight the necessity for the routine monitoring of liver function and status in the case that MdP is to be used by females for birth control.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be requested from the corresponding author.
Authors' contributions
FAA and MNA were involved in the conceptualization and methodology of the study. FAA, MNA and MEQ were involved in the investigative aspects of the study and in the formal analysis. FAA and MNA were involved in preparation of the figures and in the writing of the original draft of the manuscript. All authors edited and revised the manuscript. All authors confirm the authenticity of all the raw data. All authors have read and agreed to the final version of the manuscript.
Ethics approval and consent to participate
The Medical Ethical Committee at the University of Mosul, Mosul, Iraq approved the study protocol with code number CCMRE-phA-22-13 on 18/10/2022.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
|
Darroch JE: Trends in contraceptive use. Contraception. 87:259–263. 2013.PubMed/NCBI View Article : Google Scholar | |
|
Tandulwadkar S, Gupta S, Singh A, Mishra S and Singhania S: Medroxyprogesterone acetate versus gonadotropin-releasing hormone antagonist for the prevention of premature luteinizing hormone surge in hyper-responder women undergoing controlled ovarian stimulation for IVF/ICSI Cycles. JBRA Assist Reprod. 27(15)2023.PubMed/NCBI View Article : Google Scholar | |
|
Bigrigg A, Evans M, Gbolade B, Newton J, Pollard L, Szarewski A, Thomas C and Walling M: Depo Provera. Position paper on clinical use, effectiveness and side effects. Br J Fam Plann. 25:69–76. 1999.PubMed/NCBI | |
|
Phung MT, Lee AW, Wu AH, Berchuck A, Cho KR, Cramer DW, Doherty JA, Goodman MT, Hanley GE, Harris HR, et al: Depot-medroxyprogesterone acetate use is associated with decreased risk of ovarian cancer: The mounting evidence of a protective role of progestins. Cancer Epidemiol Biomarkers Prev. 30:927–935. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Heffron R, Achilles SL, Dorflinger LJ, Hapgood JP, Kiarie J, Polis CB and Steyn PS: Pharmacokinetic, biologic and epidemiologic differences in MPA-and NET-based progestin-only injectable contraceptives relative to the potential impact on HIV acquisition in women. Contraception. 99:199–204. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Abed MN, Qazzaz ME and Alassaf FA: Investigating the nephrotoxic effects of medroxyprogesterone in female albino rats. Ukr J Nephrol Dial. 2024:25–33. 2024. | |
|
Sathe A and Gerriets V: Medroxyprogesterone. In: StatPearls. StatPearls Publishing, Treasure Island, FL, 2024. Available from: http://www.ncbi.nlm.nih.gov/books/NBK559192/. | |
|
Baik SH, Baye F and McDonald CJ: Effects of hormone therapy on survival, cancer, cardiovascular and dementia risks in 7 million menopausal women over age 65: A retrospective observational study. medRxiv: May 26, 2022 (Epub ahead of print). | |
|
Ramazonovna KS: Differential diagnosis and treatment of depressive disorders in women during menopause. Eur J Innov Nonform Educ. 2:23–30. 2022. | |
|
Hosseinzadeh P and Wild R: Role of lipid management in women's health preventive care. Obstet Gynecol Clin. 48:173–191. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Özcan Ö, Den Elzen WPJ, Hillebrand JJ, Den Heijer M, Van Loendersloot LL, Fischer J, Hamer H, de Jonge R and Heijboer AC: The effect of hormonal contraceptive therapy on clinical laboratory parameters: A literature review. Clin Chem Lab Med. 62:18–40. 2024.PubMed/NCBI View Article : Google Scholar | |
|
Trinh A, Vyas A, Roselle A, Velu D, Hota L and Kadiyala M: Contraception and Cardiovascular Effects: What Should the Cardiologist Know? Curr Cardiol Rep. 25:1489–1498. 2023.PubMed/NCBI View Article : Google Scholar | |
|
Björnsson ES: Drug-induced cholestasis. In: Cholestatic Liver Disease. Carey EJ and Lindor KD (eds). Springer, New York, NY, pp13-31, 2014. Available from: https://doi.org/10.1007/978-1-4939-1013-7_2. | |
|
Kapp N: WHO provider brief on hormonal contraception and liver disease. Contraception. 80:325–326. 2009.PubMed/NCBI View Article : Google Scholar | |
|
Sitruk-Ware R: Reprint of pharmacological profile of progestins. Maturitas. 61:151–157. 2008.PubMed/NCBI View Article : Google Scholar | |
|
Mukherjea M, Mukherjee P, Biswas R, Chakraborty AS and Kushari J: Effect of medroxyprogesterone acetate contraception on human serum enzymes. Int J Fertil. 26:35–39. 1981.PubMed/NCBI | |
|
Connolly TJ and Zuckerman AL: Contraception in the patient with liver disease. Semin Perinatol. 22:178–182. 1998.PubMed/NCBI View Article : Google Scholar | |
|
Curtis KM, Tepper NK, Jatlaoui TC, Berry-Bibee E, Horton LG, Zapata LB, Simmons KB, Pagano HP, Jamieson DJ and Whiteman MK: US medical eligibility criteria for contraceptive use, 2016. MMWR Recomm Rep. 65:1–103. 2016.PubMed/NCBI View Article : Google Scholar | |
|
Itriyeva K: Use of Long-acting reversible contraception (LARC) and the Depo-provera shot in adolescents. Curr Probl Pediatr Adolesc Health Care. 48:321–332. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Sathe A and Gerriets V: Medroxyprogesterone. In: StatPearls. StatPearls Publishing, Treasure Island, FL, 2022. Available from: http://www.ncbi.nlm.nih.gov/books/NBK559192/. | |
|
Abd-Elkareem M, Alnasser SM, Meshal A, Abdullah RI and Ali AU: The effect of Norethisterone acetate on the uterus of albino rats: Histological, histochemical and ultrastructure study. BMC Vet Res. 20(384)2024.PubMed/NCBI View Article : Google Scholar | |
|
Chisholm NC and Juraska JM: Effects of long-term treatment with estrogen and medroxyprogesterone acetate on synapse number in the medial prefrontal cortex of aged female rats. Menopause. 19:804–811. 2012.PubMed/NCBI View Article : Google Scholar | |
|
Chane E, Wondifraw H, Hadgu R and Fasil A: Assessment of liver function tests of women taking hormonal contraceptives at University of Gondar comprehensive specialized hospital and Family Guidance Association of Gondar (FGAE), 2022; a comparative cross-sectional study. PLoS One. 18(e0289746)2023.PubMed/NCBI View Article : Google Scholar | |
|
Ebele JI, Emeka EN, Ignatius CM, Peace UN and Ebele E: Effects of duration of use of hormonal contraceptives on liver function. Res J Med Sci. 3:52–55. 2009. | |
|
Faddah L, Al-Rehany M, Abdel-Hamid N and Bakeet A: Oxidative stress, lipid profile and liver functions in average Egyptian long term depo medroxy progesterone acetate (DMPA) users. Molecules. 10:1145–1152. 2005.PubMed/NCBI View Article : Google Scholar | |
|
Iluz-Freundlich D, Zhang M, Uhanova J and Minuk GY: The relative expression of hepatocellular and cholestatic liver enzymes in adult patients with liver disease. Ann Hepatol. 19:204–208. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Ribeiro AJS, Yang X, Patel V, Madabushi R and Strauss DG: Liver microphysiological systems for predicting and evaluating drug effects. Clin Pharmacol Ther. 106:139–147. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Kowalska K, Ściskalska M, Bizoń A, Śliwińska-Mossoń M and Milnerowicz H: Influence of oral contraceptives on lipid profile and paraoxonase and commonly hepatic enzymes activities. J Clin Lab Anal. 32(e22194)2018.PubMed/NCBI View Article : Google Scholar | |
|
Rahim SA: Activities of some liver enzymes in serum of woman receiving oral contraceptives, injection and Cu-IUD. Eur Asian J Biosci: 14, 2020. | |
|
Taheri M, Rahimi M, Naderi M, Ghavami S, Mokhtari M, Rashidi H and Hashemi M: Effects of a subdermal levonorgestrel contraceptive implant (Norplant) on serum cholesterol, triglycerides, ALT and AST in Iranian women. Contraception. 73:56–58. 2006.PubMed/NCBI View Article : Google Scholar | |
|
Oh RC, Hustead TR, Ali SM and Pantsari MW: Mildly elevated liver transaminase levels: Causes and evaluation. Am Fam Physician. 96:709–715. 2017.PubMed/NCBI | |
|
Prati D, Taioli E, Zanella A, Torre ED, Butelli S, Del Vecchio E, Vianello L, Zanuso F, Mozzi F, Milani S, et al: Updated definitions of healthy ranges for serum alanine aminotransferase levels. Ann Intern Med. 137:1–10. 2002.PubMed/NCBI View Article : Google Scholar | |
|
Shosha HM, Ebaid HM, Toraih EA, Abdelrazek HMA and Elrayess RA: Effect of monosodium glutamate on fetal development and progesterone level in pregnant Wistar Albino rats. Environ Sci Pollut Res. 30:49779–49797. 2023.PubMed/NCBI View Article : Google Scholar | |
|
Coombes Z, Plant K, Freire C, Basit AW, Butler P, Conlan RS and Gonzalez D: Progesterone metabolism by human and rat hepatic and intestinal tissue. Pharmaceutics. 13(1707)2021.PubMed/NCBI View Article : Google Scholar | |
|
Serfaty D: Update on the contraceptive contraindications. J Gynecol Obstet Hum Reprod. 48:297–307. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Egoro ET, Tokoni OG and Anakwe CS: Studies on some plasma biochemical parameters in short and long term users of oral contraceptives containing lower doses of estrogen and progestin composition in Lagos, Nigeria. Health (NY). 1(2)2018. | |
|
Onyesom I, Osioma E, Etagar E and Ofili M: Activities of some liver enzymes in serum of humans receiving DMPA and CUPID contraceptives. Sch J App Med Sci. 1:62–64. 2013. | |
|
Bakry S and Abu-Shaeir W: Electrophoretic and histopathologica l studies on adult female rats treated with depo-provera (DMPA). Aust J Basic Appl Sci. 4:61–70. 2010. | |
|
Ahmed O, Robinson MW and O'Farrelly C: Inflammatory processes in the liver: Divergent roles in homeostasis and pathology. Cell Mol Immunol. 18:1375–1386. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Masama C, Jarkas DA, Thaw E, Daneshmend AZ, Franklyn SI, Beaurepaire C and McQuaid RJ: Hormone contraceptive use in young women: Altered mood states, neuroendocrine and inflammatory biomarkers. Horm Behav. 144(105229)2022.PubMed/NCBI View Article : Google Scholar | |
|
Chen P, Yao L, Yuan M, Wang Z, Zhang Q, Jiang Y and Li L: Mitochondrial dysfunction: A promising therapeutic target for liver diseases. Genes Dis. 11(101115)2024.PubMed/NCBI View Article : Google Scholar |