Clinical efficacy of the use of myoinositol alone compared with clomiphene citrate alone and the combined use of both medications as regards the conception rates of patients with PCOS: A systematic review and meta‑analysis
- Authors:
- Published online on: June 12, 2025 https://doi.org/10.3892/wasj.2025.360
- Article Number: 72
-
Copyright : © Saadia et al. This is an open access article distributed under the terms of Creative Commons Attribution License [CC BY 4.0].
Abstract
Introduction
In total, 10-15% of females of reproductive age suffer from polycystic ovary syndrome (PCOS) worldwide (1). The condition is characterized by hyperandrogenism, anovulation and menstrual irregularities, coupled with polycystic morphology ultrasound observations. PCOS is associated with various factors related to metabolic dysregulation, such as insulin resistance, obesity and type 2 diabetes. Furthermore, PCOS is a leading cause of anovulatory infertility (2). Clomiphene citrate (CC) has been used as a first-line treatment for inducing ovulation in this subset of the population. However, its use is frequently limited by side-effects and the relatively high incidence of resistance (3).
PCOS is commonly associated with insulin resistance and an elevated body mass index (BMI). Typical laboratory findings in patients with PCOS include increased levels of luteinizing hormone (LH) and normal follicle-stimulating hormone (FSH) levels (4). The increased levels of LH drive ovarian thecal cells into overproducing androgens, leading to anovulation. Increasing the levels of FSH either by amplifying endogenous production or administering exogenous FSH can reverse the unresolved follicles (5).
The therapeutic approach for PCOS-related subfertility encompasses reducing insulin resistance, inhibiting the influence of androgens on targeted tissues and addressing anovulation (6). Pharmacological solutions to anovulation may involve the use of CC alone or in combination with insulin-sensitizing agents, such as metformin and myoinositol (7-9). By contrast, effective non-pharmacological strategies for improving ovulation include a 10% reduction in body weight and ovarian drilling (10).
Myoinositol is a member of the B complex group of vitamins that has emerged as a potential therapeutic agent for patients with PCOS (11). It improves insulin resistance and thereby normalizes hormonal parameters in patients with PCOS (12). Furthermore, it has been proposed that myoinositol can enhance the efficacy of other methods of ovulation induction (13,14). However, evidence regarding the superiority in the efficacy of myoinositol over CC in improving fertility outcomes either when used alone or in combination in patients with PCOS remains controversial. The benefits of myoinositol, such as a decrease in insulin resistance, can improve reproductive outcomes in patients with PCOS, since increased insulin resistance is involved in the pathogenesis of PCOS.
Therefore, the present systematic review and meta-analysis was performed to assess the currently available evidence from the literature to compare the ovulation and pregnancy rates in patients with PCOS treated with either CC alone or in combination with myoinositol. The present study sought to provide an updated overview of the topic, potentially contributing to evidence-based decision-making in the clinical management of PCOS cases in terms of reproductive outcome.
Materials and methods
Review design
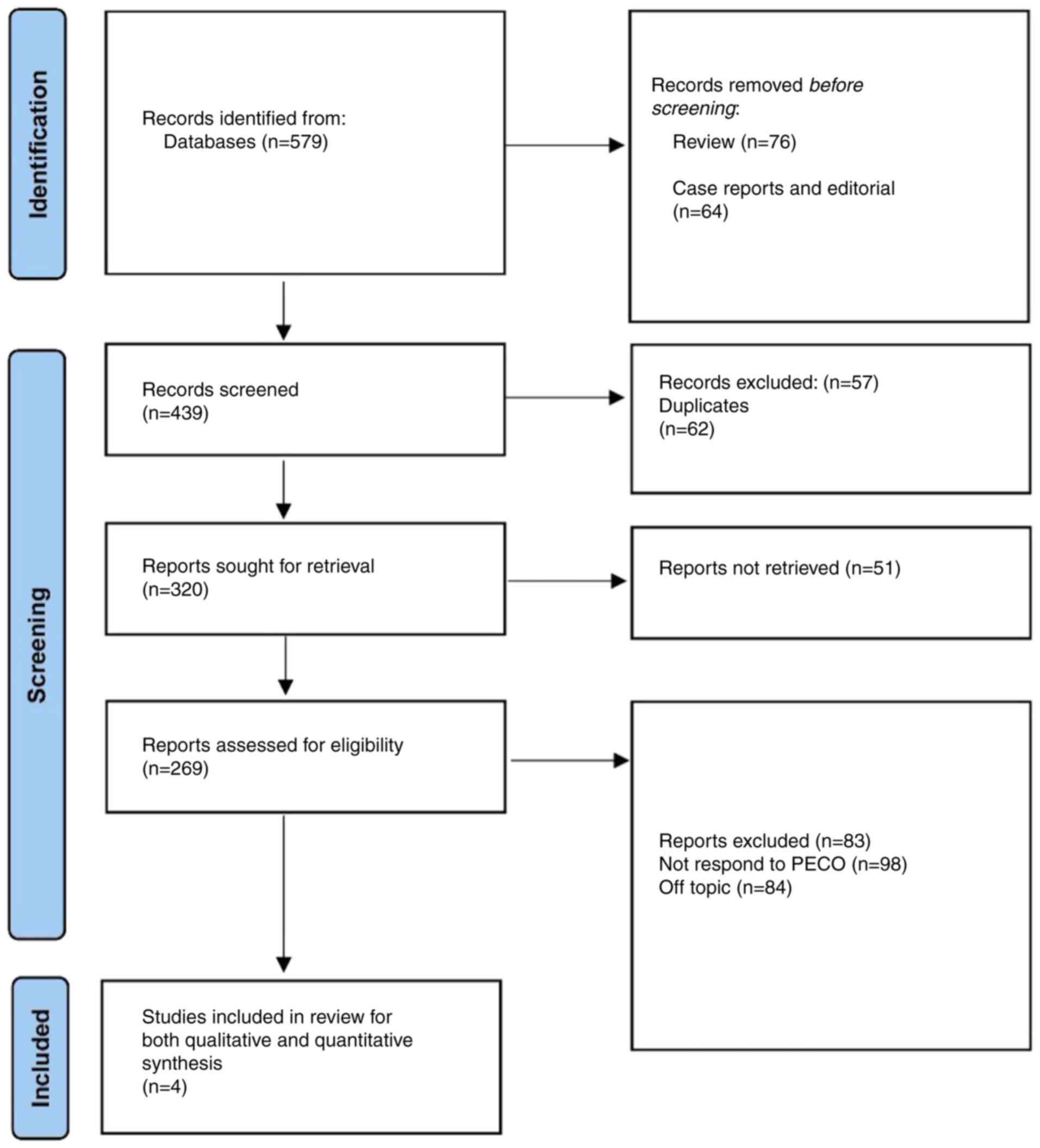
The PRISMA protocol (15) was utilized in conducting the present review, with the study selection process presented in Fig. 1. The aim of the present study was to assess the impact of these strategies on conception rates in patients with PCOS. The intention was to provide a comparative assessment, thereby allowing for a more informed choice whilst managing infertility associated with PCOS. The central research question in the present study was the relative efficacy of myoinositol, either used alone or in combination with CC, in increasing the conception rates in patients with PCOS.
Search protocol
To conduct the present study, a specific set of key words was used to streamline the search to ensure the retrieval of the most relevant articles (Table I). The databases that were searched included PubMed (https://pubmed.ncbi.nlm.nih.gov/) and PMC (https://pmc.ncbi.nlm.nih.gov/articles/PMC3049418/), as well as Google Scholar (https://scholar.google.com/). These databases were selected as they are comprehensive and widely used sources of scientific literature that include a range of peer-reviewed articles and research studies across multiple disciplines. The present meta-analysis was registered in INPLASY (NPLASY Protocol 5559; https://inplasy.com/inplasy-2023-11-0112/).
Selection criteria
The present study included studies on patients of reproductive age already diagnosed with PCOS and presenting with infertility, menstrual regulation, hirsutism and/or obesity. The exclusion criteria were studies on females presenting with infertility due to reasons other than PCOS, those with other hormonal conditions (such as hypothyroidism or hyperprolactinemia) and couples with male factor infertility.
The literature search was limited to articles published within the past 10 years (from January, 2013 to November, 2023). This timeframe was selected to ensure the inclusion of the most recent and relevant studies, reflecting up-to-date research and perspectives on the treatment strategies for patients with PCOS. The present study outcomes or endpoints focused on whether myoinositol either when used alone or in combination with CC was more effective in increasing the fertility rates in patients with PCOS. Additionally, the present study aimed to assess whether myoinositol could exert secondary beneficial effects, such as weight reduction on the symptoms of PCOS.
Data extraction protocol
The data extraction protocol for the present systematic review was developed and implemented to have reliable and valid study findings. This process was initiated with the development of a standardized data extraction form to capture all necessary information from the included studies. The form was designed to capture details pertaining to the study design, sample size, participant characteristics, interventions and comparators, outcome measures, key findings and any potential sources of bias. Each study was independently assessed by two authors (ZS and MMM) who extracted the data.
The information extracted included the authors, year of publication, study design, population characteristics (including age, BMI and PCOS diagnostic criteria), interventions (specifically, myoinositol and CC use) and outcomes (conception rate, ovulation rate and secondary PCOS symptoms). The authors performed discussions to resolve any discrepancies in data extraction. Where necessary, a third author was consulted to reach a consensus. To ensure the consistency of the data extraction process, an inter-rater reliability test would be conducted. Cohen's κ-statistic was used to measure the degree of agreement between the two reviewers. The κ-value was calculated to be 0.85, indicating a high level of agreement between the reviewers. This robust value reassured the reliability of the data extraction process.
Assessment of bias
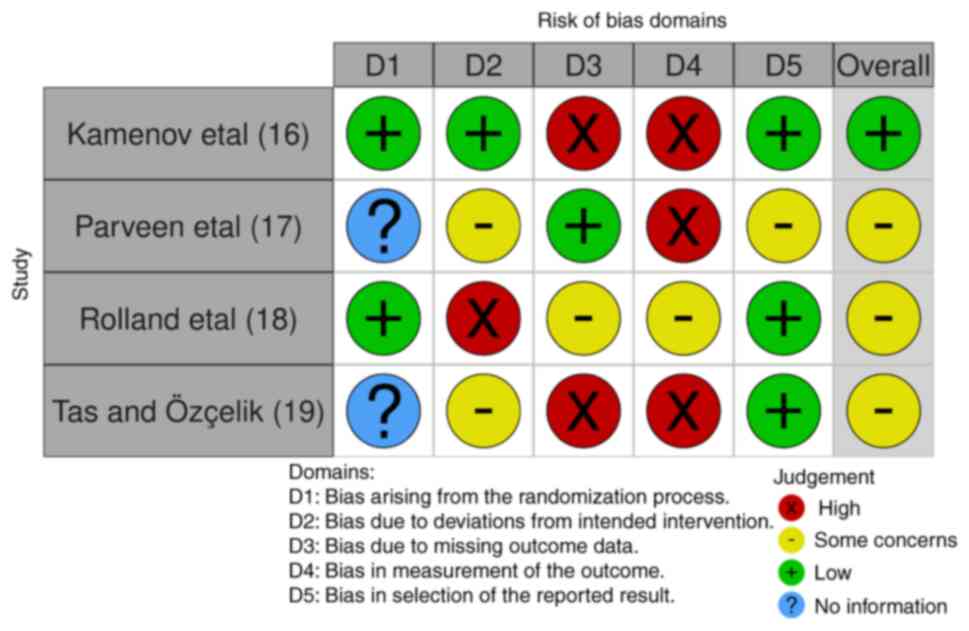
Bias assessment was performed using the Cochrane Collaboration's Risk of Bias 2.0 (RoB 2.0) tool (16). This instrument is widely recognized for its robustness in evaluating the risk of bias in randomized controlled trials and is available on the Cochrane website (Fig. 2).
Meta-analysis protocol and statistical analysis
The meta-analysis protocol was meticulously designed and implemented using Review Manager 5 (version 5.4.1), a software developed by the Cochrane Collaboration specifically for performing meta-analyses. The primary focus of the present meta-analysis was to compare the efficacy of myoinositol to CC in terms of improving ovulation and pregnancy rates in patients with PCOS. For each included study, the odds ratios (ORs) and their respective 95% confidence intervals (CIs) were extracted or calculated from the reported data. These ORs represented the odds of successful ovulation and pregnancy in the myoinositol group compared with those in the CC group. To synthesize these results, a random-effects model was applied for each meta-analysis. The results of the meta-analysis were visually represented through forest plots, which were generated using Review Manager 5. Each plot revealed the OR and 95% CI values of each study, in addition to the pooled effect estimate and its 95% CI. The heterogeneity among the included studies was assessed using the I2 statistic.
Results
Article filtering protocol
A total of 579 records were identified from the various databases. Based on the predetermined criteria, 64 records that were not deemed appropriate for inclusion into the present study, including case reports and editorials, as well as another 76 reviews, were excluded. All the records found were in the English language; thus, no study was excluded due to language. The remaining 439 records subsequently underwent a preliminary screening procedure. In total, 62 duplicate entries were found and were therefore excluded. Another 57 records were excluded as the full texts for these articles were not found. As a result, 320 records were recognized as potentially fitting the inclusion criteria for further analysis. However, 51 of these studies could not be retrieved for a thorough analysis. The admissibility of the remaining 269 reports was then determined based on their applicability to the Population, Exposure, Comparator and Outcome (PECO) issue and the subject of the systematic review. A total of 83 reports were ultimately removed at this point; in addition, a total of 98 reports failed to respond to PECO and 84 were found to be off-topic. A final set of four articles (17-20) were determined to be eligible for inclusion into the present study following this meticulous screening (Table II). The two authors (ZS and MMM) analyzed these studies and overall, two studies were deemed to be of low risk and two studies yielded an unclear score for bias (Fig. 2). The results of are presented in Table II.
Efficacy
From the selected studies, an analysis of the comparison of myoinositol and metformin, both when combined with CC, revealed that the metformin group was associated with a higher ovulation rate (18). Although the pregnancy rate was slightly higher in the metformin + CC group, this difference was not statistically significant. Notably, there were more reported side-effects in the myoinositol group. The addition of myoinositol to CC treatment appeared to have significantly enhanced the ovulation rate in another cohort of patients eligible for ovulation induction (19). Although the pregnancy rate was also higher in the combination therapy group, these differences were not statistically significant, suggesting the need for further investigations. In obese patients with PCOS with infertility (20), co-treatment with myoinositol and CC led to increased endometrial thickness and a higher number of mature follicles compared with those in the CC-alone group, suggesting potential benefits for fertility. Although the ovulation rates were similar in both groups, the pregnancy rate was higher in combination group; however, the results were not statistically significant (20).
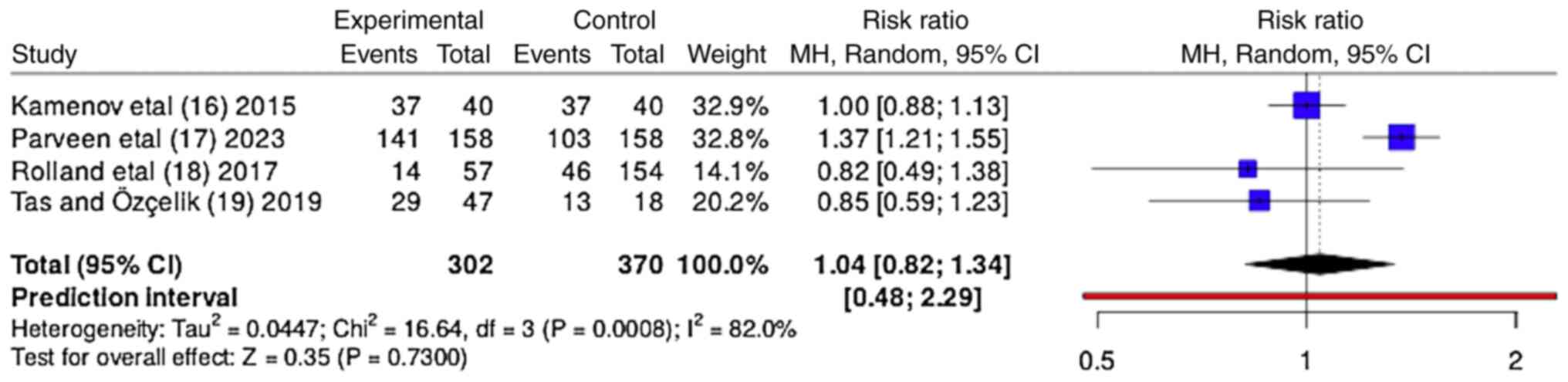
The forest plot presented in Fig. 3 provides a visualization of the OR for the efficacy of myoinositol in improving ovulation rates compared with CC. The forest plot indicates a lack of conclusive evidence for the superiority of myoinositol over CC in improving ovulation in patients with PCOS, with significant heterogeneity observed among the studies. When the results from all four studies were pooled, the total OR value was 1.04 (95% CI, 0.82-1.34), suggesting a modestly higher likelihood of improved ovulation with myoinositol compared with CC; however, the wide confidence interval crosses 1, indicating that the difference was not statistically significant. The heterogeneity among the studies was significant, with an I² value of 82%, indicating substantial variability in the effects of myoinositol on ovulation across the studies. This may be due to the differences in study design, patient characteristics or other factors. The τ² value was 0.047, further reinforcing the presence of significant heterogeneity. The overall Z-test did not detect a significant difference between myoinositol and CC in terms of ovulation (Z=0.35; P=0.7300), suggesting that the observed differences could have occurred by chance.
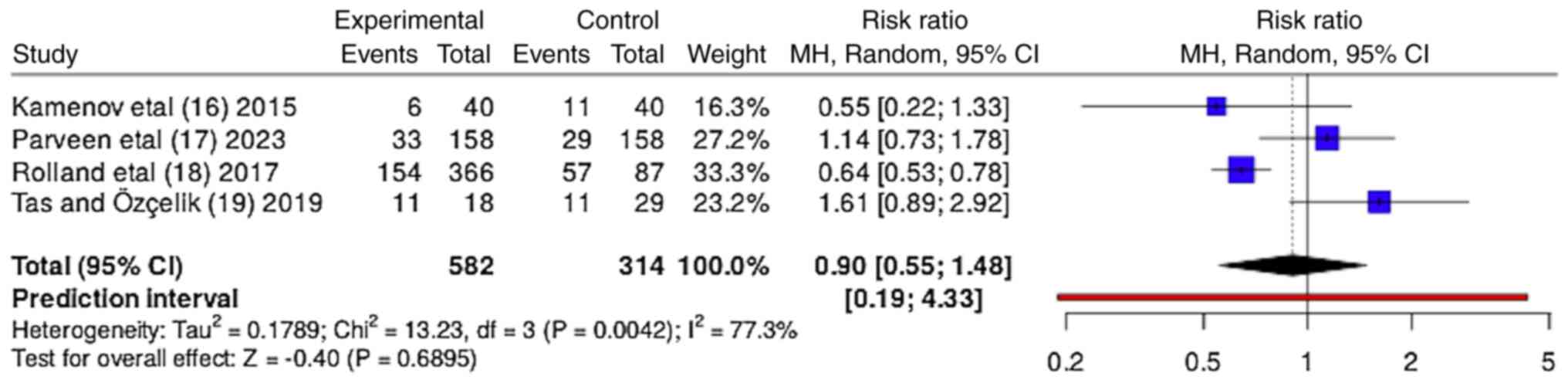
The forest plot presented in Fig. 4 compares the OR value of the efficacy of myoinositol in improving pregnancy rates compared with CC. The forest plot suggests that there is no conclusive evidence to support the superiority of myoinositol over CC in improving pregnancy rates in patients with PCOS, with significant heterogeneity observed among the studies. When all four studies were aggregated, the pooled OR value was 0.9 (95% CI, 0.55-1.48), suggesting a lower likelihood of achieving pregnancy with myoinositol compared with CC. However, the CI spanned 1, indicating that this difference was not statistically significant. The heterogeneity among the studies was moderately high, with an I² value of 77%, indicating considerable variability in the effects of myoinositol on pregnancy rates across the studies. This could be attributed to differences in study design, patient populations or other factors. The τ² value was 0.1789, further emphasizing the presence of heterogeneity. The Z-test for the overall effect was not statistically significant (Z=0.40; P=0.6895), indicating the possibility that the observed differences could have occurred by chance.
Secondary outcomes
The selected studies (17-20) collectively evaluated the effects of myoinositol, either alone or in combination with CC, on various parameters, such as ovulation and pregnancy rates, BMI, homeostatic model assessment index (HOMA) and adverse events, in patients with PCOS (Table II). In these studies, myoinositol demonstrated potential benefits for individuals with PCOS. In particular, in anovulatory patients with PCOS and insulin resistance (17), treatment with myoinositol appeared to stimulate ovulation and aid pregnancy. Additionally, it was associated with reductions in BMI and HOMA index, suggesting improvements in weight control and insulin resistance (Table II).
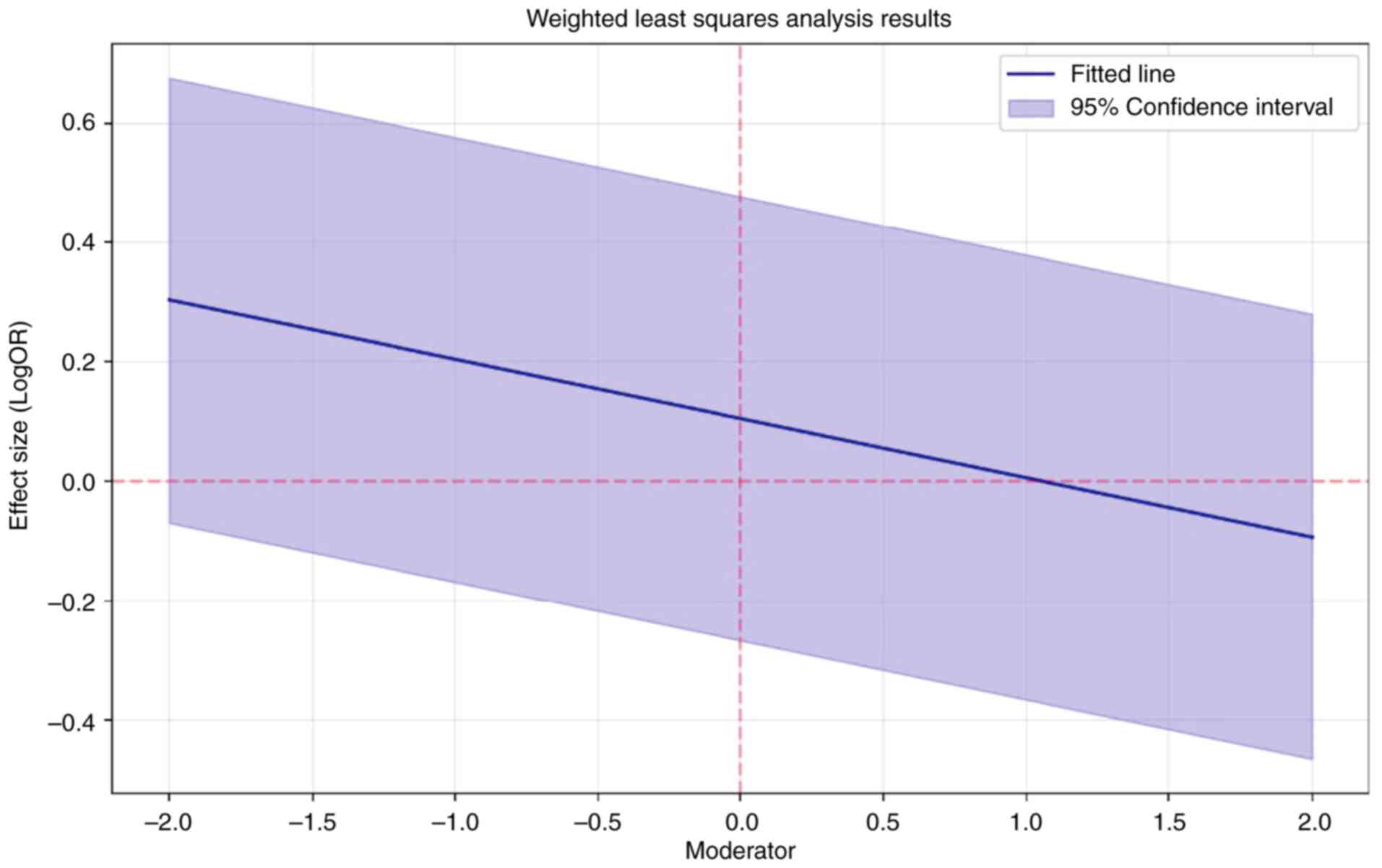
To explore potential sources of heterogenicity, weighted meta-regression analysis was performed, which revealed R2=0.12, indicating that 12% of the variability in effect sizes is explained by the moderator variable. The results were not statistically significant (P=0.654). The moderator had a small negative effect of-0.099; however, it was not significant (P=0.654). The B was 0.105 and best power weighting was 2 (Fig. 5).
Discussion
The present systematic review investigated the use of myoinositol and CC for enhancing conception rates in patients with PCOS. However, the relative efficacy of these two treatments remains ambiguous. The pooled OR value revealed that the difference in conception rates between myoinositol and CC was not significant. However, myoinositol treatment was associated with enhancements in other parameters, such as weight control and insulin resistance. The combined use of both treatments was not found to significantly elevate conception rates compared with the use of CC alone, although some studies observed trends towards increased pregnancy rates (19,20) The included studies examined exhibited significant heterogeneity.
According to Kamenov et al (16), myoinositol resulted in ovulation in 61.7% of the patients and a pregnancy rate of 37.9% among the ovulatory women. Notably, among the patients who were resistant to myoinositol, subsequent treatment with CC led to ovulation in 72.2% of the patients, of whom 42.6% became pregnant. These results underscored the potential of myoinositol to improve the ovarian response in patients with PCOS. Notably, their study also observed a reduction in both the BMI and HOMA index, highlighting the role of myoinositol in ameliorating insulin resistance and controlling body weight (17).
Parveen et al (17) compared the efficacy of metformin and myoinositol in inducing ovulation in patients with PCOS. The ovulation rate was found to be higher in the metformin group (44.6%) compared with that in the myoinositol group (32.6%). The pregnancy rates were slightly lower with myoinositol (18.4%) compared with those in the metformin (21.1%) group, though the differences were not statistically significant. Side-effects were minimal and predominantly occurred in the myoinositol group, suggesting that metformin could be a more effective first-line therapy with fewer adverse effects (18).
Rolland et al (18) further explored the synergistic effects of myoinositol and CC in patients with PCOS. They reported a significantly higher ovulation rate with the combination of myoinositol and CC (65.5%) compared with that in CC alone (42%). The cumulative pregnancy rate was also higher in the combination group, albeit not statistically significant due to the small sample size. Their study reported the advantage of the use of myoinositol in combination with CC for ovulation induction; however, there is a need for further studies using large sample sizes to validate these findings (19).
Taş and Özçelik (19) observed the effects of myoinositol in combination with CC in obese and infertile patients with PCOS. The combination therapy led to a significantly higher thickness of the endometrium and the number of mature follicles, critical for successful ovulation and pregnancy. However, the ovulation rates between the two groups were comparable. A statistically non-significant trend towards higher pregnancy rates was nevertheless observed in the combination therapy (20).
PCOS is responsible for the majority of cases of infertility secondary to anovulation (21). The coexisting endocrine and metabolic aberrations in patients with PCOS can potentially exacerbate uterine dysfunction, leading to anomalous endometrial cell proliferation, and chronic low-grade inflammation thereby posing implantation difficulties (22). Ovulation induction and assisted reproductive treatments also pose a challenge in these women as there is a higher risk of miscarriage in women suffering from obesity with PCOS and undergoing ovulation induction. Therefore, weight reduction prior to treatment for ovulation induction should be considered (23).
Furthermore, the quality and maturity of oocytes in PCOS compromises the results of assisted reproduction technology (24). Siristatidis et al (25) recommended the in vitro maturation of oocytes prior to in vitro fertilization to achieve a high clinical pregnancy rate (25). Myoinositol regulates the menstrual cycle and improves metabolic conditions and thus induces ovulation. Therefore, it may have the potential to improve clinical pregnancy (26).
CC is a selective estrogen receptor modulator that is the most widely used therapeutic agent for infertility globally. Whilst CC yields ovulation rates of ~85%, the pregnancy rates remain at 40-50% (27). This disparity is due to endometrial atrophy and thickening of cervical mucous caused by CC (28). Myoinositol has exhibited promising effects in improving fertility outcome in patients with PCOS who received gonadotrophins combined with IUI (29). Another previous study demonstrated that combined treatment with myoinositol and D-chiro inositol significantly reduced ‘LH, free testosterone, fasting insulin and HOMA index, whilst increasing 17-β-estradiol’ levels, in young overweight patients with PCOS, thereby promoting the use of myoinositol for reproductive performance (30,31). It also improves the ovulation rate and quality of oocyte (32). Given these findings, it is conceivable that integrating myoinositol with CC may enhance pregnancy outcomes achieved with CC alone, by mitigating the peripheral anti-estrogenic effects of CC.
Although the present systematic review and meta-analysis provided insight into the comparative efficacy of myoinositol and CC in enhancing conception rates among patients with PCOS, it has certain limitations. The number of studies incorporated into the meta-analysis was minimal, with only four studies meeting the inclusion criteria. This limited sample size potentially restricted the power of the study and may have affected the reliability and generalizability of the findings. Additionally, the total number of participants across the studies was relatively small, which may further limit the statistical power and the ability to detect significant differences between treatment groups. In addition, significant heterogeneity was observed among the included studies. This heterogeneity could arise from differences in study design, patient populations, treatment protocols or outcome measurements, which can confound the results and make interpretation a challenge. The application of the random-effects models in the analysis attempted to mitigate this issue; however, it cannot completely eliminate the potential biases introduced by heterogeneity. The present study also focused on the conception rates as the primary outcome. Although myoinositol was found to mediate beneficial effects on weight control and insulin resistance, these were secondary outcomes and may not have been as comprehensively assessed across all studies. Therefore, the true potential of myoinositol in improving these other aspects of PCOS may not be fully captured in this analysis.
Future research is thus required to address these limitations by incorporating larger sample sizes and more standardized study designs. This would enhance the robustness of the findings and provide clearer insight into the optimal therapeutic strategies for the management if PCOS-related infertility. Additionally, exploring the long-term effects of myoinositol and its potential benefits on metabolic parameters in a broader PCOS population would be valuable for developing comprehensive treatment protocols.
The quantitative and qualitative analysis undertaken in the present review presented no significant difference in conception rates between the use of myoinositol and CC. Additionally, the combined administration of both treatments did not significantly enhance conception rates in comparison to CC alone. However, the research did unveil an association between myoinositol treatment and improvements in auxiliary parameters, such as weight control and insulin resistance. These findings underscore the potential benefits of myoinositol beyond conception rates in the management of PCOS. Therefore, it would be pertinent to conclude that whilst myoinositol, alone or in combination with CC, did not significantly augment conception rates compared with CC alone, it may provide additional health benefits for patients with PCOS. This underlines the complex nature of PCOS and the need for a comprehensive treatment approach that addresses not only fertility issues, but also other health concerns associated with this condition. These findings, however, need to be interpreted with cognizance of the inherent limitations of the study, notably the small number of studies and participants, the significant heterogeneity among the included studies and the focus on conception rates as the primary outcome. The necessity for further research, particularly robust, large-scale and homogeneous studies, to confirm these findings and determine the most effective treatment strategy for patients with PCOS seeking conception is therefore evident.
The clinical implications of the present systematic review and meta-analysis are multifaceted, particularly given the prevalence and complexity of PCOS. The present study provides insight for healthcare providers, particularly for those involved in the management of infertility in patients with PCOS. The present study found no significant difference in conception rates between myoinositol and CC when used individually or in combination. This indicates that myoinositol alone may not provide a superior alternative to CC in enhancing conception rates for patients with PCOS. For clinical practice, this suggests that while myoinositol can be considered as an adjunct treatment, it should not be relied upon solely for achieving higher conception rates compared with CC. Physicians should continue using CC as the first-line treatment for ovulation induction in PCOS.
Although the combination of myoinositol and CC did not significantly improve conception rates compared with CC alone, a number of studies (13,16,19) did indicate a trend towards higher pregnancy rates with combination therapy, suggesting potential benefits that warrant further investigation. Clinicians may consider using combination therapy in specific patient populations, particularly those who do not respond adequately to CC alone. However, this approach should be based on individual patient characteristics and response to treatment.
Myoinositol was also found to be associated with improvements in various secondary parameters, such as weight control and insulin resistance, as evidenced by reductions in BMI and HOMA index. These findings support the use of myoinositol for its metabolic benefits in patients with PCOS. Clinicians should consider prescribing myoinositol to improve overall metabolic health, which can indirectly support fertility treatment outcomes.
The present study highlighted minimal side-effects associated with myoinositol compared with CC. This is a significant consideration for patient tolerability and long-term adherence to treatment. In clinical practice, myoinositol may be preferred for patients who experience adverse effects from CC or those with a higher risk of metabolic syndrome, since it offers a safer profile with added metabolic benefits.
The present review synthesized currently available evidence on the comparative effectiveness of myoinositol and CC, filling a gap in the literature regarding their relative efficacy in treating infertility in patients with PCOS. It provided a comprehensive overview of the available studies, helping to inform clinical decision-making. However, the significant heterogeneity observed among the included studies emphasizes the variability in study designs, patient populations and treatment protocols. This finding underscores the need for standardized protocols in future research to enable more consistent and comparable results. Future studies are required to focus on conducting large-scale, well-designed randomized controlled trials to confirm the findings of the present meta-analysis. These studies should aim to reduce heterogeneity by using standardized patient selection criteria, treatment protocols, and outcome measures. In addition, future studies should also investigate the long-term outcomes of myoinositol and CC treatment, including not only conception rates but also live birth rates, pregnancy complications and long-term metabolic health of mothers and offspring.
Given the trend towards higher pregnancy rates with combination therapy, further research should explore the optimal dosage, timing and patient populations for combined myoinositol and CC treatment. Understanding the mechanisms underlying the potential synergistic effects could also provide deeper insights.
Future studies are required to explore personalized medicine approaches, identifying patient subgroups who may benefit the most from myoinositol, CC or their combination. Genetic, metabolic and phenotypic profiling could help tailor treatments to individual patient needs.
The present study used a robust and systematic approach using PRISMA protocol for transparency. The application of the Cochrane Collaboration's Risk of Bias 2.0 (RoB 2.0) tool provided a systematic and standardized method for evaluating potential biases in the included studies, ensuring a thorough and objective assessment. However, the present meta-analysis was limited to four studies that fitted the inclusion criteria, limiting the sample size and restricting the statistical power of the study. There was substantial heterogenicity among the included studies. While the study highlighted a degree of benefit in administering myoinositol in terms of weight control and insulin resistance, these secondary outcomes were not the primary focus of the present study. Additional comprehensive studies are required to explore these aspects fully.
In conclusion, whilst the present systematic review and meta-analysis provided valuable insight into the relative efficacy of myoinositol and CC in enhancing conception rates among patients with PCOS, the findings should be interpreted with caution due to the inherent limitations. The necessity for further research, particularly robust, large-scale and homogeneous studies, is evident to confirm these findings and determine the most effective treatment strategy for patients with PCOS seeking conception.
In conclusion, the present study used a random effects model, and conducted meta-regression analysis; however, heterogenicity could not be eliminated. This may be due to different study protocols, study populations and intervention protocols. Therefore, it is recommended that the findings be interpreted with caution and the pooled estimate taken as indicative and not definite. This highlights the need for further uniform protocol studies on this topic in the future.
Acknowledgements
The authors would like to acknowledge the services of Miss Hafsa Intikhab, an IGCSE student, at Pakistan International School, Al-Qassim, Buraydah, Saudi Arabia for volunteering to assist with the typing and formatting of this manuscript.
Funding
Funding: The present study was supported by the Deanship of graduate studies and Scientific research at Qassim University for financial support (grant no. QU-APC-2025).
Availability of data and materials
The data generated in the present study may be requested from the corresponding author.
Authors' contributions
ZS and MMM designed the study, analyzed the data and drafted the manuscript. MM collected the data from the included studies, spell-checked and formatted the manuscript. All authors revised, and have read and approved the final manuscript. ZS and MMM confirms the authenticity of all the raw data.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
|
Bellver J, Rodríguez-Tabernero L, Robles A, Muñoz E, Martínez F, Landeras J, García-Velasco J, Fontes J, Álvarez M, Álvarez C, et al: Polycystic ovary syndrome throughout a woman's life. J Assist Reprod Genet. 35:25–39. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Tanbo T, Mellembakken J, Bjercke S, Ring E, Åbyholm T and Fedorcsak P: Ovulation induction in polycystic ovary syndrome. Acta Obstet Gynecol Scand. 97:1162–1167. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Palomba S, Falbo A, Russo T and Zullo F: Ovulation induction in anovulatory patients with polycystic ovary syndrome. Current Drug Therapy. 1:91–105. 2006. | |
|
Escobar-Morreale HF: Polycystic ovary syndrome: Definition, aetiology, diagnosis and treatment. Nat Rev Endocrinol. 14:270–284. 2018. | |
|
Zahra M, Shah M, Ali A and Rahim R: Effects of metformin on endocrine and metabolic parameters in patients with polycystic ovary syndrome. Horm Metab Res. 49:103–108. 2017.PubMed/NCBI View Article : Google Scholar | |
|
Morley LC, Tang T, Yasmin E, Norman RJ and Balen AH: Insulin-sensitising drugs (metformin, rosiglitazone, pioglitazone, D-chiro-inositol) for patients with polycystic ovary syndrome, oligo amenorrhoea and subfertility. Cochrane Database Syst Rev. 11(CD003053)2017.PubMed/NCBI View Article : Google Scholar | |
|
Cataldo NA, Barnhart HX, Legro RS, Myers ER, Schlaff WD, Carr BR, Diamond MP, Carson SA, Steinkampf MP, Coutifaris C, et al: Extended-release metformin does not reduce the clomiphene citrate dose required to induce ovulation in polycystic ovary syndrome. J Clin Endocrinol Metab. 93:3124–3127. 2008.PubMed/NCBI View Article : Google Scholar | |
|
Chen X, He S and Wang D: Effects of metformin on body weight in polycystic ovary syndrome patients: Model-based meta-analysis. Expert Rev Clin Pharmacol. 14:121–130. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Abu Hashim H: Twenty years of ovulation induction with metformin for PCOS; what is the best available evidence? Reprod Biomed Online. 32:44–53. 2016.PubMed/NCBI View Article : Google Scholar | |
|
Malik M, Tasnim N and Mahmud G: Effect of metformin alone compared with metformin plus simvastatin on polycystic ovarian syndrome in Pakistani women. J Coll Physicians Surg Pak. 28:184–187. 2018.PubMed/NCBI View Article : Google Scholar | |
|
DiNicolantonio JJ and O'Keefe JH: Myo-inositol for insulin resistance, metabolic syndrome, polycystic ovary syndrome and gestational diabetes. Open Heart. 9(e001989)2022.PubMed/NCBI View Article : Google Scholar | |
|
Chandrasekaran S and Sagili H: Metabolic syndrome in patients with polycystic ovary syndrome. Obstet Gynaecol. 20:245–252. 2018. | |
|
Caprio F, D'Eufemia MD, Trotta C, Campitiello MR, Ianniello R, Mele D and Colacurci N: Myo-inositol therapy for poor-responders during IVF: A prospective controlled observational trial. J Ovarian Res 8: Article. 8(37)2015.PubMed/NCBI View Article : Google Scholar | |
|
Zain MM, Jamaluddin R, Ibrahim A and Norman RJ: Comparison of clomiphene citrate, metformin, or the combination of both for first-line ovulation induction, achievement of pregnancy, and live birth in Asian women with polycystic ovary syndrome: A randomized controlled trial. Fertil Steri. 91:514–521. 2009.PubMed/NCBI View Article : Google Scholar | |
|
Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, Cates CJ, Cheng HY, Corbett MS, Eldridge SM, et al: RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ. 366(l4898)2019.PubMed/NCBI View Article : Google Scholar | |
|
Kamenov Z, Kolarov G, Gateva A, Carlomagno G and Genazzani AD: Ovulation induction with myo-inositol alone and in combination with clomiphene citrate in polycystic ovarian syndrome patients with insulin resistance. Gynecol Endocrinol. 31:131–135. 2015.PubMed/NCBI View Article : Google Scholar | |
|
Parveen S, Iram N, Saeed BZ, Yaqub U and Niazi NK: Clomiphene supported ovulation induction in subfertile polycystic ovary syndrome women: Role of different insulin sensitizers. Pak Armed Forces Med J. 73(810)2023. | |
|
Rolland AL, Peigné M, Plouvier P, Dumont A, Catteau-Jonard S and Dewailly D: Could myo-inositol soft gel capsules outperform clomiphene in inducing ovulation? Results of a pilot study. Eur Rev Med Pharmacol Sci. 21 (Suppl 2):S10–S14. 2017.PubMed/NCBI | |
|
Taş M and Özçelik B: Does the combination of myo-inositol improve pregnancy outcomes in obese polycystic ovary syndrome women undergoing ovarian stimulation with clomiphene citrate? J Surg Med. 3:707–710. 2019. | |
|
Nakamura Y, Ono M, Yoshida Y, Sugino N, Ueda K and Kato H: Effects of clomiphene citrate on the endometrial thickness and echogenic pattern of the endometrium. Fertil Steril. 67:256–260. 1997.PubMed/NCBI View Article : Google Scholar | |
|
ESHRE Capri Workshop Group. Health and fertility in World Health Organization group 2 anovulatory women. Hum Reprod Update. 18:586–599. 2012.PubMed/NCBI View Article : Google Scholar | |
|
Palomba S, Piltonen TT and Giudice LC: Endometrial function in women with polycystic ovary syndrome: A comprehensive review. Hum Reprod Update. 27:584–618. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Hamilton-Fairley D, Kiddy D, Watson H, Paterson C and Franks S: Association of moderate obesity with a poor pregnancy outcome in women with polycystic ovary syndrome treated with low dose gonadotrophin. Br J Obstet Gynaecol. 99:128–131. 1992.PubMed/NCBI View Article : Google Scholar | |
|
Kumar P, Nawani N, Malhotra N, Malhotra J, Patil M, Jayakrishnan K, Kar S, Jirge PR and Mahajan N: Assisted reproduction in polycystic ovarian disease: A multicentric trial in India. J Hum Reprod Sci. 6:49–53. 2013.PubMed/NCBI View Article : Google Scholar | |
|
Siristatidis CS, Maheshwari A, Vaidakis D and Bhattacharya S: In vitro maturation in subfertile women with polycystic ovarian syndrome undergoing assisted reproduction. Cochrane Database Syst Rev: CD006606, 2013. | |
|
Pundir J, Psaroudakis D, Savnur P, Bhide P, Sabatini L, Teede H, Coomarasamy A and Thangaratinam S: Inositol treatment of anovulation in women with polycystic ovary syndrome: A meta-analysis of randomised trials. BJOG. 125:299–308. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Kousta E, White DM and Franks S: Modern use of clomiphene citrate in induction of ovulation. Hum Reprod Update. 3:359–365. 1997.PubMed/NCBI View Article : Google Scholar | |
|
Massai MR, de Ziegler D, Lesobre V, Bergeron C, Frydman R and Bouchard P: Clomiphene citrate affects cervical mucus and endometrial morphology independently of the changes in plasma hormonal levels induced by multiple follicular recruitment. Fertil Steril. 59:1179–1186. 1993.PubMed/NCBI View Article : Google Scholar | |
|
Emekçi Özay Ö, Özay AC, Çağlıyan E, Okyay RE and Gülekli B: Myo-inositol administration positively effects ovulation induction and intrauterine insemination in patients with polycystic ovary syndrome: A prospective, controlled, randomized trial. Gynecol Endocrinol. 33:524–528. 2017.PubMed/NCBI View Article : Google Scholar | |
|
Benelli E, Del Ghianda S, Di Cosmo C and Tonacchera M: A combined therapy with Myo-Inositol and D-Chiro-Inositol improves endocrine parameters and insulin resistance in PCOS young overweight women. Int J Endocrinol. 2016(3204083)2016.PubMed/NCBI View Article : Google Scholar | |
|
Gerli S, Mignosa M and Di Renzo GC: Effects of inositol on ovarian function and metabolic factors in women with PCOS: A randomized double blind placebo-controlled trial. Eur Rev Med Pharmacol Sci. 7:151–159. 2023.PubMed/NCBI | |
|
Etrusco A, Laganà AS, Chiantera V, Buzzaccarini G and Unfer V: Myo-inositol in assisted reproductive technology from bench to bedside. Trends Endocrinol Metab. 35:74–83. 2024.PubMed/NCBI View Article : Google Scholar |