Yuanhuacine modulates lipopolysaccharide‑induced interleukin-6 through regulation of the JAK1/STAT3 pathway and prevents tubular damage in acute kidney injury
- Authors:
- Published online on: July 3, 2025 https://doi.org/10.3892/etm.2025.12918
- Article Number: 168
-
Copyright: © Park et al. This is an open access article distributed under the terms of Creative Commons Attribution License.
Abstract
Introduction
Inflammation is an immune response that protects the host from pathogenic infections or tissue damage (1). Lipopolysaccharide (LPS), a component of Gram-negative bacteria, binds to Toll-like receptor 4 and activates signaling pathways that induce the production of inflammatory cytokines, promoting immune cell-mediated inflammation (2,3). LPS-induced inflammation has been shown to be regulated by nuclear factor κB (NF-κB), Janus kinase/signal transducer and activator of transcription (JAK/STAT), and mitogen-activated protein kinase (MAPK) signaling pathways (4).
Interleukin 6 (IL-6) is a multifunctional cytokine that enhances immune responses by activating immune cells and plays a critical role in various inflammatory diseases (5). But uncontrolled IL-6 expression has been strongly associated with inflammatory and autoimmune diseases, including sepsis and rheumatoid arthritis (6,7). Therefore, effective regulation of IL-6 is essential for maintaining physiological homeostasis and preventing inflammatory disorders.
The JAK/STAT pathway is a key signaling cascade involved in IL-6 expression. This pathway is activated in several inflammatory conditions, including cancer and acute kidney injury (AKI) (8,9). Upon ligand binding, such as with IL-6 or interferon-γ, receptors activate JAK proteins, leading to the phosphorylation and subsequent activation of STAT proteins. Phosphorylated STATs translocate to the nucleus, where they induce the expression of target genes, including proinflammatory cytokines such as IL-6(10). Since many diseases may be linked to the uncontrolled JAK/STAT pathway, it needs to be tightly regulated.
AKI is a common complication in patients with sepsis, which is characterized by a sudden decline in renal function (11). Globally, approximately six million cases of septic AKI occur annually, with nearly half of patients in intensive care developing acute renal impairment, which is associated with high mortality rates (12). A recent study suggests that inflammation caused by LPS contributes to septic kidney injury (13). This inflammation is closely related to the excessive secretion of inflammatory cytokines by macrophages, causing tubular damage. When inflammation occurs, circulating monocytes migrate to the kidneys and differentiate into macrophages to repair damaged tissue by secreting various cytokines (14,15). However, excessive cytokine production can lead to detrimental effects, contributing to tissue damage. In particular, IL-6 has been identified as a key mediator in the progression of kidney injury following AKI, where its overexpression exacerbates the inflammatory response and worsens kidney damage (16,17). Furthermore, the JAK/STAT pathway has been implicated in AKI pathogenesis (8,18). Thus, targeting the IL-6 and JAK/STAT pathways represents a potential strategy for mitigating kidney disease progression.
Yuanhuacine (YC), a diterpene compound isolated from Daphne genkwa, has been reported to exert potent anticancer effects in various cancer cell lines and tumor models (19). Recent studies have demonstrated that YC functions as a topoisomerase 1 inhibitor with potent anticancer effects in BL2 triple-negative breast cancer and non-small cell carcinoma cell and animal models (20-22). Additionally, several signaling pathways have been implicated in the anti-tumor activity of YC. However, the modulation of cytokines and the anti-inflammatory potential of YC in AKI remain unexplored.
In this study, we aimed to investigate the regulatory effects of YC on pro-inflammatory cytokine production in LPS-stimulated human macrophages and evaluate its protective role in an animal model of AKI.
Materials and methods
Cell cultivation and treatment
Human THP-1 cells were obtained from the Korea Cell Line Bank and maintained in RPMI 1640 medium supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin at 37˚C in a 5% CO2 incubator. To induce macrophage-like differentiation, THP-1 monocytes were treated with 10 nM phorbol 12-myristate 13-acetate (Sigma-Aldrich) for 24 h (23). Differentiated cells were then stimulated with 5 ng/ml LPS (Sigma-Aldrich). This LPS concentration was established under conditions that did not saturate cytokine expression (24). YC (MedChem Express) was administered at varying concentrations for experimental analyses. Based on cytotoxicity tests and previous reports (22), YC showed no cytotoxicity up to 10 µM when used alone, but a reduction in cell viability was observed at 10 µM when co-treated with LPS. Possible saturation effects were noted at concentrations above 1 µM (data not shown). Therefore, in this report, concentrations below 1 µM were selected to avoid cytotoxicity and ensure accurate assessment of pharmacological activity. YC was dissolved in dimethyl sulfoxide (DMSO) and used at a final concentration of ≤0.1%; 0.1% DMSO treatment was used as a negative control, which did not affect cell activation. To assess JAK inhibition, cells were treated with 2.5, 5, or 10 µM filgotinib, while STAT3 inhibition was evaluated using 25, 50, or 100 µM S3I-201 (Axon Medchem) as comparative groups.
Cell viability assay [Cell Counting Kit (CCK)-8]
THP-1 cells were seeded at a density of 5x104 cells/well in 96-well plates and differentiated into macrophages. Differentiated cells were treated with YC at concentrations ranging from 0.1 nM to 10 µM for 24 h, with or without co-treatment with LPS (5 ng/ml). Following incubation, a CCK-8 viability assay (Donginbio) was performed according to the manufacturer's instructions. The plates were incubated at 37˚C for 1 h, and absorbance was measured at 450 nm using an EMax Plus Microplate Reader (Molecular Devices).
Reverse transcription polymerase chain reaction (RT-PCR)
Total RNA was extracted from differentiated and activated THP-1 cells using TRI reagent (Molecular Research Center), according to the manufacturer's protocol. RNA concentrations were adjusted to 1 µg per sample, and complementary DNA (cDNA) synthesis was performed using Moloney murine leukemia virus (M-MLV) reverse transcriptase (Promega) and oligo(dT) primers. Conventional PCR was carried out using a PCR premix (Bioneer). PCR amplification was performed in a C1000 Touch Thermal Cycler (Bio-Rad Laboratories) under the following conditions: initial denaturation at 95˚C for 5 min; followed by 26 cycles of denaturation at 95˚C for 30 sec, annealing at 50-65˚C for 30 sec, and extension at 72˚C for 30 sec; with a final extension at 72˚C for 5 min and holding at 4˚C. The primer sequences used are listed in Table I.
ELISA for IL-6 detection
THP-1 cells (5x105 cells/ml) were seeded in 48-well plates for IL-6 measurement. After 24 h of LPS or YC treatment, the cell culture supernatants were collected by centrifugation at 10,000 x g for 5 min. The levels of IL-6 in the supernatants were quantified using an ELISA kit (BD Biosciences; cat. no. 555220), according to the manufacturer's instructions. Optical density was measured at 450 nm using an EMax Plus Microplate Reader (Molecular Devices), and cytokine concentrations were determined based on a standard curve.
Western blotting analysis
For in vitro experiments, cells were lysed using RIPA buffer (LPS Solution) supplemented with a protease inhibitor cocktail (Roche) and phosphatase inhibitors 2 and 3 (Sigma-Aldrich) to prevent protein degradation. For in vivo experiments, tissues were lysed using NP-40 buffer. Protein concentrations were determined using the Bradford assay with reagents purchased from Takara (Takara Bio Inc.), following the standard protocol described by Bradford. Lysates were mixed with 5X loading buffer, heated at 100˚C for 10 min, and separated on a 10% Tris-glycine gel. Proteins were transferred onto polyvinylidene difluoride (PVDF) membranes (Millipore). To block nonspecific binding, membranes were incubated in 5% skim milk for 1 h, followed by washing with phosphate-buffered saline containing 0.05% Tween-20 (PBST; Duchefa Biochemie). Membranes were then incubated overnight at 4˚C with primary antibodies (listed in Table II). Detection was performed using horseradish peroxidase-conjugated secondary antibodies (Enzo Life Sciences), and signals were visualized using a chemiluminescent substrate (Cyanagen Srl, Bologna, Italy).
Nuclear and cytoplasmic protein fractionation for mobile protein identification
Differentiated THP-1 cells were treated with YC (8, 40, and 200 nM), with or without LPS (5 ng/ml), for 2 h to investigate protein translocation. Cells were detached using Accutase solution (Innovative Cell Technologies), and nuclear and cytoplasmic protein fractions were extracted using the NE-PER Nuclear and Cytoplasmic Extraction Kit (Thermo Fisher Scientific, Inc.; cat. no. 78833), according to the manufacturer's instructions. Subsequently, the intracellular positions of phosphorylated STAT3 (p-STAT3) and total STAT3 were confirmed by western blotting analysis as described above.
Immunofluorescence staining
Macrophage-like THP-1 cells were treated with varying concentrations of YC and LPS for 2 h, fixed onto slide glass, permeabilized with methanol, and blocked with 1% bovine serum albumin (BSA; bioWORLD). Fixed cells were incubated overnight at 4˚C with primary antibodies against p-STAT3 and STAT3 (both at 1:200 dilution; Cell Signaling Technology). Alexa Fluor 488-conjugated goat anti-rabbit secondary antibody (1:500 dilution; Life Technologies) was used for fluorescence detection. After washing, drying, and mounting with Roche mounting media, slides were examined at room temperature using a Zeiss LSM 510 Meta confocal microscope (Zeiss). Images were analyzed using Zeiss microscopy software (Zeiss).
Mice and housing
Male C57BL/6N mice (6-8 weeks old, weighing 24-27 g) were purchased from Koatech and maintained under specific pathogen-free conditions with free access to food and water (25); 4-5 mice were housed per cage. All animal procedures were approved by the Institutional Animal Care and Use Committee (IACUC) of the Korea Research Institute of Bioscience and Biotechnology (KRIBB; approval no.: KRIBB-AEC-24265) and conducted in accordance with Korean national animal welfare regulations and the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals.
Establishment of an LPS-induced AKI mouse model and treatment evaluation
The mice were divided into four groups for the experiment: Control, LPS, YC (0.2 µg/kg) + LPS, and YC (5 µg/kg) + LPS. On day 0, the mice were administered YC intraperitoneally 30 min prior to LPS injection. Two doses of YC (0.2 and 5 µg/kg) were chosen based on previous studies investigating the toxicity and effects of YC on renal function (26,27). Thirty minutes later, the mice received an intraperitoneal injection of LPS (10 mg/kg; Sigma-Aldrich) dissolved in PBS (28,29), while the control group was given vehicle treatment. Blood samples (~500 µl per mouse) were collected via retro-orbital bleeding under 2-3% isoflurane anesthesia in accordance with protocols approved by the IACUC and international animal welfare guidelines. The mice were euthanized immediately after blood collection by cervical dislocation to minimize pain. This procedure was performed quickly and skillfully by trained personnel. Kidneys were promptly harvested, then either stored at -80˚C for later experiments or fixed in 10% formalin for subsequent analysis. Acute kidney injury (AKI) was confirmed within 48 h by an increase in baseline serum creatinine (sCr) of 2.3-fold according to the KDIGO guidelines (30).
Blood biochemistry analysis
Serum urea nitrogen and creatinine levels in the AKI mouse model were analyzed by KP&T as a commissioned service.
RT-quantitative (q)PCR analysis in mouse kidney tissue
Total RNA was extracted from mouse kidney tissues using TRI reagent (Molecular Research Center) according to the manufacturer's instructions. RNA concentrations were normalized to 1 µg per sample for cDNA synthesis. qPCR was performed using the QuantStudio 3 Real-Time PCR System (Applied Biosystems) and PowerUp SYBR Green Master Mix (Applied Biosystems), according to the manufacturer's protocol. The thermal cycling conditions included an initial uracil-DNA glycosylase activation step at 50˚C for 2 min, followed by polymerase activation and initial denaturation at 95˚C for 10 min. This was followed by 50-60 cycles of denaturation at 95˚C for 15 sec and annealing/extension at 60˚C for 1 min. A melt curve analysis was conducted following amplification to confirm the specificity of the PCR products. Target gene expression levels were normalized to GAPDH expression and quantified using the comparative 2-ΔΔCq method (31). Primer sequences used for quantitative PCR are listed in Table III.
Renal immunostaining in the AKI mouse model
For histological analysis, mouse kidneys were fixed in 10% formalin for 24 h, embedded in paraffin, and sectioned at a thickness of 4 µm. Tissue sections were stained with hematoxylin and eosin (H&E) for histopathological evaluation. For immunohistochemistry (IHC), the sections were permeabilized with 1% Triton X-100 solution (Sigma-Aldrich) and treated with peroxidase-blocking solution (Dako) to reduce nonspecific background staining. To block nonspecific binding, sections were incubated with 1% BSA (bioWORLD) for 10 min. After blocking, sections were incubated overnight at 4˚C with primary antibodies against p-STAT3 (1:200 dilution; Cell Signaling Technology) and p-JAK1 (1:200 dilution; Invitrogen; Thermo Fisher Scientific, Inc.). After washing, slides were incubated with a secondary antibody (1:500 dilution; Enzo Life Sciences) for 1 h at room temperature. The slides were counterstained with hematoxylin, followed by bluing using a bluing buffer, and then immersed in distilled water. Finally, slides were mounted with mounting solution (Sigma-Aldrich), and images were captured using a light microscope (Leica).
Renal histological assessment in the AKI mouse model
Based on a previous study (32), the H&E-stained kidney slides were histologically graded for tubular injury. Histological evaluation was performed according to three criteria of kidney injury: tubular necrosis, tubular vacuolization, and tubular cast formation. Severity was graded on a scale from 0 to 3 as follows: 0 (damage in <5% of tubules), 1 (damage in 5-33% of tubules), 2 (damage in 34-66% of tubules), and 3 (damage in >66% of tubules). Twenty random fields per kidney sample were analyzed at 20x magnification.
Statistical analysis
Statistical analysis was performed using GraphPad Prism version 10.0 (GraphPad Software, Inc.). Data are presented as the mean ± standard deviation. One-way ANOVA followed by Tukey's or Dunnett's multiple comparisons test, or Kruskal-Wallis test followed by Dunn's multiple comparisons test were performed depending on data distribution. Two-way ANOVA followed by Šidák post hoc test was also performed for multiple comparisons. P<0.05 was considered to indicate a statistically significant difference. Image processing and quantification were conducted using ImageJ software (National Institutes of Health).
Results
YC co-treatment effectively attenuates LPS-induced IL-6 expression in differentiated THP-1 cells
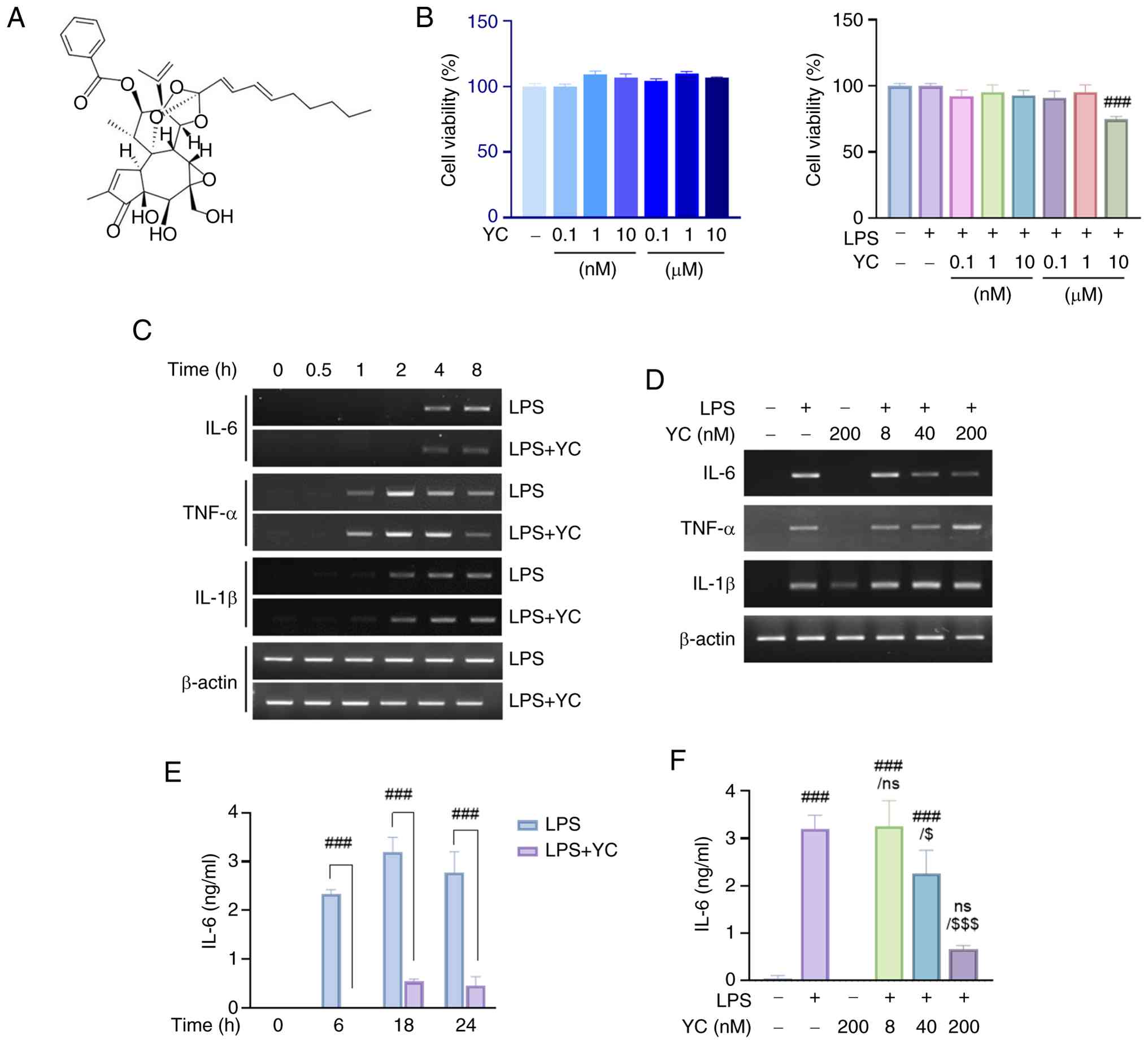
YC, a diterpene compound (Fig. 1A), was evaluated for cytotoxicity by treating cells with various concentrations (0.1 nM-10 µM) for 24 h in the presence or absence of LPS (5 ng/ml). As shown in Fig. 1B, YC alone did not exhibit cytotoxicity up to 10 µM (left panel). However, co-treatment with LPS resulted in approximately a 30% reduction in cell viability at 10 µM (right panel). Since a saturation effect was observed at concentrations above 1 µM (data not shown), subsequent experiments were conducted using YC at concentrations below 1 µM to avoid both cytotoxic effects and potential saturation associated with higher concentrations.
To assess the effects of YC, we examined its impact on LPS-induced cytokine expression using RT-PCR and ELISA. THP-1 cells were treated with 5 ng/ml LPS and 200 nM YC. We found that TNF-α was initially expressed with LPS induction at 1 h; subsequently, TNF-α expression appeared to increase in the YC group at 4 h. IL-1β expression started at 2 h after treatment, and there was minimal variation in comparison to the LPS treatment group. However, the IL-6 expression was decreased by YC, following 4 h of administration, in contrast to the LPS treatment group (Fig. 1C).
To further validate these results, YC was administered at varying concentrations (8, 40, and 200 nM) at 4 h, at which time the presence of IL-6 was confirmed. RT-PCR analysis showed a dose-dependent decrease in IL-6 mRNA expression after LPS stimulation, but not in TNF-α and IL-1β expression, by YC (Fig. 1D). Moreover, to confirm the effect on IL-6 inhibition at the protein level, THP-1 cells were treated with 5 ng/ml LPS and 200 nM YC, and their effects on the cells were recorded over time (0, 6, 18, and 24 h). As with previous RT-PCR data, the expression of IL-6 induced by LPS treatment was significantly suppressed by YC (Fig. 1E). Additionally, ELISA results for IL-6 protein levels treated with various concentrations of YC confirmed dose-dependent inhibition after 24 h of treatment (Fig. 1F).
YC inhibits phosphorylation of the JAK1/STAT3 pathway in LPS-treated THP-1 macrophages
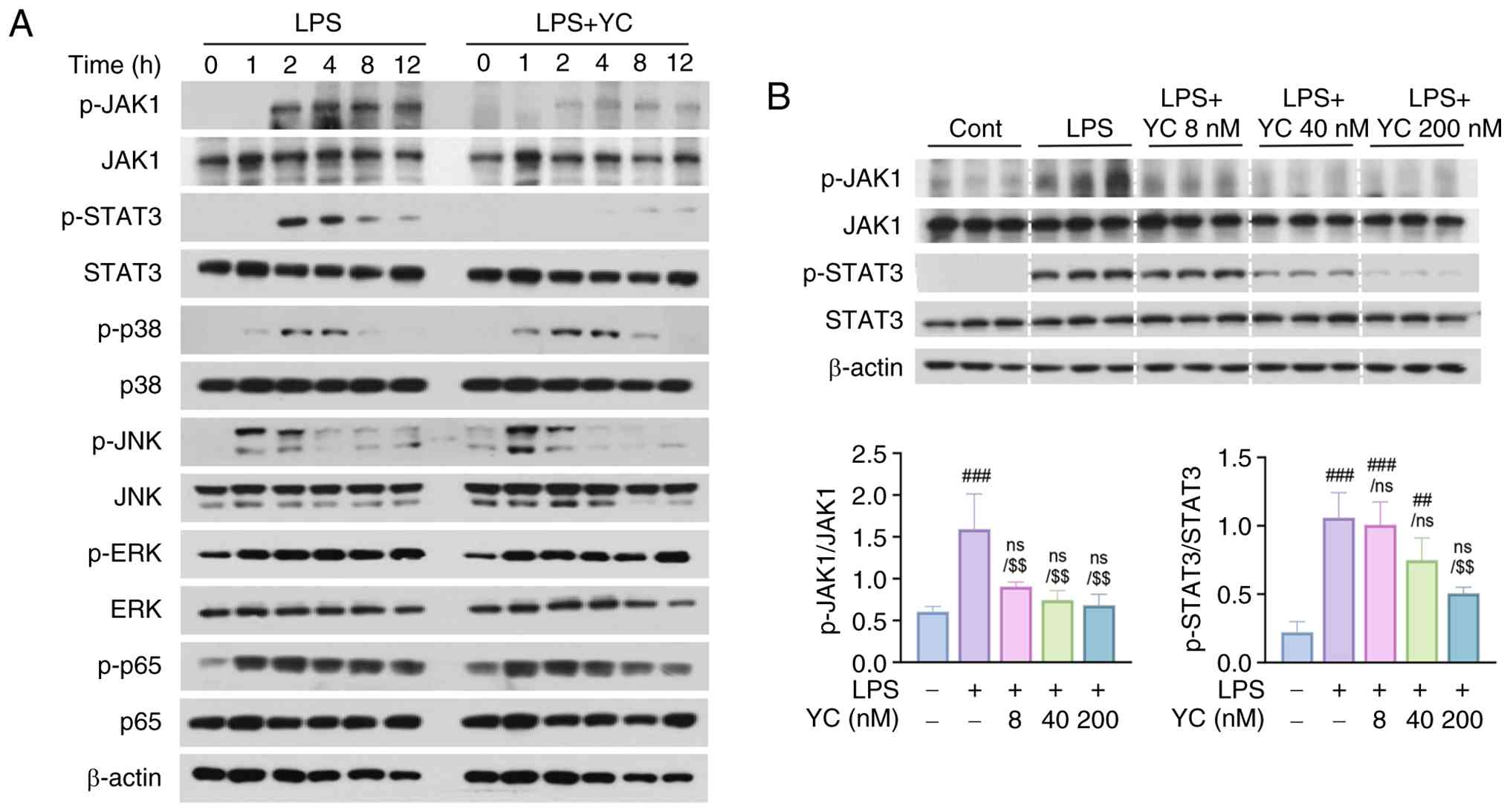
To determine how YC specifically regulates LPS-induced IL-6 expression, we analyzed LPS-related pathways using western blotting. The total protein levels of key signaling molecules, including NF-κB, MAPK, and JAK/STAT3, remained unchanged in LPS- and 200 nM YC-treated cells over 12 h. Phosphorylation of p65, p38, extracellular signal-regulated kinase, and c-Jun N-terminal kinase was detected within 1 h of LPS treatment; however, YC co-treatment did not significantly affect the phosphorylation of these molecules. In contrast, phosphorylation of JAK1 and STAT3 was observed at 2 h of LPS treatment, and YC co-treatment effectively inhibited the phosphorylation of these proteins (Fig. 2A). The JAK/STAT pathway is a critical signaling cascade involved in IL-6 production (10). To further elucidate the concentration-dependent effects of YC, an additional experiment was conducted to assess its impact on the expression and phosphorylation levels of JAK1 and STAT3. The results confirmed that YC suppressed JAK1 and STAT3 phosphorylation in a concentration-dependent manner (Fig. 2B). These findings suggest that the YC-mediated inhibition of JAK1/STAT3 activation is a key mechanism underlying the suppression of IL-6 expression.
YC inhibits STAT3 transcriptional activity by preventing its nuclear translocation
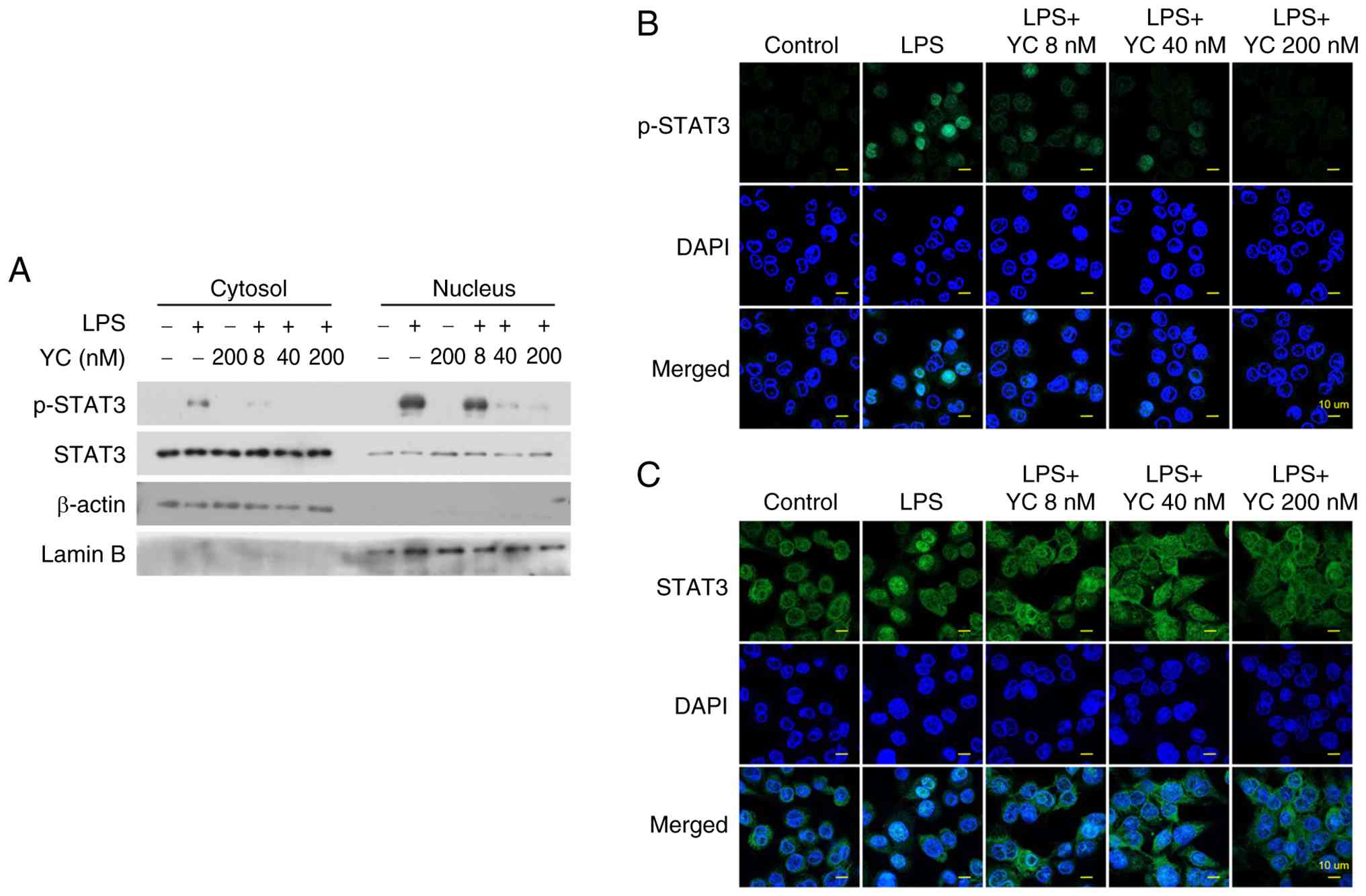
To investigate whether YC prevents STAT3 nuclear translocation, we analyzed cytoplasmic and nuclear fractions of THP-1 cells treated with 5 ng/ml LPS and various concentrations of YC. Protein immunoblotting revealed that activated STAT3 translocated to the nucleus in response to LPS stimulation. However, in cells treated with YC, phosphorylated STAT3 levels were reduced in both the cytoplasmic and nuclear fractions in a dose-dependent manner (Fig. 3A). To further confirm these findings, confocal microscopy was performed to visualize STAT3 localization. In unstimulated cells, STAT3 was primarily localized in the cytoplasm. Following LPS treatment, phosphorylated STAT3 accumulated in the nucleus. However, YC treatment effectively blocked STAT3 phosphorylation, leading to a significant reduction in nuclear translocation (Fig. 3B and C).
Comparative efficacy of YC and JAK/STAT inhibitors in IL-6 suppression
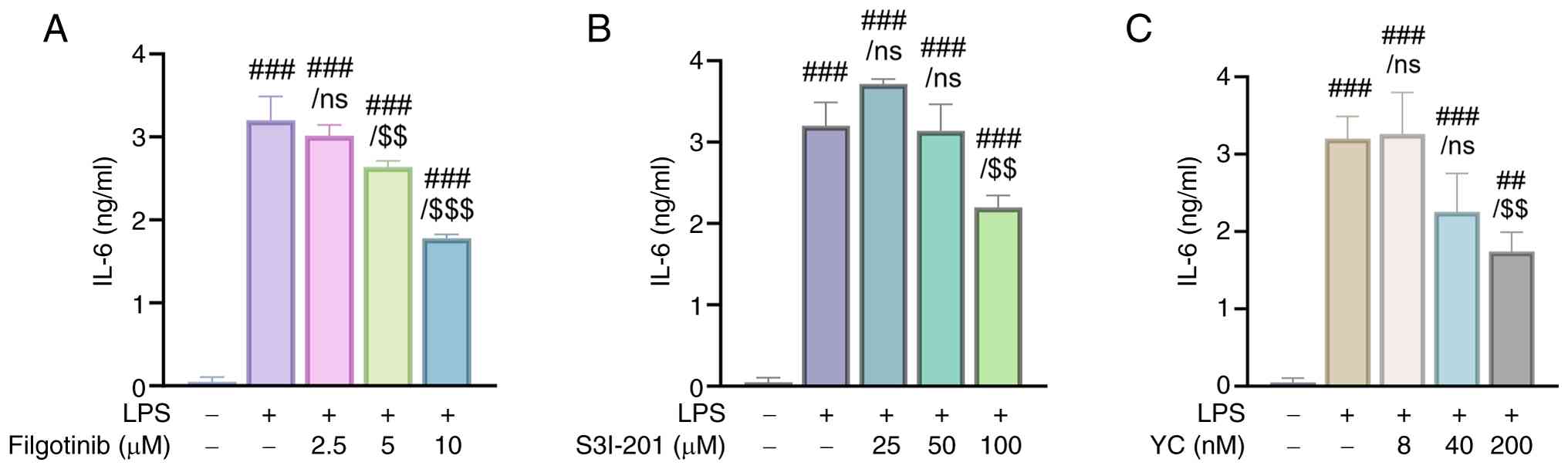
To evaluate the efficacy of YC in suppressing LPS-induced IL-6 expression via inhibition of JAK1 and STAT3, we compared its effects with those of known inhibitors. Specifically, filgotinib, a selective JAK1 inhibitor, and S3I-201, a STAT3 inhibitor, were used for comparison. The results showed that LPS stimulation significantly increased IL-6 expression in THP-1 cells. However, treatment with filgotinib, S3I-201, or YC reduced IL-6 expression in a dose-dependent manner (Fig. 4A-C). In particular, YC exhibited similar inhibitory effects at markedly lower concentrations than those inhibitors. These findings suggest that LPS-induced IL-6 expression in THP-1-derived macrophages is regulated via inhibition of JAK and STAT3, and that YC is a potential therapeutic agent for various diseases associated with IL-6 and the JAK1/STAT3 pathway.
YC alleviates severe inflammation in an AKI mouse model
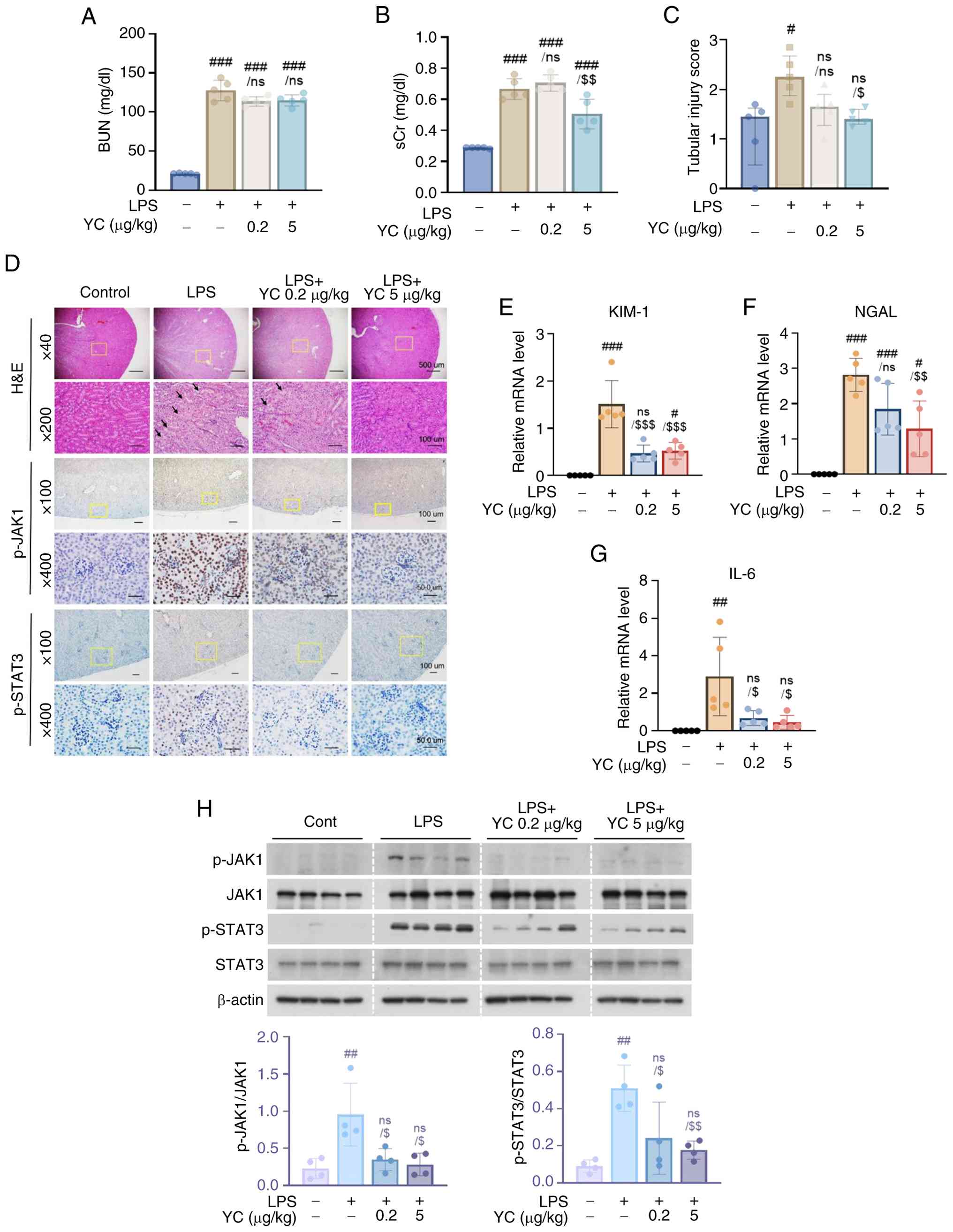
Through the above experiments, we found that YC inhibits the expression of LPS-induced IL-6 in human macrophages. Therefore, we hypothesized that YC could alleviate tubular damage caused by macrophages in an AKI disease model, a frequent complication of LPS-induced sepsis (33). AKI results in an abrupt deterioration in renal function (11). To evaluate the efficacy of YC in an LPS-induced AKI mouse model, we identified renal function markers and evaluated histopathological changes. As shown in Fig. 5A and B, YC treatment at 5 µg/kg slightly reduced sCr levels, though no significant decrease in blood urea nitrogen was observed. These findings suggest that YC confers partial protection against kidney dysfunction (34). To evaluate the impact of YC on kidney tubular damage, histological changes in H&E-stained kidney tissues were examined following LPS and YC treatment (32). Compared to kidneys treated with LPS alone, YC treatment alleviated renal pathological damage, including tubular necrosis, vacuolization, and cast formation (Fig. 5C and D).
The mRNA expression levels of neutrophil gelatinase-associated lipocalin (NGAL) and kidney injury molecule-1 (KIM-1), well-established biomarkers of renal injury (35), were analyzed by RT-qPCR. The results showed that LPS-induced kidney injury led to a marked increase in NGAL and KIM-1 expression, whereas YC treatment reduced their mRNA levels in a dose-dependent manner (Fig. 5E and F). These results confirm that LPS effectively induces septic AKI and that YC treatment mitigates renal injury.
Inflammation significantly contributes to renal injury in sepsis, with IL-6 serving as a valuable prognostic marker for AKI (36). Therefore, we assessed IL-6 expression in kidney tissues using RT-qPCR. As shown in Fig. 5G, LPS administration significantly increased IL-6 mRNA levels in kidney tissue, whereas YC treatment suppressed IL-6 expression.
YC suppresses JAK1 and STAT3 phosphorylation in the kidney tissues of the AKI mouse model
To determine whether the regulation of the JAK1/STAT3 axis in macrophages by YC also applies to renal tissues, we investigated the expression of JAK1/STAT3 in renal tissues in the AKI mouse model by western blotting and IHC. As shown in Fig. 5H, YC pretreatment effectively reduced JAK1 and STAT3 phosphorylation in LPS-treated kidneys. IHC analysis further confirmed that JAK1/STAT3 phosphorylation was significantly elevated in LPS-treated kidneys compared to normal controls, whereas YC treatment attenuated JAK1 activation and restored STAT3 activation to basal levels (Fig. 5D). These findings suggest that YC exerts prophylactic effects against septic AKI by inhibiting JAK1/STAT3 signaling and IL-6 expression.
Discussion
Our study demonstrates that YC suppresses LPS-induced IL-6 production by inhibiting the JAK1/STAT3 signaling pathway. In a mouse model of AKI, a condition worsened by LPS-induced pro-inflammatory cytokines, YC treatment significantly reduced tubular damage and inhibited IL-6 expression mediated by the JAK1/STAT3 pathway. While previous research on YC has primarily focused on its anticancer effects and mechanisms within tumor cells (19), its role in macrophage biology and inflammatory diseases has remained largely unexplored.
According to a previous study (21), YC treatment induces the differentiation of THP-1 monocytes into macrophage-like cells via PKC activation, similar to the effect of PMA. This differentiation is characterized by morphological changes and the upregulation of pro-inflammatory cytokines such as TNF-α, IL-1β, and IL-6. Notably, the C-6,7 epoxide moiety of YC has been identified as a key pharmacophore responsible for this effect. Consistent with these findings, we also observed similar morphological changes in our study (data not shown). This may explain the slight early-phase increase in TNF-α and IL-1β mRNA levels observed in our experiments. Due to its structural characteristics, YC activates immune cells but does not exacerbate inflammation. Rather, early cytokine induction may promote pathogen clearance, supporting the potential role of YC as a pro-resolution agent in inflammation, in line with the concept proposed by Fullerton and Gilroy (37).
In the same previous study (21), it was also reported that YC activates genes related to the NF-κB signaling pathway while inhibiting the expression of IL-10, a STAT3-mediated cytokine. Similar effects were observed in the murine macrophage cell line Raw 264.7. Although STAT3 itself was not directly examined in that study, the results suggest that YC may regulate STAT3-dependent cytokine production in macrophages, which aligns with our current findings.
However, the precise molecular mechanisms by which YC specifically regulates IL-6 remain unclear. To address this, we evaluated the expression of SOCS3, a well-characterized negative regulator of STAT3(38). However, no significant changes in SOCS3 expression were observed following YC treatment, suggesting that SOCS3 may not play a critical role in the selective inhibition of STAT3 by YC. Given that our study demonstrates STAT3 inhibition by YC occurs in the cytoplasm, several underlying mechanisms may be proposed. Similar to known inhibitors such as Stattic, YC may target the SH2 domain of STAT3, thereby disrupting its dimerization (39). Another possibility is that YC may promote rapid dephosphorylation of STAT3 by activating specific phosphatases or facilitate its degradation via ubiquitination (40,41). To validate these hypotheses and elucidate the precise molecular targets of YC, further structural and biochemical analyses will be necessary.
In summary, our findings extend the therapeutic potential of YC beyond its previously recognized anticancer properties to include significant anti-inflammatory effects. By modulating macrophage-mediated immune responses through inhibition of the JAK1/STAT3/IL-6 axis, YC emerges as a promising candidate for the treatment of inflammatory diseases, particularly those driven by dysregulated STAT3 and IL-6 signaling pathways. These results suggest that YC may play a crucial role not only in oncology but also in inflammation therapy, providing valuable foundational data for the development of new treatments targeting STAT3 and IL-6-related pathologies.
Acknowledgements
Not applicable.
Funding
Funding: This work was supported by the Korea Research Institute of Bioscience and Biotechnology Research Initiative Program (grant no. KGM1232511).
Availability of data and materials
The data generated in the present study may be requested from the corresponding author.
Authors' contributions
UJP drafted the original manuscript, and was responsible for the design and execution of the experimental investigation, data analysis, curation and methodology. JWK supervised the study, contributed to the conceptualization, managed the project, secured funding, and reviewed and edited the manuscript. UJ and JW confirm the authenticity of all the raw data. All authors read and approved the final manuscript.
Ethics approval and consent to participate
All animal care and experimental protocols were conducted in accordance with the regulations of the Korea Research Institute of Bioscience and Biotechnology (KRIBB). The Institutional Animal Care and Use Committee of KRIBB approved the animal care and experimental protocols (approval no. KRIBB-AEC-24265). The authors confirm that all methods were performed in compliance with the relevant guidelines and regulations.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Use of artificial intelligence tools
During the preparation of this work, AI tools were used to improve the readability and language of the manuscript, and subsequently, the authors revised and edited the content produced by the AI tools as necessary, taking full responsibility for the ultimate content of the present manuscript.
References
|
Marshall JS, Warrington R, Watson W and Kim HL: An introduction to immunology and immunopathology. Allergy Asthma Clin Immunol. 14 (Suppl 2)(S49)2018.PubMed/NCBI View Article : Google Scholar | |
|
Page MJ, Kell DB and Pretorius E: The role of lipopolysaccharide-induced cell signalling in chronic inflammation. Chronic Stress (Thousand Oaks). 6(24705470221076390)2022.PubMed/NCBI View Article : Google Scholar | |
|
Zhang X, Tian X, Wang Y, Yan Y, Wang Y, Su M, Lv H, Li K, Hao X, Xing X and Song S: Application of lipopolysaccharide in establishing inflammatory models. Int J Biol Macromol. 279(135371)2024.PubMed/NCBI View Article : Google Scholar | |
|
Alanazi FJ, Alruwaili AN, Aldhafeeri NA, Ballal S, Sharma R, Debnath S, Sinha A, Rekha A, Khan NH, Alrashoud MM, et al: Pathological interplay of NF-κB and M1 macrophages in chronic inflammatory lung diseases. Pathol Res Pract. 269(155903)2025.PubMed/NCBI View Article : Google Scholar | |
|
Aliyu M, Zohora FT, Anka AU, Ali K, Maleknia S, Saffarioun M and Azizi G: Interleukin-6 cytokine: An overview of the immune regulation, immune dysregulation, and therapeutic approach. Int Immunopharmacol. 111(109130)2022.PubMed/NCBI View Article : Google Scholar | |
|
Hirano T: IL-6 in inflammation, autoimmunity and cancer. Int Immunol. 33:127–148. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Šundalić S, Košuta I, Baršić Lapić I, Rako I, Rogić D, Radonić R and Vujaklija Brajković A: Interleukin-6 and leukocyte cell population data in newly diagnosed sepsis-a prospective study. Medicina (Kaunas). 61(468)2025.PubMed/NCBI View Article : Google Scholar | |
|
Zhang Z, Zhao Z, Qi C, Zhang X, Xiao Y, Chen C, Zou Y, Chen X, Gu L, Huang J, et al: Butyrolactone I blocks the transition of acute kidney injury to chronic kidney disease in mice by targeting JAK1. MedComm (2020). 6(e70064)2025.PubMed/NCBI View Article : Google Scholar | |
|
Rose-John S: Interleukin-6 signalling in health and disease. F1000Res 9: F1000 Faculty Rev-1013, 2020. | |
|
Hu X, Li J, Fu M, Zhao X and Wang W: The JAK/STAT signaling pathway: From bench to clinic. Signal Transduct Target Ther. 6(402)2021.PubMed/NCBI View Article : Google Scholar | |
|
Kellum JA, Romagnani P, Ashuntantang G, Ronco C, Zarbock A and Anders HJ: Acute kidney injury. Nat Rev Dis Primers. 7(52)2021.PubMed/NCBI View Article : Google Scholar | |
|
Soukup J and Pliquett RU: Acute kidney injury during sepsis and prognostic role of coexistent chronic heart failure. J Clin Med. 14(964)2025.PubMed/NCBI View Article : Google Scholar | |
|
Baek JH: The impact of versatile macrophage functions on acute kidney injury and its outcomes. Front Physiol. 10(1016)2019.PubMed/NCBI View Article : Google Scholar | |
|
Zhu XX, Zheng GL, Lu QB, Su JB, Liu Y, Wang M, Sun QY, Hu JY, Bao N, Xiao PX, et al: Cichoric acid ameliorates sepsis-induced acute kidney injury by inhibiting M1 macrophage polarization. Eur J Pharmacol. 976(176696)2024.PubMed/NCBI View Article : Google Scholar | |
|
Hu X, Zhou W, Wu S, Wang R, Luan Z, Geng X, Xu N, Zhang Z, Ruan Z, Wang Z, et al: Tacrolimus alleviates LPS-induced AKI by inhibiting TLR4/MyD88/NF-κB signalling in mice. J Cell Mol Med. 26:507–514. 2022.PubMed/NCBI View Article : Google Scholar | |
|
Privratsky JR, Ide S, Chen Y, Kitai H, Ren J, Fradin H, Lu X, Souma T and Crowley SD: A macrophage-endothelial immunoregulatory axis ameliorates septic acute kidney injury. Kidney Int. 103:514–528. 2023.PubMed/NCBI View Article : Google Scholar | |
|
González-Lafuente L, Mercado-García E, Vázquez-Sánchez S, González-Moreno D, Boscá L, Fernández-Velasco M, Segura J, Kuro-O M, Ruilope LM, Liaño F and Ruiz-Hurtado G: Interleukin-6 as a prognostic marker in acute kidney injury and its klotho-dependent regulation. Nefrologia (Engl Ed). 44:818–829. 2024.PubMed/NCBI View Article : Google Scholar | |
|
Ibrahim H, Sharawy MH, Hamed MF and Abu-Elsaad N: Peficitinib halts acute kidney injury via JAK/STAT3 and growth factors immunomodulation. Eur J Pharmacol. 984(177020)2024.PubMed/NCBI View Article : Google Scholar | |
|
Bailly C: Yuanhuacin and related anti-inflammatory and anticancer daphnane diterpenes from Genkwa Flos-An overview. Biomolecules. 12(192)2022.PubMed/NCBI View Article : Google Scholar | |
|
Zhang S, Li X, Zhang F, Yang P, Gao X and Song Q: Preparation of yuanhuacine and relative daphne diterpene esters from Daphne genkwa and structure-activity relationship of potent inhibitory activity against DNA topoisomerase I. Bioorg Med Chem. 14:3888–3895. 2006.PubMed/NCBI View Article : Google Scholar | |
|
Fermaintt CS, Peramuna T, Cai S, Takahashi-Ruiz L, Essif JN, Grant CV, O'Keefe BR, Mooberry SL, Cichewicz RH and Risinger AL: Yuanhuacine is a potent and selective inhibitor of the basal-like 2 subtype of triple negative breast cancer with immunogenic potential. Cancers (Basel). 13(2834)2021.PubMed/NCBI View Article : Google Scholar | |
|
Kang JI, Hong JY, Lee HJ, Bae SY, Jung C, Park HJ and Lee SK: Anti-tumor activity of yuanhuacine by regulating AMPK/mTOR signaling pathway and actin cytoskeleton organization in non-small cell lung cancer cells. PLoS One. 10(e0144368)2015.PubMed/NCBI View Article : Google Scholar | |
|
Baxter EW, Graham AE, Re NA, Carr IM, Robinson JI, Mackie SL and Morgan AW: Standardized protocols for differentiation of THP-1 cells to macrophages with distinct M(IFNγ+LPS), M(IL-4) and M(IL-10) phenotypes. J Immunol Methods. 478(112721)2020.PubMed/NCBI View Article : Google Scholar | |
|
Park EK, Jung HS, Yang HI, Yoo MC, Kim C and Kim KS: Optimized THP-1 differentiation is required for the detection of responses to weak stimuli. Inflamm Res. 56:45–50. 2007.PubMed/NCBI View Article : Google Scholar | |
|
Sun X, Wang H, Liu Y, Yang Y, Wang Y, Liu Y, Ai S, Shan Z and Luo P: 5-methoxytryptophan alleviates lipopolysaccharide-induced acute kidney injury by regulating Nrf2-mediated mitophagy. J Inflamm Res. 17:9857–9873. 2024.PubMed/NCBI View Article : Google Scholar | |
|
Chen YY, Guo JM, Qian YF, Guo S, Ma CH and Duan JA: Toxicity of daphnane-type diterpenoids from Genkwa Flos and their pharmacokinetic profile in rat. Phytomedicine. 21:82–89. 2013.PubMed/NCBI View Article : Google Scholar | |
|
Yu JG, Guo J, Zhu KY, Tao W, Chen Y, Liu P, Hua Y, Tang Y and Duan JA: How impaired efficacy happened between Gancao and Yuanhua: Compounds, targets and pathways. Sci Rep. 7(3828)2017.PubMed/NCBI View Article : Google Scholar | |
|
Lee SH, Kim KH, Lee SM, Park SJ, Lee S, Cha RH, Lee JW, Kim DK, Kim YS, Ye SK and Yang SH: STAT3 blockade ameliorates LPS-induced kidney injury through macrophage-driven inflammation. Cell Commun Signal. 22(476)2024.PubMed/NCBI View Article : Google Scholar | |
|
Ban KY, Nam GY, Kim D, Oh YS and Jun HS: Prevention of LPS-induced acute kidney injury in mice by bavachin and its potential mechanisms. Antioxidants (Basel). 11(2096)2022.PubMed/NCBI View Article : Google Scholar | |
|
Kellum JA and Lameire N: KDIGO AKI Guideline Work Group. Diagnosis, evaluation, and management of acute kidney injury: A KDIGO summary (Part 1). Crit Care. 17(204)2013.PubMed/NCBI View Article : Google Scholar | |
|
Livak KJ and Schmittgen TD: Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.PubMed/NCBI View Article : Google Scholar | |
|
Gupta KK, Donahue DL, Sandoval-Cooper MJ, Castellino FJ and Ploplis VA: Abrogation of plasminogen activator inhibitor-1-vitronectin interaction ameliorates acute kidney injury in murine endotoxemia. PLoS One. 10(e0120728)2015.PubMed/NCBI View Article : Google Scholar | |
|
Chen H, Liu N and Zhuang S: Macrophages in renal injury, repair, fibrosis following acute kidney injury and targeted therapy. Front Immunol. 13(934299)2022.PubMed/NCBI View Article : Google Scholar | |
|
Wang W, Zhou PH, Hu W, Xu CG, Zhou XJ, Liang CZ and Zhang J: Cryptotanshinone hinders renal fibrosis and epithelial transdifferentiation in obstructive nephropathy by inhibiting TGF-β1/Smad3/integrin β1 signal. Oncotarget. 9:26625–26637. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Yousef Almulhim M: The efficacy of novel biomarkers for the early detection and management of acute kidney injury: A systematic review. PLoS One. 20(e0311755)2025.PubMed/NCBI View Article : Google Scholar | |
|
Balci Ç, Özcan MS, Aşci H, Karabacak P, Kuruşçu O, Taner R, Özmen Ö, Tepebaşi MY, İlhan İ and Çömlekçi S: Radiofrequency electromagnetic and pulsed magnetic fields protected the kidney against lipopolysaccharide-induced acute systemic inflammation, oxidative stress, and apoptosis by regulating the IL-6/HIF1α/eNOS and Bcl2/Bax/Cas-9 pathways. Medicina (Kaunas). 61(238)2025.PubMed/NCBI View Article : Google Scholar | |
|
Fullerton JN and Gilroy DW: Resolution of inflammation: A new therapeutic frontier. Nat Rev Drug Discov. 15:551–567. 2016.PubMed/NCBI View Article : Google Scholar | |
|
Sobah ML, Liongue C and Ward AC: SOCS proteins in immunity, inflammatory diseases, and immune-related cancer. Front Med (Lausanne). 8(727987)2021.PubMed/NCBI View Article : Google Scholar | |
|
Schust J, Sperl B, Hollis A, Mayer TU and Berg T: Stattic: A small-molecule inhibitor of STAT3 activation and dimerization. Chem Biol. 13:1235–1242. 2006.PubMed/NCBI View Article : Google Scholar | |
|
Liu X, Chen J and Liu L: DUSP2 inhibits the progression of lupus nephritis in mice by regulating the STAT3 pathway. Open Life Sci. 18(20220649)2023.PubMed/NCBI View Article : Google Scholar | |
|
Chen S, Zhang J, Sun D, Wu Y, Fang J, Wan X, Li S, Zhang S, Gu Q, Shao Q, et al: SYVN1 promotes STAT3 protein ubiquitination and exerts antiangiogenesis effects in retinopathy of prematurity development. Invest Ophthalmol Vis Sci. 64(8)2023.PubMed/NCBI View Article : Google Scholar |