Paraplegia following intra‑thecal therapy in patients with acute lymphoblastic leukaemia: A report of two cases
- Authors:
- Published online on: July 30, 2025 https://doi.org/10.3892/wasj.2025.381
- Article Number: 93
-
Copyright : © Zahid et al. This is an open access article distributed under the terms of Creative Commons Attribution License [CC BY 4.0].
Abstract
Introduction
Acute lymphoblastic leukaemia (ALL) is the most common paediatric cancer, primarily affecting young children, with a peak incidence between the ages of 2 and 4 years (1,2). Characterized by the overproduction of immature lymphoblasts, ALL disrupts normal haematopoiesis and weakens immune function (3,4). Advances in treatment, including intrathecal therapy (IT therapy), have significantly improved the survival rates of patients by effectively preventing central nervous system (CNS) relapses, which occur in 5-10% of ALL cases, despite treatment (5). IT therapy, particularly with methotrexate, cytarabine and corticosteroids, is essential for the management of ALL and for CNS prophylaxis; however, it is associated with a significant risk of developing neurological complications (6).
The incidence of acute neurotoxicity from IT methotrexate in paediatric patients ranges from 3 to 12%, with complications such as seizures, encephalopathy, neuropathy and paraplegia being particularly concerning (7,8). Cases of paraplegia following IT therapy, although rare, highlight the potential for severe and long-lasting neurological impairment. Rison (9) described a 3-year-old patient who developed lower extremity paraparesis and areflexia after receiving methotrexate, cytarabine and hydrocortisone, with findings suggesting that ventral nerve roots may be particularly vulnerable to neurotoxic effects. Similarly, Rolf et al (10) reported cases of ascending motor paraplegia, underscoring the critical need for vigilant monitoring of neurological status in paediatric patients with ALL receiving IT therapy.
Other neurotoxic complications beyond paraplegia have been documented, including acute encephalomyelitis and posterior reversible encephalopathy syndrome (PRES), which manifest with seizures, headaches and visual disturbances (11). These symptoms suggest that methotrexate and other chemotherapeutic agents can cause direct damage to the CNS, potentially leading to long-term cognitive and functional impairments in survivors (12). The mechanisms underlying these complications may be related to the pharmacological properties of methotrexate and its metabolites, with cumulative dosing and concurrent therapies increasing the risk (8,13). The present study describes the cases of 2 paediatric patients with ALL who developed paraplegia following IT therapy. These cases highlight the need for awareness of neurotoxic complications in CNS-directed chemotherapy and emphasize the importance of timely intervention to improve patient outcomes.
Case report
Case 1
A 17-year-old male adolescent diagnosed with Philadelphia chromosome-positive precursor B-cell ALL by flow cytometric analysis (performed at another hospital) presented to Mayo Hospital, Lahore, Pakistan with the first bone remission. His most recent haematological evaluation revealed a white blood cell count of 67.91x103/µl, comprising 94% blast cells and 6% lymphocytes, a red blood cell (RBC) count of 2.57x106/µl and a platelet count of 85x103/µl (Table I). Additionally, a histological report indicated platelet anisocytosis. Molecular biological analysis demonstrated the absence of BCR-ABL translocations (data not shown). The patient commenced treatment following the St. Jude protocol for the induction of bone marrow remission. After ~2.5 months, the patient completed the second St. Jude maintenance and IT therapy cycle. At 5 days post-IT therapy, the patient developed urinary retention, followed by sudden-onset paraplegia and stool incontinence 1 day later. A whole-body bone scan, anterior and posterior projections, was performed following 3 h of intravenous injection of 600 MBq Tc-99m MDP, which revealed bone lesions suggestive of marrow infiltration in the pelvis, femora, tibia and right knee joint. Cerebrospinal fluid (CSF) analysis indicated the absence of microorganisms, with lactate dehydrogenase levels of 25 U/l, glucose levels of 76 mg/dl and protein levels of 32.3 mg/dl. Cytological analysis revealed a light proteinaceous background and no atypical or malignant cells (Table II).
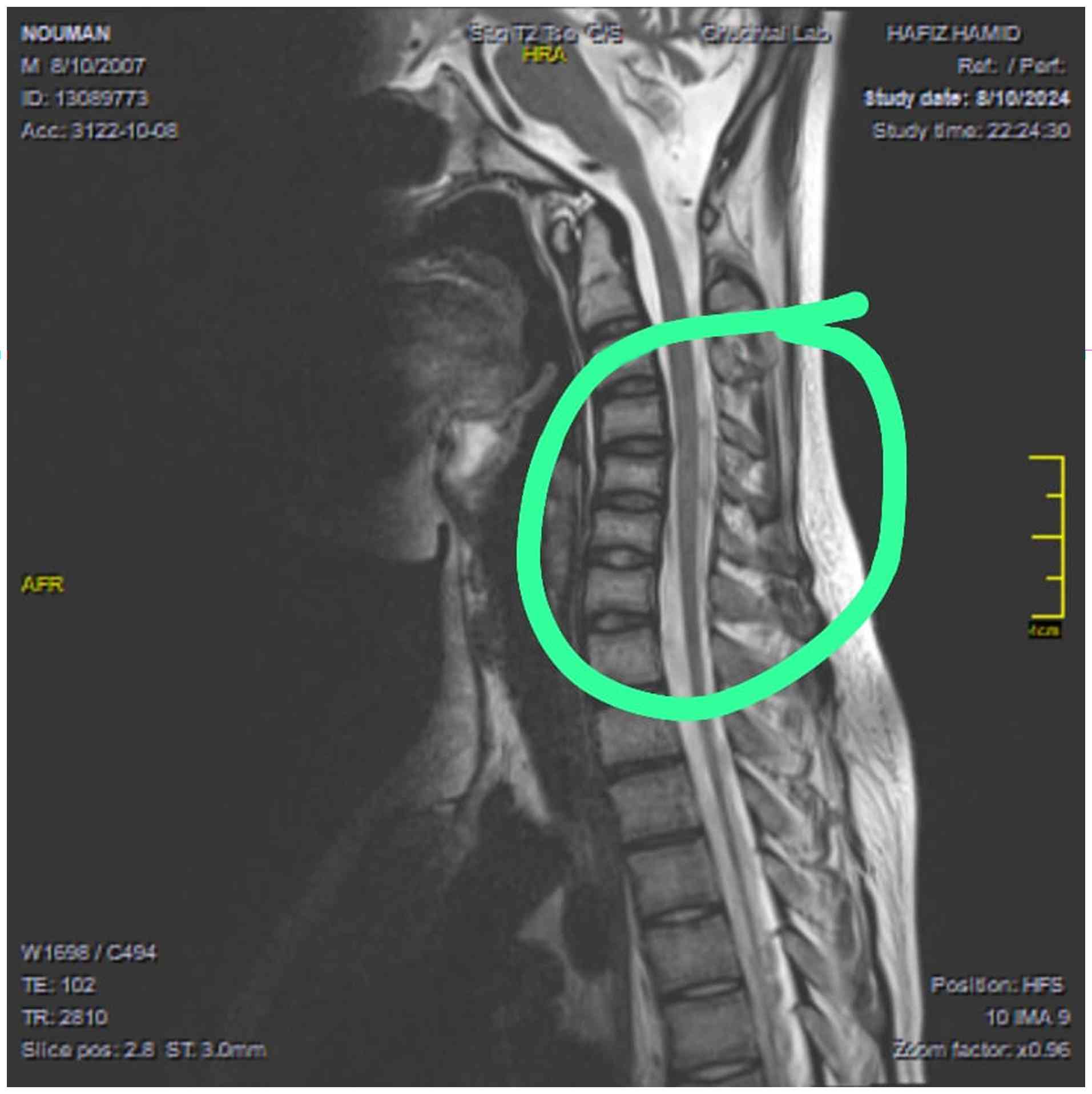
Further imaging analyses, including an MRI of the lumbosacral and dorsal spines, revealed atrophy of the spine in the thoracic region and below some side effects of chemotherapy-subacute combined degeneration (which involves spinal cord atrophy) (Fig. 1). The other notable finding was a few hyperintense signals in the cervical cord at the C3, C4 and C5 levels, that mimicked transverse myelitis and discrepancy in size of cervical cord at this level (Fig. 2). However, a nerve conduction analysis performed on the 7th day following the onset of weakness revealed evidence of lumbosacral (L5-S1) radiculopathy. In the subsequent management, the patient underwent five sessions of plasmapheresis, each with a 3-day gap. At 1 month thereafter, follow-up nerve conduction analyses demonstrated absent motor responses in both the tibial and peroneal nerves. By contrast, the median and ulnar nerves exhibited normal distal latencies, reduced compound muscle action potential amplitudes, typical conduction velocities and absent F-waves. Sensory nerve conduction analyses revealed absent sural and normal responses in both median nerves. Additionally, electromyography findings indicated the absence of motor units in the right tibialis anterior, gastrocnemius and rectus femoris muscle samples, with mildly reduced recruitment in the right first dorsal interosseous muscle. These findings indicate acute axonal motor sensory neuropathy characterized by denervation potentials that predominantly affect the lower limbs. The patient is currently undergoing regular follow-up with continued chemotherapy as per the St. Jude protocol with close monitoring of neurological status. Neurological deficits, including paraplegia and stool incontinence, have shown no significant improvement to date. A working diagnosis of possible transverse myelitis secondary to intrathecal chemotherapy is being considered, although differential diagnoses, such as paraneoplastic or treatment-induced neurotoxicity remain under evaluation.
Case 2
A 17-year-old male came to Mayo Hospital, Lahore, Pakistan and was diagnosed with T-cell ALL after a histopathology report revealed the presence of T-cell markers, such as CD3, CD4, CD5 and CD7 (Table III). The viability index of the lysed peripheral blood sample was 90%. A bone marrow examination revealed Sudan Black B-negative ALL (T-cell ALL) with >90% blast cells, suppressed erythropoiesis, leukopoiesis and the absence of megakaryocytes. The bone scan did not reveal any active bone pathology. The cardiac function of the patient was as expected, with good biventricular systolic function. The patient was scheduled to receive two cycles of hyperfractionated cyclophosphamide, vincristine, doxorubicin and prednisolone (hyper-CVAD) treatment, with reassessment planned after each cycle. At 4 days after completing both cycles of hyper-CVAD treatment, the patient underwent IT therapy. After 3 days, he developed urinary retention and underwent catheterization. At 2 days thereafter, the patient experienced stool incontinence. Following 10 days of IT therapy, he began to experience weakness in his lower limbs, starting from the hip joint and progressing distally.
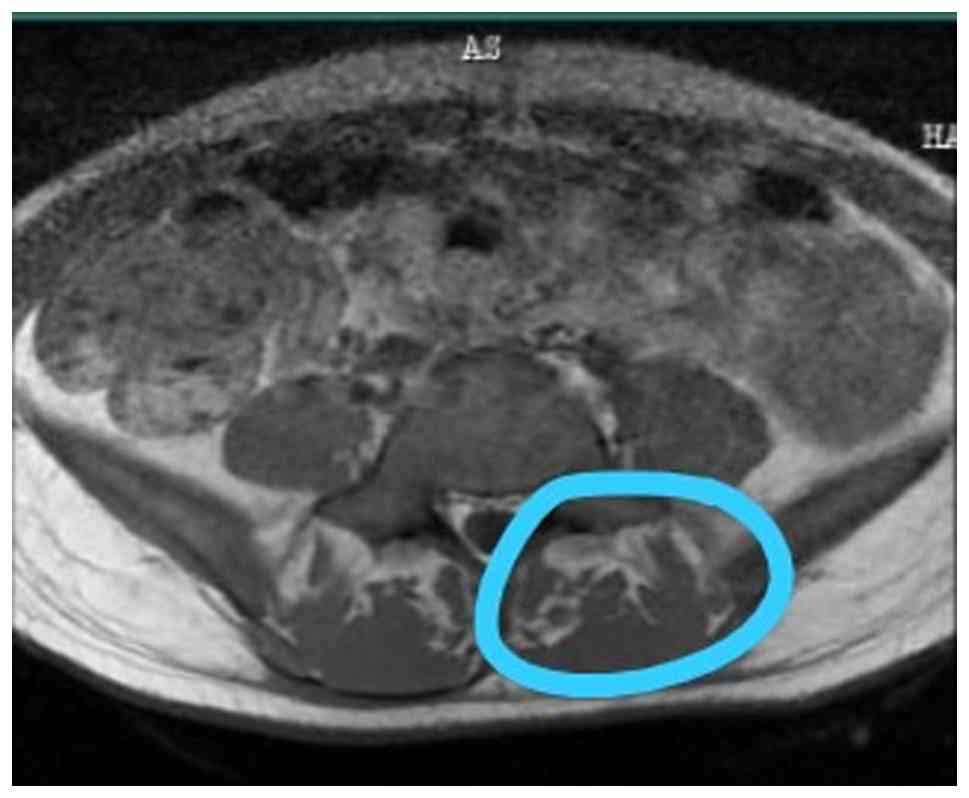
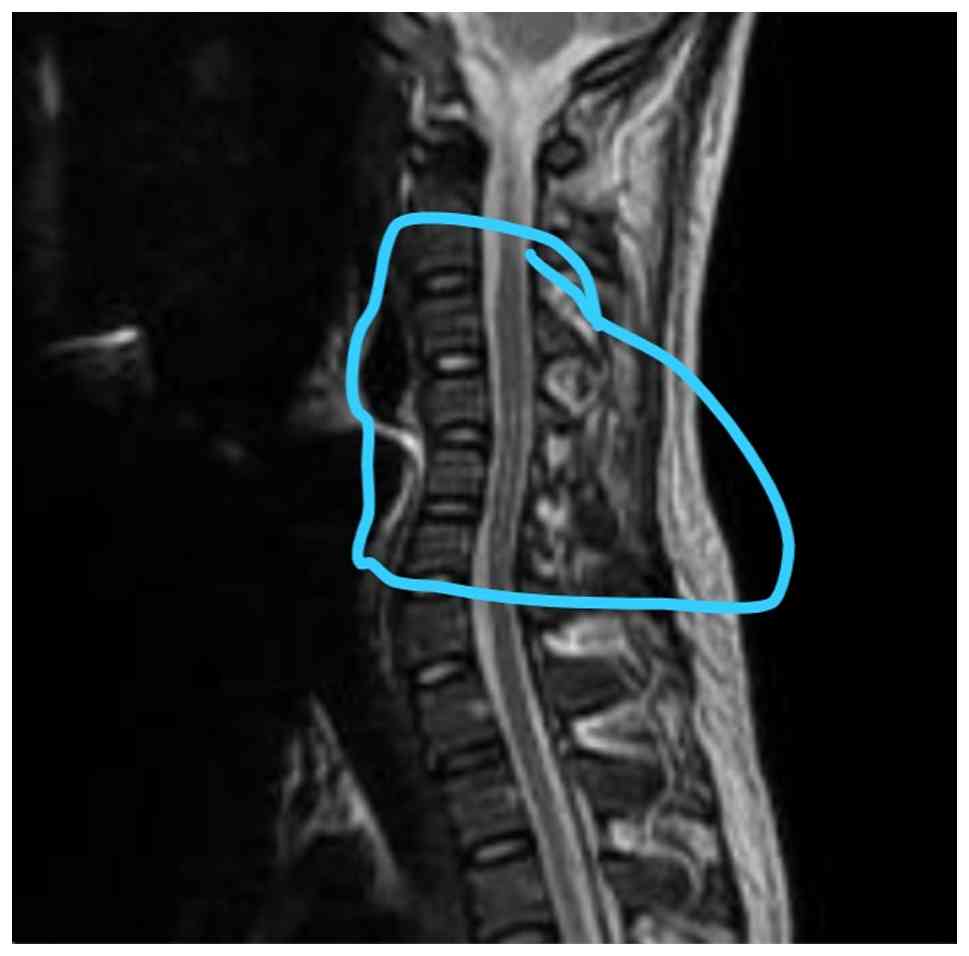
Nerve conduction analyses revealed reduced amplitudes of the bilateral common peroneal nerves, absent F-wave responses and reduced amplitudes of the bilateral tibial nerves. These findings suggested lumbosacral (L5-S1) radiculopathy. The MRI thoracolumbar spine report documented mild straightening of the spine and disc bulges at L3-L4 and L4-L5, resulting in mild indentation of the anterior thecal sac and fatty atrophy in back muscles indicating reduced mobility state (Fig. 3). There were hyperintense signals in the T2 sagittal cervical spine along central canal; however, no evidence of spondylolisthesis was found (Fig. 4). CSF analysis revealed increased glucose and protein levels and cytological examination of smears revealed a light proteinaceous background with barely visible red RBCs and lymphocytes (Table IV). Atypical or malignant cells were not observed. An MRI of the cervical spine revealed non-compressive disc bulges at C4-C5, L3-L4 and L4-L5. A further examination revealed an intact sensory input and 5/5 upper limb power. However, the lower limb power was 0/5 for both legs, and plantar reflexes were absent in both legs (Table V). The thyroid profile exhibited decreased serum T3 and TSH levels (Table VI). The patient remains under regular follow-up, with intrathecal chemotherapy ongoing as per protocol. Neurological impairments, including paraplegia and bowel incontinence, persist without notable improvement.
Discussion
Paraplegia following IT therapy is a rare, yet severe complication of paediatric ALL treatment. IT chemotherapy, primarily with methotrexate, cytarabine and corticosteroids, is essential for preventing CNS relapse in ALL; however, it also poses a risk of neurotoxicity. The cases presented herein underscore the need for vigilance in identifying and managing neurotoxic effects, particularly given the severe impact that paraplegia can have on the quality of life and functional outcomes of patients.
Neurological complications associated with IT therapy in ALL range from mild symptoms, such as headaches and seizures, to severe outcomes, including myelopathy, encephalopathy and progressive paraplegia. The neurotoxic effects of IT methotrexate have been reported in 3-12% of paediatric patients with ALL, with potential mechanisms including direct neurotoxicity, chemical arachnoiditis and spinal cord injury (7,8). Mauler et al (14) reported acute neurotoxicity due to IT methotrexate overdose, while Pan et al (15) documented transverse myelopathy, both underscoring the capacity for significant neurological impairment following treatment. Similarly, Rison (9) described a case of polyradiculoneuritis in a 3-year-old patient, suggesting that the ventral nerve roots may be particularly susceptible to neurotoxic damage from IT agents.
The timing and dosage of IT chemotherapy play a critical role in developing neurotoxic effects. Hirakawa et al (16) noted that higher doses and cumulative administration frequencies were associated with an increased risk of myelopathy, seizures and encephalopathy. Richards et al (13) also noted a dose-dependent association, which necessitates careful dosing strategies, particularly in paediatric patients whose neurological systems are still developing. This is further supported by the study by Brock and Jennings (17), who reported a fatal case of acute encephalomyelitis following a single dose of IT methotrexate, highlighting the risks associated with short-term exposure. A high CSF protein level has been recognized as a possible marker of IT chemotherapy-induced neurotoxicity, particularly in ALL (18). High CSF protein levels can result from the disruption of the blood-brain barrier, inflammatory processes, or direct cytotoxic action on neural tissue, and all of these factors lead to dysfunction in the spinal cord (19). Studies have reported that elevated CSF protein levels are associated with demyelination (20), arachnoiditis (21) and myelopathy, leading to symptoms, such as weakness of the lower limbs or paraplegia (22). In the patients in the present study, a significant increase in CSF protein levels (case 2, 123.8 mg/dl) was noted in the days following IT therapy. Based on the lack of other aetiologies on neuroimaging and the temporal association with treatment, it can be hypothesized that the elevated CSF protein level was due to chemotherapy-induced neurotoxicity and the probable pathophysiological mechanism resulting in paraplegia in this case.
Beyond paraplegia, other severe neurotoxic conditions have been associated with IT chemotherapy, including PRES, which manifests as seizures, headaches and visual disturbances (11). PRES and related syndromes illustrate a broad range of CNS effects that can result from ALL treatment, with neurotoxicity potentially leading to lasting cognitive and motor impairments in survivors (12). Given these risks, continuous monitoring and individualized dosing regimens are essential for patient management.
In the cases presented herein, interventions such as plasmapheresis were utilized in response to severe neurotoxic symptoms. Plasmapheresis has been suggested as a therapeutic option to reduce the neurotoxic effects by removing circulating neurotoxic agents. However, the efficacy of plasmapheresis for treating IT chemotherapy-induced paraplegia remains unclear and warrants further investigation. Future research is needed to understand the mechanisms underlying these complications and refine preventive and therapeutic approaches, especially regarding dosage adjustments and early recognition of neurotoxic symptoms. These cases highlight the importance of awareness and preparedness among clinicians to promptly identify neurotoxic complications associated with IT chemotherapy in paediatric ALL. Understanding the risk factors and mechanisms of neurotoxicity could lead to better prevention and management strategies, ultimately enhancing patient safety and outcomes of patients with ALL.
In conclusion, the cases reported herein highlight the rare, yet serious risk of paraplegia and other neurotoxic effects following IT therapy for the treatment of paediatric patients with ALL. Early identification and prompt management of neurotoxicity are crucial to minimize long-term complications and improve functional outcomes. Clinicians should remain vigilant for neurological symptoms in patients with ALL undergoing IT therapy to ensure timely intervention. Future research is required to better elucidate the mechanisms of neurotoxicity in IT chemotherapy, including prospective studies examining its safety profile in paediatric patients with ALL. Investigations focusing on optimal dosing strategies, early detection of markers and effective therapeutic interventions are essential to enhance patient safety and reduce the neurotoxic risks associated with this life-saving treatment.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be requested from the corresponding author.
Authors' contributions
MZ, SI and MAh contributed to the conception and design of the study. MZ, MAb and OT were involved in the initial conception of the study and case selection. MW, OT, MU and SU contributed to the drafting of the manuscript and participated in the interpretation of the clinical data of the patients. MZ, MW, MU, and SU reviewed and edited the manuscript. SI, MAi and MAb were responsible for acquiring the patients' data and medical history, and assisted in clinical decision-making. SI, MAi and MAb provided the patient's data and history. SI and MAb confirmed the authenticity of all the raw data. All authors have read and approved the final version of the manuscript.
Ethics approval and consent to participate
The study protocol was approved by the King Edward Medical University Institutional Review Board (reference no. 400/RC/KEMU). Written informed consent was obtained from the patients' guardians for participation in the present study.
Patient consent for publication
Written informed consent for the publication of clinical data and images was obtained from the legal guardians of both underaged patients.
Competing interests
The authors declare that they have no competing interests.
References
|
Inaba H, Greaves M and Mullighan CG: Acute lymphoblastic leukaemia. Lancet. 381:1943–1955. 2013.PubMed/NCBI View Article : Google Scholar | |
|
Pui CH, Relling MV and Downing JR: Acute lymphoblastic leukemia. N Engl J Med. 350:1535–1548. 2004.PubMed/NCBI View Article : Google Scholar | |
|
Radadiya A, Zhu W, Coricello A, Alcaro S and Richards NGJ: Improving the treatment of acute lymphoblastic leukemia. Biochemistry. 59:3193–3200. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Ramya LN, Doble M, Rekha VPB and Pulicherla KK: L-Asparaginase as potent anti-leukemic agent and its significance of having reduced glutaminase side activity for better treatment of acute lymphoblastic leukaemia. Appl Biochem Biotechnol. 167:2144–2159. 2012.PubMed/NCBI View Article : Google Scholar | |
|
Paul S, Kantarjian H, Sasaki K, Marx K, Jain N, Savoy JM, DiPippo A, Jammal N, Bravo GM, Kadia T, et al: Intrathecal prophylaxis with 12 versus 8 administrations reduces the incidence of central nervous system relapse in patients with newly diagnosed Philadelphia chromosome positive acute lymphoblastic leukemia. Am J Hematol. 98:E11–E14. 2023.PubMed/NCBI View Article : Google Scholar | |
|
Kwong YL, Yeung DYM and Chan JCW: Intrathecal chemotherapy for hematologic malignancies: Drugs and toxicities. Ann Hematol. 88:193–201. 2009.PubMed/NCBI View Article : Google Scholar | |
|
Khan RB, Hudson MM, Ledet DS, Morris EB, Pui CH, Howard SC, Krull KR, Hinds PS, Crom D, Browne E, et al: Neurologic morbidity and quality of life in survivors of childhood acute lymphoblastic leukemia: A prospective cross-sectional study. J Cancer Surviv. 8:688–696. 2014.PubMed/NCBI View Article : Google Scholar | |
|
Taylor OA, Brown AL, Brackett J, Dreyer ZE, Moore IK, Mitby P, Hooke MC, Hockenberry MJ, Lupo PJ and Scheurer ME: Disparities in neurotoxicity risk and outcomes among pediatric acute lymphoblastic leukemia patients. Clin Cancer Res. 24:5012–5017. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Rison RA: Ascending sensory motor polyradiculoneuropathy with cranial nerve involvement following administration of intrathecal methotrexate and intravenous cytarabine in a patient with acute myelogenous leukemia: A case report*. Cases J. 1(255)2008.PubMed/NCBI View Article : Google Scholar | |
|
Rolf N, Boehm H, Kaindl AM, Lauterbach I and Suttorp M: Acute ascending motoric paraplegia following intrathecal chemotherapy for treatment of acute lymphoblastic leukemia in children: Case reports and review of the literature. Klin Padiatr. 218:350–354. 2006.PubMed/NCBI View Article : Google Scholar | |
|
Tang JH, Tian JM, Sheng M, Hu SY, Li Y, Zhang LY, Gu Q and Wang Q: Study of posterior reversible encephalopathy syndrome in children with acute lymphoblastic leukemia after induction chemotherapy. J Child Neurol. 31:279–284. 2016.PubMed/NCBI View Article : Google Scholar | |
|
Mateos MK, Marshall GM, Barbaro PM, Quinn MCJ, George C, Mayoh C, Sutton R, Revesz T, Giles JE, Barbaric D, et al: Methotrexate-related central neurotoxicity: Clinical characteristics, risk factors and genome-wide association study in children treated for acute lymphoblastic leukemia. Haematologica. 107:635–643. 2022.PubMed/NCBI View Article : Google Scholar | |
|
Richards S, Pui CH and Gayon P: Childhood Acute Lymphoblastic Leukemia Collaborative Group (CALLCG). Systematic review and meta-analysis of randomized trials of central nervous system directed therapy for childhood acute lymphoblastic leukemia. Pediatr Blood Cancer. 60:185–195. 2013.PubMed/NCBI View Article : Google Scholar | |
|
Mauler DJ, R Richter K, Merrill S, Valencia-Sánchez C, Krishna C and M Mrugala M: Troubleshooting an unusual complication following intrathecal chemotherapy delivered via Ommaya catheter: A case report. Mol Clin Oncol. 13:76–79. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Pan Y, Wang C, Wang H, Tao Q, Xiong S and Zhai Z: Transverse myelopathy occurring with intrathecal administration of methotrexate and cytarabine chemotherapy: A case report. Oncol Lett. 11:4066–4068. 2016.PubMed/NCBI View Article : Google Scholar | |
|
Hirakawa Y, Kitao A, Watanabe M, Matsumoto S, Komaki R, Sakai R, Morimoto K, Yakushijin K and Minami H: Irreversible intrathecal chemotherapy-induced myelopathy in a patient with diffuse large B-cell lymphoma. Intern Med. 63:547–551. 2024.PubMed/NCBI View Article : Google Scholar | |
|
Brock S and Jennings HR: Fatal acute encephalomyelitis after a single dose of intrathecal methotrexate. Pharmacotherapy. 24:673–676. 2004.PubMed/NCBI View Article : Google Scholar | |
|
Werner RA: Paraplegia and quadriplegia after intrathecal chemotherapy. Arch Phys Med Rehabil. 69:1054–1056. 1988.PubMed/NCBI | |
|
Laviv Y, Kasper BS and Kasper EM: Vascular hyperpermeability as a hallmark of phacomatoses: Is the etiology angiogenesis comparable with mechanisms seen in inflammatory pathways? Part I: historical observations and clinical perspectives on the etiology of increased CSF protein levels, CSF clotting, and communicating hydrocephalus: A comprehensive review. Neurosurg Rev. 41:957–968. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Lamers KJB, Vos P, Verbeek MM, Rosmalen F, van Geel WJA and van Engelen BGM: Protein S-100B, neuron-specific enolase (NSE), myelin basic protein (MBP) and glial fibrillary acidic protein (GFAP) in cerebrospinal fluid (CSF) and blood of neurological patients. Brain Res Bull. 61:261–264. 2003.PubMed/NCBI View Article : Google Scholar | |
|
Zhao M, Qiao L, Shi J, Huang F, Zhang M, Lin X, Wang J, Geng T and Zuo H: Arachnoid involved in idiopathic hypertrophic pachymeningitis. J Neurol Sci. 346:227–230. 2014.PubMed/NCBI View Article : Google Scholar | |
|
Weiss S and Kahn Y: Intrathecal methotrexate causing paraplegia in a middle-aged woman. Acta Haematol. 60:59–61. 1978.PubMed/NCBI View Article : Google Scholar |