Impact of artificial intelligence and digital twin technology on cardiovascular disease diagnosis and management challenges and future directions (Review)
- Authors:
- Published online on: June 16, 2025 https://doi.org/10.3892/wasj.2025.363
- Article Number: 75
-
Copyright : © Sharon John et al. This is an open access article distributed under the terms of Creative Commons Attribution License [CC BY 4.0].
Abstract
1. Introduction
Cardiovascular diseases (CVDs) are heart and blood vessel disorders. Among these are hypertension (high blood pressure), coronary artery disease (CAD; expressed as heart attacks), cerebrovascular diseases (such as strokes), heart failure and other vascular-related ailments. CVD comprises heart muscle and circulatory system diseases that supply the heart, brain and other organs (1,2). Despite being a non-communicable disease, CVD continues to be the number one cause of mortality worldwide, as well as the leading cause of high morbidity and mortality rates worldwide (3,4). Hypertension is a chronic condition characterized by consistently elevated arterial blood pressure, which exerts excessive force on the vascular walls. It is often asymptomatic during the early stages, but progressively damages the cardiovascular system, contributing to complications such as left ventricular hypertrophy, atherosclerosis, renal dysfunction, and increased risk of stroke and myocardial infarction. CAD arises due to the accumulation of atherosclerotic plaques within the coronary arteries, leading to restricted blood flow to the myocardium. Clinically, it manifests as angina pectoris and, in the advanced stages, as myocardial infarction (heart attack), which occurs when a plaque ruptures and forms a thrombus, causing complete arterial occlusion. Cerebrovascular diseases, such as ischemic and haemorrhagic strokes, result from disruption of blood supply to the brain. Ischemic stroke is caused by arterial blockage, whereas haemorrhagic stroke is due to vessel rupture. Both can lead to irreversible neurological damage and are often precipitated by longstanding hypertension or atherosclerosis. Heart failure is a clinical syndrome in which the heart becomes unable to pump sufficient blood to meet the metabolic demands of the body. It may arise from chronic hypertension, myocardial infarct, or cardiomyopathies. Affected patients usually present with fatigue, dyspnoea and oedema. Heart failure reduces the quality of life of patients and has a high potential for hospitalization and mortality (5).
CVD is the number one cause of mortality globally. Estimates by the World Health Organization (WHO) indicate that CVDs are the cause of ~17.9 million deaths eachyear, which translates to ~32% of total global deaths (6). Of note, >75% of such deaths occur in low- and moderate-income countries, reflecting intensive differences in access to healthcare and preventive interventions (7). The burden is complemented by those modifiable risk factors such as unhealthy eating habits, physical inactivity, smoking and excessive alcohol consumption. In addition, the incidence of diseases such as hypertension, diabetes and overweight, heart attack and stroke also increases. The Global Burden of Disease Study also reported that global deaths due to CVD increased from 12.4 million in 1990 to 19.8 million in 2022, underscoring the escalating nature of this public health challenge (8).
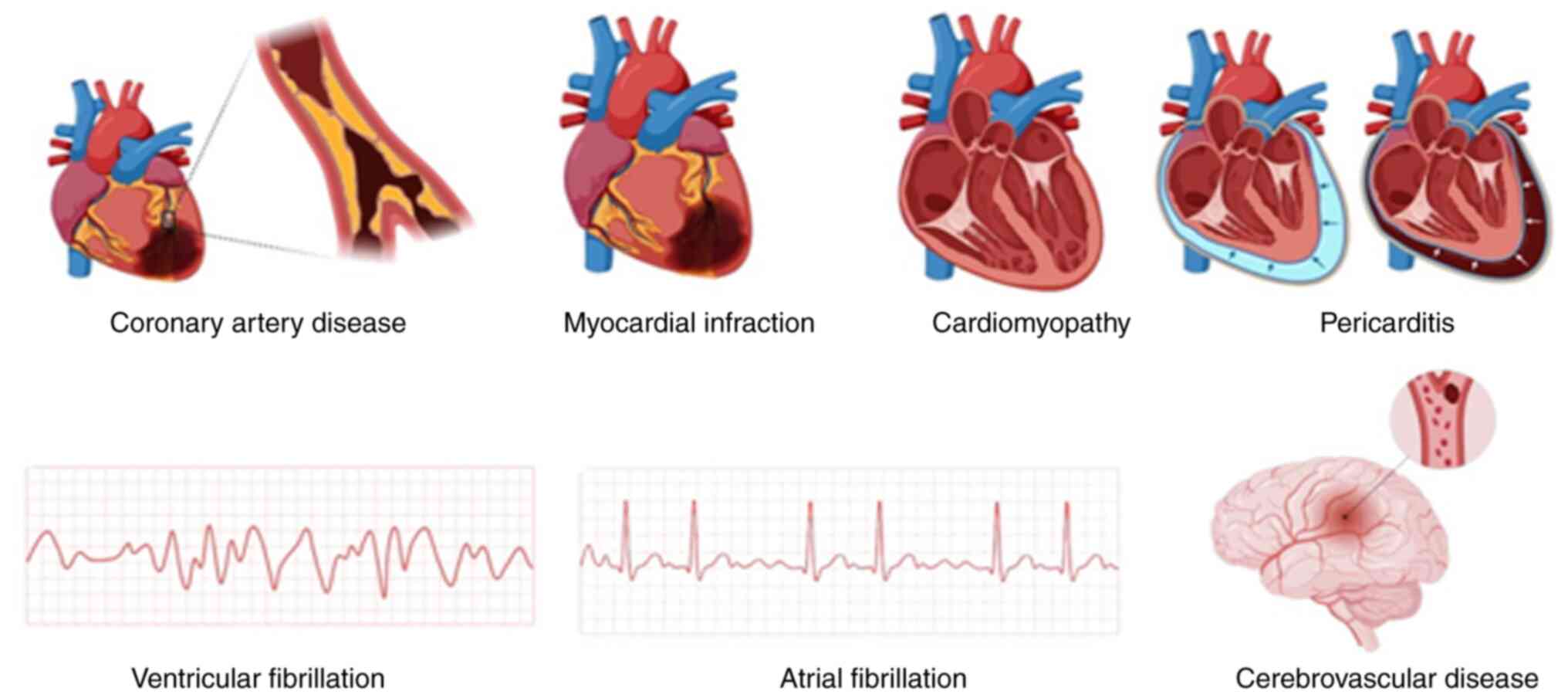
Ischemic heart disease (IHD), stroke, and Congestive heart failure (CHF) collectively contribute to ~80% of CVD-related deaths, while other conditions are presented in Fig. 1. IHD, also known as CAD, is characterized by a low blood supply to the heart muscle due to the narrowing or blockage of coronary arteries. This leads to chest pain (angina) and, if left untreated, can result in myocardial infarction (heart attack). It is the most common form of CVD and a major contributor to sudden cardiac death globally. Stroke is a cerebrovascular disorder that occurs when the blood supply to part of the brain is interrupted or reduced, depriving brain tissue of oxygen and nutrients (9). It can be classified into ischemic stroke (caused by blockage of a cerebral artery) and haemorrhagic stroke (caused by rupture of a blood vessel). Stroke is a main cause of disability and mortality, particularly among the aging population.CHF, also referred to simply as heart failure, is a chronic, progressive disease where the heart cannot effectively pump blood enough to meet the needs of the body. It usually results in conditions, such as IHD and high blood pressure. CHF is characterized by the presence of signs and symptoms such as fatigue, shortness of breath and fluid overload, and an important cause of entry into the hospital among the elderly (10,11).
There is a significant requirement for more accurate, effective and individual clinical and medical strategies with the increasing stress and diversity of heart disease, such as IHD, stroke and heart failure. The conventional risk models are usually inadequate in representing the heterogeneity of CVD presentations and patient outcomes. In the current context, novel technologies, such as artificial intelligence (AI) and digital twin (DT) technology have the potential to transform cardiovascular care by facilitating data-driven, patient-specific treatment. The present review discusses the inclusion of AI and DT technologies within cardiovascular medicine, and their ability to enhance diagnosis, individualize treatment and meet the significant challenges in CVD management.
Genetic aetiology for CVD is currently regarded as being crucial to its pathogenesis, both with monogenic and polygenic input determining the susceptibility of the individual. It has been found that a number of ‘key genes’ have contributed to different CVD conditions. Apolipoprotein E is associated with lipid metabolism and the risk of atherosclerosis, specifically with the ε4 allele, increasing levels of low-density lipoprotein (LDL) cholesterol and contributing to the development of CAD (12). LDL receptor gene mutations are associated with familial hypercholesterolemia, a disease-causing premature atherosclerosis. Proprotein convertase subtilisin/kexin type 9, another gene of lipid metabolism, regulates LDL receptor degradation, and gain-of-function mutations lead to hyperlipidaemia, but loss-of-function variants are protective against CAD (13). In addition, endothelial nitric oxide synthase, which controls vascular tone and endothelial function, is implicated in hypertension and endothelial dysfunction (14). Angiotensin-converting enzyme gene polymorphisms affect blood pressure control and myocardial remodelling, and the I/D polymorphism is most significantly associated with hypertensive heart disease and myocardial infarction (15). Additionally, myosin heavy chain 7 and troponin T2 are critical sarcomeric genes implicated in cardiomyopathies and heart failure. The discovery of these genetic determinants is critical for understanding disease pathophysiology, risk stratification and further developing precision medicine strategies in cardiovascular diseases (16).
Personalized medicine is a strategy aimed at personalizing treatment and interventions tailored to the individual by different factors, such as the genetic makeup, lifestyle habits and environmental exposures of the patient, since these significantly determine outcomes and responses to treatment (16). Personalized medicine in cardiology has immense potential for the identification and treatment of various conditions, such as cardiomyopathies and arrhythmias. Additionally, it can improve drug selection and assist in reducing adverse drug reactions. In the age of personalized medicine, digital twin technology is an innovative strategy with huge potential to transform the delivery of health services (17).
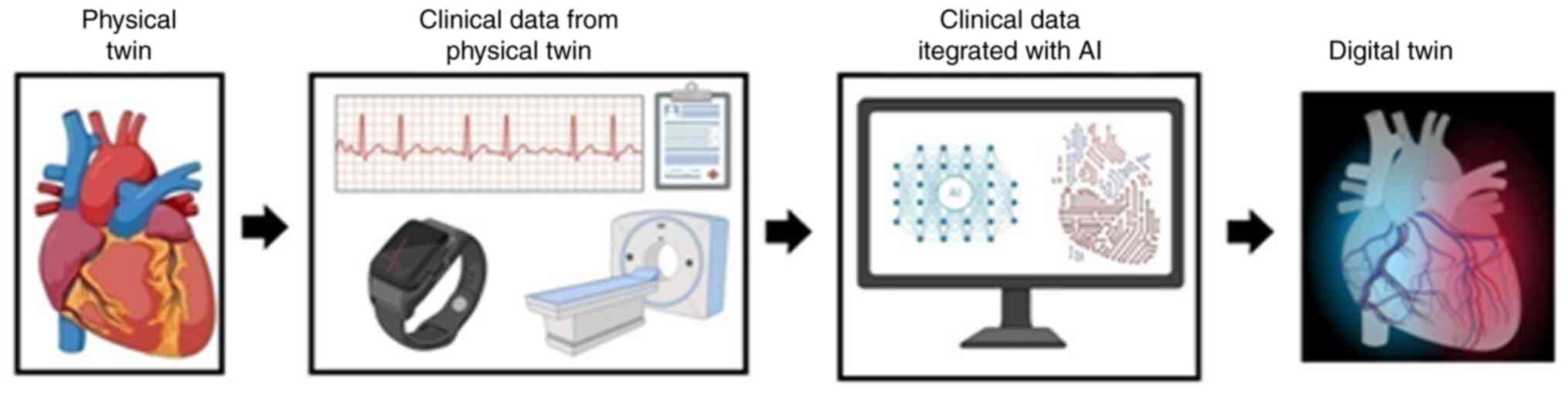
DT technology originated from the aviation industry and is currently applied to numerous industries, such as medicine. A DT a computer model of a real counterparty, used for predicting treatment outcomes and prognosis for the physical twin. DTs provide doctors with a image of the patient's health, making it possible to have personalized treatment plans and interventions. AI-powered DT technology can refine accuracy in the treatment of CVDs (18,19). DT technology is continuously reinforced with information from its physical counterpart. Perhaps the greatest strength of DT technology is their capacity to incorporate and process various datasets, such as wearable devices, genetic information, electronic health records (EHRs) and patient-reported data (20,21).
2. Digital twin technology
Definition and origins of DTs
DT technology is rapidly evolving and is used across numerous industries and domains. A digital twin is a multi-dimensional virtual representation of a physical object or system (real-life twin) that integrates data to enable stimulation, monitoring, optimization and predictive maintenance (22-24). DT technology was first introduced by Michael Grieves in 2002 and was initially applied in the aerospace industry at NASA for product lifecycle management (24,25). Over time, this technology has evolved and diversified across numerous industries, becoming a cornerstone of the Fourth industrial Revolution (Industry 4.0) (26). The integration of cutting-edge technologies, such as internet of things (IoT), AI and big data has driven the growth and widespread application of digital twins across various industries (27,28). The development of DTs for CVDs is illustrated in Fig. 2.
General applications in healthcare and other industries
DT technology has been widely and efficiently employed in various sectors, including healthcare system management, public health and disease management, and personalized medicine. It aids in developing patient-specific models that support accurate diagnosis and tailored treatment planning, thereby advancing the effectiveness of personalized medicine. Additionally, DTs are used to model public health processes, predict disease spread, and managing resources during pandemics (29-33). Furthermore, this technology is employed in urban planning, manufacturing products, logistics and supply chain by optimizing production processes, resource use, and infrastructure modelling, reducing downtime in manufacturing operations and improving urbanization. It also enhances logistics operations by simulating processes, predicting disruptions and optimizing delivery routes (34,35).
A growing body of research is currently applying DT technology across various medical specialties, including cardiovascular medicine. Several case studies have highlighted DT application and potential clinical impact. For instance, researchers from King's College London, Imperial College London, and the Alan Turing Institute created >3,800 anatomically accurate digital heart models using real patient data to examine how age, sex and lifestyle factors affect cardiac function (36). These digital twins are being used to improve treatments and guide drug development. Another case study from the Carle Illinois College of Medicine involved building DTs of patients with heart failure by integrating genomic and proteomic data, enabling personalized simulations of treatment responses (37). At Johns Hopkins University, patient-specific DTs of the heart have been developed to improve the precision of catheter ablation in atrial fibrillation. Additionally, the Turing Institute is working on cardiovascular DTs for patients with pulmonary arterial hypertension to simulate heart and blood flow dynamics for better monitoring and intervention planning. These studies underscore the growing clinical relevance of DT technology in cardiovascular care (38).
Relevance of DTs in cardiovascular medicine
DTs in cardiovascular medicine provide immense potential for advancing personalized care, enhance disease predication, and optimizing clinical decision making. DTs are virtual twins that replicate the anatomy and physiology of an individual's heart and circulatory system. DTs in cardiovascular medicine have exceptional applications including enhanced diagnostic workflows by generating precise replicas to enhance disease phenotyping and diagnostic processes, personalized stimulations and prognostication to predict disease risk and improve procedural planning, clinical decision support and non-invasive planning using Electrocardiographic Imaging (ECGI) to guide interventions such as ablations (34,39,40).
Rationale for applying DTs to CVDs
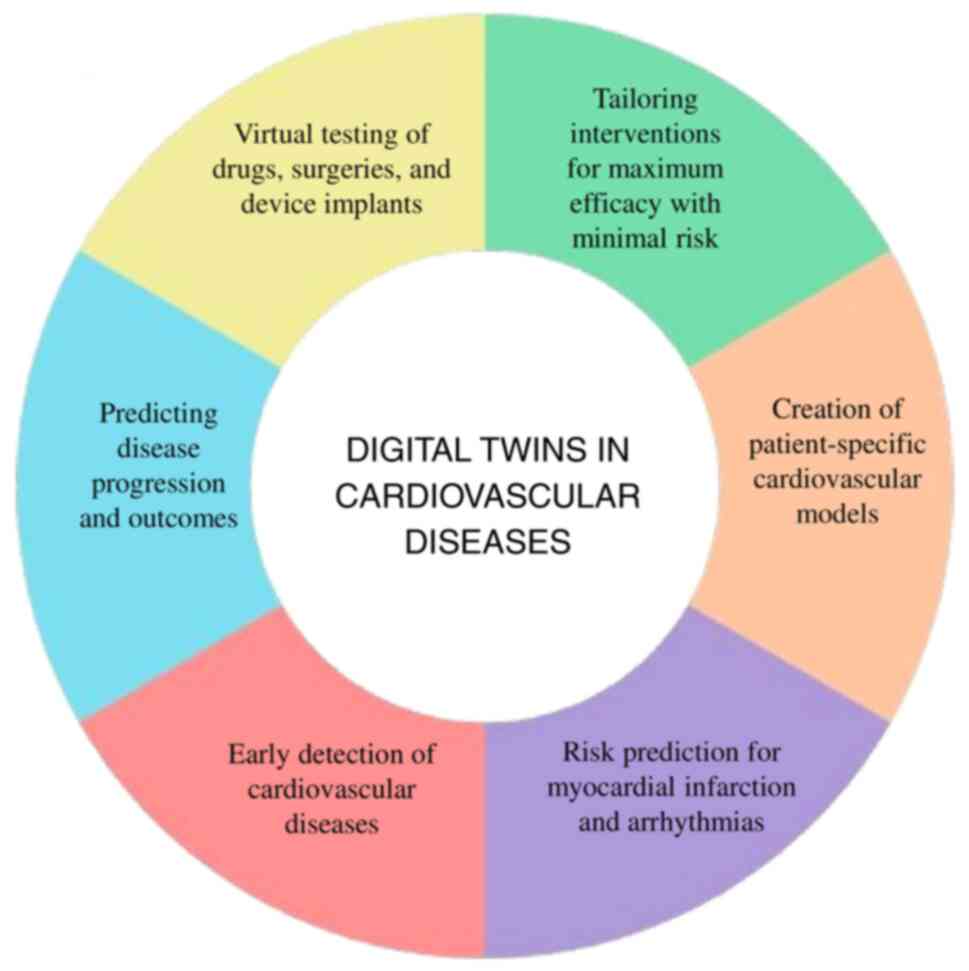
DTs can mimic how cardiovascular systems operate in real-time, facilitating data-driven decision-making and the development of personalized treatment strategies. This technology is essential for tailoring patient-specific solutions and cost-effective treatment, rendering it an asset in the management of CVDs. The integration of big data analytics with DTs will significantly improve the monitoring of cardiovascular health and help anticipate complications before they arise, enabling timely interventions and effective management of CVDs (41,42). DT technology applications in CVD are illustrated in Fig. 3.
Methodology for DT technology in CVDs
The development of a DT for cardiovascular applications begins with the collection of different patient data, including electronic health records, medical imaging, wearable sensor data and genetic information. These data are then pre-developed and integrated through cleaning, harmonization and standardization. High-level modelling, such as physiological modelling and machine learning-based modelling, is utilized to establish a virtual twin (DT) of the cardiovascular system of the patient. The model is calibrated and validated by comparing its predictions with actual patient outcomes. Following validation, the DT is employed for clinical use, such as diagnosis support, risk prediction and personalized treatment planning. Over time, a feedback loop is maintained by updating the model with new patient data to increase accuracy over time (43).
3. Personalized disease modelling
To create patient-specific disease models based on DTs, patient data are originally obtained from different sources, including cardiac imaging [magnetic resonance imaging (MRI), CT scans], ECG data, genetic information and clinical history. This information is combined in computer simulations with the help of sophisticated tools such as AI and machine learning (ML). These technologies provide researchers and physicians with an electronic copy of the cardiovascular system of the patient to model heart functions, predict the development of the disease and use it with customized treatment options. The models are able to change the future with new information, give them dynamic and more accurate (44).
Creation of patient-specific cardiovascular models
DTs are the virtual model that accepts different types of modalities, such as imaging data, genomics data, or physiological monitoring make simulations representing cardiac function and disease progression accurately. The creation of a DT can be categorized into active, passive and semi-active. Thus, the first type of active DT model tracks the systemic circulation continuously at points that can be accessed and updates itself in real-time through the integration of data obtained in real-time. A passive DT describes the model of which creation had been offline, using a set of pre-collected data. A semi-active DT includes elements that exhibit a degree of dynamism. Recent innovations and advances in AI and ML have enhanced the capabilities of DT models, paving the way for real-time patient-specific simulations (45). Automated pipelines can efficiently extract fibre orientations and anatomical annotations of the heart from clinical data, streamlining the process, minimizing manual effort and reducing errors. Patient-specific models can also aid in optimizing procedures, such as cardiac ablation by forecasting long-term recurrence rates based on unique anatomical and physiological features. The integration of these models into broader healthcare systems is being investigated, particularly in relation to collaborative frameworks that could enable a virtual human twin project (46-48).
Risk prediction for myocardial infarction and arrhythmias
Myocardial infarction is defined as the death of myocardial cells due to prolonged ischemia. Worldwide, myocardial infarction presents as a silent killer, taking the lives of patients within seconds in the case that no medical attention reaches the victim. For diagnostic purposes, an ECG can serve to confirm a myocardial infarction, followed by ST-segment shifts or T-wave inversions, and elevated biochemical markers such as cardiac troponin, representing myocardial injury. Together with these, diagnosis is aided by other imaging techniques, such as radionuclide ventriculography, myocardial perfusion scintigraphy employing single photon emission computed tomography, and MRI (49,50). DTs couple clinical data with mechanistic models and data-driven approaches to improve training, optimally with personalized diagnosis and treatment planning. They further refine the inferred precision of myocardial tissue properties by taking cardiac MRI and ECG as examples of multi-modal data, which are instrumental to building accurate models of myocardial infarction (51). Cardiac arrhythmias are irregularities or disruptions in the electrical activity of the heart. DTs allow for the analysis of electrical signals by simulating how arrhythmias develop based on the anatomy and physiology of an individual. DTs have the potential to predict the occurrence and pathways of ventricular tachycardia in patients with ischemic cardiomyopathy, as well as the risk of arrhythmia in patients with myocardial infarction. By evaluating the likelihood of arrhythmias, personalized heart models built from cardiac imaging data can predict future arrhythmic occurrences more accurately than current clinical measurements. This capability could enhance the identification of patients suitable for preventive ablation, ultimately lowering mortality and morbidity rates (40,52-54).
4. Diagnosis and predictive analytics
Early detection of CVDs
DTs create a virtual entity, not only of the physiological conditions of the real twin, but also of environmental and behavioural factors affecting heart health (55). An ECG is a widely used traditional method for diagnosing CVDs. DTs can simulate specific heart diseases using personalized ECG data. This method enhances early diagnosis and intervention by producing high-fidelity, patient-specific ECG signals; DTs improve the sensitivity of cardiac disease detection models (56). The integration of AT and ML with DTs enables real-time patient predictions and dynamic simulations, enhancing disease detection accuracy and clinical outcomes. Advanced techniques are employed to model blood flow and detect conditions, such as abdominal aortic aneurysms, including inverse analysis using recurrent neural networks. This approach enhances the identification and treatment of cardiovascular disorders through on-invasive data analysis (57).
Predicting disease progression and outcomes
DT technology exhibits promising results in predicting disease progression by integrating various data sources. The SynTwin method builds DTs to help precision medicine using network science and synthetic data. The notion that this approach improves clinical endpoint predictions by studying patient similarities and putting such similarities into a network community structure is proved. It demonstrates a higher predictive value with synthetic data than obtaining results directly from real datasets (58). In a previous observational study, a DT of patients with type 2 diabetes mellitus over a period of1year demonstrated significant changes in cardiovascular risk markers, including body weight, QRISK3 scores and A1c values. With personalized health counselling, digital twins can assist patients in migrating into lower-risk categories for cardiovascular conditions (59).
5. Treatment simulation and optimization
Virtual testing of medications, surgeries and implanted devices
DTs have been used to successfully test anti-arrhythmic medications (AADs) on patients with atrial fibrillation, including amiodarone, sotalol, dronedarone, flecainide, and propafenone. Digital twins may be able to assess the effectiveness of different AADs that could lessen the likelihood that atrial fibrillation would return following catheter ablation, according to research. This method opens the door for controlled and economical research into different drug combinations (60). Surgeons can model and plan surgical procedures based on the anatomy of each patient using the digital twin technique. Through simulation, the method enables healthcare professionals to anticipate possible challenges or issues that may arise during the procedure, enabling them to develop plans that lower risks and improve surgical results. In order to evaluate the impact that anatomical variations have on the functioning of cardiovascular devices, DTs are also utilized to model their deployment. This capability helps with medical device design and testing in terms of risk reduction and outcome optimization. DTs use simulation to maximize procedural planning and optimize the diagnostic process. To better understand the features of DTs and how they may be incorporated into clinical practice, further empirical research required (61,62).
Customizing interventions for optimal effectiveness, while minimizing risks
Through modelling how various drugs interact with the cardiovascular system of a patient, clinicians can determine the optimal dosing regimens, while minimizing side-effects. For example, DTs can estimate the way a patient will respond to various antihypertensive agents and thus prescribed pharmacotherapy to optimize blood pressure control without complications (63). DTs provide the benefit of testing intervention in a virtual environment before delivery. This capacity enables strict assessment of all possible treatment effects and risks and thus leads to ensuring clinical practice. DTs can be used to make accurate risk assessments of a variety of possible future health courses based on multiple treatment options. This strong predictive ability is crucial for determining the myriad of factors that can lead to adverse health outcomes, enabling healthcare professionals to anticipate and create strong contingency plans specific to the needs of each individual patient (64).
6. AI techniques integrated with digital twins for CVDs
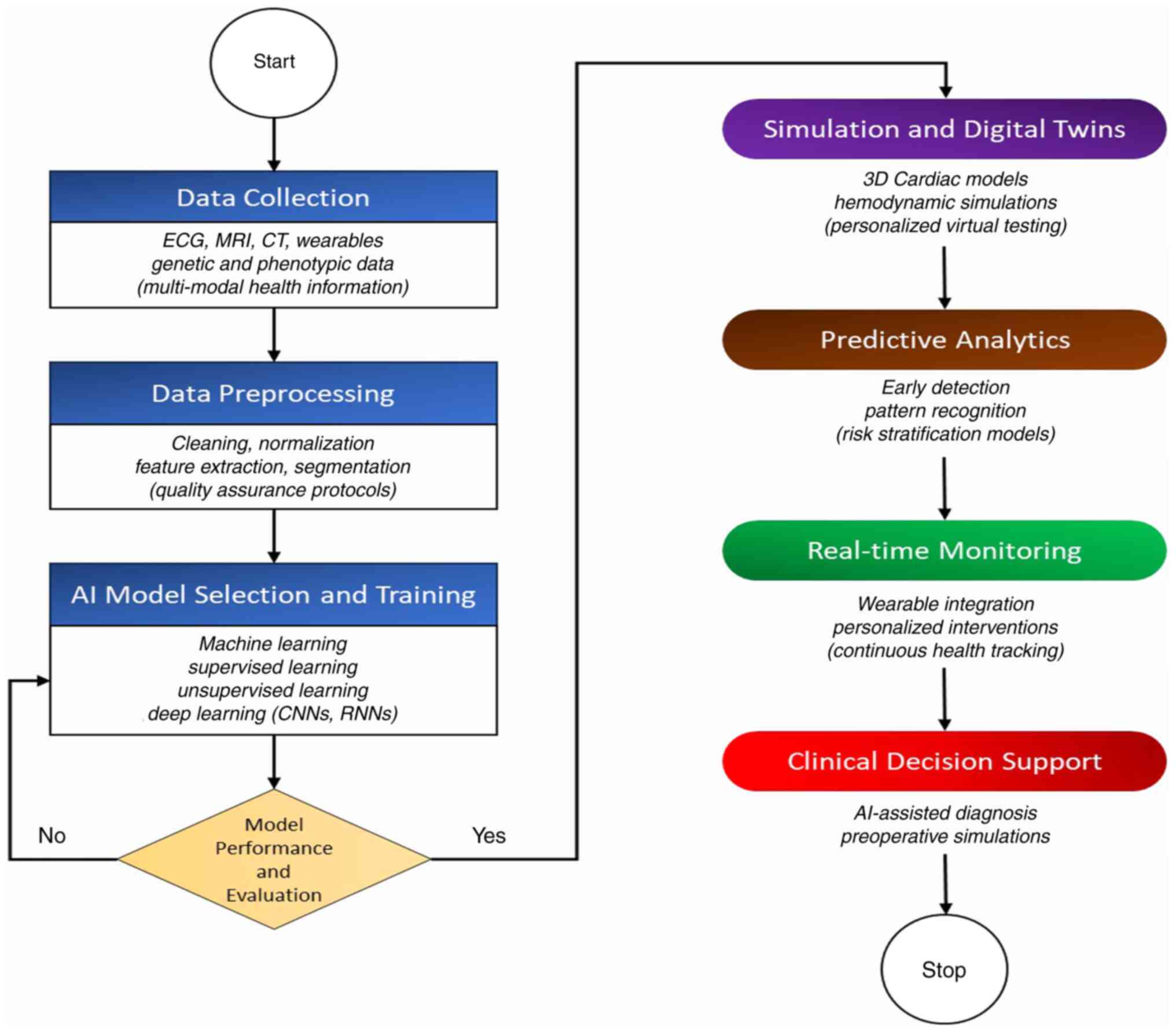
Combining AI methods with DT technology for cardiovascular conditions yields substantial improvements in personalization, predictive analytics and decision-making in healthcare. This integration enables more advanced patient care through the early detection of risk factors, the simulation of disease progression, personalized treatment strategies, adaptive treatment monitoring and preoperative stimulations (65-67). By employing ML and deep learning methods, DTs are set to revolutionize cardiovascular patient care (68). AI powered DTs are automated, providing objective assessments and continuous health monitoring, which can help with early diagnosis and intervention, and reduce the dependence on human interpretation. For instance, the lung-DT framework classifies chest X-rays and tracks lung health in real-time using AI, providing insight that complement traditional diagnostic workflows, but do not replace them (69). DTs are utilized for synthesizing complex clinical data in CVDs to produce personalized models that guide therapy and inform prognosis. In order to support clinical decisions, these models provide a more thorough image of the patient's state by estimating parameters that are otherwise impossible to measure and addressing discrepancies in clinical measurements (70). Current research highlights that DTs can enhance and supplement present healthcare practices rather than completely replace them even though they can automate some diagnostic procedures and increase productivity. They are not considered stand-alone substitutes for conventional diagnostic techniques, but rather instruments to maximize resource allocation, enhance patient outcomes and lower expenses (71).
ML
ML is a branch of AI that focuses on the development of algorithms and statistical models to enable computers to learn from experience and create predictions (72). ML techniques are crucial in speeding up the efficiency of DT models. Through systematic processing and analysis of the huge amounts of data produced by monitoring patients, ML algorithms are able to identify key patterns and insights as a guide and improve clinical decision making. The capability of ML algorithms to predict CVD has been highly promising. Support vector machines and boosting algorithms have shown high predictive power for heart failure, cardiac arrhythmias, coronary artery disease and stroke, with combined area under the curve (AUC) values of 0.92 and 0.93, respectively (73,74). The two major areas of cardiovascular medicine where ML is widely used are echocardiography and electrocardiography, which are used to create predictive models for early disease identification and treatment optimization. These models enable timely interventions by assisting physicians in identifying individuals at higher risk of developing heart failure. The integration of machine learning algorithms with wearable sensors and digital stethoscopes further enhances the identification and treatment of cardiovascular diseases (75).
Supervised learning for predictive modelling
Supervised learning algorithms are trained using input data paired with their respective labels, which allows them to identify and model the underlying associations within the data. Bzdok et al (76) explored the impact of sample size and predictor count on logistic regression, random forests, and other supervised learning algorithms. Baessler et al (77) identified ML-based texture analysis that enabled the diagnosis of myocardial infarction on non-contrast-enhanced cardiac cine MRI, despite the absence of visible signal abnormalities. Ali et al (78) demonstrated the high accuracy of ML algorithms, including random forest (RF), decision tree (DT) and K-nearest neighbors (KNN), in heart disease prediction. Notably, RF, DT and KNN demonstrated exceptional performance on certain datasets, achieving 100% accuracy, sensitivity, and specificity, highlighting their potential for early and accurate heart disease diagnosis (79). Gosling et al (80) demonstrated the feasibility of using coronary CT angiography with fractional flow reserve (CT-FFR) to create DTs of patient-specific coronary arteries. These 3D models, combined with hemodynamic simulations, allow for the non-invasive assessment of coronary artery stenos is and virtual planning of percutaneous coronary interventions, providinga potential paradigm shift in cardiovascular care (80).
Unsupervised learning for pattern recognition and anomaly detection
The fundamental strength of unsupervised learning lies in its ability to autonomously explore data and identify hidden structures without the need for predefined labels or human intervention, rendering it a powerful tool for exploratory data analysis (81). Unsupervised learning can be divided into four categories: Association, auto encoders, clustering and anomaly detection. Finding data points in a dataset that considerably differ from expected or typical behaviour is the goal of anomaly detection (82). A new temporal convolutional network (TCN) auto encoder (TCN-AE) architecture was presented by Thill et al (83) for the automated identification of cardiac arrhythmias in ECG recordings. TCN-AEs reliably identify cardiac arrhythmias by recording the temporal dependencies in heartbeats using dilated convolutions. This innovative method outperformed current methods in arrhythmia diagnosis, exhibiting greater accuracy and efficacy (83). Using a modified DBSCAN (density-based spatial clustering of applications with noise) algorithm with adaptive parameter selection, Nanehkaran et al (84) presented a novel unsupervised method for the diagnosis of heart disease. Their approach effectively distinguishes between healthy and ill individuals by finding patterns in patient data. With a high accuracy of ~95%, their results demonstrated how well this method works to find patterns and anomalies in the patient data (84). Using only normal images for training, Nakao et al (85) successfully implemented an unsupervised auto-encoding generative adversarial network (AE-GAN) based method to detect anomalies, such as cardiomegaly and pleural effusion, in chest radiographs. Unsupervised ML for coronary artery disease was investigated by Flores et al (86) in 2021. Their approach discovered the subgroups hidden in patient by analysing both genetic and phenotypic data. This method may outperform conventional risk stratification models since it offers a more complex understanding of the illness and risk factors (86).
Deep learning
As an advanced development of ML, deep learning uses sophisticated algorithms to simulate human cognitive processes. To analyse data and make inferences, these algorithms create deep neural networks that are modelled after the structure of the human brain and nervous system. The most popular and effective deep learning models in clued convolutional neural networks (CNNs), recurrent neural networks (RNNs) and GANs, each suited for different types of data and tasks (87-89). Deep learning has shown promise in medical imaging, particularly in tasks, such as cardiac image classification, segmentation and anomaly detection with a higher accuracy from cardiac images generated from MRI, CT and ultrasound (90-92). Subramani et al (93) reported 96% accuracy in predicting cardiovascular disease outcomes using a combined deep learning and machine learning approach. Deep learning algorithms are being utilized more in predicting CVDs; however, addressing the biases, particularly for diverse populations remains a critical challenge. These models employ several strategies to reduce bias; however, effectiveness varies and disparities can persist across sex and racial groups. Bias in cardiovascular datasets often arises from imbalanced data, underrepresentation of minority groups, and systematic differences in data collection (94). The strategies to address bias include data balancing using techniques such as the synthetic minority over-sampling technique (SMOTE) and resampling are used to address class imbalance, improving model performance for underrepresented group. Synthetic data generation can supplement scarce patient records, improving model generalizability and performance across diverse populations (95). The use of data from multiple centres and varied populations is recommended to reduce bias and improve model robustness.
CNNs for segmenting and analysing images
CNNs are commonly used to analyse images, detect objects and segments in cardiovascular diseases. CNNs have been applied to various imaging modalities, including X-rays, CT and MRI, and they have been shown to increase the precision and effectiveness of the diagnosis of cardiovascular disease (96). In order to enhance cardiac image segmentation, more complex CNN architectures have been proposed, such as residual convolutional neural networks and dual-stream convolutional neural networks. These models use both intra-slice and inter-slice information to increase the segmentation accuracy of cardiac magnetic resonance (CMR) images. They have demonstrated encouraging outcomes in cardiac anatomy segmentation and disease diagnosis. Deep Dual-Stream Confusion Network Court has increased the accuracy of the cardiac image segmentation in the left and right ventricular cavity and myocardium compared to other techniques (97). The effectiveness of a fully convolutional neural network (FCN) in the automated evaluation of right atrium and left atrium CMR images in both long-axis and short-axis CMR images was demonstrated by Bai et al (98). FCN-based myocardial segmentation in CMR images has been proposed by Romaguera et al (99). Integrating image saliency and shape priors with CNNs has significantly improved segmentation results. Methods improve visual clarity in heart tissue and help with accurate location of cardiac areas, both are necessary for an accurate diagnosis (100,101).
RNNs for prediction and analysing time-series
A class of deep learning algorithms known as RNNs is designed to process sequential data efficiently. The ability to incorporate the memory of previous inputs when processing input sequences is the primary characteristic that sets RNNs apart (102,103). In a new predictive modelling framework for the early detection of heart failure, Choi et al (104) demonstrated the effectiveness of gated recurrent unit (GRU) deep learning techniques (104). An RNN model termed the deep heart failure trajectory model (DHTM) was developed by Lu et al (105) to forecast the long-term course of recurrent heart failure. An RNN-based method for predicting disease onset and risk based on EHR data was introduced by Rasmy et al (106) as the REverse Time AttentIoN (RETAIN) model. Shahi et al (107) proved the efficacy of RNNs in conjunction with echo state networks from reservoir computing in cardiac action potential predictions for at least 15-20 beats. The models, important for the comprehension of arrhythmic conditions, are of high accuracy in forecasting cardiac voltage time series, allowing for potential early intervention strategies (107). RNNs, particulalry bidirectional GRUs, have been used to classify ECG signals for biometric authentication and abnormal cardiovascular rhythm detection with high classification accuracy (108,109). The principal branches of AI utilized in the development and improvement of digital twins for the heart are illustrated in Fig. 4.
7. Challenges in digital twin technology
DT technology holds particular promise in transforming the delivery of CVD care through personalized medicine. Yet various critical challenges currently stand in the way of its universal acceptance. These are technical constraints in combining heterogeneous and multiform datasets, ethical considerations related to patient confidentiality and data integrity and a dearth of universal confidence based on inadequate verification of the digital twin models. The resolution of these critical issues, such as enhancing data integration, having stringent privacy controls and establishing trust through stringent validation is imperative for the adoption of this revolutionary technology in cardiovascular medicine. Conventional inverse analysis techniques fail to work for non-linear systems such asblood circulation in the cardiovascular system and require the use of more sophisticated techniques like recurrent neural networks (29). The exchange of real-time data among physical and virtual twins is a major challenge, demanding highly advanced technological infrastructure and expertise. In addition, the skilled hands necessary for operating and interpreting digital twin systems are a barrier to universal adaptation, restricting the target user base and enhancing the need for specialized education. Moral concerns regarding patient information privacy and monitoring also pose significant hurdles to the use of digital twin technologies. Overcoming this concern involves strict verification, validation, and uncertainty quantification to ensure that the simulations by the DTs are trustworthy (62).
Practical challenges
AI-powered DTs hold ample promise for improved patient outcomes and tailored therapy when incorporated into clinical procedures. Before these technologies can be extensively and successfully implemented in actual healthcare settings, a number of practical issues need to be resolved. DTs require the seamless integration of diverse data sources including electronic health records, wearable devices, imaging and multi omics data. The process of establishing interoperability among these disparate systems is difficult and frequently complicated by disparate data formats and standards. Especially in the context of current healthcare IT infrastructures, real-time data interchange and dynamic digital twin updating are crucial yet technically challenging. Ensuring accuracy and reliability of AI-driven DTs is critical. Strict validation studies are required to confirm that DT predictions and recommendations are clinically safe for patient care and building trust among clinicians and patients require transparent algorithms and clear-cut demonstration of clinical benefits (110-112).
Technical limitations
The technical limitations include data integration, real-time exchange, model robustness, validation and computational demands. The integration of vast, intricate and varied data streams such as imaging, physiological, and environmental data is necessary for digital twins. Real-time data interchange and system interoperability is difficult to achieve technically and continue to be a significant obstacle. Accuracy, dependability, and validation of digital twin models for clinical usage are difficult to achieve. Empirical studies on the characteristics and effectiveness of the paradigm in various patient populations are scarce. Advanced techniques and substantial computational resources are needed to create high-fidelity digital twins, and they may not always be accessible in clinical settings (113).
Ethical considerations
The use of patient data in DT raises concerns about privacy, data protection and potential misuse. Strong security measures are essential to maintain patient trust. When data are utilized for purposes other than direct clinical care, ongoing data collecting and monitoring may raise ethical concerns about patient permission and the possibility of surveillance. If DT technology is only available to well-resourced organizations or individuals, there is a chance that it will exacerbate health inequities (114).
Training and usability
DT implementation and interpretation frequently require for specific knowledge that may not be widely available among today's healthcare practitioners. It is a realistic difficulty to integrate digital twins into current healthcare workflows without causing disturbance. Adoption must be facilitated by user-friendly interfaces and precise clinical guidelines. In clinical practice, training and consistent use may be hindered by the lack of established protocols for the creation and use of digital twins (113).
8. Synopsis and considerations
The integration of multi-modal data, sophisticated computational infrastructure and specialized software and hardware, such as GPU-accelerated systems for real-time cardiac modelling are among the significant upfront expenditures associated with implementing digital twin technology. Maintaining the model, managing data and hiring qualified staff to run and interpret DT outputs are ongoing costs. DTs can reduce the need for physical clinical trials which would potentially lower long-term costs and improve cost effective patient management. Some of the digital health initiatives have shown cost savings in cardiovascular care. DT technology is more likely to be affordable and used by high-resource hospitals and research facilities because it requires sophisticated IT infrastructure and knowledge. Widespread adoption in lower resource settings is currently limited by technical, financial and workforce barriers. Integration with the existing healthcare systems and data privacy are the main challenges.
The ability of DT technology to integrate behavioural and environmental factors with physiological and clinical data in cardiovascular disease is growing. For precise disease phenotyping, risk assessment and individualized treatment, these elements are acknowledged as essential. DTs can better replicate real-world effects on cardiovascular health by incorporating information about a patient's surroundings, including climate, air quality and pollution exposure. By combining ambient data with physiological and medical data, advances in generative AI and ML make it possible to create more dynamic and customized simulations and predictions. Additionally, DT models are being used to incorporate lifestyle elements including food, exercise and other health-related habits. For instance, models that diagnose and predict CVDs using patient lifestyle information, observed symptoms and medical history have been developed, enabling users to self-monitor and receive tailored advice. Continuous monitoring of behavioural patterns and environmental exposures is made possible by real-time information from wearable technology and IoT sensors, promoting proactive and preventive care.
9. Conclusion and future perspectives
The application of DT technology in cardiovascular medicine is a paradigm shift towards personalized healthcare. Through the use of AI and ML, DTs facilitate accurate diagnosis, optimized treatment plans, and real-time monitoring of patients. Nevertheless, overcoming current challenges with data integration, privacy, and validation is essential for wider clinical adoption. Future research is required to focus on developing robust AI-driven models, which will enhance interoperability, and establish ethical frameworks for DT implementation. Collaborative efforts between academia, healthcare institutions and industry will be crucial part in translating DTs from research prototypes into accessible clinical tools. DTs could revolutionize the treatment and management of CVDs.
Collecting and analysing datasets which includes the patient specific cardiovascular parameters is the key to advance research in this field. The Cancer Imaging Archive (TCIA), UK Biobank and PhysioNet, are publicly available repositories which provide valuable imaging and physiological datasets. The clinical data from EHRs and real-time data from wearable devices will further enhance DT models. Collaborations with hospitals and research institutions can facilitate access to clinical data, while computational modelling tools like OpenSim and SimVascular can assist in synthesizing data. With the integration of these sources of information with machine learning will make DTs more predictive, leading to more accurate, patient-specific cardiovascular treatment.
Acknowledgements
The authors acknowledge and appreciate the Department of Research, Meenakshi Academy of Higher Education and Research (Kanchipuram, India) for its support and encouragement. The authors would also like to express their deepest gratitude to Professor E.M. Shankar and Dr Sivadoss Raju from the Infectious Diseases Society of India (IDSI) (https://idsi.org.in/) for their unwavering logistic support extended throughout the project tenure.
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
All authors (ASSJ, SA, BS, MKMR, KS, SR, PVC, NR and SS) were involved in the writing of the original draft, and in the reviewing and editing of the manuscript. ASSJ, SA, BS and SS were also involved in the investigative aspects of the study. All authors have read and approved the final manuscript. Data authentication is not applicable.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
|
Frąk W, Wojtasińska A, Lisińska W, Młynarska E, Franczyk B and Rysz J: Pathophysiology of cardiovascular diseases: New insights into molecular mechanisms of atherosclerosis, arterial hypertension, and coronary artery disease. Biomedicines. 10(1938)2022.PubMed/NCBI View Article : Google Scholar | |
|
Yuyun MF, Sliwa K, Kengne AP, Mocumbi AO and Bukhman G: Cardiovascular diseases in sub-saharan africa compared to high-income countries: An epidemiological perspective. Glob Heart. 15(15)2020.PubMed/NCBI View Article : Google Scholar | |
|
Thiriet M: Cardiovascular Disease: An Introduction. In: Vasculopathies. Biomathematical and Biomechanical Modeling of the Circulatory and Ventilatory Systems. Vol. 8. Springer, Cham, 2018. | |
|
Mensah GA, Roth GA and Fuster V: The global burden of cardiovascular diseases and risk factors: 2020 and beyond. J Am Coll Cardiol. 74:2529–2532. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Gaziano T, Reddy KS, Paccaud F, Horton S and Chaturvedi V: Chapter 33 cardiovascular disease'. In Jamison DT, Breman J, Measham AR, Alleyne G, Claeson M, Evans DB, Jha P, Mills A and Musgrove P (eds). Disease control priorities in developing countries, 2nd edition. Washington: World Bank, pp645-662, 2006. | |
|
Di Cesare M, Perel P, Taylor S, Kabudula C, Bixby H, Gaziano TA, McGhie DV, Mwangi J, Pervan B, Narula J, et al: The heart of the world. Glob Heart. 19(11)2024.PubMed/NCBI View Article : Google Scholar | |
|
Global Cardiovascular Risk Consortium. Magnussen C, Ojeda FM, Leong DP, Alegre-Diaz J, Amouyel P, Aviles-Santa L, De Bacquer D, Ballantyne CM, Bernabé-Ortiz A, et al: Global effect of modifiable risk factors on cardiovascular disease and mortality. N Engl J Med. 389:1273–1285. 2023.PubMed/NCBI View Article : Google Scholar | |
|
Lindstrom M, DeCleene N, Dorsey H, Fuster V, Johnson CO, LeGrand KE, Mensah GA, Razo C, Stark B, Varieur Turco J and Roth GA: Global burden of cardiovascular diseases and risks collaboration, 1990-2021. J Am Coll Cardiol. 80:2372–2425. 2022.PubMed/NCBI View Article : Google Scholar | |
|
World Health Organization (WHO): The Global Health Observatory. Explore a World of Health Data. WHO, Geneva, 2021. | |
|
Johansen H, Thillaiampalam S, Nguyen D and Sambell C: Diseases of the circulatory system-hospitalization and mortality. Health Rep. 17:49–53. 2005.PubMed/NCBI | |
|
Saito I, Yamagishi K, Kokubo Y, Yatsuya H, Iso H, Sawada N, Inoue M and Tsugane S: Impact of cardiovascular disease on the death certificate diagnosis of heart failure, ischemic heart disease, and cerebrovascular disease-The Japan public health center-based prospective study. Circ J. 87:1196–1202. 2023.PubMed/NCBI View Article : Google Scholar | |
|
Day IN and Wilson DI: Science, medicine, and the future: Genetics and cardiovascular risk. BMJ. 323:1409–1412. 2001.PubMed/NCBI View Article : Google Scholar | |
|
Grejtakova D, Boronova I, Bernasovska J and Bellosta S: PCSK9 and lipid metabolism: Genetic variants, current therapies, and cardiovascular outcomes. Cardiovasc Drugs Ther: Jun 22, 2024 (Epub ahead of print). | |
|
Coorey G, Figtree GA, Fletcher DF, Snelson VJ, Vernon ST, Winlaw D, Grieve SM, McEwan A, Yang JYH, Qian P, et al: The health digital twin to tackle cardiovascular disease-a review of an emerging interdisciplinary field. NPJ Digit Med. 5(126)2022.PubMed/NCBI View Article : Google Scholar | |
|
Moiseev VS, Demurov LM, Kobalava ZD, Chistiakov DA, Tereshchenko SN, Kondrat'ev II, Korovina EA and Nosikov VV: The polymorphism of the angiotensin-converting enzyme gene in patients with hypertension, left ventricular hypertrophy and the development of a myocardial infarct at a young age. Preliminary report. Ter Arkh. 69:18–23. 1997.PubMed/NCBI(In Russian). | |
|
Sheikhy A, Fallahzadeh A, Aghaei Meybodi HR, Hasanzad M, Tajdini M and Hosseini K: Personalized medicine in cardiovascular disease: Review of literature. J Diabetes MetabDisord. 20:1793–1805. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Vallée A: Envisioning the future of personalized medicine: Role and realities of digital twins. J Med Internet Res. 26(e50204)2024.PubMed/NCBI View Article : Google Scholar | |
|
Thangaraj PM, Benson SH, Oikonomou EK, Asselbergs FW and Khera R: Cardiovascular care with digital twin technology in the era of generative artificial intelligence. Eur Heart J. 45:4808–4821. 2024.PubMed/NCBI View Article : Google Scholar : (Epub ahead of print). | |
|
Sel K, Osman D, Zare F, Masoumi Shahrbabak S, Brattain L, Hahn JO, Inan OT, Mukkamala R, Palmer J, Paydarfar D, et al: Building digital twins for cardiovascular health: From principles to clinical impact. J Am Heart Assoc. 13(e031981)2024.PubMed/NCBI View Article : Google Scholar | |
|
Manocha A, Bhatia M and Kumar G: Smart monitoring solution for dengue infection control: A digital twin-inspired approach. Comput Methods Programs Biomed. 257(108459)2024.PubMed/NCBI View Article : Google Scholar | |
|
Banerjee S, Das D, Chatterjee P and Ghosh U: Blockchain-enabled digital twin technology for next-generation transportation systems. In: 2023 IEEE 26th International Symposium on Real-Time Distributed Computing (ISORC). IEEE, pp224-229, 2023. | |
|
Liu J, Zhang T, Fan J and Lang S: Applications of digital twin technology in AUV. In: 2023 IEEE 11th International Conference on Computer Science and Network Technology (ICCSNT). IEEE, pp293-297, 2023. | |
|
Botín-Sanabria DM, Mihaita AS, Peimbert-García RE, Ramírez-Moreno MA, Ramírez-Mendoza RA and Lozoya-Santos JDJ: Digital twin technology challenges and applications: A comprehensive review. Remote Sens. 14(1335)2022. | |
|
Wagner T, Kittl C, Jakob J, Hiry J and Häger U: Digital twins in power systems: A proposal for a definition. IEEE Power Energy Mag. 22:16–23. 2024. | |
|
Sado K, Peskar J, Downey A, Khan J and Booth K: A digital twin based forecasting framework for power flow management in DC microgrids. Sci Rep. 15(6430)2025.PubMed/NCBI View Article : Google Scholar | |
|
Bahrin MAK, Othman MF, Azli NHN and Talib MF: Industry 4.0: A review on industrial automation and robotic. J Teknol. 78:137–143. 2016. | |
|
Fuller A, Fan Z, Day C and Barlow C: Digital twin: Enabling technologies, challenges, and open research. IEEE Access. 8:108952–108971. 2020. | |
|
De Benedictis A, Mazzocca N, Somma A and Strigaro C: Digital twins in healthcare: An architectural proposal and its application in a social distancing case study. IEEE J Biomed Health Inform. 27:5143–5154. 2023.PubMed/NCBI View Article : Google Scholar | |
|
Sun T, He X and Li Z: Digital twin in healthcare: Recent updates and challenges. Digit Health. 9(20552076221149651)2023.PubMed/NCBI View Article : Google Scholar | |
|
Vallée A: Digital twin for healthcare systems. Front Digit Health. 5(1253050)2023.PubMed/NCBI View Article : Google Scholar | |
|
Ghatti S, Yurish LA, Shen H, Rheuban K, Enfield KB, Facteau NR, Engel G and Dowdell K: Digital twins in healthcare: A survey of current methods. Arch Clin Biomed Res. 7:365–338. 2023. | |
|
Ying L, Zhang L, Yang Y, Zhou L, Ren L, Wang F, Liu R, Pang Z and Deen MJ: A novel cloud-based framework for elderly healthcare services using digital twin. IEEE Access. 7:49088–49101. 2019. | |
|
Lehtola VV, Koeva M, Elberink SO, Raposo P, Virtanen JP, Vahdatikhaki F and Borsci S: Digital twin of a city: Review of technology serving city needs. Int J Appl Earth Obs Geoinf. 114(102915)2022. | |
|
Corral-Acero J, Margara F, Marciniak M, Rodero C, Loncaric F, Feng Y, Gilbert A, Fernandes JF, Bukhari HA, Wajdan A, et al: The ‘digital twin’ to enable the vision of precision cardiology. Eur Heart J. 41:4556–4564. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Gillette K, Gsell MAF, Prassl AJ, Karabelas E, Reiter U, Reiter G, Grandits T, Payer C, Štern D, Urschler M, et al: A Framework for the generation of digital twins of cardiac electrophysiology from clinical 12-leads ECGs. Med Image Anal. 71(102080)2021.PubMed/NCBI View Article : Google Scholar | |
|
Lamata P: King's College London. Thousands of cardiac ‘digital twins’ offer new insights into the heart, 2025. | |
|
Dorbala P and Iyer R: Carle Illinois College of Medicine. Digital twinning: New machine learning research tracks heart failure development for targeted treatment, 2024. | |
|
Sakata K, Bradley RP, Prakosa A, Yamamoto CAP, Ali SY, Loeffler S, Tice BM, Boyle PM, Kholmovski EG, Yadav R, et al: Assessing the arrhythmogenic propensity of fibrotic substrate using digital twins to inform a mechanisms-based atrial fibrillation ablation strategy. Nat Cardiovasc Res. 3:857–868. 2024.PubMed/NCBI View Article : Google Scholar | |
|
Rudnicka Z, Proniewska K, Perkins M and Pręgowska A: Cardiac healthcare digital twins supported by artificial intelligence-based algorithms and extended reality: A systematic review. Electronics. 13(866)2024. | |
|
de Lepper AGW, Buck CMA, van 't Veer M, Huberts W, van de Vosse FN and Dekker LRC: From evidence-based medicine to digital twin technology for predicting ventricular tachycardia in ischaemic cardiomyopathy. J R Soc Interface. 19(20220317)2022.PubMed/NCBI View Article : Google Scholar | |
|
Martin CH, Reventos-Presmanes J, Guichard JB, Mont L, Guillem MS, Climent AM and Hernandez I: Generation of cardiac digital twins based on noninvasive cardiac mapping. EP Europace. 25 (Suppl 1)(euad122.643)2023. | |
|
Jones D, Snider C, Nassehi A, Yon J and Hicks B: Characterising the digital twin: a systematic literature review. CIRP J Manuf Sci Technol. 29:36–52. 2020. | |
|
Katsoulakis E, Wang Q, Wu H, Shahriyari L, Fletcher R, Liu J, Achenie L, Liu H, Jackson P, Xiao Y, et al: Digital twins for health: A scoping review. NPJ Digit Med. 7(77)2024.PubMed/NCBI View Article : Google Scholar | |
|
Papachristou K, Katsakiori PF, Papadimitroulas P, Strigari L and Kagadis GC: Digital twins' advancements and applications in healthcare, towards precision medicine. J Pers Med. 14(1101)2024.PubMed/NCBI View Article : Google Scholar | |
|
Lim KYH, Zheng P and Chen CH: A state-of-the-art survey of digital twin: Techniques, engineering product lifecycle management and business innovation perspectives. J Intell Manuf. 31:1313–1337. 2019. | |
|
Chakshu NK, Sazonov I and Nithiarasu P: Towards enabling a cardiovascular digital twin for human systemic circulation using inverse analysis. Biomech Model Mechanobiol. 20:449–465. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Roney CH, Sim I, Yu J, Beach M, Mehta A, Alonso Solis-Lemus J, Kotadia I, Whitaker J, Corrado C, Razeghi O, et al: Predicting atrial fibrillation recurrence by combining population data and virtual cohorts of patient-specific left atrial models. Circ Arrhythm Electrophysiol. 15(010253)2022.PubMed/NCBI View Article : Google Scholar | |
|
Viceconti M, De Vos M, Mellone S and Geris L: Position paper From the digital twins in healthcare to the virtual human twin: A moon-shot project for digital health research. IEEE J Biomed Health Inform: Oct 11, 2023 (Epub ahead of print). | |
|
Zheng T, Azzolin L, Sánchez J, Dössel O and Loewe A: An automated pipeline for generating fiber orientation and region annotation in patient-specific atrial models. Curr Dir Biomed Eng. 7:136–139. 2021. | |
|
Fathima SN: An update on myocardial infarction. Curr Res Trends Med Sci Technol. 1(95)2021. | |
|
Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR and White HD: Writing Group on behalf of the Joint ESC/ACCF/AHA/WHF Task Force for the Universal Definition of Myocardial Infarction. Third universal definition of myocardial infarction. Glob Heart. 7:275–295. 2012.PubMed/NCBI View Article : Google Scholar | |
|
Li L, Camps J, Jenny Wang Z, Beetz M, Banerjee A, Rodriguez B and Grau V: Toward enabling cardiac digital twins of myocardial infarction using deep computational models for inverse inference. IEEE Trans Med Imaging. 43:2466–2478. 2024.PubMed/NCBI View Article : Google Scholar | |
|
Fu DG: Cardiac arrhythmias: Diagnosis, symptoms, and treatments. Cell Biochem Biophys. 73:291–296. 2015.PubMed/NCBI View Article : Google Scholar | |
|
Deng D, Arevalo HJ, Prakosa A, Callans DJ and Trayanova NA: A feasibility study of arrhythmia risk prediction in patients with myocardial infarction and preserved ejection fraction. EP Europace. 18 (Suppl 4):iv60–iv66. 2016.PubMed/NCBI View Article : Google Scholar | |
|
Arevalo HJ, Vadakkumpadan F, Guallar E, Jebb A, Malamas P, Wu KC and Trayanova NA: Arrhythmia risk stratification of patients after myocardial infarction using personalized heart models. Nat Commun. 7(11437)2016.PubMed/NCBI View Article : Google Scholar | |
|
Ahmadova AA: Applications of digital twins in medicine and the ontological model of medical digital twins. Probl Inf Soc. 15:98–105. 2024. | |
|
Hu Y, Chen J, Hu L, Li D, Yan J, Ying H, Liang H and Wu J: Personalized heart disease detection via ECG digital twin generation. arXiv [Preprint]: 11171, 2024. | |
|
Moore JH, Li X, Chang JH, Tatonetti NP, Theodorescu D, Chen Y, Asselbergs FW, Venkatesan M and Wang ZP: SynTwin: A graph-based approach for predicting clinical outcomes using digital twins derived from synthetic patients. Pac Symp Biocomput. 29:96–107. 2024.PubMed/NCBI | |
|
Joshi S, Dharmalingam M, Vadavi A, Thajudeen M, Keshavamurthy A, Bhonsley A and Shamanna P: Abstract P278: 1-year outcomes of A1c reduction, weight loss, and lowered QRISK3 scores in type 2 diabetes remission: Insights from an RCCT leveraging whole-body digital twin technology. Circulation. 149 (Suppl 1)(P278)2024. | |
|
Hwang T, Kwon O, Lim B, Jin Z, Yang S, Kim D, Park J, Yu H, Kim T, Uhm J, et al: Clinical application of virtual antiarrhythmic drug test using digital twins in patients who recurred atrial fibrillation after catheter ablation. EP Europace. 25(euad122.076)2023. | |
|
Kadry K, Gupta S, Nezami FR and Edelman ER: Probing the limits and capabilities of diffusion models for the anatomic editing of digital twins. NPJ Digit Med. 7(354)2024.PubMed/NCBI View Article : Google Scholar | |
|
Winter PD and Chico TJA: Using the non-adoption, abandonment, scale-up, spread, and sustainability (NASSS) framework to identify barriers and facilitators for the implementation of digital twins in cardiovascular medicine. Sensors (Basel). 23(6333)2023.PubMed/NCBI View Article : Google Scholar | |
|
Lareyre F, Adam C, Carrier M and Raffort J: Using digital twins for precision medicine in vascular surgery. Ann Vasc Surg. 67:e577–e578. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Erol T, Mendi AF and Doğan D: The digital twin revolution in healthcare, 2020 4th International Symposium on Multidisciplinary Studies and Innovative Technologies (ISMSIT); Istanbul, Turkey. IEEE, pp1-7, 2020. | |
|
Shu H, Liang R, Li Z, Goodridge A, Zhang X, Ding H, Nagururu N, Sahu M, Creighton FX, Taylor RH, et al: Twin-S: A digital twin for skull base surgery. Int J Comput Assist Radiol Surg. 18:1077–1084. 2023.PubMed/NCBI View Article : Google Scholar | |
|
An Q, Rahman S, Zhou J and Kang JJ: A comprehensive review on machine learning in healthcare industry: classification, restrictions, opportunities and challenges. Sensors (Basel). 23(4178)2023.PubMed/NCBI View Article : Google Scholar | |
|
Rathore MM, Shah SA, Shukla D, Bentafat E and Bakiras S: The role of AI, machine learning, and big data in digital twinning: A systematic literature review, challenges, and opportunities. IEEE Access. 9:32030–32052. 2021. | |
|
Bezborodova OE, Bodin ON, Gerasimov AI, Kramm MN, Rahmatullov RF and Ubiennykh AG: ‘Digital Twin’ technology in medical information systems. J Phys Conf Ser. 1515(052022)2020. | |
|
Vinuesa R and Brunton SL: Enhancing computational fluid dynamics with machine learning. Nat Comput Sci. 2:358–366. 2022.PubMed/NCBI View Article : Google Scholar | |
|
Cuocolo R, Caruso M, Perillo T, Ugga L and Petretta M: Machine learning in oncology: A clinical appraisal. Cancer Lett. 481:55–62. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Krittanawong C, Virk HUH, Bangalore S, Wang Z, Johnson KW, Pinotti R, Zhang H, Kaplin S, Narasimhan B, Kitai T, et al: Machine learning prediction in cardiovascular diseases: A meta-analysis. Sci Rep. 10(16057)2020.PubMed/NCBI View Article : Google Scholar | |
|
Dalal S, Goel P, Onyema E, Alharbi A, Mahmoud A, Algarni M and Awal H: Application of machine learning for cardiovascular disease risk prediction. Comput Intell Neurosci. 2023(9418666)2023. | |
|
Yarasuri VK, Reddy DS, Muneesh PS, Kaushik RVS, Vardhan TN and Nisha KL: Developing machine learning models for cardiovascular disease prediction. 2022 2nd Asian Conference on Innovation in Technology (ASIANCON). IEEE, pp1-6, 2022. | |
|
Brites ISG, da Silva LM, Barbosa JLV, Rigo SJ, Correia SD and Leithardt VRQ: Machine learning and IoT applied to cardiovascular diseases identification through heart sounds: A literature review. Informatics. 8(73)2021. | |
|
Zhou J, You D, Bai J, Chen X, Wu Y, Wang Z, Tang Y, Zhao Y and Feng G: Machine learning methods in real-world studies of cardiovascular disease. Cardiovasc Innov Appl. 7(25)2023. | |
|
Bzdok D, Krzywinski M and Altman N: Machine learning: Supervised methods. Nat Methods. 15:5–6. 2018. | |
|
Baessler B, Mannil M, Oebel S, Maintz D, Alkadhi H and Manka R: Subacute and chronic left ventricular myocardial scar: Accuracy of texture analysis on nonenhanced cine MR images. Radiology. 286:103–112. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Ali MM, Paul BK, Ahmed K, Bui FM, Quinn JMW and Moni MA: Heart disease prediction using supervised machine learning algorithms: Performance analysis and comparison. Comput Biol Med. 136(104672)2021.PubMed/NCBI View Article : Google Scholar | |
|
Naeem S, Ali A, Anam S and Ahmed MM: An unsupervised machine learning algorithm: Comprehensive review. Int J Com Dig Sys. 13:911–921. 2023. | |
|
Gosling RC, Morris PD, Silva Soto DA, Lawford PV, Hose DR and Gunn JP: Virtual coronary intervention: A treatment planning tool based upon the angiogram. JACC Cardiovasc Imaging. 12:865–872. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Usmani UA, Happonen A and Watada J: A review of unsupervised machine learning frameworks for anomaly detection in industrial applications. In: Arai K (eds) Intelligent Computing. SAI 2022. Lecture Notes in Networks and Systems. Vol. 507. Springer, Cham, pp158-189, 2022. | |
|
Cholevas C, Angeli E, Sereti Z, Mavrikos E and Tsekouras GE: Anomaly Detection in Blockchain Networks Using Unsupervised Learning: A survey. Algorithms. 17(201)2024. | |
|
Thill M, Konen W, Wang H and Bäck T: Temporal convolutional autoencoder for unsupervised anomaly detection in time series. Appl Soft Comput. 112(107751)2021. | |
|
Nanehkaran YA, Licai Z, Chen J, Jamel AAM, Shengnan Z, Navaei YD and Aghbolagh MA: Anomaly detection in heart disease using a density-based unsupervised approach. Wirel Commun Mob Comput. 2022(6913043)2022. | |
|
Nakao T, Hanaoka S, Nomura Y, Murata M, Takenaga T, Miki S, Watadani T, Yoshikawa T, Hayashi N and Abe O: Unsupervised deep anomaly detection in chest radiographs. J Digit Imaging. 34:418–427. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Flores AM, Schuler A, Eberhard AV, Olin JW, Cooke JP, Leeper NJ, Shah NH and Ross EG: Unsupervised learning for automated detection of coronary artery disease subgroups. J Am Heart Assoc. 10(e021976)2021.PubMed/NCBI View Article : Google Scholar | |
|
Manakitsa N, Maraslidis GS, Moysis L and Fragulis GF: A review of machine learning and deep learning for object detection, semantic segmentation, and human action recognition in machine and robotic vision. Technologies. 12(15)2024. | |
|
Fan J, Ma C and Zhong Y: A selective overview of deep learning. Stat Sci. 36:264–290. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Shrestha A and Mahmood A: Review of deep learning algorithms and architectures. IEEE Access. 7:53040–53065. 2019. | |
|
Ravi D, Wong C, Deligianni F, Berthelot M, Andreu-Perez J, Lo B and Yang GZ: Deep learning for health informatics. IEEE J Biomed Health Inform. 21:4–21. 2017.PubMed/NCBI View Article : Google Scholar | |
|
Esteva A, Chou K, Yeung S, Naik N, Madani A, Mottaghi A, Liu Y, Topol E, Dean J and Socher R: Deep learning-enabled medical computer vision. NPJ Digit Med. 4(5)2021.PubMed/NCBI View Article : Google Scholar | |
|
Chen C, Qin C, Qiu H, Tarroni G, Duan J, Bai W and Rueckert D: Deep learning for cardiac image segmentation: A review. Front Cardiovasc Med. 7(25)2020.PubMed/NCBI View Article : Google Scholar | |
|
Subramani S, Varshney N, Anand MV, Soudagar MEM, Al-Keridis LA, Upadhyay TK, Alshammari N, Saeed M, Subramanian K, Anbarasu K and Rohini K: Cardiovascular diseases prediction by machine learning incorporation with deep learning. Front Med (Lausanne). 10(1150933)2023.PubMed/NCBI View Article : Google Scholar | |
|
Xia B, Innab N, Kandasamy V, Ahmadian A and Ferrara M: Intelligent cardiovascular disease diagnosis using deep learning enhanced neural network with ant colony optimization. Sci Rep. 14(21777)2024.PubMed/NCBI View Article : Google Scholar | |
|
Shahul Hameed MA, Qureshi AM and Kaushik A: Bias mitigation via synthetic data generation: A review. Electronics. 13(3909)2024. | |
|
Liu T, Tian Y, Zhao S, Huang X and Wang Q: Residual convolutional neural network for cardiac image segmentation and heart disease diagnosis. IEEE Access. 8:82153–8216. 2020. | |
|
Hu H, Fang B, Ran Y, Wei X, Xian W, Zhou M and Kwong S: Deep dual-stream convolutional neural networks for cardiac image semantic segmentation. IEEE Trans Industr Inform. 20:7440–7448. 2024. | |
|
Bai W, Sinclair M, Tarroni G, Oktay O, Rajchl M, Vaillant G, Lee AM, Aung N, Lukaschuk E, Sanghvi MM, et al: Automated cardiovascular magnetic resonance image analysis with fully convolutional networks. J Cardiovasc Magn Reson. 20(65)2018.PubMed/NCBI View Article : Google Scholar | |
|
Romaguera LV, Romero FP, Costa Filho CFF and Costa MGF: Myocardial segmentation in cardiac magnetic resonance images using fully convolutional neural networks. Biomed Signal Process Control. 44:48–57. 2018. | |
|
Liu D, Jia Z, Jin M, Liu Q, Liao Z, Zhong J, Ye H and Chen G: Cardiac magnetic resonance image segmentation based on convolutional neural network. Comput Methods Programs Biomed. 197(105755)2020.PubMed/NCBI View Article : Google Scholar | |
|
Zotti C, Luo Z, Lalande A and Jodoin P: Convolutional neural network with shape prior applied to cardiac MRI segmentation. IEEE J Biomed Health Inform. 23:1119–1128. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Mienye ID, Swart TG and Obaido G: Recurrent neural networks: A comprehensive review of architectures, variants, and applications. Information. 15(517)2024. | |
|
Baruah RD and Organero MM: Explicit context integrated recurrent neural network for applications in smart environments. Expert Syst Appl. 255(124752)2024. | |
|
Choi E, Schuetz A, Stewart WF and Sun J: Using recurrent neural network models for early detection of heart failure onset. J Am Med Inform Assoc. 24:361–370. 2017.PubMed/NCBI View Article : Google Scholar | |
|
Lu XH, Liu A, Fuh SC, Lian Y, Guo L, Yang Y, Marelli A and Li Y: Recurrent disease progression networks for modelling risk trajectory of heart failure. PLoS One. 16(e0245177)2021.PubMed/NCBI View Article : Google Scholar | |
|
Rasmy L, Wu Y, Wang N, Geng X, Zheng WJ, Wang F, Wu H, Xu H and Zhi D: A study of generalizability of recurrent neural network-based predictive models for heart failure onset risk using a large and heterogeneous EHR data set. J Biomed Inform. 84:11–16. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Shahi S, Marcotte CD, Herndon CJ, Fenton FH, Shiferaw Y and Cherry EM: Long-time prediction of arrhythmic cardiac action potentials using recurrent neural networks and reservoir computing. Front Physiol. 12(734178)2021.PubMed/NCBI View Article : Google Scholar | |
|
Lynn HM, Pan SB and Kim P: A deep bidirectional GRU network model for biometric electrocardiogram classification based on recurrent neural networks. IEEE Access. 7:145395–145405. 2019. | |
|
Jovanovic L, Zivkovic M, Bacanin N, Bozovic A, Bisevac P and Antonijevic M: Metaheuristic optimized electrocardiography time-series anomaly classification with recurrent and long-short term neural networks. Int J Hybrid Intell Syst. 20:275–300. 2024. | |
|
Łukaniszyn M, Majka Ł, Grochowicz B, Mikołajewski D and Kawala-Sterniuk A: Digital twins generated by artificial intelligence in personalized healthcare. Appl Sci. 14(9404)2024. | |
|
Vallée A: Challenges and directions for digital twin implementation in otorhinolaryngology. Eur Arch Otorhinolaryngol. 281:6155–6159. 2024.PubMed/NCBI View Article : Google Scholar | |
|
Vidovszky AA, Fisher CK, Loukianov AD, Smith AM, Tramel EW, Walsh JR and Ross JL: Increasing acceptance of AI-generated digital twins through clinical trial applications. Clin Transl Sci. 17(e13897)2024.PubMed/NCBI View Article : Google Scholar | |
|
Meijer C, Uh HW and El Bouhaddani S: Digital twins in healthcare: Methodological challenges and opportunities. J Pers Med. 13(1522)2023.PubMed/NCBI View Article : Google Scholar | |
|
Weerarathna IN, Kumar P, Verma P, Raymond D, Luharia A and Mishra G: Leveraging digital twin technology to combat cardiovascular disease: A comprehensive review. In: Proceedings of the 2024 2nd DMIHER International Conference on Artificial Intelligence in Healthcare, Education and Industry (IDICAIEI). IEEE, Wardha, pp1-6, 2024. |