The impact of exercise on telomere length dynamics: Molecular mechanisms and implications in athletes (Review)
- Authors:
- Published online on: April 10, 2025 https://doi.org/10.3892/wasj.2025.344
- Article Number: 56
-
Copyright : © Baliou et al. This is an open access article distributed under the terms of Creative Commons Attribution License [CC BY 4.0].
Abstract
1. Introduction
Biological age provides a reliable way for identifying disease onset and mortality than chronological age (1). Indeed, a multiorgan analysis has been performed to estimate the biological age of particular tissues, confirming its divergence from chronological age (1).
The molecular mechanisms underlying the biological aging process are linked and synergistically exert their effects. In a molecular setting, the major driving forces of aging are senescence, stem cell depletion, impaired metabolic function, dysregulated nutrient sensing, loss of proteostasis, genomic instability, telomere shortening, and changes in the epigenetic landscape (2). Concisely, genomic instability and telomere shortening emerge through the accumulation of DNA damage, thereby disrupting cellular function. During altered nutrient sensing, the loss of proteostasis and mitochondrial dysfunction, metabolism becomes dysregulated, thus providing low energy levels to cells. The chronic appearance of these hallmarks accounts for the excessive production of free radicals and the respective release of inflammatory mediators from senescent cells, known as senescence-associated secretory phenotype (SASP) (3). The interconnectedness of these aforementioned processes substantiates that the aging process is multifaceted (4). Indeed, telomere dysfunction is not only a key hallmark of aging, but can also amplify other hallmarks of aging, accelerating the aging process and leading to the onset of age-related diseases (5). Recently, deficient RNA processing, attenuated autophagy, defects of the microbiome, changes in driving mechanical forces and inflammation have been proposed to as novel integrative hallmarks of aging. All these hallmarks accelerate the onset of several age-related disorders (6).
The escalating number of diseases has placed senescence at the focus of research on aging. Senescence can be triggered by oncogene stimulation, DNA damage, telomere shortening, mitochondrial disturbance, or chromatin alterations as a defense mechanism to stimuli (7). Even though senescence is central to aging, it does not reflect aging alone (8). Telomere shortening is required for an age-related decline in cellular function, causing genome instability and senescence (8). Moreover, telomere length is considered a valuable biomarker for evaluating biological aging with further implications regarding age-related disorders (9,10). Indeed, telomere shortening can increase the susceptibility to several age-related disorders, including metabolic diseases, diseases of cardiovascular or cerebrovascular system, cancer, infertility (11-18).
Research has unraveled the function and structure of telomeres, ensuring genome stability. Telomeres are nucleoprotein structures located at chromosomal ends, comprising repetitive DNA sequences associated with proteins of the shelterin complex (5). In particular, the shelterin protein complex consists of six following proteins: Telomere repeat binding factor (TRF)1, TRF2, and TRF-1 and TRF-2 interacting nuclear protein 2 with different roles in sustaining telomere length values (19). For example, TRF1 upregulation is sufficient to drive telomere shortening owing to hindering telomerase action at telomeres (20). However, TRF1 cannot bind to the bloom syndrome protein (BLM) helicase, which, in turn, prevents BLM from accomplishing repair during errors in replication. In this regard, the loss of TRF1 additionally results in the generation of damage in telomeres (21).
Telomeres are known for their pleiotropic role in aging, as they are implicated in genome stability and the regulation of stress-dependent pathways and gene expression (22,23). In this manner, chromosomes are protected from telomeres by fusing them through recombination or non-homologous end joining, ensuring genome integrity (24). In particular, telomeres coated with shelterin complex proteins hinder the recognition of chromosomal ends as double-strand breaks (24). By contrast, the shortest telomeres can cause the activation of DNA damage machinery, which is recruited and the subsequent senescence occurs (25).
Telomere shortening occurs typically during aging and can be accentuated due to certain parameters. The insufficient DNA replication or oxidative stress can accelerate the telomere shortening rate (26). On the one side, the telomeres shorten with each cell division (27). On the other side, oxidative damage accelerates telomere shortening. Guanine-rich regions at telomeres are mainly affected in oxidative stress conditions, mediating further mitochondrial disturbance and oxidative burst (28). Telomere dysfunction can impair mitochondrial function, inactivating the p53 transcription factor, thereby downregulating mitochondrial biogenesis through the inhibition of peroxisome proliferator-activated receptor gamma co-activator 1a/b (PGC-1a/b) (29,30). Alternatively, mitochondrial malfunction can be mediated by telomere shortening through the suppression of nicotinamide adenine dinucleotide-dependent sirtuin 1 deacetylase, which in turn suppresses the action of PGC-1a (31). As a result, telomere shortening, mitochondrial dysfunction, and senescence are interrelated. In a molecular setting, telomeres become too short, and DNA damage response is activated, leading to senescence or apoptosis (32). In particular, either protective p53/p21 or the p16Ink4a signaling pathway is activated, resulting in cell cycle progression arrest (33). Due to telomere dysfunction, the senescent phenotype of cells becomes apparent through limited cell replication and the respective release of SASP mediators (34).
Furthermore, telomere shortening can be reversed through the action elicited by telomerase reverse transcriptase (TERT), which uses the telomerase RNA component as a scaffold to extend telomeric DNA. In differentiated cells, telomerase is inactivated, whereas it is stimulated in germ cells (35-37).
In summary, host genetics and environmental parameters contribute to variation in telomere length between shortening and elongation (38). Obesity, a lack of exercise, smoking and alcohol consumption patterns accelerate the aging trajectory of individuals, leading to a higher rate of age-associated complications (37). On the other hand, a healthy diet and exercise can ameliorate the progression of age-related disorders related to telomere shortening (39-41).
Overall, notable advancements have been made in exercise. The effect of exercise on the aging process has been evaluated by measuring telomere length values. The present review discusses the benefits of exercise on telomere length dynamics and the factors that determine the positive effects of exercise in both general populations and athletes. The novelty of the present review is its focus on the circumstances under which exercise can benefit the aging process.
2. Effect of exercise on telomere dynamics
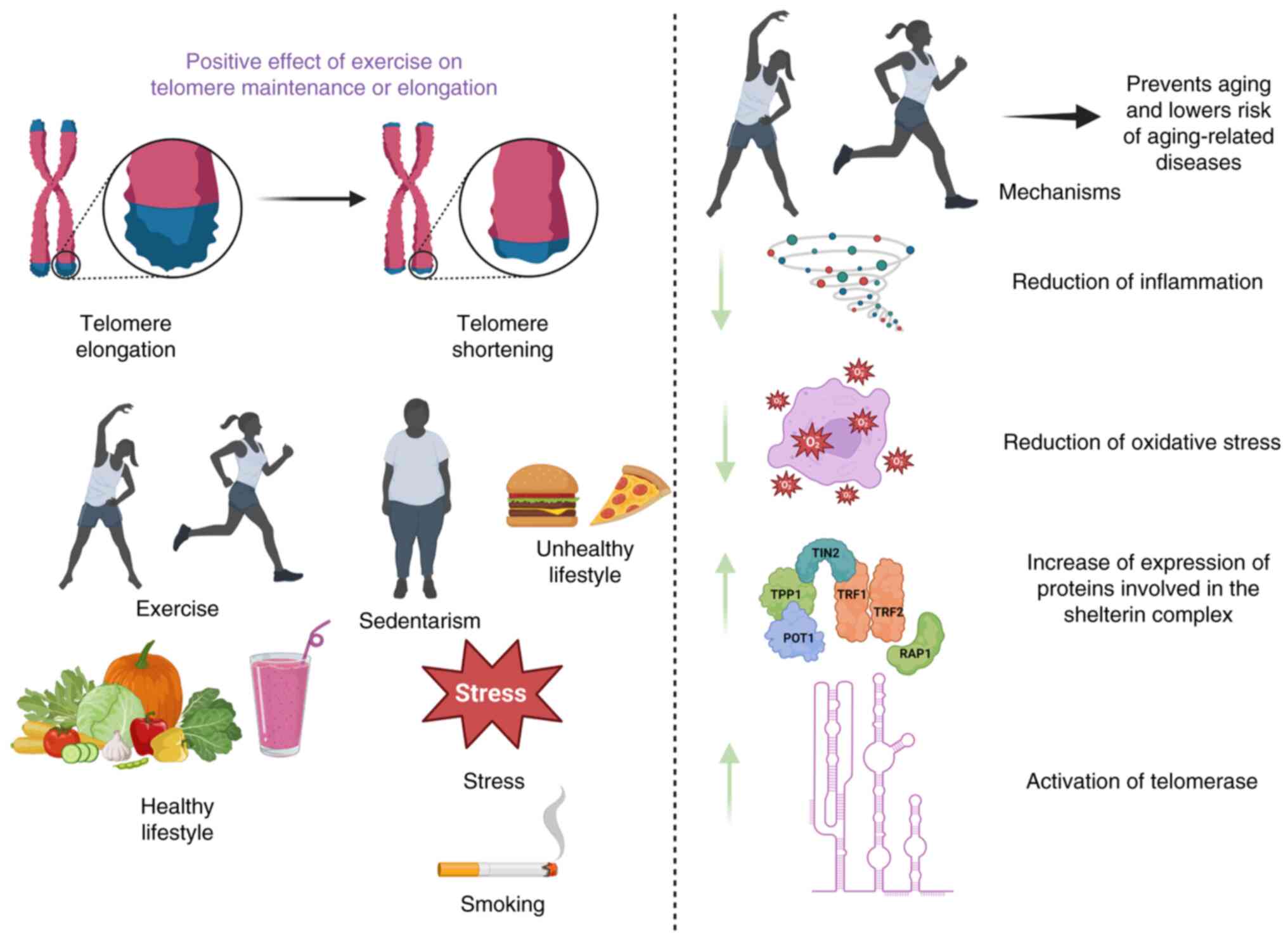
Physical exercise undoubtedly plays a crucial role in determining healthy aging (42). Indeed, exercise can compromise the molecular processes driving the aging hallmarks, thus attenuating the potential risk of developing aging-related diseases (43,44). From an epidemiological perspective, exercise is strongly connected with preserving telomere length, which aligns with its importance for general wellness (45). To support this, a recent comprehensive study with 36,383 participants aged 62 years has demonstrated that moderate to intense exercise substantially lowers the hazard ratio for mortality (46).
The past decade has demonstrated a growing interest in understanding the impact of exercise on telomere length. Numerous systematic reviews have extensively highlighted the positive correlation between exercise and telomere dynamics. Vyas et al (47) conducted a study which demonstrated that 749 physically active adults had longer telomeres than those in the control group, irrespective of sex and ethnicity factors. Similarly, Valente et al (48) identified an association between physical activity and the elongation of telomere length, based on data from 30 studies involving 7,418 participants. In a previous systematic review, a healthy lifestyle that includes exercise was found to be associated with the maintenance or elongation of telomere length, according to a comprehensive meta-analysis of 20 studies involving 2,995 individuals, regardless of the characteristics of an individual (49). Another systematic review, which also included 27 observational studies, eight randomized controlled trials (RCTs) and eight interventional studies with a restricted number of individuals, yielded the most recent findings (50). This systematic research demonstrates how aerobic and moderate-intensity exercise significantly contributes to sustaining telomere length values (50). In parallel, another systematic review which included seven RCTs involving 939 individuals indicated that performing aerobic exercise for >6 months preserved telomeres against their degeneration (51). In that systematic review, the majority of studies demonstrated minimal heterogeneity (51). However, five of the eight RCTs provided the findings of a comprehensive review of how exercise affected telomere length (51). Of note, three studies were not included in the meta-analysis, since the demographic factors of these three studies were different from the following factors: Age, sex, body mass index and level of exercise (51). Accordingly, another systematic review highlighted that a healthy lifestyle involving diet and physical activity can induce telomere length elongation (49). More recently, a meta-analysis of nine trials illustrated that high-intensity interval training positively impacts telomere length in individuals (52). High-intensity exercise improved telomere length values in healthy individuals compared to the control group. Moreover, there was a high probability of bias in approximately half of the studies (52).
From a clinical perspective, the protective nature of exercise on telomere length dynamics was shown in a clinical trial of healthy volunteers. The leukocyte telomere length (LTL) values in the most active participants were greater compared to those of inactive individuals (53). Compared to less active twins, the LTL values of more active twins were 88 nucleotides longer than those of inactive ones (53). In another study on 548 Danish twins of the same sex, the increase in leukocyte telomere length values was associated with outstanding physical ability scores (54). Accordingly, a 10-year longitudinal study demonstrated that a decline in grip strength was linked to telomere shortening to a greater extent, which was driven by higher levels of inflammatory markers (55) (Table I).
According to recent research, exercise is beneficial in sustaining telomere length homeostasis in a sex-independent manner. Interestingly, a positive association between exercise and telomere length values has been underlined. In one cross-sectional study, 1,476 older Caucasian and African American women were enrolled, highlighting that moderate to intense exercise can contribute to telomere length elongation (56). In a 10-year prospective follow-up study which enrolled elderly women from the Helsinki Birth Cohort Study (HBCS), it was proven that the absence of exercise was associated with an increased telomere shortening rate (57). In another study conducted on elderly adults from Northern Finland, the beneficial effects of moderate-intensity exercise were proven to be more pronounced in males in a statistically significant manner (58) (Table I).
To provide insight into the effects of exercise on telomere length, inflammation and oxidative stress are the most critical denominators linking aging with habitual physical exercise (Fig. 1). Chronic low-grade inflammation accounts for an increased white blood cell turnover, triggering hematopoietic stem cell division and ultimately resulting in telomere shortening (59). In this direction, regular exercise alleviates inflammation, prolonging the health span (60). For example, regular exercise has been reported to drive natural killer-mediated cytotoxicity or neutrophil phagocytosis or enhance the recruitment of T-cells at targeted sites or prevent the populations of senescent and exhausted T-cells, confirming its protective nature against the immune system impairment (59). By reducing the expression levels of pro-inflammatory mediators, such as RAP-1, NF-κB, interleukin (IL)-6, PARP-1 and tumor necrosis factor-α (TNFα), exercise is considered to reduce inflammation (60).
Oxidative stress is the other mechanism by which telomere shortening emerges in cells, thus triggering cell death (61). The accumulation of free radicals increases the prevalence of 8-oxoguanine lesions at telomeres, thereby impairing the function of the shelterin protein complex and preventing the action of base excision repair mechanism (62). Aside from the oxidation of guanine bases at telomeres, oxidative damage causes the accumulation of single-strand breaks, hindering the replication fork progression (63). Due to this situation, the increased formation of multi-telomeric foci at chromatid ends, known as fragile telomeres, emerge (64). Mounting evidence has supported that regular exercise compromises the excessive generation of reactive oxygen species (ROS) (65,66). Consistent with this, physical activity can also increase antioxidant response, as shown by elevated expression levels of superoxide dismutase (SOD) and catalase (CAT) (67). Notably, moderate-intensity exercise is inversely associated with oxidative and pro-inflammatory markers (68). As a result, mounting evidence has supported that inflammation and oxidative stress are the key parameters driving telomere shortening, and exercise appears to counteract telomere erosion by counteracting oxidative stress and inflammation (Fig. 1).
Another facet of the beneficial effects of regular exercise on the maintenance of telomere length is that regular aerobic exercise can upregulate the TERT component of telomerase, driving telomere length maintenance (69,70). In another case, it has been shown that long-term exercise confers a remarkable increase in telomerase action, as observed in the heart, skeletal muscle, brain and peripheral blood mononuclear cells (PBMCs) (71). In an in vivo setting, the telomerase activity of endurance athletes who consistently participate in intense aerobic exercise is 2.5-fold higher than that of young and middle-aged inactive individuals, respectively (72). In athletes, this increase in telomerase activity seems to be associated with increased levels of telomere-stabilizing proteins (telomere repeat-binding factor 2 and Ku70) and low expression levels of cell-cycle inhibitors (cell-cycle-checkpoint kinase 2, p16 and p53), highlighting the vasculoprotective effects of exercise (72). Notably, the action of telomerase is accelerated in conditions of attenuated oxidative stress and inflammation (68). Moreover, gene expression in the subtelomeric regions has been observed to be modified due to the reduced binding of the shelterin protein complex (73). Another notable aspect of the association between physical activity and telomere length dynamics can be attributed to the changed levels of the shelterin protein complex. In this direction, exercise prevents telomere erosion by upregulating TRF2 shelterin protein, since TRF2 shields telomeres against cellular senescence and chromosomal end-to-end fusion (72,74). Consistent with this, 3 weeks of voluntary wheel running positively affects telomere length homeostasis by increasing TRF2 levels of the shelterin protein complex in PBMCs, heart, and aortic tissues of mice (75). Accordingly, the overall response to endurance training on telomere length dynamics has been proved in endurance-trained athletes who show longer telomeres due to higher telomerase in combination with increased PBMC TRF2 mRNA and protein expression (72). As a result, shelterin protein complex-mediated telomerase recruitment closely regulates telomere length in conjunction with cellular proliferative activity, which is exacerbated by elevated oxidative stress and inflammation (Fig. 1).
In chronic diseases, exercise can be associated with prolonged telomere length values. It prevents the potential onset and progression of age-related metabolic illnesses, such as obesity (76), type 2 diabetes (T2D) (77) and cardiovascular disease (78), contributing to telomere length elongation through attenuating oxidative burst and inflammation. Several examples of diseases compromised by exercise are analyzed in this section (Table I). For example, compared with participants with high level of physical fitness, those with a low level of physical fitness were at a 2-fold greater risk of having a shorter telomere length (79) (Table I). In an NHANES study of obese individuals, it was proven that exercise in overweight or obese individuals attenuated their telomere shortening rates; however, long-term obesity can counteract the beneficial nature of exercise (80). Considering that telomere shortening is a characteristic of diabetic patients, it was illustrated that exercise can confer protection against telomere shortening present in patients with diabetic nephropathy (81).
Although exercise is positively linked to sustaining excellent human health, its effect on telomere length values is dependent on several factors, including duration, intensity and type. The duration of exercise may significantly influence telomere length. For example, in patients with T2D, acute exercise can increase glucose uptake through the increased translocation of glucose transporter type 4 to the muscle plasma membrane, either through the accumulation of ROS or the increased release of calcium ions or upregulation of AMP-activated protein kinase signaling cascade, resulting in an improved insulin signaling-mediated glucose uptake (82). By contrast, chronic exercise ameliorates insulin signaling by improving mitochondrial dysfunction in muscle cells (82). Apart from muscle cells, acute exercise induces a wide range of responses that parallel those involved in the aging process (83). In particular, acute exercise causes a significant inflammatory response and impairs cognitive, musculoskeletal and cardiovascular performance in circumstances that correspond to aging or age-related cellular dysfunction (83). In addition to the above, exercise type (endurance or resistance) can play a crucial role in determining telomere length values. For example, individuals performing endurance training have been shown to have longer telomeres compared to those of individuals performing resistance training due to the activation of telomerase enzyme in their leukocytes (84). However, some other studies present inconsistent results on the effect of resistance training on telomere length values. Resistance training diminishes age-related muscle loss and systemic inflammation in the long-term, explaining the beneficial effects of resistance training on telomere length values (85,86). Intensity is another parameter that determines the outcome of the exercise. In a recent meta-analysis, high-intensity interval training appeared to have a beneficial effect on telomere length values in a healthy population when compared to other forms of exercise, such as resistance training or aerobic exercise (52). In another meta-analysis, small-moderate exercise appeared to be beneficial on telomere length dynamics, which appeared to depend on the type of physical activity (52). Several studies have highlighted the intensity of exercise in a sex-dependent manner. According to cross-sectional studies, postmenopausal women who engaged in resistance and aerobic moderate exercise for 60 min more than three times a week during 19 months presented telomere length elongation in their PBMCs compared with their peers who were sedentary (87). In addition, postmenopausal women with stage I-III breast cancer who exercised in a moderate to intense manner exhibited longer PBMC telomere length values (69). Of note, men who participated in moderate physical activity had longer leukocyte telomeres and a lower proportion of short telomeres than those who participated in low or high levels of activity in the long-term (88).
In addition to the above, a recent systematic review provided convincing evidence that physical capacity can serve as a predictive marker for assessing aging trajectory, relying on evaluating leukocyte telomere length or DNA methylation levels (89). In particular, the selection of the experiential technique measuring telomere length dynamics can provide different insights into the effect of exercise on aging. For example, the leukocyte telomere length can be determined through quantitative polymerase chain reaction (qPCR) or fluorescence in situ hybridization (FISH) (90). The qPCR method can provide measurements regarding the average telomere length in the blood cell population in absolute terms (91). Due to its low cost and minimal DNA amount required, qPCR is widely used, and it can be used for benefits for high-throughput studies (92). However, different DNA extraction methods account for the inconsistent results that emerge across different labs (92). The main drawback of the qPCR method is determining the mean telomere length in each chromosome end in a diploid cell.
By contrast, the Q-FISH method can precisely provide the median value of asymmetric telomere length distribution. In general, the Q-FISH method is considered superior to the previous one since Q-FISH can accurately identify telomere length values in a single cell and at a chromosome-specific level at high-resolution (93-95). In particular, Q-FISH is the only methodology that can provide accurate measurements regarding the median value of an individual's telomere length distribution as well as the percentages of short, critical short, and long, critical long telomeres (94). In addition to the above, the flow-FISH technique has proven that telomere length varies according to cell type or tissue (96). Considering the limitations of methodologies, no causal association between exercise and longevity has been observed (97). Despite the use of laboratory techniques to estimate the beneficial contribution of physical exercise to longevity, more biomarkers are required to yield reliable results.
3. Mechanisms underlying the effect of exercise on telomere dynamics in athletes
Focusing on athletes, the present review provides a concise update on the influence of exercise on aging, given that the cardiac function and metabolic parameters of athletes are better than those of non-athletes (98). Indeed, epidemiological studies and systematic reviews have supported that elite athletes present mortality at lower levels and lower susceptibility to diseases than the general population (99).
The majority of research has focused on health-related traits in endurance athletes. It is known that endurance physical activity induces long-term adaptations in the metabolism of athletes, relying on mitochondrial respiration, thus ameliorating the cellular function of the athletes cardiorespiratory system (100). For this reason, endurance athletes present a significantly lower disease risk due to their long telomere length values (101).
Furthermore, the molecular mechanisms underlying the interaction between endurance exercise and the immune system have been emphasized (102). When comparing sprint/power (SPW) and endurance athletes, research has shown that the anti-inflammatory defense was upregulated in SPW athletes due to increased IL-10 expression levels in these athletes. By contrast, a pro-inflammatory status was activated in endurance athletes through an increase in IL-6 expression levels in respective athletes (102). When inflammation occurred in athletes, they exhibited accentuated telomere shortening (102). By contrast, the telomere length maintenance or elongation was associated with the anti-inflammatory response in athletes (102). Consistent with this, the prolonged athletes' aerobic training showed the downregulation of the CAT/TBARS ratio of anti-oxidant molecule catalase (CAT) to thiobarbituric acid reactive substances (TBARS), which is considered the primary byproduct of lipid peroxidation (102). In two types of training, a positive relationship emerged between the relative performance (RP) of athletes and aging regardless of the training mode (endurance or SPW) (102). The qPCR analysis showed that the average telomere length of leukocytes was reduced in athletes who presented either inflammation or oxidative stress (102). In addition, endurance athletes were characterized by improved endothelial function by elevating nitric oxide (NO) levels (103). Consequently, the improved NO bioavailability of endurance athletes was associated with telomere length elongation and improved redox ratios compared to age-matched controls, with the results being more pronounced in the middle-aged groups (104). As a result, the mechanisms linking aging and RP of SPW and endurance athletes relied on attenuating inflammation, oxidative stress and telomere shortening rate.
In addition to the above, the upregulation of the proteins of the shelterin complex or telomerase can prevent telomere shortening. In previous research, the increased telomerase expression was considered the underlying mechanism by which endurance athletes possessed longer telomeres than their inactive peers (72,75,105,106). For example, the effect of exercise in endurance athletes on aging was attributed to telomere length maintenance, either increasing telomerase or proteins of shelterin complex, such as TRF2 or preventing the action of cell-cycle inhibitors (72). The results proved that high telomerase expression and activity in combination with a low Chk2 expression discriminated athletes from the controls, irrespective of age (72). Of note, there was no difference in telomere length between young and aged endurance athletes, excluding age as a significant confounder (72). In another study, endurance athletes exhibited a higher whole-blood leukocyte TERT expression following long-term aerobic exercise, compared to healthy controls (105). In line with the above, a systematic review highlighted that the physical activity of endurance athletes delayed telomere erosion through a telomerase-dependent mechanism (107). In particular, endurance athletes exhibited an upregulated leukocyte TERT expression and activity (107). However, eight professional marathon runners did not exhibit any difference in telomerase expression levels in PBMCs before and after running marathons for 7 days, implying that the effect of exercise on telomerase can be neutral in marathon runners (108). No difference was detected in terms of telomere length and telomerase in marathon runners, but enrichment of shelterin complex components was presented in PBMCs (108).
As regards master athletes across various disciplines, few studies have assessed the relationship between oxidative stress and inflammatory markers with aging biomarkers (104,109,110). Master athletes are middle-aged individuals recognized for their competitive sports training and healthy lifestyles (111). They generally experience the advantages of exercise due to stress management, controlled metabolic profiling, and positive clinical health indicators (111). Master athletes (from 100m to marathon) demonstrate normal biological aging due to their distinct metabolic profiles and levels of physical fitness, which correlate with specific adaptations in their pulmonary systems (111). It is well-established that master sprinters demonstrate a better redox balance, enhanced anti-inflammatory status, and telomere length elongation compared to age-matched untrained controls (104,110,112).
In this perspective, it has been shown that master sprinters improve their inflammatory status more than age-matched controls, reducing the effects of aging and exhibiting longer telomeres than their counterparts (103). Similar findings regarding inflammation have been observed in endurance athletes (103). Master sprinters also enhance their antioxidant defenses more than endurance athletes, supporting a reduced aging rate due to a better redox balance (103). Notably, Aguiar et al (113) provided compelling evidence that master athletes have longer telomeres than sedentary individuals, based on a meta-analysis of 11 studies. From the molecular perspective, the telomere length elongation of master athletes is attributed to reduced inflammation, an improved antioxidant defense, the enhanced binding of shelterin complex proteins, elevated mitochondrial biogenesis and telomerase activity (113). By upregulating essential enzymes involved in antioxidant defense, such as CAT and the SOD/thiobarbituric acid reactive substances (TBARS) ratio, master athletes have altered the equilibrium between oxidants and antioxidants (113). Nonetheless, there was a moderate bias risk in the studies involved in that meta-analysis of master athletes (113).
Based on the fact that master athletes maintain a crucial antioxidant and anti-inflammatory status, the immune repertoire of master athletes infected by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus and its association with aging was examined (114). Simões et al (114) emphasized that master athletes preserve immune homeostasis, sustaining the proportions of T effector cells without inducing senescence in the T-cell population following exposure to the SARS-CoV2 virus. Master athletes possess longer telomeres associated with well-balanced immune homeostasis, which reduces the risk of developing COVID-19(114). Even when master athletes are infected with SARSCoV-2, they combat the disease more effectively than others (114). Consistent with this finding, it has been observed that the general population has an improved prognosis in the event of SARS-CoV-2 infection due to long telomeres (115). In parallel, master athletes exhibit elevated NO levels. This information is noteworthy, since NO has been proven to exert antibacterial and antiviral properties against the coronavirus in vitro and hepatitis virus (116). Accordingly, elite athletes sustain the highest performance following infection with SARS-CoV-2 virus (117). However, master athletes present telomere shortening when engaged in highly intense competitive training activities (87,88). Indeed, telomere shortening has been observed in master athletes who experienced ‘fatigued myopathic athlete syndrome’ (118,119), a condition that includes muscle damage due to oxidative burst (68,118).
In this context, when analyzing telomere length dynamics in athletes, the focus should be directed towards elite athletes who demonstrate the most notable performance in a particular sport (120). Initially, a previous meta-analysis demonstrated that sustainable engagement in regular exercise for at least 10 years can cause telomere length elongation in elite athletes >45 year of age (121). Subsequently, Muniesa et al (122) supported the concept that young elite athletes attenuate their aging process through telomere length elongation. Accordingly, Simoes et al (123) proved that exercise confers to elite sprinters telomere length elongation compared to their inactive peers. The results from the biochemical analyses of elite athletes have confirmed the attenuation of the aging process, not only by hindering telomere shortening, but also by reducing body fat and ameliorating lipid profiles (123). To verify the above, a recent systematic review demonstrated that the physical activity of elite athletes provides benefits for telomere length maintenance, regardless of exercise type (109). To understand the molecular mechanisms underlying the positive effect of exercise on telomere length dynamics of elite athletes, another study recruited elite athletes who followed all-intensity sports, measuring telomere length, oxidative stress and inflammation markers at athletes in an age-dependent manner (124). Indeed, that study provided insight into the effects of exercise on aging in two groups of elite athletes: Those <25 and those >25 years of age. In parallel, elite athletes were categorized into those participating in low-intensity sports, moderate-intensity sports, high-intensity sports and high-intensity endurance sports (124). For this reason, that study examined the impact of exercise on elite athletes and their inflammatory responses. The results revealed no statistically significant differences in the IL-8 levels among the different sport intensities between the two age groups of elite athletes. Of note, the IL-10 levels were increased in young elite athletes participating in moderate or high-intensity sports (124). In addition, TNFα was the only cytokine that was upregulated in elite athletes >25 years of age, along with increased activity of the antioxidant, CAT, regardless of sport intensity (124). In line with those results regarding elite athletes inflammation, the telomere length elongation was observed in all young elite athletes participating in all types of intensity sports, with the most significant increase noted among those in high-intensity sports (124). By contrast, older athletes engaging in high-intensity sports, exhibited an increase in IL-10 levels compared to that of age-matched athletes and those who belonged to low- and moderate-intensity sports. Notably, IL-6 levels were only elevated in older athletes >25 years of age (124). In fact, athletes >25 years of age experienced diminished immune responses characterized by a concurrent expression of pro-inflammatory and anti-inflammatory cytokines (124). Thus, the older athletes enrolled in the study did not experience the beneficial effects of exercise on their telomere length, owing to their age (124). In this regard, it was evident that high-intensity sports primarily contribute to a slower pace of aging, although their effects appear to be more compromised in older athletes >25 years of age (124). Within this context, the combination of telomere analysis, genotype/phenotype, metabolome, echocardiography and biochemical examinations in athletes has been shown to enhance athletic performance and overall wellness (125).
Nevertheless, scientific literature has proposed that the lifestyle of elite athletes was the central moderator of the relationship between physical exercise and aging. Initially, Rae et al pointed out that there was a divergence in the impact of exercise on telomere maintenance due to either lifestyle or competition-mediated stress (126). For example, the highly intensive training of elite athletes was linked to ‘overtraining syndrome’, in which the repair in response to injuries was inadequate (126). Indeed, elite athletes coped with various stressors before, during and after sports, suggesting stress was one underlying mechanism driving aging in athletes (127). Other longitudinal studies have demonstrated that athletes could not face stress, exhibiting a high risk of developing several diseases and negatively affecting telomere length maintenance (128-130). Accordingly, the higher cortisol reactivity of athletes exhibited an inverse association with the immune system function of athletes, expediting the aging of the immune system (131). Apart from the association of the stress response of athletes with their immune system, the increased cortisol secretion during rigorous training appeared to cause defects in their metabolism, thus compromising the welfare and telomere length maintenance of athletes (131,132). As a result, in elite athletes, rigorous training negatively affected telomere length by expediting their aging process (132).
In the assessment of athletes, a precise understanding of the performance of athletes can be provided by combining traditional biochemical and ergophysiological analysis with cutting-edge techniques, such as telomere analysis, genotyping/phenotypic profiling and metabolomics (125). Indeed, combining -omic and telomere technologies may provide a revolutionary process for improving the performance of athletes. Each factor provides essential insight and contributes to an the accurate understanding of the health and well-being of athletes (125). In addition, biochemical tests associated with energy metabolism and inflammation can be used to increase the effectiveness of recovery strategies in athletes (125). In this direction, telomere analysis can be used to optimize the training course of athletes to reduce injury risk, since telomere analysis is a robust biomarker of evaluating biological aging (125).
However, several ethical limitations should be considered when using telomere analysis. The ethical considerations are focused on predicting the performance and the potential of disease due to exercise in athletes. On the one hand, different individuals may decide to undergo telomere analysis to select which sport they wish to participate in. On the other hand, telomere analysis can become detrimental due to its use by athletes in terms of competition. For example, athletes can conduct telomere analysis to accomplish individualized training and avoid the probability of being injured. A multifaceted image of the health of an athlete can be obtained by performing telomere analysis in combination with genetic analysis, biochemical tests, metabolomics and echocardiography, thus enabling tailored interventions in selecting optional training, diet, and nutritional supplementation (125). Likewise, the misuse of genetic data (doping) should not be recommended due to ethics (133). Furthermore, biochemical tests associated with energy metabolism and inflammation can be used to increase the effectiveness of recovery needs in athletes (125). In this direction, telomere analysis can be used to optimize the training course of athletes to reduce injury risk since telomere analysis is a robust biomarker for evaluating biological aging.
4. Conclusions and future perspectives
Individuals in middle age and beyond, whether in good health or facing illness, may find that engaging in exercise contributes positively to maintaining telomere length values. The positive impact of exercise on telomere length homeostasis is associated with either increased telomerase action, upregulation of the shelterin complex protein, or amelioration of oxidative and inflammatory status. The molecular mechanism underlying the beneficial effect of exercise on telomere length dynamics is affected by some confounding factors like duration, intensity and type of exercise. This area is also analyzed in athletes who compete professionally and follow a healthy lifestyle. However, several limitations need to be addressed. First, contradicting results have been generated by the different factors (type, duration and intensity) that confound the protective nature of exercise. The methods used for measuring telomere length values are an additional consideration that accounts for inconsistent results. preventing the establishment of telomere length as an accurate measure of biological aging and cellular damage. Last but not least, the calculation of telomere length values from different tissues with varied cellular composition can cause the emergence of obscure results. Longitudinal studies are required to elucidate the effect of exercise on athletes.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
All authors (SB, MS, MMA, PI, PF, IF, ER, EV, MNT, AEN and AT) were involved in the conception and design of the study. SB performed the literature search, wrote the manuscript and critically analyzed the existing knowledge. MΜA designed the figure. SB, MS, PI, PF, IF, ER, EV, MNT, AEN and AT contributed to the editing of the manuscript. All authors have read and approved the final manuscript. Data authentication is not applicable.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
AT is an Editorial Advisor of the journal, but had no personal involvement in the reviewing process, or any influence in terms of adjudicating on the final decision, for this article. The other authors declare that they have no competing interests.
References
|
Tian YE, Cropley V, Maier AB, Lautenschlager NT, Breakspear M and Zalesky A: Heterogeneous aging across multiple organ systems and prediction of chronic disease and mortality. Nat Med. 29:1221–1231. 2023.PubMed/NCBI View Article : Google Scholar | |
|
López-Otín C, Blasco MA, Partridge L, Serrano M and Kroemer G: The hallmarks of aging. Cell. 153:1194–1217. 2013.PubMed/NCBI View Article : Google Scholar | |
|
Campisi J and d'Adda Di Fagagna F: Cellular senescence: When bad things happen to good cells. Nat Rev Mol Cell Biol. 8:729–740. 2007.PubMed/NCBI View Article : Google Scholar | |
|
Schmauck-Medina T, Molière A, Lautrup S, Zhang J, Chlopicki S, Madsen HB, Cao S, Soendenbroe C, Mansell E, Vestergaard MB, et al: New hallmarks of ageing: A 2022 Copenhagen ageing meeting summary. Aging (Albany NY). 14:6829–6839. 2022.PubMed/NCBI View Article : Google Scholar | |
|
Chakravarti D, LaBella KA and DePinho RA: Telomeres: history, health, and hallmarks of aging. Cell. 184:306–322. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Tenchov R, Sasso JM, Wang X and Zhou QA: Aging hallmarks and progression and age-related diseases: A landscape view of research advancement. ACS Chem Neurosci. 15:1–30. 2024.PubMed/NCBI View Article : Google Scholar | |
|
McHugh D and Gil J: Senescence and aging: Causes, consequences, and therapeutic avenues. J Cell Biol. 217:65–77. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Zhu Y, Liu X, Ding X, Wang F and Geng X: Telomere and its role in the aging pathways: Telomere shortening, cell senescence and mitochondria dysfunction. Biogerontology. 20:1–16. 2019.PubMed/NCBI View Article : Google Scholar | |
|
de Lange T: Shelterin: The protein complex that shapes and safeguards human telomeres. Genes Dev. 19:2100–2110. 2005.PubMed/NCBI View Article : Google Scholar | |
|
Marioni RE, Harris SE, Shah S, McRae AF, von Zglinicki T, Martin-Ruiz C, Wray NR, Visscher PM and Deary IJ: The epigenetic clock and telomere length are independently associated with chronological age and mortality. Int J Epidemiol. 45:424–432. 2016.PubMed/NCBI View Article : Google Scholar | |
|
Fragkiadaki P, Nikitovic D, Kalliantasi K, Sarandi E, Thanasoula M, Stivaktakis PD, Nepka C, Spandidos DA, Tosounidis T and Tsatsakis A: Telomere length and telomerase activity in osteoporosis and osteoarthritis. Exp Ther Med. 19:1626–1632. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Kakridonis F, Pneumatikos SG, Vakonaki E, Berdiaki A, Tzatzarakis MN, Fragkiadaki P, Spandidos DA, Baliou S, Ioannou P, Hatzidaki E, et al: Telomere length as a predictive biomarker in osteoporosis (Review). Biomed Rep. 19(87)2023.PubMed/NCBI View Article : Google Scholar | |
|
Sampson MJ, Winterbone MS, Hughes JC, Dozio N and Hughes DA: Monocyte telomere shortening and oxidative DNA damage in type 2 diabetes. Diabetes Care. 29:283–289. 2006.PubMed/NCBI View Article : Google Scholar | |
|
Fitzpatrick AL, Kronmal RA, Gardner JP, Psaty BM, Jenny NS, Tracy RP, Walston J, Kimura M and Aviv A: Leukocyte telomere length and cardiovascular disease in the cardiovascular health study. Am J Epidemiol. 165:14–21. 2007.PubMed/NCBI View Article : Google Scholar | |
|
Wiemann SU, Satyanarayana A, Tsahuridu M, Tillmann HL, Zender L, Klempnauer J, Flemming P, Franco S, Blasco MA, Manns MP and Rudolph KL: Hepatocyte telomere shortening and senescence are general markers of human liver cirrhosis. FASEB J. 16:935–942. 2002.PubMed/NCBI View Article : Google Scholar | |
|
Shay JW: Role of telomeres and telomerase in aging and cancer. Cancer Discov. 6:584–593. 2016.PubMed/NCBI View Article : Google Scholar | |
|
Vasilopoulos E, Fragkiadaki P, Kalliora C, Fragou D, Docea AO, Vakonaki E, Tsoukalas D, Calina D, Buga AM, Georgiadis G, et al: The association of female and male infertility with telomere length (Review). Int J Mol Med. 44:375–389. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Vakonaki E, Tsiminikaki K, Plaitis S, Fragkiadaki P, Tsoukalas D, Katsikantami I, Vaki G, Tzatzarakis MN, Spandidos DA and Tsatsakis AM: Common mental disorders and association with telomere length. Biomed Rep. 8:111–116. 2018.PubMed/NCBI View Article : Google Scholar | |
|
de Lange T: Shelterin-mediated telomere protection. Annu Rev Genet. 52:223–247. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Smogorzewska A, van Steensel B, Bianchi A, Oelmann S, Schaefer MR, Schnapp G and de Lange T: Control of human telomere length by TRF1 and TRF2. Mol Cell Biol. 20:1659–1668. 2000.PubMed/NCBI View Article : Google Scholar | |
|
Yang Z, Takai KK, Lovejoy CA and de Lange T: Break-induced replication promotes fragile telomere formation. Genes Dev. 34:1392–1405. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Blackburn EH: Telomeres: Structure and synthesis. J Biol Chem. 265:5919–5921. 1990.PubMed/NCBI | |
|
Blackburn EH: Telomeres and telomerase: Their mechanisms of action and the effects of altering their functions. FEBS Lett. 579:859–862. 2005.PubMed/NCBI View Article : Google Scholar | |
|
Bandaria JN, Qin P, Berk V, Chu S and Yildiz A: Shelterin protects chromosome ends by compacting telomeric chromatin. Cell. 164:735–746. 2016.PubMed/NCBI View Article : Google Scholar | |
|
Fumagalli M, Rossiello F, Clerici M, Barozzi S, Cittaro D, Kaplunov JM, Bucci G, Dobreva M, Matti V, Beausejour CM, et al: Telomeric DNA damage is irreparable and causes persistent DNA-damage-response activation. Nat Cell Biol. 14:355–365. 2012.PubMed/NCBI View Article : Google Scholar | |
|
Baird DM: Telomere dynamics in human cells. Biochimie. 90:116–121. 2008.PubMed/NCBI View Article : Google Scholar | |
|
Olovnikov AM: A theory of marginotomy. The incomplete copying of template margin in enzymic synthesis of polynucleotides and biological significance of the phenomenon. J Theor Biol. 41:181–190. 1973.PubMed/NCBI View Article : Google Scholar | |
|
Radak Z and Boldogh I: 8-Oxo-7,8-dihydroguanine: Links to gene expression, aging, and defense against oxidative stress. Free Radic Biol Med. 49:587–596. 2010.PubMed/NCBI View Article : Google Scholar | |
|
Dabrowska A, Venero JL, Iwasawa R, Hankir MK, Rahman S, Boobis A and Hajji N: PGC-1alpha controls mitochondrial biogenesis and dynamics in lead-induced neurotoxicity. Aging (Albany NY). 7:629–647. 2015.PubMed/NCBI View Article : Google Scholar | |
|
Fang EF, Scheibye-Knudsen M, Chua KF, Mattson MP, Croteau DL and Bohr VA: Nuclear DNA damage signalling to mitochondria in ageing. Nat Rev Mol Cell Biol. 17:308–321. 2016.PubMed/NCBI View Article : Google Scholar | |
|
Fang EF, Scheibye-Knudsen M, Brace LE, Kassahun H, SenGupta T, Nilsen H, Mitchell JR, Croteau DL and Bohr VA: Defective mitophagy in XPA via PARP-1 hyperactivation and NAD(+)/SIRT1 reduction. Cell. 157:882–896. 2014.PubMed/NCBI View Article : Google Scholar | |
|
Armanios M: The role of telomeres in human disease. Annu Rev Genomics Hum Genet. 23:363–381. 2022.PubMed/NCBI View Article : Google Scholar | |
|
Gorgoulis V, Adams PD, Alimonti A, Bennett DC, Bischof O, Bishop C, Campisi J, Collado M, Evangelou K, Ferbeyre G, et al: Cellular senescence: Defining a path forward. Cell. 179:813–827. 2019.PubMed/NCBI View Article : Google Scholar | |
|
d'Adda di Fagagna F, Reaper PM, Clay-Farrace L, Fiegler H, Carr P, Von Zglinicki T, Saretzki G, Carter NP and Jackson SP: A DNA damage checkpoint response in telomere-initiated senescence. Nature. 426:194–198. 2003.PubMed/NCBI View Article : Google Scholar | |
|
Pech MF, Garbuzov A, Hasegawa K, Sukhwani M, Zhang RJ, Benayoun BA, Brockman SA, Lin S, Brunet A, Orwig KE and Artandi SE: High telomerase is a hallmark of undifferentiated spermatogonia and is required for maintenance of male germline stem cells. Genes Dev. 29:2420–2434. 2015.PubMed/NCBI View Article : Google Scholar | |
|
Artandi SE and DePinho RA: Telomeres and telomerase in cancer. Carcinogenesis. 31:9–18. 2010.PubMed/NCBI View Article : Google Scholar | |
|
Lee HW, Blasco MA, Gottlieb GJ, Horner JW, Greider CW and DePinho RA: Essential role of mouse telomerase in highly proliferative organs. Nature. 392:569–574. 1998.PubMed/NCBI View Article : Google Scholar | |
|
Andreu-Sánchez S, Aubert G, Ripoll-Cladellas A, Henkelman S, Zhernakova DV, Sinha T, Kurilshikov A, Cenit MC, Jan Bonder M, Franke L, et al: Genetic, parental and lifestyle factors influence telomere length. Commun Biol. 5(565)2022.PubMed/NCBI View Article : Google Scholar | |
|
Navarro C, Salazar J, Díaz MP, Chacin M, Santeliz R, Vera I, D Marco L, Parra H, Bernal MC, Castro A, et al: Intrinsic and environmental basis of aging: A narrative review. Heliyon. 9(e18239)2023.PubMed/NCBI View Article : Google Scholar | |
|
Tsoukalas D, Fragkiadaki P, Docea A, Alegakis AK, Sarandi E, Vakonaki E, Salataj E, Kouvidi E, Nikitovic D, Kovatsi L, et al: Association of nutraceutical supplements with longer telomere length. Int J Mol Med. 44:218–226. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Baliou S, Ioannou P, Apetroaei MM, Vakonaki E, Fragkiadaki P, Kirithras E, Tzatzarakis MN, Arsene AL, Docea AO and Tsatsakis A: The impact of the mediterranean diet on telomere biology: implications for disease management-A narrative review. Nutrients. 16(2525)2024.PubMed/NCBI View Article : Google Scholar | |
|
Wade KH, Richmond RC and Davey Smith G: Physical activity and longevity: How to move closer to causal inference. Br J Sports Med. 52:890–891. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Bennie JA, Shakespear-Druery J and De Cocker K: Muscle-strengthening exercise epidemiology: A new frontier in chronic disease prevention. Sports Med Open. 6(40)2020.PubMed/NCBI View Article : Google Scholar | |
|
Rebelo-Marques A, De Sousa Lages A, Andrade R, Ribeiro CF, Mota-Pinto A, Carrilho F and Espregueira-Mendes J: Aging hallmarks: The benefits of physical exercise. Front Endocrinol (Lausanne). 9(258)2018.PubMed/NCBI View Article : Google Scholar | |
|
Semeraro MD, Smith C, Kaiser M, Levinger I, Duque G, Gruber HJ and Herrmann M: Physical activity, a modulator of aging through effects on telomere biology. Aging (Albany NY). 12:13803–13823. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Ekelund U, Tarp J, Steene-Johannessen J, Hansen BH, Jefferis B, Fagerland MW, Whincup P, Diaz KM, Hooker SP, Chernofsky A, et al: Dose-response associations between accelerometry measured physical activity and sedentary time and all cause mortality: Systematic review and harmonised meta-analysis. BMJ. 366(l4570)2019.PubMed/NCBI View Article : Google Scholar | |
|
Vyas CM, Ogata S, Reynolds CF, Mischoulon D, Chang G, Cook NR, Manson JE, Crous-Bou M, De Vivo I and Okereke OI: Telomere length and its relationships with lifestyle and behavioural factors: Variations by sex and race/ethnicity. Age Ageing. 50:838–846. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Valente C, Andrade R, Alvarez L, Rebelo-Marques A, Stamatakis E and Espregueira-Mendes J: Effect of physical activity and exercise on telomere length: Systematic review with meta-analysis. J Am Geriatr Soc. 69:3285–3300. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Buttet M, Bagheri R, Ugbolue UC, Laporte C, Trousselard M, Benson A, Bouillon-Minois JB and Dutheil F: Effect of a lifestyle intervention on telomere length: A systematic review and meta-analysis. Mech Ageing Dev. 206(111694)2022.PubMed/NCBI View Article : Google Scholar | |
|
Schellnegger M, Lin AC, Hammer N and Kamolz LP: Physical activity on telomere length as a biomarker for aging: A systematic review. Sports Med Open. 8(111)2022.PubMed/NCBI View Article : Google Scholar | |
|
Song S, Lee E and Kim H: Does exercise affect telomere length? A systematic review and meta-analysis of Randomized controlled trials. Medicina (Kaunas). 58(242)2022.PubMed/NCBI View Article : Google Scholar | |
|
Sánchez-González JL, Sánchez-Rodríguez JL, Varela-Rodríguez S, González-Sarmiento R, Rivera-Picón C, Juárez-Vela R, Tejada-Garrido CI, Martín-Vallejo J and Navarro-López V: Effects of physical exercise on telomere length in healthy adults: Systematic review, meta-analysis, and meta-regression. JMIR Public Health Surveill. 10(e46019)2024.PubMed/NCBI View Article : Google Scholar | |
|
Cherkas LF, Hunkin JL, Kato BS, Richards JB, Gardner JP, Surdulescu GL, Kimura M, Lu X, Spector TD and Aviv A: The association between physical activity in leisure time and leukocyte telomere length. Arch Intern Med. 168:154–158. 2008.PubMed/NCBI View Article : Google Scholar | |
|
Bendix L, Gade MM, Staun PW, Kimura M, Jeune B, Hjelmborg JV, Aviv A and Christensen K: Leukocyte telomere length and physical ability among Danish twins age 70+. Mech Ageing Dev. 132:568–572. 2011.PubMed/NCBI View Article : Google Scholar | |
|
Baylis D, Ntani G, Edwards MH, Syddall HE, Bartlett DB, Dennison EM, Martin-Ruiz C, von Zglinicki T, Kuh D, Lord JM, et al: Inflammation, telomere length, and grip strength: A 10-year longitudinal study. Calcif Tissue Int. 95:54–63. 2014.PubMed/NCBI View Article : Google Scholar | |
|
Shadyab AH, LaMonte MJ, Kooperberg C, Reiner AP, Carty CL, Manini TM, Hou L, Di C, Macera CA, Gallo LC, et al: Leisure-time physical activity and leukocyte telomere length among older women. Exp Gerontol. 95:141–147. 2017.PubMed/NCBI View Article : Google Scholar | |
|
Jantunen H, Wasenius NS, Guzzardi MA, Iozzo P, Kajantie E, Kautiainen H, Salonen MK and Eriksson JG: Physical activity and telomeres in old age: A longitudinal 10-year follow-up study. Gerontology. 66:315–322. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Stenbäck V, Mutt SJ, Leppäluoto J, Gagnon DD, Mäkelä KA, Jokelainen J, Keinänen-Kiukaanniemi S and Herzig KH: Association of physical activity with telomere length among elderly adults - the oulu cohort 1945. Front Physiol. 10(444)2019.PubMed/NCBI View Article : Google Scholar | |
|
Simpson RJ, Lowder TW, Spielmann G, Bigley AB, LaVoy EC and Kunz H: Exercise and the aging immune system. Ageing Res Rev. 11:404–420. 2012.PubMed/NCBI View Article : Google Scholar | |
|
Zhang J, Rane G, Dai X, Shanmugam MK, Arfuso F, Samy RP, Lai MK, Kappei D, Kumar AP and Sethi G: Ageing and the telomere connection: An intimate relationship with inflammation. Ageing Res Rev. 25:55–69. 2016.PubMed/NCBI View Article : Google Scholar | |
|
Ludlow AT, Spangenburg EE, Chin ER, Cheng WH and Roth SM: Telomeres shorten in response to oxidative stress in mouse skeletal muscle fibers. J Gerontol A Biol Sci Med Sci. 69:821–830. 2014.PubMed/NCBI View Article : Google Scholar | |
|
Opresko PL, Fan J, Danzy S, Wilson DM and Bohr VA: Oxidative damage in telomeric DNA disrupts recognition by TRF1 and TRF2. Nucleic Acids Res. 33:1230–1239. 2005.PubMed/NCBI View Article : Google Scholar | |
|
von Zglinicki T: Oxidative stress shortens telomeres. Trends Biochem Sci. 27:339–344. 2002.PubMed/NCBI View Article : Google Scholar | |
|
Sfeir A, Kosiyatrakul ST, Hockemeyer D, MacRae SL, Karlseder J, Schildkraut CL and de Lange T: Mammalian telomeres resemble fragile sites and require TRF1 for efficient replication. Cell. 138:90–103. 2009.PubMed/NCBI View Article : Google Scholar | |
|
Chistiakov DA, Sobenin IA, Revin VV, Orekhov AN and Bobryshev YV: Mitochondrial aging and age-related dysfunction of mitochondria. Biomed Res Int. 2014(238463)2014.PubMed/NCBI View Article : Google Scholar | |
|
Sahin E and DePinho RA: Axis of ageing: Telomeres, p53 and mitochondria. Nat Rev Mol Cell Biol. 13:397–404. 2012.PubMed/NCBI View Article : Google Scholar | |
|
Radak Z, Chung HY, Koltai E, Taylor AW and Goto S: Exercise, oxidative stress and hormesis. Ageing Res Rev. 7:34–42. 2008.PubMed/NCBI View Article : Google Scholar | |
|
Arsenis NC, You T, Ogawa EF, Tinsley GM and Zuo L: Physical activity and telomere length: Impact of aging and potential mechanisms of action. Oncotarget. 8:45008–45019. 2017.PubMed/NCBI View Article : Google Scholar | |
|
Garland SN, Johnson B, Palmer C, Speck RM, Donelson M, Xie SX, DeMichele A and Mao JJ: Physical activity and telomere length in early stage breast cancer survivors. Breast Cancer Res. 16(413)2014.PubMed/NCBI View Article : Google Scholar | |
|
Voisin S, Eynon N, Yan X and Bishop DJ: Exercise training and DNA methylation in humans. Acta Physiol (Oxf). 213:39–59. 2015.PubMed/NCBI View Article : Google Scholar | |
|
Denham J, O'Brien BJ and Charchar FJ: Telomere length maintenance and cardio-metabolic disease prevention through exercise training. Sports Med. 46:1213–1237. 2016.PubMed/NCBI View Article : Google Scholar | |
|
Werner C, Fürster T, Widmann T, Pöss J, Roggia C, Hanhoun M, Scharhag J, Büchner N, Meyer T, Kindermann W, et al: Physical exercise prevents cellular senescence in circulating leukocytes and in the vessel wall. Circulation. 120:2438–2447. 2009.PubMed/NCBI View Article : Google Scholar | |
|
Koering CE, Pollice A, Zibella MP, Bauwens S, Puisieux A, Brunori M, Brun C, Martins L, Sabatier L, Pulitzer JF and Gilson E: Human telomeric position effect is determined by chromosomal context and telomeric chromatin integrity. EMBO Rep. 3:1055–1061. 2002.PubMed/NCBI View Article : Google Scholar | |
|
van Steensel B, Smogorzewska A and de Lange T: TRF2 protects human telomeres from end-to-end fusions. Cell. 92:401–413. 1998.PubMed/NCBI View Article : Google Scholar | |
|
Denham J, Nelson CP, O'Brien BJ, Nankervis SA, Denniff M, Harvey JT, Marques FZ, Codd V, Zukowska-Szczechowska E, Samani NJ, et al: Longer leukocyte telomeres are associated with ultra-endurance exercise independent of cardiovascular risk factors. PLoS One. 8(e69377)2013.PubMed/NCBI View Article : Google Scholar | |
|
Slentz CA, Houmard JA and Kraus WE: Modest exercise prevents the progressive disease associated with physical inactivity. Exerc Sport Sci Rev. 35:18–23. 2007.PubMed/NCBI View Article : Google Scholar | |
|
Sanz C, Gautier JF and Hanaire H: Physical exercise for the prevention and treatment of type 2 diabetes. Diabetes Metab. 36:346–351. 2010.PubMed/NCBI View Article : Google Scholar | |
|
Schuler G, Adams V and Goto Y: Role of exercise in the prevention of cardiovascular disease: Results, mechanisms, and new perspectives. Eur Heart J. 34:1790–1799. 2013.PubMed/NCBI View Article : Google Scholar | |
|
Krauss J, Farzaneh-Far R, Puterman E, Na B, Lin J, Epel E, Blackburn E and Whooley MA: Physical fitness and telomere length in patients with coronary heart disease: Findings from the heart and soul study. PLoS One. 6(e26983)2011.PubMed/NCBI View Article : Google Scholar | |
|
Dankel SJ, Loenneke JP and Loprinzi PD: The impact of overweight/obesity duration and physical activity on telomere length: An application of the WATCH paradigm. Obes Res Clin Pract. 11:247–252. 2017.PubMed/NCBI View Article : Google Scholar | |
|
Testa R, Olivieri F, Sirolla C, Spazzafumo L, Rippo MR, Marra M, Bonfigli AR, Ceriello A, Antonicelli R, Franceschi C, Castellucci C, et al: Leukocyte telomere length is associated with complications of type 2 diabetes mellitus. Diabet Med. 28:1388–1394. 2011.PubMed/NCBI View Article : Google Scholar | |
|
Carapeto PV and Aguayo-Mazzucato C: Effects of exercise on cellular and tissue aging. Aging (Albany NY). 13:14522–14543. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Lefferts WK, Davis MM and Valentine RJ: Exercise as an aging mimetic: A new perspective on the mechanisms behind exercise as preventive medicine against age-related chronic disease. Front Physiol. 13(866792)2022.PubMed/NCBI View Article : Google Scholar | |
|
Werner CM, Hecksteden A, Morsch A, Zundler J, Wegmann M, Kratzsch J, Thiery J, Hohl M, Bittenbring JT, Neumann F, et al: Differential effects of endurance, interval, and resistance training on telomerase activity and telomere length in a randomized, controlled study. Eur Heart J. 40:34–46. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Mayer F, Scharhag-Rosenberger F, Carlsohn A, Cassel M, Müller S and Scharhag J: The intensity and effects of strength training in the elderly. Dtsch Arztebl Int. 108:359–364. 2011.PubMed/NCBI View Article : Google Scholar | |
|
Dimauro I, Scalabrin M, Fantini C, Grazioli E, Beltran Valls MR, Mercatelli N, Parisi A, Sabatini S, Di Luigi L and Caporossi D: Resistance training and redox homeostasis: Correlation with age-associated genomic changes. Redox Biol. 10:34–44. 2016.PubMed/NCBI View Article : Google Scholar | |
|
Kim JH, Ko JH, Lee D, Lim I and Bang H: Habitual physical exercise has beneficial effects on telomere length in postmenopausal women. Menopause. 19:1109–1115. 2012.PubMed/NCBI View Article : Google Scholar | |
|
Savela S, Saijonmaa O, Strandberg TE, Koistinen P, Strandberg AY, Tilvis RS, Pitkälä KH, Miettinen TA and Fyhrquist F: Physical activity in midlife and telomere length measured in old age. Exp Gerontol. 48:81–84. 2013.PubMed/NCBI View Article : Google Scholar | |
|
Tzemah-Shahar R, Hochner H, Iktilat K and Agmon M: What can we learn from physical capacity about biological age? A systematic review. Ageing Res Rev. 77(101609)2022.PubMed/NCBI View Article : Google Scholar | |
|
Ferreira MSV, Kirschner M, Halfmeyer I, Estrada N, Xicoy B, Isfort S, Vieri M, Zamora L, Abels A, Bouillon AS, et al: Comparison of flow-FISH and MM-qPCR telomere length assessment techniques for the screening of telomeropathies. Ann NY Acad Sci. 1466:93–103. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Cawthon RM: Telomere length measurement by a novel monochrome multiplex quantitative PCR method. Nucleic Acids Res. 37(e21)2009.PubMed/NCBI View Article : Google Scholar | |
|
Lin J, Smith DL, Esteves K and Drury S: Telomere length measurement by qPCR - Summary of critical factors and recommendations for assay design. Psychoneuroendocrinology. 99:271–278. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Gutierrez-Rodrigues F, Santana-Lemos BA, Scheucher PS, Alves-Paiva RM and Calado RT: direct comparison of flow-FISH and qPCR as diagnostic tests for telomere length measurement in humans. PLoS One. 9(e113747)2014.PubMed/NCBI View Article : Google Scholar | |
|
Tsatsakis A, Tsoukalas D, Fragkiadaki P, Vakonaki E, Tzatzarakis M, Sarandi E, Nikitovic D, Tsilimidos G and Alegakis AK: Developing BIOTEL: A semi-automated spreadsheet for estimating telomere length and biological age. Front Genet. 10(84)2019.PubMed/NCBI View Article : Google Scholar | |
|
Canela A, Vera E, Klatt P and Blasco MA: High-throughput telomere length quantification by FISH and its application to human population studies. Proc Natl Acad Sci USA. 104:5300–5305. 2007.PubMed/NCBI View Article : Google Scholar | |
|
Tigchelaar EF, Zhernakova A, Dekens JA, Hermes G, Baranska A, Mujagic Z, Swertz MA, Muñoz AM, Deelen P, Cénit MC, et al: Cohort profile: LifeLines DEEP, a prospective, general population cohort study in the northern Netherlands: Study design and baseline characteristics. BMJ Open. 5(e006772)2015.PubMed/NCBI View Article : Google Scholar | |
|
Kujala UM: Is physical activity a cause of longevity? It is not as straightforward as some would believe. A critical analysis. Br J Sports Med. 52:914–918. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Gabrys L, Baumert J, Heidemann C, Busch M and Finger JD: Sports activity patterns and cardio-metabolic health over time among adults in Germany: Results of a nationwide 12-year follow-up study. J Sport Health Sci. 10:439–446. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Lemez S and Baker J: Do elite athletes live longer? A systematic review of mortality and longevity in elite athletes. Sports Med Open. 1(16)2015.PubMed/NCBI View Article : Google Scholar | |
|
Beattie K, Kenny IC, Lyons M and Carson BP: The effect of strength training on performance in endurance athletes. Sports Med. 44:845–865. 2014.PubMed/NCBI View Article : Google Scholar | |
|
Garatachea N, Santos-Lozano A, Sanchis-Gomar F, Fiuza-Luces C, Pareja-Galeano H, Emanuele E and Lucia A: Elite athletes live longer than the general population: A meta-analysis. Mayo Clin Proc. 89:1195–1200. 2014.PubMed/NCBI View Article : Google Scholar | |
|
Sousa CV, Silva Aguiar S, Deus LA, Barbosa LP, Dos Santos PA, Neves RVP, Maciel LA, Moraes MR, Moreira SR, Grubert Campbell CS, et al: Faster and healthier: Relationship between telomere and performance in master athletes. Int J Sports Med. 41:339–344. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Rosa TS, Neves RVP, Deus LA, Sousa CV, da Silva Aguiar S, de Souza MK, Moraes MR, Rosa ÉCCC, Andrade RV, Korhonen MT and Simões HG: Sprint and endurance training in relation to redox balance, inflammatory status and biomarkers of aging in master athletes. Nitric Oxide. 102:42–51. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Sousa CV, Aguiar SS, Santos PA, Barbosa LP, Knechtle B, Nikolaidis PT, Deus LA, Sales MM, Rosa ECCC, Rosa TS, et al: Telomere length and redox balance in master endurance runners: The role of nitric oxide. Exp Gerontol. 117:113–118. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Denham J, O'Brien BJ, Prestes PR, Brown NJ and Charchar FJ: Increased expression of telomere-regulating genes in endurance athletes with long leukocyte telomeres. J Appl Physiol (1985). 120:148–158. 2016.PubMed/NCBI View Article : Google Scholar | |
|
Hagman M, Werner C, Kamp K, Fristrup B, Hornstrup T, Meyer T, Böhm M, Laufs U and Krustrup P: Reduced telomere shortening in lifelong trained male football players compared to age-matched inactive controls. Prog Cardiovasc Dis. 63:738–749. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Denham J and Sellami M: Exercise training increases telomerase reverse transcriptase gene expression and telomerase activity: A systematic review and meta-analysis. Ageing Res Rev. 70(101411)2021.PubMed/NCBI View Article : Google Scholar | |
|
Laye MJ, Solomon TPJ, Karstoft K, Pedersen KK, Nielsen SD and Pedersen BK: Increased shelterin mRNA expression in peripheral blood mononuclear cells and skeletal muscle following an ultra-long-distance running event. J Appl Physiol (1985). 112:773–781. 2012.PubMed/NCBI View Article : Google Scholar | |
|
Abrahin O, Cortinhas-Alves EA, Vieira RP and Guerreiro JF: Elite athletes have longer telomeres than sedentary subjects: A meta-analysis. Exp Gerontol. 119:138–145. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Minuzzi LG, Chupel MU, Rama L, Rosado F, Muñoz VR, Gaspar RC, Kuga GK, Furtado GE, Pauli JR and Teixeira AM: Lifelong exercise practice and immunosenescence: Master athletes cytokine response to acute exercise. Cytokine. 115:1–7. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Kusy K and Zieliński J: Sprinters versus long-distance runners: How to grow old healthy. Exerc Sport Sci Rev. 43:57–64. 2015.PubMed/NCBI View Article : Google Scholar | |
|
Benedini S, Dozio E, Invernizzi PL, Vianello E, Banfi G, Terruzzi I, Luzi L and Corsi Romanelli MM: Irisin: A potential link between physical exercise and metabolism-an observational study in differently trained subjects, from elite athletes to sedentary people. J Diabetes Res. 2017(1039161)2017.PubMed/NCBI View Article : Google Scholar | |
|
Aguiar SS, Sousa CV, Santos PA, Barbosa LP, Maciel LA, Coelho-Júnior HJ, Motta-Santos D, Rosa TS, Degens H and Simões HG: Master athletes have longer telomeres than age-matched non-athletes. A systematic review, meta-analysis and discussion of possible mechanisms. Exp Gerontol. 146(111212)2021.PubMed/NCBI View Article : Google Scholar | |
|
Simões HG, Rosa TS, Sousa CV, Aguiar SDS, Motta-Santos D, Degens H, Korhonen MT and Campbell CSG: Does longer leukocyte telomere length and higher physical fitness protect master athletes from consequences of coronavirus (SARS-CoV-2) infection? Front Sports Act Living. 2(87)2020.PubMed/NCBI View Article : Google Scholar | |
|
Liu S, Wang C, Green G, Zhuo H, Liu KD, Kangelaris KN, Gomez A, Jauregui A, Vessel K, Ke S, et al: Peripheral blood leukocyte telomere length is associated with survival of sepsis patients. Eur Respir J. 55(1901044)2020.PubMed/NCBI View Article : Google Scholar | |
|
Keyaerts E, Vijgen L, Chen L, Maes P, Hedenstierna G and Van Ranst M: Inhibition of SARS-coronavirus infection in vitro by S-nitroso-N-acetylpenicillamine, a nitric oxide donor compound. Int J Infect Dis. 8:223–226. 2004.PubMed/NCBI View Article : Google Scholar | |
|
Siopis G: Elite athletes maintain peak performance after testing positive for SARS-CoV-2. J Sci Med Sport. 25:195–196. 2022.PubMed/NCBI View Article : Google Scholar | |
|
Collins M, Renault V, Grobler LA, St Clair Gibson A, Lambert MI, Wayne Derman E, Butler-Browne GS, Noakes TD and Mouly V: Athletes with exercise-associated fatigue have abnormally short muscle DNA telomeres. Med Sci Sports Exerc. 35:1524–1528. 2003.PubMed/NCBI View Article : Google Scholar | |
|
Wan JJ, Qin Z, Wang PY, Sun Y and Liu X: Muscle fatigue: General understanding and treatment. Exp Mol Med. 49(e384)2017.PubMed/NCBI View Article : Google Scholar | |
|
Burns L, Weissensteiner JR, Cohen M and Bird SR: A survey of elite and pre-elite athletes' perceptions of key support, lifestyle and performance factors. BMC Sports Sci Med Rehabil. 14(2)2022.PubMed/NCBI View Article : Google Scholar | |
|
Saßenroth D, Meyer A, Salewsky B, Kroh M, Norman K, Steinhagen-Thiessen E and Demuth I: Sports and exercise at different ages and leukocyte telomere length in later life-data from the berlin aging study II (BASE-II). PLoS One. 10(e0142131)2015.PubMed/NCBI View Article : Google Scholar | |
|
Muniesa CA, Verde Z, Diaz-Ureña G, Santiago C, Gutiérrez F, Díaz E, Gómez-Gallego F, Pareja-Galeano H, Soares-Miranda L and Lucia A: Telomere length in elite athletes. Int J Sports Physiol Perform. 12:994–996. 2017.PubMed/NCBI View Article : Google Scholar | |
|
Simoes HG, Sousa CV, Dos Santos Rosa T, da Silva Aguiar S, Deus LA, Rosa ECCC, Amato AA and Andrade RV: Longer telomere length in elite master sprinters: Relationship to performance and body composition. Int J Sports Med. 38:1111–1116. 2017.PubMed/NCBI View Article : Google Scholar | |
|
Sellami M, Al-muraikhy S, Al-Jaber H, Al-Amri H, Al-Mansoori L, Mazloum NA, Donati F, Botre F and Elrayess MA: Age and sport intensity-dependent changes in cytokines and telomere length in elite athletes. Antioxidants (Basel). 10(1035)2021.PubMed/NCBI View Article : Google Scholar | |
|
Spanakis M, Fragkiadaki P, Renieri E, Vakonaki E, Fragkiadoulaki I, Alegakis A, Kiriakakis M, Panagiotou N, Ntoumou E, Gratsias I, et al: Advancing athletic assessment by integrating conventional methods with cutting-edge biomedical technologies for comprehensive performance, wellness, and longevity insights. Front Sports Act Living. 5(1327792)2023.PubMed/NCBI View Article : Google Scholar | |
|
Rae DE, Vignaud A, Butler-Browne GS, Thornell LE, Sinclair-Smith C, Derman EW, Lambert MI and Collins M: Skeletal muscle telomere length in healthy, experienced, endurance runners. Eur J Appl Physiol. 109:323–330. 2010.PubMed/NCBI View Article : Google Scholar | |
|
Epel ES, Blackburn EH, Lin J, Dhabhar FS, Adler NE, Morrow JD and Cawthon RM: Accelerated telomere shortening in response to life stress. Proc Natl Acad Sci USA. 101:17312–17315. 2004.PubMed/NCBI View Article : Google Scholar | |
|
Epel E, Daubenmier J, Moskowitz JT, Folkman S and Blackburn E: Can meditation slow rate of cellular aging? Cognitive stress, mindfulness, and telomeres. Ann N Y Acad Sci. 1172:34–53. 2009.PubMed/NCBI View Article : Google Scholar | |
|
Purcell R, Gwyther K and Rice SM: Mental health in elite athletes: Increased awareness requires an early intervention framework to respond to athlete needs. Sports Med Open. 5(46)2019.PubMed/NCBI View Article : Google Scholar | |
|
Seib C, Whiteside E, Humphreys J, Lee K, Thomas P, Chopin L, Crisp G, O'Keeffe A, Kimlin M, Stacey A and Anderson D: A longitudinal study of the impact of chronic psychological stress on health-related quality of life and clinical biomarkers: Protocol for the Australian healthy aging of women study. BMC Public Health. 14(9)2014.PubMed/NCBI View Article : Google Scholar | |
|
Jiang Y, Da W, Qiao S, Zhang Q, Li X, Ivey G and Zilioli S: Basal cortisol, cortisol reactivity, and telomere length: A systematic review and meta-analysis. Psychoneuroendocrinology. 103:163–172. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Mehrsafar AH, Serrano Rosa MA, Moghadam Zadeh A and Gazerani P: Stress, professional lifestyle, and telomere biology in elite athletes: A growing trend in psychophysiology of sport. Front Psychol. 11(567214)2020.PubMed/NCBI View Article : Google Scholar | |
|
Hurst P, King A, Massey K, Kavussanu M and Ring C: A national anti-doping education programme reduces doping susceptibility in British athletes. Psychol Sport Exerc. 69(102512)2023.PubMed/NCBI View Article : Google Scholar |