Erythrocyte sedimentation reaction order, neutrophil‑lymphocyte ratio and platelet‑lymphocyte ratio in patients with breast cancer: Development of an early detection method for cancer
- Authors:
- Published online on: July 31, 2025 https://doi.org/10.3892/wasj.2025.382
- Article Number: 94
-
Copyright : © Ngadikun et al. This is an open access article distributed under the terms of Creative Commons Attribution License [CC BY 4.0].
Abstract
Introduction
The most frequently diagnosed type of cancer and the main causal of cancer-related mortality worldwide is breast cancer (BC), with an incidence of 11.7% (ranked first) and a mortality of rate of 6.9% (ranked fifth) among all cancer cases in 2020(1). It is also a leading type of cancer in developing countries, such as Indonesia, where it ranks first in the total new cancer cases (16.6%), as well as in mortality rates among females with cancer (30.8%) (2). Patients with cancer in developing countries tend to have a worse prognosis compared to those in developed countries; this is mainly due to late diagnosis (first diagnosed at a late stage) (3). Therefore, the development of early detection methods for cancer is a necessity. Recently, studies on the early diagnosis of cancer have focused on the study of biomarkers at the molecular level, such as nucleic acids and proteins through tissue biopsy or blood, saliva and urine (4). One of the molecules that can be used as a marker is miRNA, as it regulates gene expression (5). A popular method for the development of early detection strategies for cancer is cancer proteomics. This method involves the qualitative and quantitative analysis of the differences in protein expression in cancer tissue compared to normal tissue, thus providing markers for cancer diagnosis (6). Inflammatory proteins can function as targets for proteomics analysis in early cancer detection. This is due to the fact that the inflammatory process is associated with various cancer types. Chronic inflammation can lead to the development or growth of cancer, as the inflammatory cells along with their secretions greatly contributed to tumor growth, survivability and potential migration (7).
Based on the findings of a recent study, various components of the inflammatory system, such as platelets, neutrophils and lymphocytes, are known to play a crucial role in carcinogenesis and the development of tumor severity (8). In chronic inflammation, cytokines and inflammatory cells can cause tumor cells to survive, proliferate, invade, or even induce angiogenesis (8). To date, the neutrophil-lymphocyte ratio (NLR) and platelet-lymphocyte ratio (PLR), among various peripheral blood-derived inflammation-based scores, have been suggested to be prognostic markers of cancer. Evidence reinforcing the use of PLR as prognostic factor is most clearly observed in non-small cell lung cancer (9). Both scores can be obtained from total blood count data; thus, they can be used as an inexpensive alternative for assessing the prognosis of patients with cancer. However, both cannot be used as standalone diagnostic method for BC, as they are not specific for cancer. Therefore, the identification of inexpensive and easily available prognostic markers is urgently required to help establish a diagnosis; one of these markers may be the erythrocyte sedimentation rate (ESR).
The measurement of the ESR is an action that is frequently performed in clinical settings, as it is very simple and inexpensive. As the ESR measurement has often been performed, this method can be an alternative to disease monitoring, even though it has a fairly low sensitivity and specificity. Moreover, a higher ESR level has been found to be associated with an increased risk of malignancy in patients with dermatomyositis with transcriptional intermediary factor 1 antibody (10). The most common method used to measure the ESR the Westergren method. The ESR is determined by measuring distance between the plasma surface and the upper limit of blood cells that have been given anticoagulants (EDTA) and left in the pipette for 1 h, measured in millimeters per hour (mm/h). The boundary between red blood cells (erythrocytes) and the surrounding blood plasma is termed the erythrocyte-plasma interface (EPI) (11,12). The periodical measurement of the EPI reveals pattern of blood sedimentation over time. The blood sedimentation pattern is influenced by the components in the blood, including the existence of marker proteins in the blood of patients with cancer (13). Therefore, observing the ESR every minute can produce specific ESR patterns for patients with cancer. From the ESR variable, a new variable can be derived, namely the reaction order from the ESR curve, which is known as ESR order (ESRO). A previous study on ESRO for the detection of ovarian epithelial cancer revealed high sensitivity and specificity values (83.3 and 90.6%, respectively) (13). Therefore, the present study aimed to compare the receiver operating characteristic (ROC) curve, as well as the area under the curve (AUC), and the sensitivity and specificity of ESRO compared to that of NLR and PLR for the early detection of BC.
Patients and methods
Patient samples
The present study used the whole blood of patients and normal subjects. Samples from both groups were obtained at the same time, between March 15 to June 30, 2019. A total of 3.0 ml blood was taken from the vein of each subject, then placed in an EDTA-containing tube where 1.0 ml blood was used for ESR measurements carried out immediately after the samples were collected.
Study design and study subjects
The present study used a cross-sectional study design. Subjects were patients with BC who were treated at RSUP Dr. Sardjito Yogyakarta (Yogyakarta, Indonesia). Subjects were assigned to two as follows: Group 1 consisted of subjects with BC (n=27); and group 2 consisted of normal subjects as the control group (n=29). The study was performed at three sites, namely RSUP Dr. Sardjito Yogyakarta, the Clinical Pathology Laboratory, Faculty of Medicine, Public Health and Nursing, Gadjah Mada University (Yogyakarta, Indonesia) and the Inter-University Center of Gadjah Mada University (Yogyakarta, Indonesia). Patients who were treated at RSUP Dr. Sardjito and were diagnosed by specialist doctor via a histopathology examination and other supporting examinations were included in the BC patient group. Subjects who had never had cancer or had a relative who had cancer were set as the control group. Moreover, patients with a history of or were suffering from more than one type of cancer or other diseases were excluded from the BC group. Healthy subjects who had recently suffered sepsis and/or inflammation were also excluded. The protocol of the present study met the ethical qualifications of the Medical and Health Ethics Review Committee of the Faculty of Medicine Universitas Gadjah Mada with ethical clearance no. KE/FK/0965/EC/2018. All test subjects stated in writing that they were willing to be the subjects of the study, and samples from both groups were taken in the same time frame.
A total of 3.0 ml blood was taken from the vein of each subject and then placed in a tube containing EDTA. A total of 1.0 m of this blood was used for the ESRO examination in a Westergren tube, carried out as soon as the blood was obtained. ESR observations were carried out by reading the EPI every 1 min for 2 h. It was then derived into a new variable, namely ESRO, that was obtained through the integral method of the ESR variable based on the general equation of reaction rate kinetics (14). A total of 2.0 ml EDTA-blood obtained was used for routine blood tests, which also included platelet, neutrophil and lymphocyte absolute counts. From these data, two new variables were established, namely NLR, obtained from absolute neutrophil count divided by absolute lymphocyte count, and PLR, obtained from the absolute platelet count divided by the absolute lymphocyte count.
Statistical analysis
Different tests of each parameter (ESRO, NLR and PLR) were carried out between the normal and cancer groups using the t-test, with Matrix Laboratory (MatLab) software version r2016 for Windows. A P-value <0.01 was considered to indicate a statistically significant difference. In addition, ROC curves, AUC from ROC, and standard error (SE) tests were also carried out to determine the accuracy of each test parameter. Moreover, ROC analysis was used to obtain sensitivity and specificity values from each parameter at certain cut-offs. Parameter accuracy values based on AUC were then categorized into five categories, namely excellent (AUC, 0.9-1), considerable (AUC, 0.8-0.9), fair (AUC, 0.7-0.8), poor (AUC, 0.6-0.7) and failed (AUC, 0.5-0.6) (15).
Results
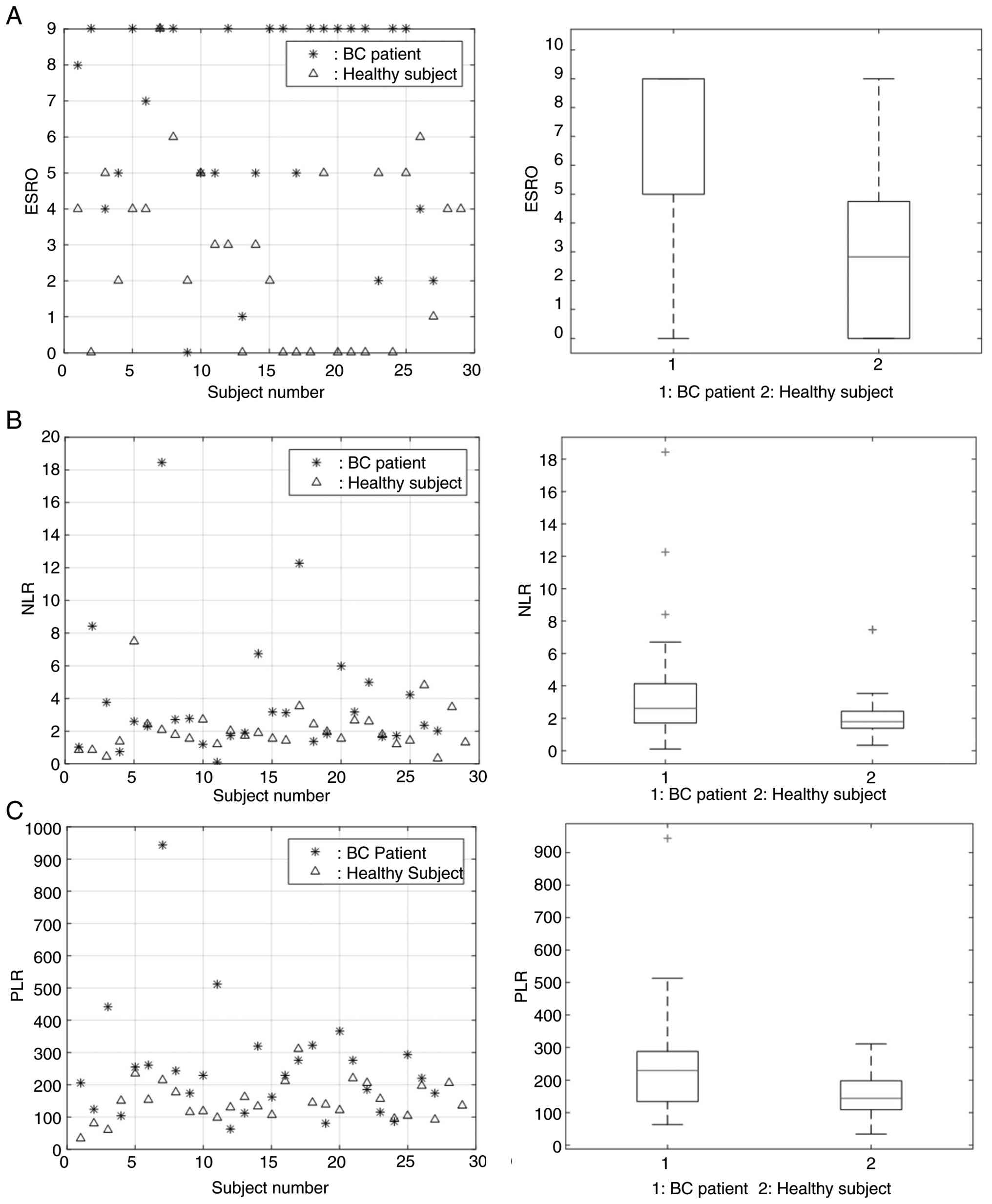
The present study involved 56 women in total. There were 29 healthy women in the normal group, and 27 women with BC in the BC group. The normal subjects themselves had a median age of 21 years (range, 19-44 years), while the median age in the BC group was 55 years (range, 33-68 years). All participants in the BC group had undergone chemotherapy. There were 15 patients (55.56%) who had undergone chemotherapy in <1 year, 10 patients (37.04%) in 1-2 years, and 2 patients (7.41%) in >2 years. Moreover, the majority of the healthy subjects had a normal body mass index (48.28%), 2 subjects (6.90%) were underweight, 12 subjects (41.38%) were overweight and 1 subject (3.45%) was obese (Table I).
The ESRO, NLR and PLR in the BC group were 6.63, 3.79 and 250.95, respectively, while these values in the control group were 2.83, 2.08 and 148.19, respectively. From these data, the BC group had higher average values of ESRO, NLR and PLR compared to the control group (Fig. 1 and Table II). Furthermore, the sensitivity and specificity of ESRO was the highest compared to NLR and PLR, with 96.6% of sensitivity and 59.3% of specificity (Fig. 2 and Table III).
Table IIIComparison of AUC, sensitivity and specificity values between patients with breast cancer and normal subjects. |
Discussion
There are several markers in the blood that can be used as markers of cancer prior to the clinical diagnosis; thus, these markers can be developed as a screening method for certain types of cancer. Such an example is the combination of carcinoembryonic antigen (CEA) and CA125, which results in high sensitivity and specificity for lung cancer detection compared to CEA alone (16). Recently, several markers for BC have been proposed based on molecular hallmarks, such as immunohistochemical markers [for example HER2 (ERBB2) and protein Ki-67 (MKI67)], genomic markers (for example BRCA1, BRCA2 and PIK3CA), and immunomarkers (for example tumor-infiltrating lymphocytes and PD-L1). However, complex diagnostic algorithms are required to combine these parameters for the detection of BC (17). The present study provides a novel panel of markers, in which markers of BC are detected through the analysis of changes in ESR using the Westergren method. The principle of the present study was as follows: Blood that has been inserted into the tube/column undergoes aggregation, where the top column contains plasma. Over time, the collection of erythrocytes in the blood tends to stick together and form macromolecular aggregates, also known as rouleaux. The presence of specific proteins (markers) affects the balance of ions in the plasma in the compact and diffuse layers. The movement of ions in and out of the compact layer into the diffuse layer affects erythrocyte zeta potential (ZPE), which ultimately affects the erythrocyte aggregation-disaggregation pattern (18,19), according to the following reaction equation:
(Equation 1)
where ‘E’ represents erythrocytes, and ‘n’ is the number of erythrocytes. From the reaction equation, changes in ZPE due to the presence of biomarkers affect erythrocyte aggregations. When the balance shifts to the right there will be aggregation followed by sedimentation. In other words, the presence of specific markers in the blood of cancer patients can be detected by analyzing erythrocyte aggregation-disaggregation patterns (13). This method has advantages over current detection methods, as the majority of cancer protein biomarkers exist in a low concentration among human plasma proteomes (17). Therefore, proteomics assay would be extremely challenging due to that matter. The sedimentation mechanism in the present study was analyzed by modifying the ESR measurement variable with the integral method into a new variable, namely ESRO. A new equation can be formulated if it is assumed the speed of aggregation is proportional to the number of red blood cells, which is shown as follows (13):
(Equation 2)
where ‘R’ is the rate of erythrocyte aggregation, ‘k’ is the aggregation rate constant, ‘n’ is the ESRO value, ‘a’ is the number of erythrocytes before aggregation occurs, and ‘x’ is the number of erythrocytes undergoing aggregation.
Proteins are chains of amino acids, where each amino acid has a different acid dissociation constant (pKa) value (20). This pKa affects the ability of each amino acid to repel or attract ion. The variation in the type of amino acid in each protein will certainly affect the tendency of the protein to attract or reject H+ ions. These ions will affect the balance of the compact layer and diffuse layer around the erythrocytes. The change in balance between the compact and diffuse layers will affect the zeta potential of erythrocytes (ZPE) and will also affect the change of aggregation-disaggregation patterns. Different proteins, including specific biomarkers, must have different erythrocyte aggregation-disaggregation patterns due to the different amino acids content. The pattern will be more obvious after conducting a reaction order analysis of the erythrocyte sedimentation rate (13). From this equation, a new parameter can be obtained, namely ESRO, which is obtained through the integration of (equation 1) into (equation 2), with the number of erythrocytes before sedimentation as variable ‘a’, and the number of erythrocytes that undergoes aggregation as variable ‘x’.
The two blood parameters studied, namely NLR and PLR, generally indicate inflammation in the tumor area. However, the mechanism of their influence in the prognosis of BC itself have not been fully determined. Some studies have reported that the increase in neutrophils and platelets can be related to various pro-tumor in vivo activities, such as increased angiogenesis, which support increased proliferation and potential metastasis of tumor cells (21,22). Moreover, lymphocytes themselves are considered to play a crucial role in suppressing tumor cell maturation, thus affecting surveillance of tumor cells themselves. Such an example is CD8+ cells that assist CD4+ cells in their antitumor immunity (23). Based on these data, it can be hypothesized that the analysis of changes in the ratio of neutrophils/platelets to lymphocytes has the potential to be developed into a method to determine tumor progression and the prognosis of patients with cancer. In other words, the two ratio parameters, namely NLR and PLR, have the potential to be developed as predictive markers in patients with BC.
In the present study, from the three parameters, namely ESRO, NLR and PLR, it was found that ESRO and PLR could distinguish between BC and normal groups, indicated by a value of P<0.01. Moreover, the NLR could distinguish the two groups (P>0.01). From the results of the t-test, it was found that the ESRO could distinguish the two groups better than PLR (P-value of 2.41x10-6 compared to 0.0044 (Fig. 1 and Table II). These results demonstrate that the ESRO was the optimal parameter able to differentiate the cancer group from normal group, even though it did not indicate that the cancer type was BC as the values are also different in other type of cancer.
The ESRO parameter also shows the best performance in distinguishing BC and N groups indicated by the AUC value on the ROC curve of ESRO, which is 0.8212 compared to 0.6513, and 0.7261 in the NLR and PLR, respectively (Fig. 2 and Table III). From these data, ESRO had the best ability to detect the presence of markers in the blood of patients with BC compared to the other two parameters. In general, the sensitivity of all parameters (ESRO, NLR and PLR) was higher than their specificity (sensitivity, 96.6, 86.2 and 89.7%, respectively; and specificity, 59.3, 40.7 and 55.6%, respectively). From the aforementioned data, the sensitivity and specificity of ESRO were greater than those of NLR and PLR. The sensitivity of ESRO, NLR and PLR in the present study this research was also higher compared to that of another study using POSTN, CEA and CA153 to differentiate between BC and normal samples, which were 71.7, 49.1 and 45.7% respectively (24). The ESRO also exhibited better sensitivity compared to combination of POSTN + CEA (96.6 vs. 74%), POSTN + CA153 (96.6 vs. 76.3%), and even POSTN + CEA + CA153 (96.6 vs. 70.5%), but had lower specificity compared to POSTN + CEA (59.3 vs. 78.3%), POSTN + CA153 (59.3 vs. 72.5%), and POSTN + CEA + CA153 (59.3 vs. 81.1%). Despite the lower specificity, ESRO still possess far superior sensitivity, with comparable specificity (24). Compared to previous study in epithelial ovarian cancer, ESRO in BC showed with higher sensitivity (96.6 vs. 83.3%), but lower specificity (59.3 vs. 90.6%) (13). Other than having good sensitivity and specificity, ESRO parameter was way easier to be obtained compared to proteomic analysis, showing its potential to be developed as a biomarker detection parameter in BC patients.
The inflammatory condition of cancer patients is closely related to the severity of cancer, which leads to its influence on patient survival. Inflammatory cells are also considered to be a trigger for cancer, allegedly through several mechanisms such as increasing cell proliferation so that DNA damage accumulates and induction of angiogenesis, which increases the potential for cancer cell metastasis. This indicates the importance of easy and inexpensive inflammatory detection methods that they can be applied to health units in remote areas that are not as sophisticated as in large cities, in the hope that they can detect inflammation as early as possible so that proper treatment can be done immediately. Thus, the progression of cancer can be suppressed and can have an impact on increasing patient survival. Compared to other methods, ESR examination has been widely performed in clinical settings in assessing the inflammatory condition of cancer patients. This method is also very inexpensive and simple; thus, it can be applied to health service units with minimal facilities. However, the present study still has limitations as the number of patients was minimal. Therefore, further research is required with a greater number of patients in various health care units in order to be able to generalize the results of the present study. Another limitation of the present study was the difference in age between the patients in the BC and normal group; thus, further research is warranted with similar ages between the BC and normal groups.
In conclusion, in the present study, it was found that patients with BC had significantly higher ESRO values compared to the normal healthy subjects. Based on the ROC, AUC, SE, sensitivity and specificity values, ESRO parameters exhibit a superior performance in the presence of BC cells compared with NLR or PLR.
Acknowledgements
The authors would like to thank Mrs. Tri Purwanti (a laboratory worker at the Inter-University Center, Universitas Gadjah Mada, Yogyakarta, Indonesia), for her technical assistance with the ESR observations.
Funding
Funding: The present study was funded by Community Funding Grant from Faculty of Medicine, Public Health, and Nursing UGM, with reference no. 2019.1485/UNI/KK.KMK/PP/PT/2019.
Availability of data and materials
The data generated in the present study may be requested from the corresponding author.
Authors' contributions
N, KAN and MP were involved in the conception and design of the study, and in the drafting of the manuscript. US, KAN and MP were involved in data acquisition. N was involved in data analysis and interpretation, in the critical revision of the manuscript, in the statistical analysis and in the provision of funding. NG, US, KAN and MP were involved in administrative duties, and technical and material support. N supervised the study. All authors confirm the authenticity of all raw data. All authors have read and approved the final manuscript.
Ethics approval and consent to participate
The present study was ethically approved by the Ethical Committee of Faculty of Medicine, Public Health, and Nursing Universitas Gadjah Mada on September 13, 2018, with reference no. of KE/FK/0965/EC/2018, followed by the amendment that was approved by the same ethical committee on April 23, 2019. All test subjects stated in writing that they were willing to be the subjects of this study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
|
Globocan, ‘Breast: Fact sheet,’ The Global Cancer Observatory. Accessed on March 31, 2024. Available from: https://gco.iarc.who.int/media/globocan/factsheets/cancers/20-breast-fact-sheet.pdf. | |
|
Globocan, ‘Indonesia: Fact sheets,’ The Global Cancer Observatory. Accessed on March 31,. 2024, from https://gco.iarc.who.int/media/globocan/factsheets/populations/360-indonesia-fact-sheet.pdf. | |
|
Damsees R, Jaghbir M, Salam M, Al-Omari A and Al-Rawashdeh N: Unravelling the predictors of late cancer presentation and diagnosis in Jordan: A cross-sectional study of patients with lung and colorectal cancers. BMJ Open. 13(e069529)2023.PubMed/NCBI View Article : Google Scholar | |
|
Sarhadi VK and Armengol G: Molecular biomarkers in cancer. Biomolecules. 12(1021)2022.PubMed/NCBI View Article : Google Scholar | |
|
Chakrabortty A, Patton DJ, Smith BF and Agarwal P: miRNAs: Potential as biomarkers and therapeutic targets for cancer. Genes (Basel). 14(1375)2023.PubMed/NCBI View Article : Google Scholar | |
|
Kwon YW, Jo HS, Bae S, Seo Y, Song P, Song M and Yoon JH: Application of proteomics in cancer: Recent trends and approaches for biomarkers discovery. Front Med (Lausanne). 8(747333)2021.PubMed/NCBI View Article : Google Scholar | |
|
Nigam M, Mishra AP, Deb VK, Dimri DB, Tiwari V, Bungau SG, Bungau AF and Radu AF: Evaluation of the association of chronic inflammation and cancer: Insights and implications. Biomed Pharmacother. 164(115015)2023.PubMed/NCBI View Article : Google Scholar | |
|
Zhao H, Wu L, Yan G, Chen Y, Zhou M, Wu Y and Li Y: Inflammation and tumor progression: Signaling pathways and targeted intervention. Signal Transduct Target Ther. 6(263)2021.PubMed/NCBI View Article : Google Scholar | |
|
Zhou K, Cao J, Lin H, Liang L, Shen Z, Wang L, Peng Z and Mei J: Prognostic role of the platelet to lymphocyte ratio (PLR) in the clinical outcomes of patients with advanced lung cancer receiving immunotherapy: A systematic review and meta-analysis. Front Oncol. 12(962173)2022.PubMed/NCBI View Article : Google Scholar | |
|
Tang KY, Zhang HL, Zhang XY and Jin HZ: Clinical and laboratory features between anti-TIF1γ dermatomyositis with and without malignancy: 37 case series and a review. J Dermatol. 51:1646–1657. 2024.PubMed/NCBI View Article : Google Scholar | |
|
Zhbanov A and Yang S: Effects of aggregation on blood sedimentation and conductivity. PLoS One. 10(e0129337)2015.PubMed/NCBI View Article : Google Scholar | |
|
Piva E, Stoppa A, Pelloso M and Plebani M: The VES-Matic 5 system: Performance of a novel instrument for measuring erythrocyte sedimentation rate. Clin Chem Lab Med. 607:1081–1090. 2022.PubMed/NCBI View Article : Google Scholar | |
|
Ngadikun Pradjatmo H, Nugroho KA, Pasala M and Prasetyastuti : Analysis of erythrocyte sedimentation rate order in epithelial ovarian cancer. J Cancer. 14:2173–2180. 2023.PubMed/NCBI View Article : Google Scholar | |
|
Ford IJ: Measures of thermodynamic irreversibility in deterministic and stochastic dynamics. New J Phys. 17(075017)2015. | |
|
Çorbacıoğlu SK and Aksel G: Receiver operating characteristic curve analysis in diagnostic accuracy studies: A guide to interpreting the area under the curve value. Turk J Emerg Med. 23:195–198. 2023.PubMed/NCBI View Article : Google Scholar | |
|
Saad HM, Tourky GF, Al-Kuraishy HM, Al-Gareeb AI, Khattab AM, Elmasry SA, Alsayegh AA, Hakami ZH, Alsulimani A, Sabatier JM, et al: The potential role of MUC16 (CA125) biomarker in lung cancer: A magic biomarker but with adversity. Diagnostics (Basel). 12(2985)2022.PubMed/NCBI View Article : Google Scholar | |
|
Loibl S, Poortmans P, Morrow M, Denkert C and Curigliano G: Breast cancer. Lancet. 397:1750–1769. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Landgraf L, Christner C, Storck W, Schick I, Krumbein I, Dähring H, Haedicke K, Heinz-Herrmann K, Teichgräber U, Reichenbach JR, et al: A plasma protein corona enhances the biocompatibility of Au@Fe3O4 Janus particles. Biomaterials. 68:77–88. 2015.PubMed/NCBI View Article : Google Scholar | |
|
Müller LK, Simon J, Schöttler S, Landfester K, Mailänder V and Mohr K: Pre-coating with protein fractions inhibits nano-carrier aggregation in human blood plasma. RSC Adv. 6:96495–96509. 2016. | |
|
Pahari S, Sun L and Alexov E: PKAD: A database of experimentally measured pKa values of ionizable groups in proteins. Database (Oxford). 1(baz024)2019.PubMed/NCBI View Article : Google Scholar | |
|
Poto R, Cristinziano L, Modestino L, de Paulis A, Marone G, Loffredo S, Galdiero MR and Varricchi G: Neutrophil extracellular traps, angiogenesis and cancer. Biomedicines. 10(431)2022.PubMed/NCBI View Article : Google Scholar | |
|
Stoiber D and Assinger A: Platelet-leukocyte interplay in cancer development and progression. Cells. 9(855)2020.PubMed/NCBI View Article : Google Scholar | |
|
Kushekhar K, Chellappa S, Aandahl EM and Taskén K: Role of lymphocytes in cancer immunity and immune evasion mechanisms. In: Biomarkers of the Tumor Microenvironment. Springer International Publishing, Cham, pp159-182, 2022. doi: 10.1007/978-3-030-98950-7_10. | |
|
Jia L, Li G, Ma N, Zhang A, Zhou Y, Ren L and Dong D: Soluble POSTN is a novel biomarker complementing CA153 and CEA for breast cancer diagnosis and metastasis prediction. BMC Cancer. 22(760)2022.PubMed/NCBI View Article : Google Scholar |