Research on influenza A (H1N1): Advances and future directions (Review)
- Authors:
- Published online on: April 9, 2025 https://doi.org/10.3892/wasj.2025.343
- Article Number: 55
-
Copyright : © Elumalai et al. This is an open access article distributed under the terms of Creative Commons Attribution License [CC BY 4.0].
Abstract
1. Introduction
Influenza A/H1N1pdm09, commonly known as swine flu, emerged as a novel virus in April 2009, marking the onset of the first pandemic of the 21st century (1). This virus is a subtype of the influenza A virus, classified within the Orthomyxoviridae family and characterized by its negative sense RNA genome, which encodes 11 proteins (2). Influenza viruses are categorized into four types: A, B, C and D, with type A being the most significant in causing widespread epidemics due to its ability to undergo antigenic drift and shift mechanisms that contribute to its genetic variability and evasion of host immunity (3).
The H1N1pdm09 virus is distinguished by its unique genetic characteristics that set it apart from both seasonal influenza strains and previously circulating viruses (4). In particular, the virus exhibits a susceptibility for mutations in the hemagglutinin (HA) gene, essential for viral entry into host cells, and in the neuraminidase (NA) gene, which facilitates the release of newly formed virions. These surface proteins are primary targets for neutralizing antibodies; their variability is a critical factor in the virus's ability to evade vaccine-induced immunity (1). Since its emergence in 2009, H1N1pdm09 has continued to circulate globally, leading to seasonal outbreaks and posing ongoing challenges to public health systems (4).
Influenza A encompasses multiple subtypes, each with distinct epidemiological significance. While H3N2 remains a predominant seasonal strain and H5N1 presents a pandemic threat, H1N1pdm09 warrants focused analysis due to its sustained global circulation since 2009. Unlike H5N1, which has limited human-to-human transmission, H1N1pdm09 continues to cause annual epidemics, rendering it a persistent public health challenge. Moreover, its genetic evolution and patterns of antigenic drift provide crucial insights applicable to broader influenza control strategies. Understanding the evolution of H1N1pdm09 can aid in developing more effective antiviral and vaccine strategies that may also be relevant for managing other influenza subtypes. Future research is required however, to further explore the implications of the antigenic shift and reassortment events involving H3N2 and emerging avian influenza strains (5).
As of 2024, the World Health Organization reported that H1N1pdm09 remains a significant public health concern, accounting for ~20% of all influenza A virus detection worldwide [World Health Organization (WHO), 2024]. In India, this virus has been associated with considerable morbidity and mortality, highlighting the need for continuous surveillance and research into its epidemiological trends and genetic evolution. The ability of the virus to reassort with other influenza strains, particularly those of avian and swine origin, raises concerns about potential future pandemics, echoing historical pandemics linked to antigenic shifts (5). Effective surveillance and characterization of circulating H1N1pdm09 variants are vital for understanding their epidemiological impact and guiding the development of effective vaccines and antiviral therapies. The present review aimed to summarize recent findings on the molecular characterization, surveillance strategies and therapeutic approaches associated with influenza A/H1N1pdm09, focusing on its impact from during the period between 2009 to 2024.
2. Surveillance and molecular characterization of influenza A/H1N1pdm09
Epidemiology and genetic variants. The study of influenza A/H1N1pdm09 in India has revealed marked genetic diversity among the circulating strains. To understand the implications of this genetic diversity, it is essential to examine the epidemiological trends associated with H1N1pdm09. Epidemiologically, H1N1pdm09 has been shown to affect younger age groups more severely than typical seasonal influenza viruses. This age distribution may be attributed to older individuals possessing partial immunity due to prior exposure to related influenza strains (6). However, severe cases and fatalities have also occurred in older populations, particularly among those with underlying health conditions or immunocompromised states, which increase susceptibility across all age groups (7).
The increase in publications and research surrounding influenza since the 2009 pandemic is encouraging, reflecting a growing awareness of the disease and the importance of mitigating its burden. This growing body of literature highlights not only the need for continued research, but also underscores the importance of robust surveillance systems to monitor emerging strains and their impacts. Implementing regional standardization in data collection would facilitate direct comparisons and enhance understanding of the impact of influenza in the region. In addition to monitoring existing influenza strains, it is crucial to remain vigilant for emerging subtypes and any changes in virulence or transmission dynamics (8).
Globally, annual influenza epidemics account for ~3 to 5 million cases of severe illness and between 250,000 to 500,000 deaths (6). Influenza A (H1N1) pdm09 is currently classified as a seasonal influenza virus that co-circulates with other seasonal strains, including H3N2 and influenza B (9). Understanding this context is vital as it provides insight into the mechanisms driving the rapid genetic and antigenic evolution of influenza viruses. The ability of these viruses to undergo rapid genetic and antigenic evolution is primarily due to point mutations in the genome, particularly in the HA and NA genes (antigenic drift), and reassortment of gene segments from intra-species and inter-species influenza viruses (antigenic shift) (10).
Antigenic drift and antigenic shift are key drivers of influenza virus evolution, influencing vaccine efficacy and pandemic potential. Antigenic drift involves gradual mutations in HA and NA proteins, allowing immune evasion over time; this is evident in the frequent updates to the seasonal influenza vaccine. By contrast, the antigenic shift, a more abrupt genetic reassortment event, can lead to novel pandemic strains, such as the 2009 H1N1pdm09 virus, which originated from swine, avian and human influenza strains. Understanding these mechanisms is crucial for anticipating future influenza threats and improving vaccine development strategies (11).
Influenza A viruses are categorized into various subtypes based on the variation of surface glycoproteins, HA and NA, with 18 HA (H1-H18) and 11 NA (N1-N11) subtypes identified (6). This classification underscores the complexity of the genetic landscape of H1N1pdm09. The pdmH1N1 viruses and their descendants responsible for seasonal H1N1 epidemics continue to evolve within the human population. In particular, H1N1 viruses have accumulated mutations associated with enhanced replication rates (12). The genetic variability of H1N1pdm09 also extends to patterns of antiviral resistance. Recognizing these patterns is essential for developing effective treatment strategies.
While the virus is generally susceptible to neuraminidase inhibitors, such as oseltamivir (Tamiflu), sporadic cases of resistance have been documented (13). This genetic diversity encompasses not only structural proteins but also variations in other genomic regions that influence viral replication, pathogenesis, and host adaptation. For instance, mutations in polymerase genes (PB1, PB2 and PA) can affect viral replication efficiency and contribute to host range expansion or adaptation to different environments (14). Similarly, mutations in non-structural proteins (NS1 and NS2) may enhance viral immune evasion mechanisms or modulate host cell signalling pathways (10). Surveillance efforts are essential to monitor resistance patterns and inform treatment strategies, especially in severe or high-risk cases where prompt antiviral therapy can be life-saving.
Despite progress being made in this area, gaps remain that need to be addressed through comprehensive studies. There are still limited epidemiological studies focusing on the full genetic characterization of strains and the detection of co-infections with influenza A. Notable mutations in the HA protein, such as S91R, S181T, S200P, I312V, K319T, I419M and E523D, have been identified to potentially enhance the fitness and pathogenicity of the virus in new hosts (Table I). Common mutations observed include serine-to-threonine, alanine-to-threonine and lysine-to-glutamine at various regions that alter the physicochemical features of receptor-binding domains, N-glycosylation patterns and epitope-binding sites when compared with reference strains. Such mutations contribute to diversity among all Indian strains; thus, structural and functional characterization of these variants becomes essential. In this study, the scientists observed that mutational drift results in alterations within the receptor-binding domain alongside new variant N-glycosylation patterns and novel epitope-binding sites. This highlights a pressing need for innovative therapeutic strategies moving forward. Consequently, there is an urgent need to develop distinct next-generation therapeutic inhibitors targeting the HA strains of the Indian influenza A (H1N1) virus (15).
Table INotable mutations in the H1N1 virus, including the full gene names, mutations, and their effects. |
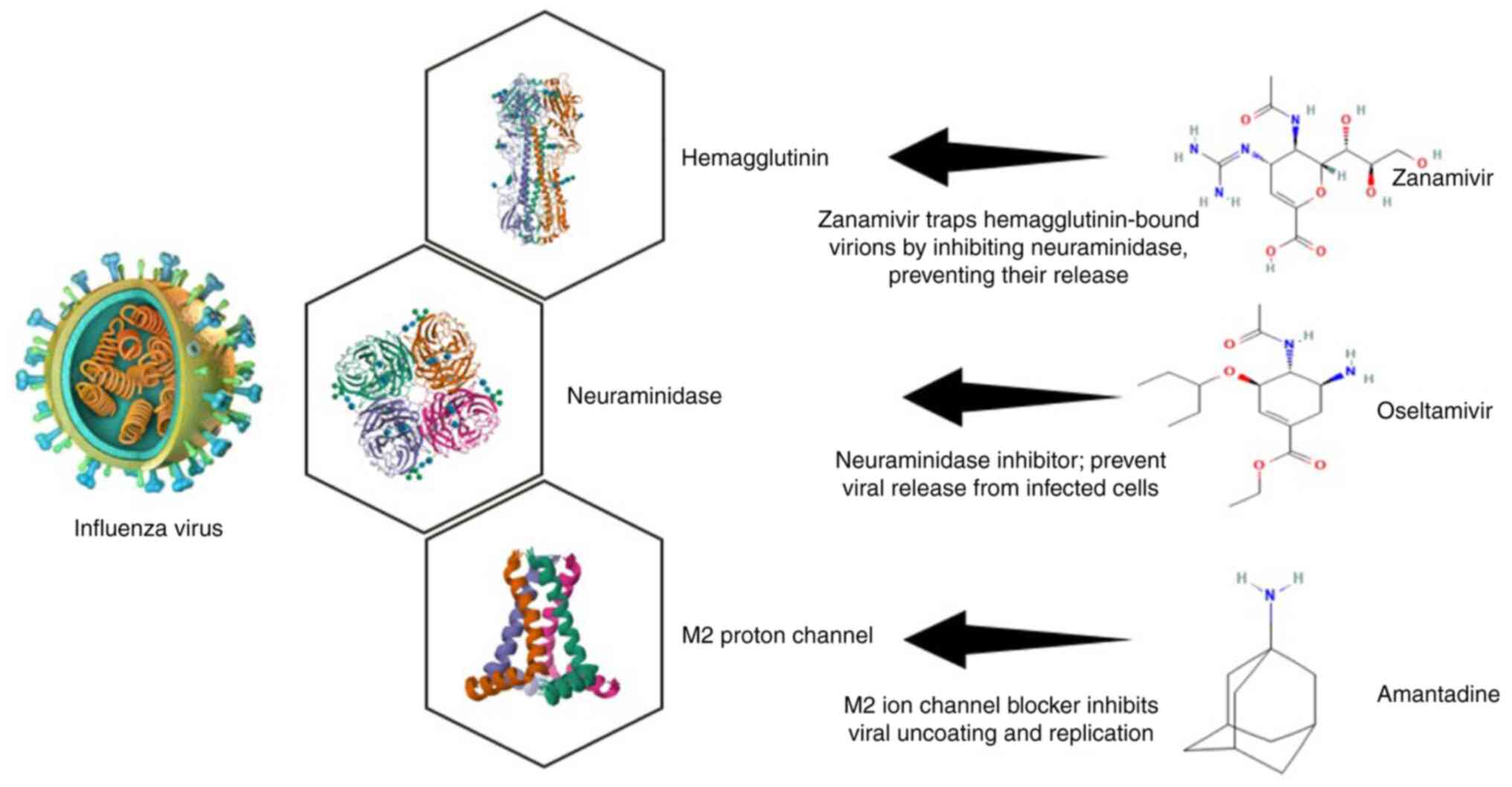
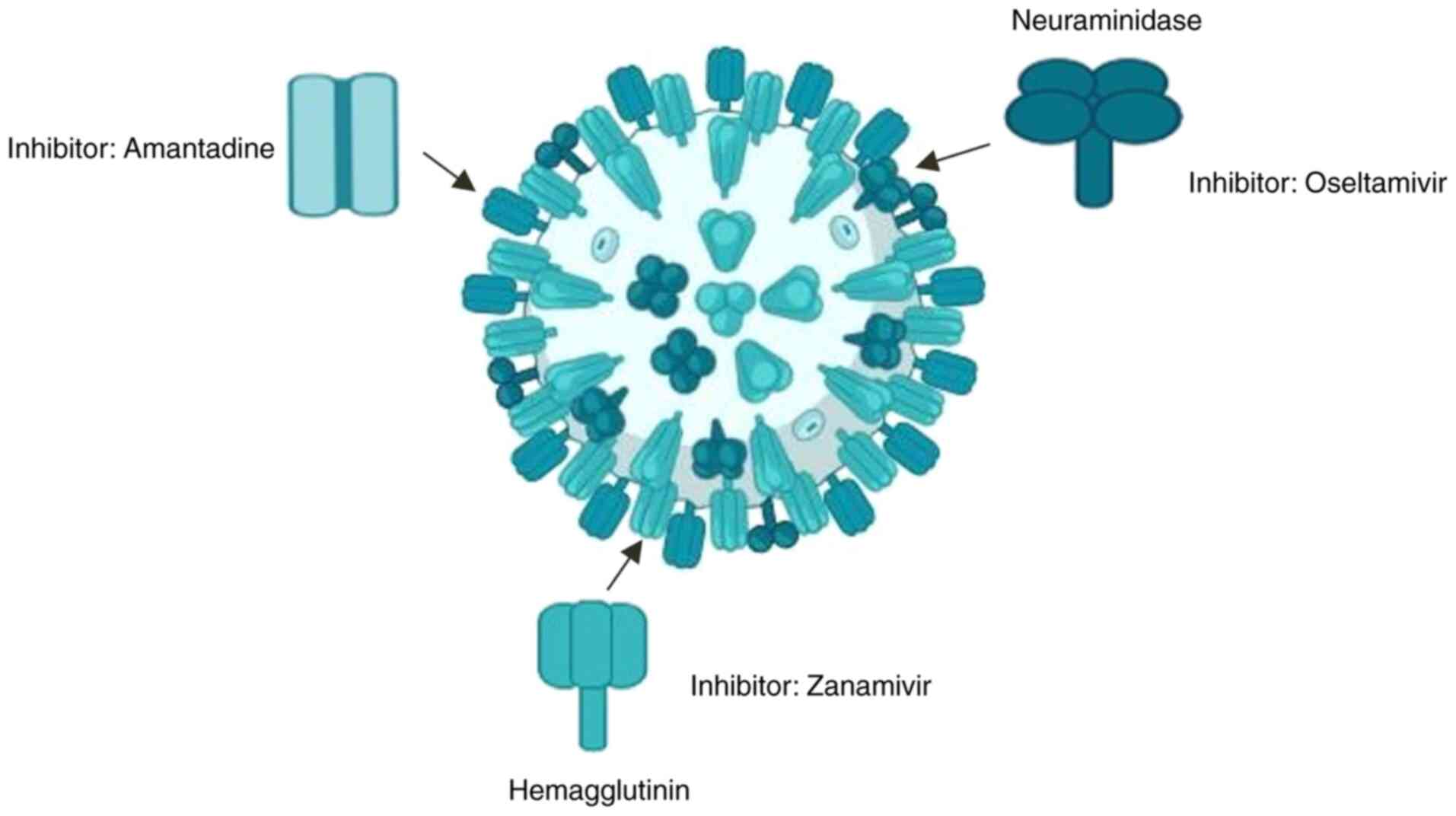
Approved antiviral drugs. Approved antiviral drugs for influenza A (H1N1) include several key medications that have been utilized to manage infections effectively. The primary class of antiviral agents used against H1N1 is neuraminidase inhibitors, which include oseltamivir (Tamiflu), zanamivir (Relenza) (Fig. 1) and peramivir (Rapivab). These drugs function by inhibiting the neuraminidase enzyme, which is essential for the release of new viral particles from infected cells, thereby limiting the spread of the virus within the respiratory tract (6). Additionally, baloxavir marboxil (Xofluza) represents a newer antiviral option that inhibits the cap-dependent endonuclease activity of the viral polymerase, providing an alternative mechanism of action compared to traditional NA inhibitors (15). While these antivirals are generally effective, concerns about resistance have emerged, particularly with oseltamivir, where sporadic cases of resistant strains have been documented (13).
An overview of the approved antiviral drugs with the mechanism of action, advantages and disadvantages is provided in Table II. Therefore, continuous surveillance and research into the efficacy and resistance patterns of these antiviral agents are crucial for optimizing treatment strategies and ensuring effective management of Influenza A (H1N1) infections (14).
3. Innovative frontiers in antiviral research
As influenza A/H1N1pdm09 continues to evolve, traditional antiviral strategies may become less effective, necessitating the development of novel therapeutic approaches. Advances in computational biology, molecular docking, viral mutation analysis and novel antiviral agents have significantly contributed to improving treatment options and designing targeted interventions against influenza infections. This section explores the potential of in silico drug discovery, the impact of HA mutations, and the development of novel antiviral compounds, including fullerene derivatives, phytochemicals and drug repurposing approaches, in combating influenza A/H1N1pdm09.
In silico analysis and molecular docking studies. Computational approaches, particularly molecular docking and molecular dynamics simulations, have revolutionized the identification of potential antiviral compounds by simulating interactions between small molecules and viral proteins. These techniques enable the rapid screening of large chemical libraries, ranking compounds based on their binding energies and predicted interactions with key viral targets. Molecular docking helps pinpoint the optimal binding poses of inhibitors within the active sites of viral proteins, offering insights into their potential efficacy against Influenza A/H1N1pdm09(16). To complement docking studies, pharmacokinetic and toxicity predictions are essential for assessing the drug-likeness of candidate molecules. Tools such as Swiss ADME and ADMETlab provide a comprehensive evaluation of Absorption, Distribution, Metabolism, and Excretion (ADME) properties, ensuring the selection of compounds with favorable bioavailability and minimal toxicity (17).
Despite the associated advantages, in silico drug discovery presents several limitations that hinder its direct clinical translation. Docking algorithms, though efficient, often produce false positives, where predicted binding interactions fail to translate into biological activity. The accuracy of scoring functions used in docking software remains a challenge, as they do not always account for protein flexibility, solvent effects, or dynamic conformational changes in biological environments (18). Moreover, high-performance computing resources are often required to conduct large-scale screenings, limiting their accessibility and efficiency in real-world applications (19).
An emerging area of computational drug discovery focuses on fullerene-based antiviral compounds, which have exhibited significant inhibitory effects against influenza A/H1N1. Polyhydrated fullerenes (fullerenols) have been identified as potential inhibitors of RNA-dependent RNA polymerase (RdRp), a critical enzyme in viral genome replication. Studies have shown that fullerenols markedly reduce H1N1 viral titers, suggesting their potential as effective antiviral candidates (20). However, moving these compounds into clinical use remains challenging due to concerns regarding toxicity, hydrophobicity, and regulatory approval hurdles. Fullerene derivatives, particularly those modified for enhanced solubility, require rigorous in vivo testing to determine their stability, pharmacokinetics and long-term safety profiles.
One significant challenge in employing in silico methods lies in translating computational predictions into actual biological activity. Although docking studies can reveal potential interactions, the effectiveness of these interactions within biological systems can vary considerably. Despite this limitation, in silico analysis and molecular docking studies remain invaluable tools in combating Influenza A/H1N1pdm09. They provide a cost-effective and efficient means for screening potential antiviral agents and accelerating the drug discovery process (17).
For instance, research conducted demonstrated that a mixture of polyhydrated fullerenes (fullerenols) exhibited significant antiviral activity against Influenza A (H1N1), markedly reducing viral infectious titers (20). These fullerenols hve been found to target RNA-dependent RNA polymerase, an enzyme critical for viral replication. The findings from that study underscore the potential of these compounds as promising leads for treating influenza A virus infections (20). Such insights could assist medicinal chemists and pharmaceutical professionals in developing and synthesizing more effective therapeutic candidates in the future. Furthermore, this research encourages the in vitro and in vivo evaluation of the proposed compounds to validate the computational findings (20).
Mutation analysis in HA protein. The HA protein plays a central role in the infectivity and antigenicity of influenza A/H1N1pdm09, mediating viral entry by binding to sialic acid receptors on host cells. Due to the selective pressure exerted by the host immune system, HA is one of the most rapidly evolving viral proteins, undergoing frequent mutations that contribute to immune evasion, altered host specificity, and changes in receptor-binding affinity (21). These mutations significantly affect the efficacy of vaccines, necessitating continuous surveillance and periodic updates of influenza vaccines. The antigenic drift, a process through which point mutations accumulate in antigenic sites of HA, leads to gradual changes that reduce the binding efficiency of pre-existing neutralizing antibodies. Over time, these mutations result in the emergence of antigenically distinct viral strains that can escape immune recognition, thereby contributing to reinfection in previously exposed or vaccinated individuals (22).
Recent genetic analyses of influenza A/H1N1pdm09 strains have identified several key HA mutations that enhance viral fitness and immune escape. These include: S91R and S181T that affect receptor-binding affinity, potentially increasing viral transmissibility; S200P which alters HA conformation, reducing antibody accessibility to neutralizing epitopes; I312V and K319T that influence antigenic site topology, enabling escape from pre-existing immunity; I419M and E523D that may affect HA stability and fusion efficiency, enhancing viral entry into host cells (23). Mutations in HA also influence glycosylation patterns, which play a critical role in shielding antigenic sites from neutralizing antibodies. The addition or removal of N-glycosylation sites at key positions can alter viral antigenicity, allowing the virus to evade immune detection. A previous study demonstrated that glycan modifications near the receptor-binding domain affect the ability of the virus to attach to host cell receptors while avoiding immune neutralization (24).
Structural modeling and molecular dynamics simulations have provided deeper insight into how HA mutations affect its functionality, as the structural organization of HA is crucial for its function. HA exists as a precursor protein (HA0), which is cleaved into two subunits: HA1, responsible for receptor binding and HA2, which is involved in membrane fusion. The antigenic sites of HA1 are particularly susceptible to mutations, influencing antibody recognition and vaccine effectiveness (25). Research mapping these mutations onto the HA trimeric crystal structure has revealed that several mutations cluster near or within known antigenic sites (Sa, Sb, Ca1, Ca2 and Cb), which are the primary targets of neutralizing antibodies (22). For example, mutations at the Ca2 and Sb sites have been linked to reduced antibody binding affinity, leading to a lower vaccine efficacy. Additionally, phylogenetic analyses have demonstrated the evolutionary trajectory of influenza A/H1N1pdm09, showing distinct clades of HA variants that exhibit differential sensitivity to monoclonal antibodies and vaccine-induced immunity. These findings underscore the importance of continuous genomic surveillance and computational modeling to predict emerging antigenic variants and optimize vaccine formulations (12).
The ability of HA to undergo antigenic drift poses a major challenge for vaccine design. Current seasonal influenza vaccines rely on pre-selected HA antigens, which may not always match the circulating strains due to ongoing HA evolution. The accumulation of escape mutations in vaccine strains reduces the effectiveness of antibody-mediated immunity, necessitating annual vaccine updates based on real-time surveillance data (13).
To address this challenge, researchers are exploring the development of universal influenza vaccines that target conserved HA epitopes less prone to mutation. Strategies include targeting the HA stem region, which is more conserved than the HA head domain; multi-epitope vaccine design, incorporating conserved antigenic determinants from multiple influenza strains; computational epitope mapping, using machine learning to identify HA regions least likely to undergo antigenic drift (26). Moreover, HA mutations also influence antiviral drug resistance, particularly against neutralizing monoclonal antibodies. The continued emergence of resistant strains highlights the need for combination therapies that target multiple viral components, reducing the likelihood of resistance development.
The ongoing genetic evolution of HA through the antigenic drift underscores the necessity for comprehensive surveillance programs, structural modeling studies and innovative vaccine strategies. Advances in bioinformatics, structural biology and immunogenetics are critical for staying ahead of rapidly evolving influenza strains and ensuring effective vaccine formulations and antiviral interventions. As the influenza virus continues to adapt, a multi-faceted approach combining genomic surveillance, computational modeling, and immunological studies will be essential to mitigate the public health impact of Influenza A/H1N1pdm09. The mutation analysis of the HA protein in influenza viruses is crucial for understanding viral evolution, pathogenicity and the mechanisms of immune escape. HA, as the primary surface glycoprotein of the influenza virus, plays a pivotal role in facilitating the entry of the virus into host cells by mediating receptor binding and promoting membrane fusion (20). Due to its status as a primary target for the host immune response, HA is subject to selective pressure that can lead to mutations enhancing viral fitness and antigenic diversity (21).
A comprehensive analysis of HA mutations involves several key steps, including sequence alignment, structural modeling and positive selection analysis. The sequence alignment of HA genes from various isolates can reveal conserved and variable regions, highlighting positions under selective pressure (22). Structural modeling further elucidates how these mutations may impact the three-dimensional conformation of the HA protein, potentially altering its interactions with host cell receptors or antibodies (12). Mutations linked to NA inhibitor treatment often occur near antigenic sites, as shown by HA trimeric crystal structure mapping. This localization affects the virus susceptibility to antiviral drugs and neutralizing antibodies, thereby influencing treatment outcomes and vaccine efficacy (23).
In the case of the influenza A/H1N1pdm09 virus, the HA protein exists as H0, which is cleaved into HA1 and HA2 by host cell proteases. The HA1 subunit contains five antigenic sites: Sb, Ca1, Ca2, and Cb. Mutations at these sites, along with changes in N-glycosylation patterns, can alter the antigenicity of the HA1 subunit, generating variants capable of evading neutralizing antibodies (20). In silico analyses, including phylogenetic studies and mutational analyses of HA amino acid sequences, provide an efficient method with which to investigate the genetic variations driving viral evolution.
Prominent mutations identified in the HA protein include S91R, S181T, S200P, I312V, K319T, I419M and E523D. These mutations may enhance viral fitness and pathogenicity in new hosts (12). The antigenic sites (Sa, Sb, Ca1, Ca2 and Cb) along with fusion peptide regions are critical for virus-host interactions. Studies utilizing tools, such as Swiss PDB Viewer and PyMOL have revealed mutations in these regions that could affect the virus antigenicity and immune evasion capabilities (23). Free-energy analysis suggests that these mutations might destabilize the protein structure, indicating a need for experimental validation.
Overall, the continuous monitoring and analysis of HA mutations are vital for anticipating and mitigating the impact of emerging influenza strains. Advances in sequencing technologies and bioinformatics tools have significantly enhanced the ability of researchers to track these mutations in real-time. This capability provides critical insight for public health interventions and vaccine development. As the influenza virus continues to evolve, understanding the molecular mechanisms underlying HA mutations will remain a key component of global influenza surveillance and control efforts.
4. Novel antiviral strategies and advancements in therapeutic agents as antiviral therapies
The continued evolution of influenza A/H1N1pdm09 and the emergence of drug-resistant strains have necessitated the exploration of innovative antiviral strategies. As traditional antiviral drugs lose efficacy due to viral mutations and resistance, alternative therapeutic approaches, including fullerene derivatives, phytochemical compounds, and drug repurposing strategies, have gained attention. These novel interventions aim to enhance antiviral efficacy while addressing the limitations of conventional treatments, such as toxicity, poor bioavailability, and high resistance development. The emergence of influenza A/H1N1pdm09 has prompted extensive research into novel therapeutic approaches and antiviral strategies to combat this persistent public health threat. As the virus continues to evolve, adapting to evade host immunity and antiviral treatments, it is crucial to explore innovative solutions to mitigate its impact. This section delves into four promising areas of research: Fullerene derivatives and their antiviral potential, the use of phytochemical compounds to inhibit influenza, the development of multi-epitope vaccines, and the repurposing of existing drugs through in silico methods. An overview of the various novel antiviral compounds and their targets is provided in Table III.
Fullerene derivatives and antiviral activity. Fullerenes are a unique class of carbon nanomaterials that have exhibited significant antiviral activity by effectively inhibiting viral replication. Their distinctive chemical and physical properties set them apart from traditional small-molecule drugs, rendering them promising candidates for both antiviral therapy and diagnostic applications. Due to their three-dimensional molecular structure, high electron affinity and extensive conjugated double bonds, fullerenes exhibit potent antioxidant, antiviral, and immunomodulatory properties (27). These features have been explored not only for treating viral infections but also for applications in cancer immunotherapy, imaging and drug delivery systems (28).
Fullerenes and their derivatives demonstrate broad-spectrum antiviral activity against multiple pathogens, including HIV, herpes simplex virus (HSV), influenza virus, Ebola virus, and cytomegalovirus (CMV) in both in vitro and in vivo models (20). Their antiviral mechanism primarily involves inhibiting viral entry and replication by interacting with viral surface proteins and enzymes.
For influenza A viruses, particularly H1N1 and H3N2, fullerenes exert their effects by inhibiting NA activity. Studies have shown that polycarboxylated C70 fullerene derivatives effectively bind to NA, preventing the cleavage of sialic acid residues on host cell surfaces. This inhibits the release of newly formed viral particles, reducing viral spread and infectivity (29). They also target RNA-Dependent RNA polymerase (RdRp), an enzyme essential for viral genome replication. This disrupts viral RNA transcription, significantly lowering viral RNA synthesis and protein expression within infected cells (20). It has also been shown that fullerenes interfere with PA endonuclease activity, thereby preventing viral RNA transcription and replication, further contributing to their antiviral activity (30). Additionally, fullerenes have been shown to modulate membrane interactions and ion transport, altering the intracellular environment required for viral replication. Their ability to cross cell membranes and interact with viral proteins enhances their effectiveness as antiviral agents.
To improve their biocompatibility, solubility and antiviral efficacy, fullerene derivatives are categorized into six major groups: Amino acid, peptide, and primary amine derivatives, piperazine and pyrrolidine derivatives, carboxyl derivatives, hydroxyl derivatives (fullerenols), glycofullerene derivatives and fullerene complexes (28). Among these, highly water-soluble polycarboxylated C70 derivatives have demonstrated broad-spectrum antiviral activity, particularly against Influenza A virus subtypes H1N1 and H3N2(20). Fullerenols, or polyhydrated fullerene derivatives, are of special interest due to their low toxicity, enhanced solubility, and compatibility with biological fluids, making them more suitable for therapeutic applications.
Despite their potential as antiviral agents, fullerenes face several challenges that hinder their clinical development. Firstly, fullerenes are intrinsically hydrophobic, rendering their aqueous solubility extremely low. This affects drug absorption, bioavailability and systemic distribution in biological environments. To overcome this issue, hydrophilic modifications, such as hydroxylation (fullerenols) or conjugation with peptides, polymers, or liposomes, have been developed to enhance their solubility (30). Secondly, while it has been indicated that pristine C60 fullerenes exhibit minimal toxicity in bacteria, fungi, human leukocytes, and rodents, functionalized derivatives may behave differently in vivo. Their interactions with cellular membranes, oxidative stress pathways and metabolic processes require further investigation to ensure their long-term safety (20). Thirdly, the metabolic fate of fullerene derivatives in the human body is not fully understood. Concerns regarding bioaccumulation in tissues and long-term clearance from the body must be addressed through further in vivo pharmacokinetic studies. Finally, the complex synthesis and functionalization of fullerenes pose challenges for large-scale pharmaceutical production. Additionally, regulatory frameworks for nanomaterial-based therapeutics are still evolving, requiring extensive safety and efficacy evaluations before fullerenes can be approved for human use.
To enhance therapeutic potential, fullerenes can be integrated into nanocarrier-based drug delivery systems. Recent research suggests that fullerene-liposome complexes exhibit significantly higher antiviral activity than conventional small-molecule inhibitors, highlighting their potential for advanced drug formulations (28). These complexes provide advantages, such as improved drug solubility and stability, enhanced target specificity and controlled drug release and reduced systemic toxicity and off-target effects (29). Additionally, fullerenes hold promise as adjunct therapies in combination with existing influenza antiviral drugs. Their ability to scavenge free radicals and modulate immune responses suggests potential synergy with neuraminidase inhibitors (e.g., oseltamivir) or RNA polymerase inhibitors (e.g., favipiravir) (31). Combining fullerenes with immunomodulatory agents may enhance host antiviral defense mechanisms, further improving treatment outcomes.
While preclinical studies on fullerene derivatives have yielded promising results, their clinical translation requires further optimization. However, future research is required to focus on: Developing more biocompatible and water-soluble fullerene derivatives to improve their therapeutic application, conducting large-scale in vivo studies to assess their pharmacokinetics, biodistribution and toxicity profiles, exploring combination therapy strategies with conventional antiviral drugs to maximize efficacy and minimize drug resistance, advancing drug delivery systems, such as fullerene-based nanoparticles, micelles, or polymer conjugates, to enhance site-specific antiviral activity and addressing regulatory challenges by establishing standardized safety guidelines for nanomaterial-based antiviral therapeutics. Given their multifunctional properties, fullerenes could revolutionize antiviral therapy by offering new strategies for targeting influenza and other viral infections.
Fullerenes inhibit viral replication effectively. Their unique properties distinguish them from traditional small-molecule drugs, rendering fullerenes as promising candidates for both the diagnosis and treatment of viral infections. For example, fullerenes and their derivatives can act as antioxidants against inflammatory diseases due to their extensive conjugated double bonds, which scavenge free radicals. Fullerene C60 has been shown to enhance tumor immunity by polarizing tumor-associated macrophages and can be combined with immune checkpoint inhibitors, such as PD-L1 monoclonal antibodies, to improve tumor immunotherapy. Additionally, fullerene C70 derivatives serve as photosensitizers that generate singlet oxygen to effectively destroy tumor cells. Endohedral metal fullerenes are also being explored as novel nuclear magnetic resonance contrast agents for treating liver steatosis and tumors (28). As a new class of broad-spectrum antiviral agents, fullerenes have gained increasing attention for their potential application in treating SARS-CoV-2. Research into the synthesis and antiviral properties of fullerenes has deepened our understanding of the relationship between their structure and bioactivity (32).
Fullerene derivatives can be classified into six categories as follows: i) Amino acid, peptide and primary amine derivatives; ii) piperazine and pyrrolidine derivatives; iii) carboxyl derivatives; iv) hydroxyl derivatives; v) glycofullerene derivatives; and vi) fullerene complexes. Numerous antiviral studies have highlighted the significant antiviral activity of fullerene C60 and its derivatives against influenza viruses. These compounds can be functionalized to enhance their solubility and biological activity. For instance, highly water-soluble polycarboxylic derivatives of C70 fullerene have demonstrated broad-spectrum antiviral activity, particularly against influenza A virus subtypes H1N1 and H3N2(30). The antiviral mechanism primarily involves inhibiting viral entry and replication by interacting with viral surface proteins, thereby preventing the virus from binding to host cells and inhibiting the PA endonuclease, an enzyme critical for viral RNA transcription. This dual action significantly reduces viral RNA synthesis and protein expression, limiting viral proliferation within host cells (28).
Moreover, the fullerene liposome complex exhibits good water solubility and low toxicity, exhibiting markedly higher anti-influenza activity in vivo compared to rimantadine. Therefore, the fullerene liposome complex presents a promising clinical candidate for treating influenza infections. Fullerene derivatives, particularly fullerenols, have shown encouraging results in inhibiting Influenza A virus replication. These compounds are characterized by low toxicity and compatibility with biological fluids, rendering them suitable candidates for antiviral therapy. The strong antiviral effects of fullerenes are attributed to their interaction with viral RNA polymerase, underscoring their potential as therapeutic agents (20).
Phytochemical compounds and influenza inhibition. Phytochemicals derived from plants have demonstrated significant potential in inhibiting influenza viruses, providing a rich resource for developing new antiviral agents. These compounds, including alkaloids, terpenoids, flavonoids and polyphenols, exhibit antiviral activities through various mechanisms that provide an alternative to traditional antiviral drugs (29). Recent analyses have identified nine phytocompounds alongside six antiviral drugs (amantadine, umifenovir, favipiravir, nitazoxanide, oseltamivir and zanamivir) that target four influenza A proteins: NA, polymerase basic protein 2, HA and the M2 ion channel protein, as depicted in Fig. 2. For instance, extracts from Schinopsis brasiliensis bark have shown biological activity against the influenza A virus. Additionally, compounds such as syringaresinol and cycloartenone have multiple targets within the influenza A virus and are considered promising phytocompounds for therapeutic use (33-35).
These phytochemicals not only directly inhibit viral activities, but also enhance host immune responses by increasing interferon production and activating macrophages that aid in viral clearance. The synergistic effects of combining phytochemicals with conventional antiviral drugs have shown enhanced efficacy in significantly reducing viral replication more than either agent alone. Such combinations can minimize side-effects, while delaying the emergence of drug-resistant viral strains, highlighting the potential of phytochemicals as complementary therapies in influenza treatment (29). As the mechanisms of action of these components are further elucidated, managing pandemics arising from influenza and similar viral diseases can be achieved more effectively. However, clinical trials are urgently required to validate these findings. The formulation of treatments may involve single-drug compounds or multi-herbal combinations that open new avenues for pharmaceutical innovation.
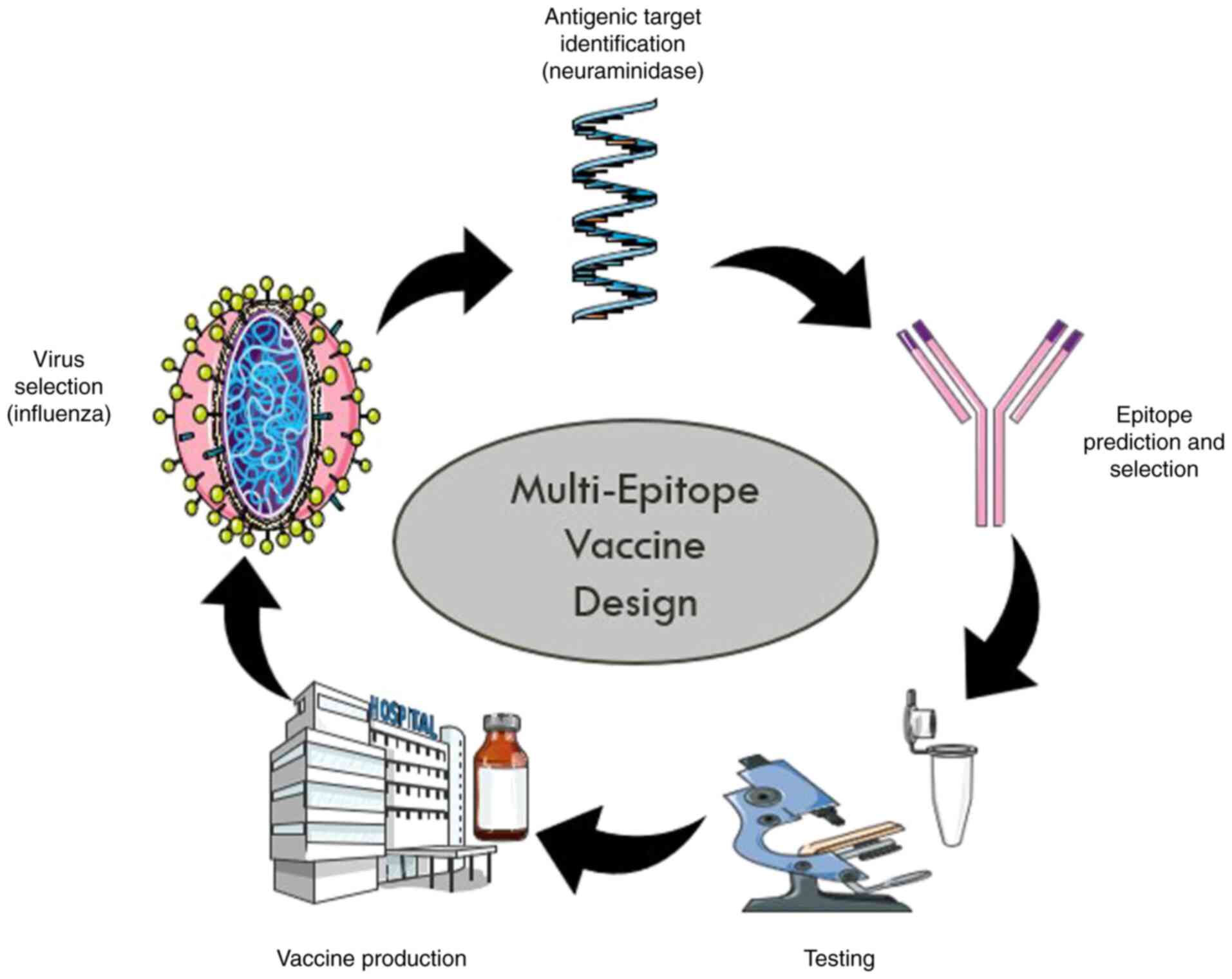
Multi-epitope vaccine design. The development of a recombinant multi-epitope vaccine based on highly conserved epitopes of HA, NA and membrane matrix proteins is gaining attention due to the fewer mutations these epitopes undergo over time (Fig. 3). Recently designed multi-epitope vaccines focus on selecting epitopes that elicit robust immune responses from B-cells, as well as cytotoxic T lymphocytes (CTLs) and CD4 T-cells. Bioinformatics tools have been utilized to evaluate the immunogenicity and chemical properties of selected epitopes effectively (34).
The Universal Immune System Simulator (UISS) computational framework has shown favorable immune responses in terms of immunoglobulin G (IgG), T-helper 1 cells (TH1), epithelial cells (EP) and interferon-gamma (IFN-γ) levels following influenza exposure alongside multi-epitope vaccine administration (35). The generation of new multi-epitope vaccines offers various advantages compared to traditional approaches; they reduce risks associated with live or attenuated vaccines since they do not rely on infectious substances or perilous sequences.
From a pharmaceutical perspective, multi-epitope vaccines demonstrate desirable properties because they consist of well-characterized peptides that can be produced efficiently at lower costs. Furthermore, these vaccines can cover a wide range of pathogens or strains, particularly important for highly variable pathogens, such as influenza virus that frequently undergo mutations leading to novel variants (36).
However, multi-epitope vaccines face limitations; one significant challenge is that the majority of epitope prediction tools do not adequately account for proper antigen processing sites which may lead to inaccurate predictions regarding epitope presentation. The composition of antigen processing mechanisms varies based on pro-inflammatory signals among different cell classes making existing prediction algorithms insufficient for assessing processing effectiveness in infected target cells. Combining epitope prediction tools with vaccine design methodologies often does not provide sufficient evidence to evaluate the global immune response elicited by a given vaccine candidate under investigation.
Despite the availability of NA inhibitors, such as oseltamivir and zanamivir, antiviral resistance remains a growing concern. Mutations such as H274Y in the NA gene have been linked to reduced drug efficacy, emphasizing the need for next-generation antivirals targeting alternative viral components. Similarly, influenza vaccines, while effective, require frequent updates due to antigenic drift. Developing a universal influenza vaccine targeting conserved viral epitopes remains a major research priority. However, challenges in epitope stability, manufacturing scalability, and immune response variability must be addressed before clinical application. Even though vaccine technology and antiviral development advanced, significant gaps remain in the management of H1N1 infections. Multi-epitope vaccines offer promising broad-spectrum immunity, yet their global deployment faces barriers including high production costs, complex storage requirements, and variable immune responses across populations. Surveillance efforts also struggle with inconsistency in data reporting, particularly in regions with limited healthcare infrastructure. The lack of a unified global database for real-time mutation tracking further complicates timely vaccine updates. These challenges highlight the need for stronger international collaboration and investment in influenza preparedness strategies.
In silico drug repurposing. Drug repurposing has emerged as a novel strategy aimed at repositioning existing drugs for new therapeutic targets. This approach is based on the observation that a single drug can affect multiple targets or activate various signaling pathways simultaneously. Drug repurposing holds significant potential by bringing medications with established safety profiles into new patient populations. Numerous examples exist where new indications for existing molecules have been identified, often stemming from serendipitous findings or focused efforts on specific modes of action associated with these drugs (26). In recent years, the need for innovative approaches within drug research has intensified alongside advancements in big data repositories coupled with analytical methods generating interest in systematic drug repurposing strategies.
Influenza remains a significant public health concern due to its high mutation rate coupled with emerging strains that often render traditional antiviral drugs ineffective over time; thus, drug repurposing serves as a strategic advantage by identifying existing medications capable of combating newly resistant strains without undergoing lengthy development processes (26). Ongoing advancements in computational biology alongside increased collaboration among researchers will further enhance successful drug repurposing efforts paving pathways towards innovative treatments, not only for influenza, but also other viral diseases. While several antiviral drugs are available for influenza treatment two out of four FDA-approved options have led to significant drug resistance highlighting an urgent need for alternative therapies aimed at reducing flu-related illnesses overall. In silico-based drug repurposing methods have identified several FDA-approved drugs exhibiting potential efficacy against influenza A viruses; examples include Promacta Tucatinib Lurasidone, which displayed high binding affinities towards viral neuraminidase enzymes suggesting their therapeutic viability against resistant strains (37). A few contemporary treatment strategies are listed in Table III along with the target and development stage.
By exploring these innovative approaches, ranging from fullerene derivatives to phytochemical compounds, researchers aim to expand available tools for preventing and treating influenza A/H1N1pdm09 infections ultimately reducing the burden of this disease on global public health.
5. Challenges in developing treatment strategies for influenza A/H1N1pdm09
Influenza A/H1N1pdm09 remains a persistent global health challenge due to its high mutation rates, antigenic variability, and resistance to antiviral therapies. The ability of the virus to undergo antigenic drift and antigenic shift allows it to evade host immune responses, making vaccine development complex and necessitating frequent vaccine updates. Additionally, challenges related to vaccine deployment, socio-economic factors, healthcare infrastructure, and policy frameworks hinder global efforts to control the virus. This section explores therapeutic limitations, vaccine surveillance difficulties and socio-economic barriers, alongside potential strategies to enhance influenza prevention and treatment.
Challenges in influenza therapy
Antigenic variation and vaccine limitations. The continuous antigenic variation of Influenza A viruses, including H1N1, remains a major obstacle for vaccine selection and effectiveness. Antigenic drift, characterized by gradual mutations in the HA and NA genes, alters antigenicity, allowing the virus to escape pre-existing immunity. A previous study identified three amino acid substitutions (190, 230 and 269) in the HA protein of Eurasian avian-like (EA) H1N1 swine influenza viruses that led to immune evasion by escaping neutralizing monoclonal antibodies (38). These findings highlight the unpredictability of influenza evolution and the need for continuous genomic surveillance to ensure vaccine efficacy.
Antiviral resistance. The emergence of antiviral-resistant influenza strains poses a significant therapeutic challenge. Numerous influenza A virus subtypes have developed resistance to M2 ion channel inhibitors, such as amantadine and rimantadine, rendering them ineffective. Resistance to adamantane-based drugs was first detected in the 1980s, but by 2003, 45% of circulating influenza A viruses exhibited resistance. Adamantane-resistant H3N2 and H1N1pdm09 strains have since become dominant, leading to the discontinuation of these drugs in influenza treatment and prevention (39).
Similarly, resistance to NA inhibitors (e.g., oseltamivir and zanamivir) is increasing, particularly in immunocompromised patients and regions with high antiviral drug use. This underscores the urgent need for alternative antiviral agents that retain efficacy against drug-resistant influenza strains while maintaining low toxicity and favorable pharmacokinetics.
Surveillance of vaccine development and deployment. Due to challenges in vaccine surveillance and global coordination, effective vaccine surveillance is essential for monitoring distribution, uptake, safety and efficacy. The Global Influenza Surveillance and Response System (GISRS), coordinated by the WHO, plays a pivotal role in influenza monitoring, providing real-time data to guide vaccine strain selection. The 2009 H1N1 pandemic tested the global capacity to detect, evaluate and report outbreaks, revealing significant gaps in epidemiological surveillance, risk assessment, and cross-country data standardization (40).
Despite advancements in influenza surveillance networks, systemic weaknesses remain. These include the absence of standardized frameworks for reporting epidemiological data, mortality rates, and risk factor statistics, delayed detection of novel influenza strains due to limited genomic sequencing capacity in resource-limited settings and gaps in global coordination, preventing rapid vaccine strain updates and timely deployment (41). To enhance influenza vaccine surveillance, stronger international partnerships, investments in genomic surveillance and standardized data-sharing protocols are required.
Advancements in vaccine technologies. Novel adjuvant technologies and vaccine platforms, including vectored vaccines, DNA-based vaccines and virus-like particles, hold promise for enhancing influenza immunity and extending vaccine durability. Research into highly conserved influenza antigens, such as the M2 protein, aims to develop a universal influenza vaccine capable of providing broad-spectrum protection against pandemic and seasonal influenza strains (42). Additionally, mammalian cell culture technology is being explored to replace egg-based vaccine production, which could accelerate vaccine manufacturing timelines and improve antigen yield consistency. Continued innovation in vaccine formulation and delivery is essential for adapting to rapidly evolving influenza viruses and enhancing protection in high-risk populations.
Socio-Economic and policy dimensions of H1N1 in resource-limited settings
Vaccine hesitancy and misinformation. Despite the proven benefits of vaccination, vaccine hesitancy remains a major public health challenge. The WHO has recognized vaccine hesitancy as a significant global health threat, particularly in regions where misinformation, cultural beliefs and distrust in healthcare systems influence vaccine uptake (43,44). Misinformation regarding vaccine safety, adverse effects, and efficacy has led to decreased immunization rates in several countries. Parental concerns, low literacy levels and socio-economic factors further contribute to low vaccination rates. Studies show that parents with lower education levels and limited healthcare access are more likely to have negative attitudes toward immunization programs, highlighting the need for targeted health education and outreach programs (43).
High treatment costs and economic burden. The economic burden of influenza extends beyond direct medical costs to include lost productivity, work absenteeism and informal caregiving burdens. In the USA, individuals aged ≥65 years account for 70-85% of influenza-related deaths, demonstrating the high medical costs associated with severe influenza cases (45). The financial impact is even greater in resource-limited settings, where the lack of government-funded immunization programs and high out-of-pocket expenditures prevent widespread vaccine accessibility. Among working-age adults (18-64 years), indirect costs due to productivity losses account for a significant portion of the economic burden, particularly in countries where paid sick leave policies are inadequate. Future research is required to focus on assessing influenza-related healthcare costs, particularly among high-risk individuals with chronic conditions (e.g., cardiovascular disease, diabetes, and immunodeficiencies).
Limited healthcare infrastructure and pandemic preparedness. Resource-limited settings often lack sufficient healthcare infrastructure, delaying early diagnosis, treatment and outbreak containment. During the H1N1 pandemic, numerous countries struggled to mobilize healthcare resources, implement surveillance protocols, and coordinate pandemic response strategies due to inadequate laboratory facilities, limited genomic sequencing capacity, and shortages of trained healthcare professionals (46). Infectious disease response requires rapid mobilization of healthcare professionals, diagnostic tools and public health resources. However, the absence of well-defined pandemic response frameworks has hindered timely intervention efforts, exacerbating disease transmission. Strengthening global pandemic preparedness strategies is essential to prevent future influenza pandemics and mitigate socio-economic disruptions.
The development of effective treatment strategies for Influenza A/H1N1pdm09 faces several significant challenges, particularly given the virus ability to rapidly mutate and evade both host immunity and antiviral therapies. These challenges include the following:
i) Viral mutation and antigenic drift: Influenza viruses, including H1N1, are known for their high mutation rates, which can lead to antigenic drift. This phenomenon complicates vaccine development and effectiveness, as the circulating strains may differ significantly from those used in vaccine formulations. As a result, vaccines may become less effective over time, necessitating frequent updates to match the most prevalent strains (21).
ii) Antiviral resistance: The emergence of antiviral resistance poses another critical challenge. While several antiviral drugs are available for treating influenza, some strains have developed resistance to commonly used medications such as oseltamivir and zanamivir. This resistance limits treatment options and underscores the urgent need for new antiviral agents that can effectively target resistant strains (35).
iii) Complexity of host immune response: The host immune response to influenza infection is complex and varies among individuals. Factors such as age, underlying health conditions and previous exposure to influenza viruses can influence the effectiveness of both vaccines and antiviral treatments. Understanding these variations is crucial for developing personalized treatment strategies (20).
iv) Challenges in drug development: The traditional drug development process is lengthy and costly, often taking years before a new antiviral agent is approved for clinical use. This timeline is problematic in the context of rapidly evolving viruses like H1N1, where immediate responses are necessary to mitigate outbreaks (26). Furthermore, translating in silico predictions into successful clinical outcomes remains a challenge, as computational models may not always accurately reflect biological activity.
Challenges in specific approaches. In addition to the overarching challenges in developing treatment strategies, each innovative approach to combating influenza A/H1N1pdm09 presents its own set of difficulties:
In silico analysis and molecular docking. While in silico methods provide valuable insight into potential antiviral compounds, one of the primary challenges is translating computational predictions into actual biological activity. The efficacy of identified interactions can vary when tested in biological systems, rendering it essential to validate findings through experimental studies (16,18). Moreover, reliance on computational models may overlook complex biological interactions that occur within living organisms.
Fullerene derivatives. Although fullerene derivatives show promise as antiviral agents due to their unique properties and mechanisms of action, challenges remain in optimizing their bioactivity and solubility for clinical use. Functionalizing these compounds effectively while maintaining low toxicity levels is crucial for their development as therapeutic agents (24). Additionally, further research is warranted to fully understand their mechanisms of action against various viral strains.
Phytochemical compounds. Phytochemicals derived from plants provide potential antiviral properties; however, the variability in composition and efficacy among different plant extracts can complicate standardization and dosage determination (29). Additionally, more rigorous clinical trials are necessary to validate the effectiveness of these compounds against influenza viruses.
Multi-epitope vaccines. While multi-epitope vaccines represent an innovative approach to providing broader protection against influenza strains, they face limitations related to epitope prediction accuracy. A number of existing prediction tools do not adequately account for proper antigen processing sites, which can lead to ineffective immune responses (26). Furthermore, ensuring that these vaccines elicit robust immune responses across diverse populations remains a challenge.
Drug repurposing. Although drug repurposing offers a strategic advantage by utilizing existing medications with established safety profiles, identifying suitable candidates that effectively target influenza A viruses can be challenging. The complexity of viral mechanisms and potential off-target effects must be carefully evaluated to ensure safety and efficacy (35).
Therefore, while there are promising avenues for developing treatment strategies against Influenza A/H1N1pdm09, significant challenges remain at both the systemic level and within specific approaches. Addressing these challenges will require continued research efforts and collaboration among scientists, clinicians and public health officials to enhance our ability to combat this persistent public health threat effectively.
6. Future research directions
The ongoing evolution of influenza A/H1N1pdm09 necessitates continuous surveillance and research to stay ahead of emerging variants. Future studies should focus on the following key areas:
Enhanced surveillance systems. Integrating multiple data sources is essential for improving real-time monitoring and forecasting of influenza outbreaks. The WHO GISRS conducts year-round global surveillance through National Influenza Centres, WHO Collaborating Centres for Reference and Research on Influenza, and Essential Regulatory Laboratories (40). These efforts aim to monitor changes in the virus genome, particularly in HA and NA. Traditional surveillance systems rely on influenza-like illness reports and virology data from healthcare providers, which typically have a reporting lag of one to two weeks. Therefore, developing simple, accurate and timely surveillance approaches is crucial. Nontraditional systems that utilize alternative data sources, such as flu prescription drug sales and school absenteeism, aim to provide more timely estimates of influenza activity. However, these systems face limitations, including low participation rates and potential overestimation of cases, highlighting the need for improved accuracy supported by clinical evidence (31).
Novel antiviral compounds. Exploring and validating new antiviral agents, particularly those derived from natural sources, through rigorous in vitro and in vivo testing is critical. Currently, two classes of anti-influenza drugs are approved for clinical treatment: M2 channel inhibitors (amantadine and rimantadine) and NA inhibitors (oseltamivir, zanamivir and peramivir). Additionally, favipiravir (T-705), the first polymerase inhibitor approved in Japan, underscores the importance of developing new generations of anti-influenza drugs targeting viral polymerase. However, most circulating influenza A viruses are resistant to adamantanes, limiting therapeutic options. One promising candidate is 1,3-dihydroxy-6-benzo(c)chromene (D715-2441), a small-molecule inhibitor that shows significant activity against various influenza A virus types, including oseltamivir-resistant strains. This molecule specifically binds to the PB2 protein, inhibiting influenza RNA polymerase activity and exhibiting a synergistic effect when used with zanamivir (47).
Vaccine development. Advancing multi-epitope and next-generation vaccines is a priority for providing broader protection against diverse influenza strains. The influenza A(H1N1)pdm09 vaccine strain remained unchanged during a five-year study period, offering a unique opportunity to study antibody dynamics with repeated exposure to identical antigens. However, circulating A(H1N1) pdm09 has undergone antigenic drift, meaning vaccine-specific antibody titers may not accurately measure protection for all individuals. Influenza A(H3N2) viruses evolve more rapidly, leading to more frequent vaccine updates. Understanding antibody development and persistence post-vaccination is crucial since A(H3N2) is typically associated with larger outbreaks and severe disease (34). The National Institute of Allergy and Infectious Diseases has published a strategic plan for developing a universal influenza vaccine (48). Analytic tools paired with longitudinal cohort studies could inform next-generation vaccine development and policy decisions for optimal use. Currently, three types of vaccines (inactivated, live attenuated, and recombinant HA vaccines) are licensed in various countries, each with its advantages and drawbacks.
In silico approaches. Utilizing computational methods for drug discovery and repurposing can significantly reduce the time and cost associated with traditional drug development. In silico genomic analysis of influenza A/H1N1pdm09 involves studying genetic sequences to identify mutations, genetic variations, and evolutionary patterns (49). Computational tools such as sequence alignment algorithms, phylogenetic analysis software, and structural modeling programs are employed to analyze the viral genome. These approaches accelerate the discovery of novel therapies, optimize vaccine design, and improve public health strategies. For example, in silico techniques have been used to evaluate the drug-likeness and molecular properties of identified phytocompounds by assessing their absorption, distribution, metabolism, excretion (ADME) and toxicity.
By focusing on these innovative research directions, enhanced surveillance systems, novel antiviral compounds, advanced vaccine development strategies and in silico approaches, researchers aim to expand the arsenal of tools available for preventing and treating influenza A/H1N1pdm09 infections. Ultimately, these efforts will help reduce the burden of this disease on global public health.
7. Conclusion
The comprehensive exploration of influenza A/H1N1pdm09 highlights the ongoing challenges and advancements in understanding and combating this persistent public health threat. As the virus evolves, enhancing surveillance, developing innovative therapeutic strategies and creating effective vaccines becomes increasingly critical. Enhanced surveillance systems that integrate multiple data sources are essential for real-time monitoring of influenza outbreaks. While traditional methods provide valuable data, they often suffer from delays that can hinder timely public health responses. Incorporating nontraditional data sources can offer more immediate insights into influenza activity, although challenges such as participation rates and accuracy remain. The exploration of novel antiviral compounds, especially those derived from natural sources, is crucial for expanding treatment options. Fullerene derivatives and phytochemicals represent innovative strategies to combat viral replication and enhance host immune responses. However, these compounds must overcome challenges related to bioactivity, solubility, and safety profiles. Advancing multi-epitope vaccines is vital for providing broader protection against diverse influenza strains. By targeting conserved epitopes, these vaccines could mitigate issues related to antigenic drift. Yet, challenges in accurately predicting epitope processing must be addressed to ensure effective immune responses. Finally, in silico approaches for drug discovery and repurposing offer strategic advantages in identifying existing medications that can combat emerging viral strains. This method accelerates the drug development process while leveraging established safety profiles. In conclusion, a multifaceted approach involving continuous research and collaboration across disciplines is necessary to enhance preparedness for future influenza outbreaks and reduce the overall burden of this disease on global public health.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
GE drafted the manuscript. DAA was involved in the drafting of the manuscript and in study supervision. SS was involved in the conceptualization of the study, as well as in the drafting of the manuscript and in study supervision. All authors were involved in the literature search. All authors have read and approved the final manuscript. Data authentication is not applicable.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
|
Salman BN, Muhammad A, Ansari UB, Shah ZU, Qureshi MF, Ahmad S and Imran R: Evolutionary analysis of influenza A(H1N1) pdm09 during the pandemic and post-pandemic period in Pakistan. Trop Biomed. 36:447–458. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Singh P, Sharma K, Bhargava A, Kumar V, Chawla R, Tiwari M, Yadav S and Gupta N: Genomic characterization of Influenza A (H1N1) pdm09 and SARS-CoV-2 from influenza like Illness (ILI) and severe acute respiratory Illness (SARI) cases reported between July-December 2022. Sci Rep. 14(10660)2024.PubMed/NCBI View Article : Google Scholar | |
|
Killingley B, Greatorex J, Cauchemez S, Morgan A and Holmes A: Virus shedding and environmental deposition of novel A(H1N1) pandemic influenza virus: Interim findings. Health Technol Assess. 14:237–354. 2012.PubMed/NCBI View Article : Google Scholar | |
|
Influenza A: (H1N1) pdm09 virus: Is it really ‘new’? Pacini Editore. 25:205–212. 2010. | |
|
Sullivan SJ, Jacobson RM, Dowdle WR and Poland GA: 2009 H1N1 influenza. Mayo Clin Proc. 85:64–76. 2010.PubMed/NCBI View Article : Google Scholar | |
|
Baldo V, Bertoncello C, Cocchio S, Fonzo M, Pillon P, Buja A and Baldovin T: The new pandemic influenza A/(H1N1)pdm09 virus: Is it really ‘new’? J Prev Med Hyg. 57:E19–E22. 2016.PubMed/NCBI | |
|
Centers for Dicease Control and Prevention (CDC): Influenza A (H1N1) pdm09 virus. CDC, Atlanta, GA, 2022. https://www.cdc.gov/flu/professionals/acip/background/references.htm. | |
|
Jain S, Kamimoto L, Bramley AM, Schmitz AM, Benoit SR, Louie J, Sugerman DE, Druckenmiller JK, Ritger KA, Chugh R, et al: Hospitalized patients with 2009 H1N1 influenza in the United States, April-June 2009. N Engl J Med. 361:1935–1944. 2009.PubMed/NCBI View Article : Google Scholar | |
|
Soudani S, Mafi A, Al Mayahi Z, Al Balushi S, Dbaibo G, Al Awaidy S and Amiche A: A systematic review of influenza epidemiology and surveillance in the Eastern Mediterranean and North African region. Infect Dis Ther. 11:15–52. 2022.PubMed/NCBI View Article : Google Scholar | |
|
Baldo V, Bertoncello C, Cocchio S, Fonzo M, Pillon P, Buja A and Baldovin T: The new pandemic influenza A/(H1N1)pdm09 virus: is it really “new”? J Prev Med Hyg. 57:E19–E22. 2016.PubMed/NCBI | |
|
Bolton JS, Klim H, Wellens J, Edmans M, Obolski U and Thompson CP: An antigenic Thrift-based approach to influenza vaccine design. Vaccines (Basel). 9(657)2021.PubMed/NCBI View Article : Google Scholar | |
|
Liang Y: Pathogenicity and virulence of influenza. Virulence. 14(2223057)2023.PubMed/NCBI View Article : Google Scholar | |
|
Caspard H, Coelingh KL, Mallory RM and Ambrose CS: Association of vaccine handling conditions with the effectiveness of live attenuated influenza vaccine against H1N1pdm09 viruses in the United States. Vaccine. 34:5066–5072. 2016.PubMed/NCBI View Article : Google Scholar | |
|
Cotter CR, Jin H and Chen Z: A single amino acid in the stalk region of the H1N1pdm influenza virus HA protein affects viral fusion, stability, and infectivity. PLoS Pathog. 10(e1003831)2014.PubMed/NCBI View Article : Google Scholar | |
|
Pushan SS, Samantaray M, Rajagopalan M and Ramaswamy A: Evolution of Indian Influenza A (H1N1) Hemagglutinin Strains: A comparative analysis of the pandemic Californian HA strain. Front Mol Biosci. 10(1111869)2023.PubMed/NCBI View Article : Google Scholar | |
|
Dar AM and Mir S: Molecular docking: Approaches, types, applications and basic challenges. J Anal Bioanal Tech. 8(356)2017. | |
|
Daina A, Michielin O and Zoete V: SwissADME: A free web tool to evaluate pharmacokinetics, Drug-likeness, and medicinal chemistry friendliness of small molecules. Sci Rep. 7(42717)2017.PubMed/NCBI View Article : Google Scholar | |
|
Ferreira LG, Dos Santos RN, Oliva G and Andricopulo AD: Molecular docking and Structure-based drug design strategies. Molecules. 20:13384–13421. 2015.PubMed/NCBI View Article : Google Scholar | |
|
Meng XY, Zhang H X, Mezei M and Cui M: Molecular docking: A powerful approach for structure-based drug discovery. Curr Comput Aided Drug Des. 7:146–157. 2011.PubMed/NCBI View Article : Google Scholar | |
|
Zaremba P, Zaremba A, Naumenko K and Koltsov A: In vitro and in silico studies of the antiviral activity of polyhydrated fullerenes against influenza A (H1N1) virus. Sci Rep. 13(10879)2023.PubMed/NCBI View Article : Google Scholar | |
|
Decker CH, Rapier-Sharman N and Pickett BE: Mutation in hemagglutinin antigenic sites in Influenza A pH1N1 viruses from 2015-2019 in the United States Mountain West, Europe, and the Northern Hemisphere. Genes (Basel). 13(909)2022.PubMed/NCBI View Article : Google Scholar | |
|
Zhang Y, Aevermann BD, Anderson TK, Burke DF, Dauphin G, Gu Z, He S, Kumar S, Larsen CN, Lee AJ, et al: Influenza research database: An integrated bioinformatics resource for influenza virus research. Nucleic Acids Res. 45:D466–D474. 2017.PubMed/NCBI View Article : Google Scholar | |
|
Mortazavi M, Pirbonyeh N, Javanmardi F and Emami A: Bioinformatics and structural analysis of antigenic variation in the hemagglutinin gene of the influenza A(H1N1) pdm09 virus circulating in Shiraz (2013 to 2015). Microbiol Spectr. 11(e0463022)2023.PubMed/NCBI View Article : Google Scholar | |
|
Ginting TE, Shinya K, Kyan Y, Makino A, Matsumoto N, Kaneda S and Kawaoka Y: Amino acid changes in hemagglutinin contribute to the replication of Oseltamivir-resistant H1N1 influenza viruses. J Virol. 86:121–127. 2012.PubMed/NCBI View Article : Google Scholar | |
|
Innocenzi P and Stagi L: Carbon-based antiviral nanomaterials: Graphene, C-dots, and fullerenes. A perspective. Chem Sci. 11:6606–6622. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Maleki A, Russo G, Parasiliti Palumbo GA, Mirjalili A and Angeletti M: In silico design of recombinant Multi-epitope vaccine against influenza A virus. BMC Bioinformatics. 22 (Suppl 14)(S617)2021.PubMed/NCBI View Article : Google Scholar | |
|
Hay AJ and McCauley JW: The WHO global influenza surveillance and response system (GISRS)-A future perspective. Influenza Other Respir Viruses. 12:551–557. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Xu PY, Li XQ, Chen WG, Deng LL, Tan YZ, Zhang Q, Xie SY and Zheng LS: Progress in antiviral fullerene research. Nanomaterials (Basel). 12(2547)2022.PubMed/NCBI View Article : Google Scholar | |
|
Peng S, Wang H, Wang Z and Wang Q: Progression of antiviral agents targeting viral polymerases. Molecules. 27(7370)2022.PubMed/NCBI View Article : Google Scholar | |
|
Sinegubova EO, Kraevaya OA, Volobueva AS, Zhilenkov AV, Shestakov AF, Baykov SV, Troshin PA and Zarubaev VV: Water-Soluble Fullerene C60 derivatives are effective inhibitors of influenza virus replication. Microorganisms. 11(681)2023.PubMed/NCBI View Article : Google Scholar | |
|
Dunning J, Baillie JK, Cao B and Hayden FG: International Severe Acute Respiratory and Emerging Infection Consortium (ISARIC). Antiviral combinations for severe influenza. Lancet Infect Dis. 14:1259–1270. 2014.PubMed/NCBI View Article : Google Scholar | |
|
Katagishi D, Yasuda D, Takahashi K, Nakamura S, Mashino T and Ohe T: Fullerene derivatives as inhibitors of the SARS-CoV-2 main protease. Bioorg Med Chem Lett. 80(129121)2023.PubMed/NCBI View Article : Google Scholar | |
|
Sette-DE-Souza PH, Costa MJF, Araújo FAC, Alencar EN and Amaral Machado L: Two phytocompounds from Schinopsis brasiliensis show promising antiviral activity with multiple targets in Influenza A virus. An Acad Bras Cienc. 93 (Suppl 4)(e20210964)2021.PubMed/NCBI View Article : Google Scholar | |
|
Song X, Li Y, Wu H, Qiu H and Sun Y: T-cell Epitope-based vaccines: A promising strategy for prevention of infectious diseases. Vaccines (Basel). 12(1181)2024.PubMed/NCBI View Article : Google Scholar | |
|
Russo G, Crispino E, Maleki A, Di Salvatore V, Stanco F and Pappalardo F: Beyond the state of the art of reverse vaccinology: Predicting vaccine efficacy with the universal immune system simulator for influenza. BMC Bioinformatics. 24(231)2023.PubMed/NCBI View Article : Google Scholar | |
|
Cha Y, Erez T, Reynolds IJ, Kumar D, Ross J, Koytiger G, Kusko R, Zeskind B, Risso S, Kagan E, et al: Drug repurposing from the perspective of pharmaceutical companies. Br J Pharmacol. 175:168–180. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Mtambo SE and Kumalo HM: In silico drug repurposing of FDA-approved drugs highlighting Promacta as a potential inhibitor of H7N9 influenza virus. Molecules. 27(4515)2022.PubMed/NCBI View Article : Google Scholar | |
|
Xu C, Zhang N, Yang Y, Liang W, Zhang Y, Wang J, Suzuki Y, Wu Y, Chen Y, Yang H, et al: Immune escape adaptive mutations in hemagglutinin are responsible for the antigenic drift of Eurasian Avian-Like H1N1 swine influenza viruses. J Virol. 96(e0097122)2022.PubMed/NCBI View Article : Google Scholar | |
|
Nelson MI, Simonsen L, Viboud C, Miller MA and Holmes EC: The origin and global emergence of adamantane resistant A/H3N2 influenza viruses. Virology. 388:270–278. 2009.PubMed/NCBI View Article : Google Scholar | |
|
Briand S, Mounts A and Chamberland M: Challenges of global surveillance during an influenza pandemic. Public Health. 125:247–256. 2011.PubMed/NCBI View Article : Google Scholar | |
|
Batool S, Chokkakula S and Song MS: Influenza treatment: Limitations of antiviral therapy and advantages of drug combination therapy. Microorganisms. 11(183)2023.PubMed/NCBI View Article : Google Scholar | |
|
Tosh PK, Jacobson RM and Poland GA: Influenza vaccines: From surveillance through production to protection. Mayo Clin Proc. 85:257–273. 2010.PubMed/NCBI View Article : Google Scholar | |
|
Salo-Tuominen K, Teros-Jaakkola T, Toivonen L, Ollila H, Rautava P, Aromaa M, Lahti E, Junttila N and Peltola V: Parental socioeconomic and psychological determinants of the 2009 pandemic influenza A(H1N1) vaccine uptake in children. Vaccine. 40:3684–3689. 2022.PubMed/NCBI View Article : Google Scholar | |
|
World Health Organization. Ten threats to global health in 2019. 2019. | |
|
De Courville C, Cadarette SM, Wissinger E and Alvarez FP: The economic burden of influenza among adults aged 18 to 64: A systematic literature review. Influenza Other Respir Viruses. 16:376–385. 2022.PubMed/NCBI View Article : Google Scholar | |
|
Lautenbach E, Saint S, Henderson DK and Harris AD: Initial response of health care institutions to emergence of H1N1 influenza: Experiences, obstacles, and perceived future needs. Clin Infect Dis. 50:523–527. 2010.PubMed/NCBI View Article : Google Scholar | |
|
Liu T, Liu M, Chen F, Chen F, Tian Y, Huang Q, Liu S and Yang J: A Small-Molecule compound has Anti-influenza A virus activity by acting as a ‘PB2 Inhibitor’. Mol Pharm. 15:4110–4120. 2018.PubMed/NCBI View Article : Google Scholar | |
|
NIAID Collaborative Influenza Vaccine Innovation Centers (CIVICs): NIAID Strategic Plan for a Universal Influenza Vaccine. https://www.niaidcivics.org/news/2019/10/niaid-strategic-plan-for-a-universal-influenza-vaccine. | |
|
Yamayoshi S and Kawaoka Y: Current and future influenza vaccines. Nat Med. 25:212–220. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Musharrafieh R, Lagarias P, Ma C, Hau R, Romano A, Lambrinidis G, Kolocouris A and Wang J: Investigation of the drug resistance mechanism of M2-S31N channel blockers through biomolecular simulations and viral passage experiments. ACS Pharmacol Transl Sci. 3:666–675. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Lee RU, Phillips CJ and Faix DJ: Seasonal influenza vaccine impact on pandemic H1N1 vaccine efficacy. Clin Infect Dis. 68:1839–1846. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Gautam K and Kakade AS: Tackling influenza A virus by M2 ion channel blockers: Latest progress and limitations. Eur J Med Chem. 267(116172)2024.PubMed/NCBI View Article : Google Scholar | |
|
Xu J, Luo Q, Huang Y, Li J, Ye W, Yan R, Zhou X, He Z, Liu G and Zhu Q: Influenza neuraminidase mutations and resistance to neuraminidase inhibitors. Emerg Microbes Infect. 13(2429627)2024.PubMed/NCBI View Article : Google Scholar | |
|
Hayden FG, Lenk RP, Stonis L, Oldham-Creamer C, Kang LL and Epstein C: Favipiravir treatment of uncomplicated influenza in adults: Results of two Phase 3, randomized, Double-blind, Placebo-controlled trials. J Infect Dis. 226:1790–1799. 2022.PubMed/NCBI View Article : Google Scholar | |
|
Rossignol JF: Nitazoxanide: A first-in-class broad-spectrum antiviral agent. Antiviral Res. 110:94–103. 2024.PubMed/NCBI View Article : Google Scholar | |
|
Kiselev OI, Maleev VV, Deeva EG, Leneva IA, Selkova EP, Osipova EA, Obukhov AA, Nadorov SA and Kulikova EV: Clinical efficacy of arbidol (umifenovir) in the therapy of influenza in adults: Preliminary results of the multicenter Double-blind randomized Placebo-controlled study ARBITR. Ter Arkh. 87:88–96. 2015.PubMed/NCBI View Article : Google Scholar : (In Russian). |