Co‑occurrence of endometriosis with psoriasis and psoriatic arthritis: Genetic insights (Review)
- Authors:
- Published online on: July 10, 2025 https://doi.org/10.3892/etm.2025.12921
- Article Number: 171
-
Copyright: © Goulielmos et al. This is an open access article distributed under the terms of Creative Commons Attribution License.
Abstract
1. Introduction
Numerous studies have shown that women with endometriosis are at a higher risk for developing various diseases as comorbidities, including asthma (1), various types of cancer (2), cardiovascular and psychiatric diseases (3,4), hypothyroidism and fibromyalgia (1). Notably, over the past decade, it was found that women with endometriosis have increased prevalence of several autoimmune diseases such as autoimmune thyroid disorder, ankylosing spondylitis (AS), coeliac disease, multiple sclerosis, rheumatoid arthritis (RA), scleroderma, Sjogren's syndrome (SS), systemic lupus erythematosus (SLE) and inflammatory bowel diseases. A recent study highlighted an association between endometriosis and both psoriasis (PS) and psoriatic arthritis (PsA) (5). The comorbidity of autoimmune diseases with endometriosis supports the current understanding of dysregulation of the immune system observed in women with endometriosis (6). However, it remains under investigation whether the disrupted immunological response and chronic inflammation characteristic of endometriosis contribute to a long-term risk of developing autoimmune diseases.
Endometriosis is a common, inflammatory, estrogen-dependent, benign gynecologic disease, affecting 8-10% of women of reproductive age (7), which results in a vast array of gynecological problems, such as deep dyspareunia, dysmenorrhea, chronic pelvic pain, heavy or irregular menstrual bleeding, pain during intercourse, urinary tract symptoms, diarrhea, subfertility and infertility, in 30% of patients (8,9). This disorder is defined by the growth of endometrial tissue outside the uterine cavity on other organs, due to the ectopic localization of endometrial cells. Endometriosis is a multifactorial disease and its susceptibility depends on the complex interaction of genetic, epigenetic, immunologic, proinflammatory, pro-angiogenic, hormonal and environmental factors (10,11). In addition to earlier theories such as metaplasia (12) and the widely accepted retrograde tubal endometrial reflux theory of Sampson (13), other immune-mediated pathways including chronic inflammation, apoptosis, angiogenesis and endothelial dysfunction have also been implicated (14,15). At present, substantial evidence indicates the significant role of the aberrant function of immune-related cells in the peritoneal environment and the consequent development of endometriosis (16,17). Accordingly, the dysregulation of the immune system leads to a chronic inflammatory response in the ectopic endometrium and influences endometriosis through the production of antibodies against various ovarian and nuclear antigens (18,19). It is worth noting that endometriosis fulfills most of the classification criteria for an autoimmune disease, such as abnormal T- and B-lymphocyte activation and function, tissue damage and multi-organ involvement (20). Consequently, endometriosis is often considered an autoimmune disease, due to the presence of autoantibodies, increased levels of cytokines and its co-occurrence and association with other autoimmune diseases (21,22).
PS is a complex, chronic, debilitating, T-cell mediated autoimmune, proliferative skin condition, related to a combination of genetic and immunological factors that interact with each other, which can be triggered by environmental factors (23). Environmental factors include chronic infections, stress, low humidity, streptococcal infections, medications, alcohol consumption, smoking, psychosocial factors and obesity (23-25). The estimated global prevalence is 2-3% (26), with peak occurrences in both males and females between the ages of 20-30 and 60-70(23), although approximately one-third of PS cases are manifested in childhood (27). PS is characterized by well-demarcated, erythematous oval plaques, with adherent silvery scalesel and hyperplasia of the epidermis, due to increased proliferation of keratinocytes, and is categorized in multiple subtypes that manifest as various phenotypes (28). The commonest form of PS is generalized plaque-type PS. In addition to the influence of PS in the appearance of the skin of the patients, due to its chronic course and recurrent attacks, it has been reported to greatly affect their psychological health as well (29). Although the exact pathogenetic mechanisms leading to the development of PS remain unclear, research has demonstrated the importance of the immunogenetic component in pathogenesis of this disorder (30). In this context, the key role of T-helper (Th)17 as well as Th1 lymphocytes and the abnormal regulation of their activity by regulatory T cells (Tregs), which are known as suppressors of lymphocytes, have been highlighted (31). In principle, the function of Tregs is related to the prevention of autoimmune diseases by suppressing the excessive immune response (32), considering that they suppress pathogenic Th1 and Th17 cells and maintain immunological homeostasis (33). However, in PS, elevated levels of Th1 and Th17 cytokines and decreased levels of Th2 and Tregs have been observed (34). As a consequence, Tregs are unable to produce sufficient amounts of suppressive cytokines such as IL-10, IL-35 and transforming growth factor (TGF-β), and cannot properly regulate the immune response (35,36). Notably, it has been suggested that inflammatory processes induce the migration of the interferon (IFN)-γ-producing Th1 lymphocytes into the skin with these cells being involved in keratinocyte and small vessel proliferation as well as inflammatory infiltration (37).
PsA is a multifactorial, seronegative for rheumatoid factor, chronic inflammatory joint disease with prevalence rates ranging between 0.3 and 1% worldwide that is associated with PS, characterized by cutaneous PS, nail changes (onycholysis, subungual hyperkeratosis) and inflammatory changes in attachments of articular capsules, tendons and ligaments to bone surface (38). PsA occurs in 10-30% of patients with PS, often developing concurrently with PS, and it manifests all the typical features of arthritis, including enthesitis (39). Although the precise etiology of PsA remains unclear, both genetic and environmental factors have been implicated. The importance of the genetic component underlying the pathogenesis of PsA has been been highlighted by previous research focusing on twins or family members, which showed the strong heritability of PsA (40).
Given the long-standing interest of our research group in the investigation of the genetics of autoimmune diseases and endometriosis, efforts in recent years have been focused on the delineation of the genetic components that are involved in the co-occurrence of endometriosis with various autoimmune diseases, including RA (41), AS (18), SS (18,41), as well as SLE (unpublished data). Thus, within this framework, the genetic basis of the recently reported association between endometriosis and the risk of PS and/or PsA (5) was analyzed in the present review, aiming to detect the relevant genetic polymorphisms that are known to date and provide insights regarding their functional significance and involvement in the underlying pathogenetic pathways, as well as the perspectives of putative immunomodulatory therapies.
2. Genetics of endometriosis, PS and PsA
Genetics of endometriosis
Strong evidence has highlighted the contribution of both genetic and epigenetic factors, which interact with environmental ones, in the pathogenesis of endometriosis and the formulation of the complex phenotype of the disease. This topic has been extensively analyzed and reviewed in various studies conducted by our group and others (11,18,41-44). Briefly, twin studies demonstrated that the sibling genetic relative risk is 2.3 and the estimated overall heritability has a rate of 51% (7,45). Despite the endometriosis-associated loci detected by independent gene association studies, the hypothesis-free approach of Genome Wide Association Studies (GWAS) as well as the high-throughput next generation sequencing (NGS) techniques offer valuable assistance in the efforts concentrated on identifying numerous novel disease susceptibility loci, although the exact functional relevance to endometriosis of most of these single nucleotide polymorphisms (SNPs) remains elusive. Notably, the largest trans-racial GWAS and replication meta-analysis of endometriosis performed to date, managed to identify 31 novel loci, in addition to the 11 previously known, which are involved in progesterone resistance, inflammatory processes, cell cycle regulation and angiogenesis (44). It has been reported that common genetic variation accounts for ~26% of the risk of the disease (46). Notably, the majority of these disease-associated SNPs, revealed by an increasing rate over the last years, confer a moderate effect in endometriosis and, according to the function of the respective proteins, were found to be involved in the immune system and autoimmunity, estrogen-induced cell growth, cell adhesion, differentiation, proliferation, apoptosis, matrix remodeling and oxidative stress (43,47-50). The importance of the epigenetic modifications, which play a pivotal role in pathogenesis of endometriosis, has been described in detail in recent studies from our group (5,51,52).
Genetics of PS
PS is a multifaceted, complex genetic disease, resulting from an interplay between genetic and environmental factors, as aforementioned. This has been demonstrated by studies involving monozygotic and dizygotic twins, where it was shown that monozygotic twins appear to have a 3-fold increased risk for the disease when compared to the dizygotic twins, while the concordance rate among monozygotic twins was never 100%, thus suggesting a role for environmental factors (53,54). Notably, the overall genetic variation accounts for 50-70% of PS susceptibility (55). Furthermore, a familial recurrence has been well established as well, considering that the incidence of the disease is greater among first- and second-degree relatives of patients than among the general population (56). Currently, advances in large-scale analysis of the human genome provide valuable insights into the genetic dissection of PS, with over 40 genomic regions of the genome and >400 SNPs associated with the disease identified through GWAS and/or NGS approaches (57-60). Most of the identified PS susceptibility genes are skin-specific, mediate epidermal differentiation, are involved in neo-vascularization or are related with adaptive immunity (61). Moreover, many of these genes are involved in antigen presentation, type 1 IFN pathways, T-cell polarization axis, negative regulation of immune response, inflammatory processes and innate immunity (57,62,63). Recently, Patel et al (64) classified the PS-associated genes into categories reflecting the pathway that they participate in and influence. These categories include interleukins, interleukin receptors, human leukocyte antigen (HLA) genes, nuclear factor-κB (NF-κB)-pathway related genes, IFNs, genes participating in the Janus κinase (JAK)-signal transducer and activator of transcription (STAT) pathway. Additional categories include genes linked to hypercholesterolemia and hypertriglyceridemia, negative regulators of T-cell activation, inflammatory markers, and nuclear hormone receptors (64).
Genetics of PsA
PS and PsA are interrelated disorders given that most patients with PsA also have PS. However, PsA represents a distinct entity having its own clinical, genetic and epidemiological features. Notably, PsA exhibits a greater heritability among first-degree relatives in comparison with PS (40), thus suggesting a marked difference regarding the genetic architecture of the two disorders and indicating a larger genetic component for PsA (65). A stronger association with PsA than PS has been observed for various loci, including HLA-B*27 (66), CSF2 (67), PTPN22 (68), KIR (69), IL-13 (70), IL-12B (71), ZNF816A (71), IL23R (57), TNFAIP3 (57), IFNLR1 (57), IFIH1 (57), NFKBIA (57), MIF (72), IL-1A (40) and some variants in LCE3A (39).
3. Shared susceptibility loci between endometriosis, PS and PsA
Recent findings have suggested a bidirectional association between endometriosis and PS as well as PsA (5), raising intriguing questions about the possible role of a partially shared genetic background in the co-occurrence of endometriosis with PS, with or without PsA. This role appears to be strengthened considering the link of these conditions with systemic inflammation (73) and, therefore, further exploration of the functional role of all shared gene loci may shed light on the mechanisms underlying these diseases. Aiming to provide a comprehensive update on the current understanding of the potential shared genetic component of the disorders under investigation, extensive research of the current literature was performed to identify gene polymorphisms associated with these diseases.
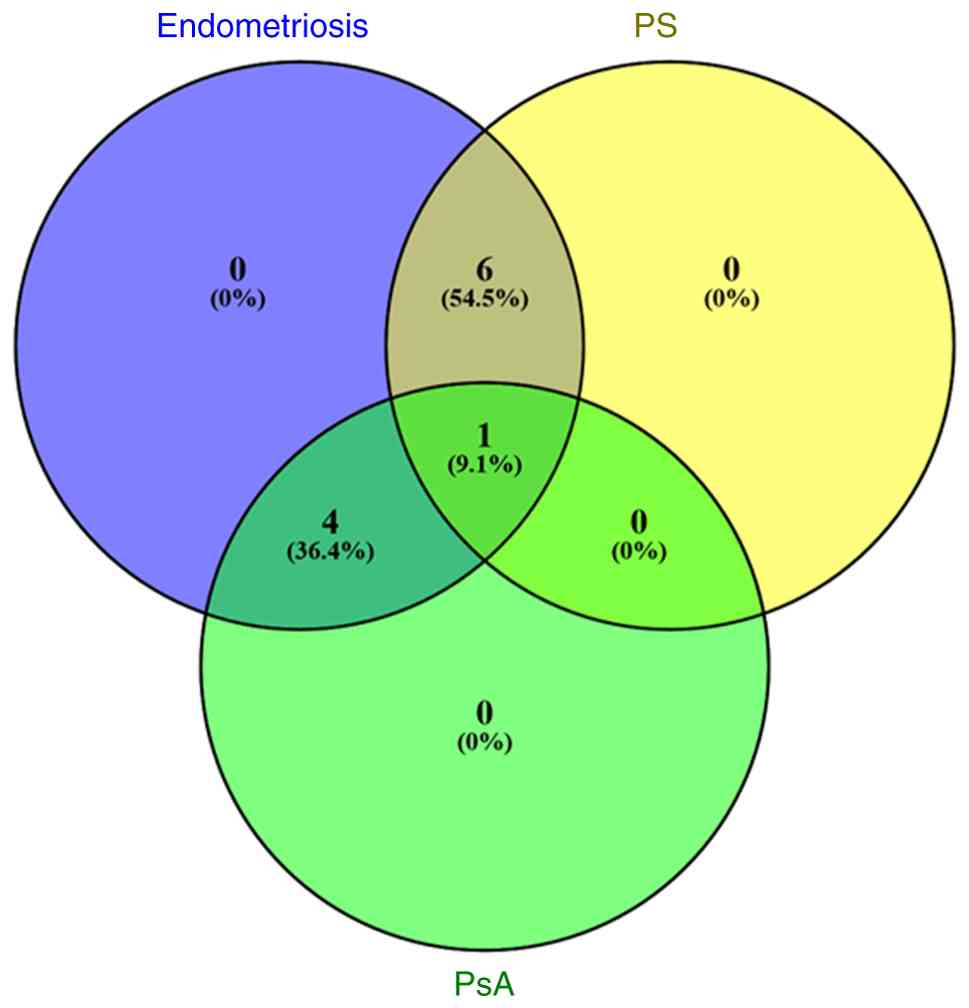
In this context, it was found that tumor necrosis factor-α (TNF-α) rs1800629 SNP is associated with the development of the three diseases (74,75). Furthermore, in the recent study by Harris et al (5) it was stated that no association was observed between PS without PsA and the risk of endometriosis. However, various gene polymorphisms were identified, including the forkhead box protein 3 (FOXP3) rs3761549 (-2383 C/T) (76,77), major histocompatibility complex (MHC), class II, DR β1 (HLA-DRB1) rs 660895 (64,78), IL-10 rs1800871 (79,80), 5,10 methylenetetrahydrofolate reductase (ΜΤΗFR) rs1801133 (Ala222Val) (81-83), STAT4 rs7574865 (84,85) and vascular endothelial growth factor (VEGF) rs1570360 (86-91) SNPs, all of which are associated with increased susceptibility to endometriosis and PS only. This observation by Harris et al (5) is indicative of the fact that other factors apart from the genetic ones are involved in the co-occurrence of endometriosis and PS without PsA.
Furthermore, the findings also showed that IL-1A rs17561 (40,92,93) and rs3783553 (40,92), macrophage migration inhibitory factor (MIF) rs755622 (72,94) and protein tyrosine phosphatase, non-receptor type 2 (PTPN22) rs2476601 (68,95,96) SNPs are associated with both endometriosis and PsA. A detailed overview of the shared gene polymorphisms is provided in Table I, while a Venn diagram illustrating the number of these shared gene polymorphisms based on the referenced studies, is presented in Fig. 1.
Table IAn overview of the genetic polymorphisms associated with the development of endometriosis, PS and PsA, as they have been confirmed by gene association and/or genome-wide association studies. |
4. Biological mechanisms related to the shared risk loci for endometriosis, PS and PsA
Polymorphisms in genes associated with endometriosis, PS and PsA
The TNF-α gene, consisting of four exons and mapped to 6p21, is a member of the class III MHC genes (97). It codes for the TNF-α cytokine, a key regulator of both autoimmunity and inflammation (98), and it has been correlated with the pathogenesis of various autoimmune, inflammatory and infectious diseases (99). The TNF-α-308 G/A rs1800629 SNP, located in the promoter region of the TNF-α gene, has been associated with an increased susceptibility to severe-stage endometriosis (74) as well as PS with PsA (100,101). It is a well-studied polymorphism from the functional viewpoint, and the presence of minor allele ‘A’ was reported to enhance the transcription levels of TNF-α, compared with allele ‘G’, thus leading to the procuction of higher protein levels in serum (102,103). Notably, this SNP has also been shown to modify the consensus sequence of the AP-2 transcription factor-binding site (104). The role of this polymorphism in endometriosis has been highlighted by the increased levels of TNF-α detected in peritoneal fluid as well as the upregulation of TNF-α gene in peritoneal macrophages and peripheral blood monocytes (105,106). Moreover, high levels of TNF-α have been correlated with the severity of endometriosis (107), while the implantation of ectopic endometrial tissue is facilitated by elevated levels of TNF-α (108). All these findings underscore the important role of this SNP in the development of endometriosis. With regard to PS, the presence of ‘A’ at position-308 in the TNF-a gene has been associated with elevated cytokine production in both psoriatic lesional skin (109,110) and the serum of patients with PS (111). Notably, the GA genotype has been associated with a later age of onset for PsA (101), while an increased frequency of the ‘A’ allele has been observed in female patients with PS (100).
Polymorphisms in genes associated with endometriosis and PS without PsA
The FOXP3 gene is positioned at the Xp11.23 locus of chromosome X (112). It encodes the FOXP3 transcription factor, a major regulatory factor involved in the T-cell activation and the development and function of Treg cells in the peripheral blood, which are considered to be inhibitors of autoimmune responses (113,114). The FOXP3 rs3761549 (-2383 C/T) SNP, located in an intronic region, has been reported to be associated with endometriosis and PS (76,77). Particularly, it was found that increased risk of PS was associated with the CC genotype and the ‘C’ allele of this SNP (77). This SNP was found to alter the FOXP3 expression pathway and affect Treg regulation in PS (115,116). Notably, an association of the rs3761549 SNP with an increased risk for endometriosis, but not with the severity or stage of the disease has been previously reported (76).
HLA molecules, which play an important role by restricting the antigenic peptides by T cells, are encoded by genes of the MHCs on chromosome 6 and help the body to discriminate between self and non-self proteins (117). These antigens exhibit the highest level of variability within the human genome and function by presenting identifying proteins to cytotoxic T cells, thereby triggering subsequent immune responses (118). The HLA-DRB1 gene belongs to the group of MHC class II genes, which are expressed on the surface of antigen-presenting cells and display peptides to Th CD4+ cells, thus inducing their activation (119). The HLA-DRB1 (A/G) rs660895 SNP, has been associated with an increased risk for moderate to severe endometriosis (78). Τhe functional significance of this SNP in endometriosis remains unclear. Patients with endometriosis exhibit reduced levels of circulating HLA class II molecules, while the proportion of HLA-DR-positive stromal and glandular epithelial cells is higher in ectopic endometrium compared with eutopic tiisue (120). Therefore, it has been hypothesized that the HLA-DR antigen may play an important role in the immunological dysregulation observed in endometriosis (78). Notably, the rs660895 SNP of the HLA-DRB1 gene has been shown to be associated with increased suseptibility to PS, particularly in early-onset cases (64,121,122). Additionally, hypomethylation of HLA-DRB1 was also found to play a role in PS pathogenesis by affecting the mRNA expression of HLA-DRB1 (123).
The IL-10 gene is located on 1q31-32, spaning a genomic region of 4.7 kb and containing 5 exons (124). It encodes IL-10, a potent anti-inflammatory cytokine that is mainly produced by T-cell subsets, including Th2 and Treg cells, but it is also produced by macrophages, monocytes, natural killer (NK) cells and B cells (125,126). The IL-10 protein modulates Th2-mediated inflammatory processes and functions as an inhibitor of the synthesis of pro-inflammatory mediators, including, cytokines and chemokines as well as adhesion and accessory molecules, thus suppressing T-cell stimulation (127,128). IL-10 rs1800871 (-819C/T), a functional promoter SNP located in a regulatory region of the promoter, has been reported to be associated with an increased risk for endometriosis and PS (79,80). It has been reported that women with the TT genotype or carrying the ‘T’ allele of rs1800871 appear to have a 2-fold increased risk for endometriosis compared with the carriers of the ‘C’ allele (79,129). Notably, the ‘C’ allele has been associated with higher levels of both IL-10 mRNA and protein compared with the ‘T’ allele in patients (130,131), suggesting that this allele may contribute to the downregulation of cell inflammation in the peritoneal cavity (79). Considering that the aforementioned SNP is located in the promoter area of IL-10 gene, it has been suggested that rs1800871 may change the binding site of a transcription factor, thus decreasing the production of IL-10, altering the immunological homeostasis and resulting in development of PS (80).
The human MTHFR gene is located on chromosome 1p36.3, a region with numerous identifiable SNPs (132) and encodes the MTHFR protein. MTHFR represents a key regulatory enzyme in the folate metabolic pathway, with its main function being the conversion of 5,10-methylenetetrahydrofolate into 5-methyltetrahydrofolate (133). The MTHFR rs1801133 (Ala222Val) SNP, located in exon 4 and substituting nucleotide C with T, has been found to be significantly associated with endometriosis (81,83) and PS (134). This SNP results in the reduced activity of the enzyme in TT homozygotes, thus resulting in a decreased production of the antioxidant glutathione, due to alterations of both gene expression levels and protein function, and increased homocysteine levels (135-138). Notably, the 677T variant, which is suggested to be unstable, may influence the thermostability and activity of the MHTFR protein (139). In endometriosis, individuals homozygous for the TT genotype have been associated with a higher susceptibility to endometriosis (81). Furthermore, the TT genotype and ‘T’ allele were found to increase the risk of PS compared with controls (82,134,140,141). Of note, it was found that rs1801133 also affects the severity of PS according to a cross-sectional study conducted by Karabacak et al (142) and a meta-analysis performed by Wu et al (143).
The STAT4 gene, which is located at 2q32.2-q32.3 and consists of 24 exons (144), codes for the STAT4 protein that is a key transcriptional factor expressed in various immune-related cells, such as macrophages, peripheral blood monocytes and dendritic cells (145). This protein, which is a member of the JAK/STAT pathway, is involved in transmission of signals from IL-12, IL-23 and type 1 IFN (146) and is necessary for the induction of IFN-γ production (147). As a consequence, STAT4 has a crucial role in the establishment of chronic inflammation (148). The intronic STAT4 rs7574865 G/T SNP has been associated with an increased risk of both endometriosis and PS (84,85). Thus, the TT genotype rs7574865 SNP was shown to be associated primarily to cases of endometriosis at minimal or mild stages of endometriosis compared with controls (84). However, the functional role of this SNP in the development of endometriosis remains unclear. A previous bioinformatics analysis was unsuccesful in identifying any activator or transcription factor whose binding sites are disrupted by the presence of the risk allele ‘T’ (149). Thus, considering the role of the STAT4 protein in IFN signaling, it can be inferred that rs7574865 SNP contributes to disease development, especially given the strong association between the Th1 response pattern and deep infiltrating endometriosis (150). Furthermore, the ‘T’ allele was previously found to be associated with the development of PS (85). Notably, STAT4 affects the optimal differentiation of Th17 cells, which are involved in the pathogenesis of both PS and RA (151,152).
The VEGF gene is located on chromosome 6p21.3, consists of 8 exons and gives rise to a family of proteins through alternative splicing events (153). It encodes the VEGF protein, which plays an important role in the regulation of angiogenesis (154) as well as endothelial cell dysfunction (155). Several transcription-factor binding sites have been detected in the 5'-UTR of this gene. Moreover, polymorphisms within this region result in an increase of the transcriptional activity of the gene (156) and may be significant in the etiology of the associated pathological conditions (157). The rs1570360 (-1154G/A) SNP, located within a transcription factor binding site in the VEGF promoter, has been associated with both endometriosis (90,158) and PS (88,89,91). Particularly, the ‘G’ allele and GG genotype have been associated with an increased risk of developing endometriosis in comparison with controls, while it has also been associated with deep infiltrating endometriosis as well as moderate to severe forms of the disease (stages III and IV) (63,90). Notably, four potential transcriptional factor binding sites were found to be created by the ‘G’ allele for EGR1, KLF4, MZF1 and SP2 factors (159). In previous studies, elevated levels of VEGF mRNA and protein have been observed in serum and peritoneal fluid of women with endometriosis (90,160,161). Angiogenic activity has been found to be dysregulated in the eutopic endometrium of patients with endometriosis (162,163). Considering that VEGF is a regulator of various processes contributing to the ectopic implantation and growth of the endometrial tissue (164), including neoangiogenesis and tissue remodeling (153), it has been suggested that any alteration in the levels of VEGF may promote the initiation of endometriosis (63,90). Of note, rs1570360 has been associated with the development of PS and the ‘G’ allele was observed significantly more frequently among patients with PS (88). Furthermore, significantly increased VEGF serum levels were found in patients with PS compared with healthy controls, while a positive correlation between the serum levels of VEGF and the severity of the disease was also detected (88). Collectively, this SNP may alter the function of the immune system and increase the susceptibility to PS.
Polymorphisms in genes associated with endometriosis and PsA
The IL-1 gene cluster, located on the chromosomal 2q12-13 locus (165), consists of nine inflammation-related genes. The encoded protein, IL-1A, is a pro-inflammatory cytokine that is primarily produced by activated macrophages, and it promotes the activation of macrophages as well as T- and B-lymphocytes (166). Both IL-1A rs17561 (p.A114S) and rs3783553 SNPs, located in exon 5 and the 3'-UTR of the gene, respectively, have been shown to be associated with susceptibility to endometriosis and PsA (40,92,93,167). With respect to endometriosis, it has been suggested that the impairment of the IL-1 family network may result in a disrupted activation of the immune system in the peritoneal cavity of patients with endometriosis compared with the serum of affected women, thus leading to the establishment of chronic inflammation (168). Moreover, the increased expression levels of IL-1A in the peritoneal fluid and serum of women with endometriosis (169,170) may be involved in the implantation of menstrual endometrium on peritoneal surfaces (9). Thus, functional alterations of IL-1A probably play a role in the pathogenesis of endometriosis. In this context, it has been suggested that rs3783553, located in the 3'-UTR, may impair mRNA stability and lead to an alteration of the expression levels of IL-1A (171). A strong association of rs17561, which results in an A114S substitution, and endometriosis has been reported, especially among women with moderate to severe forms of the disease (92). Although, a three-dimensional model of the mature IL-1A protein has been constructed (172), the biological significance of the p.114S variant remains unclear given that no functional analyses have been performed in endometriotic cells thus far. However, by using the bioinformatics tool, PolyPhen-2 (http://genetics.bwh.harvard.edu/pph2/) (173), it was suggested that the risk variant might affect processing efficiency at the protein level (92). An increased expression of IL-1A has been detected in the serum synovial fluid and skin of patients with PsA, but no extensive studies have been performed to date, clarifying the protein function in patients with PsA (174,175). Genetic variants of IL-1A may induce inflammation in PsA through intracellular effects, thus contributing to the development of the disease.
The MIF gene, located on chromosome 22q11.2 region and consisting of three exons (176), encodes the MIF protein, a key pro-inflammatory cytokine produced by T lymphocytes and activated macrophages (177). Furthermore, MIF is involved in the upregulation of IL-6, IFN-γ and TNF-α genes (178). Notably, MIF has been implicated in the pathogenesis of various autoimmune diseases, such as RA, multiple sclerosis, inflammatory bowel disease, PsA and juvenile rheumatoid arthritis (94,179). This protein has also been found to activate several molecular pathways in ectopic endometrial tissue, including immune-inflammatory processes, angiogenesis, and the growth, development and implantation of endometriotic tissue (180). It has been reported that the rs755622-173 G>C SNP, located in the promoter region of the MIF gene, has been associated with an increased risk of endometriosis and PsA (72,94). The increased transcriptional activity of the MIF gene in women with endometriosis, due to the presence of ‘C’ allele, leads to an increased synthesis of local estrogen in ectopic tissues, which has been suggested to be involved in both maintenance and progression of endometriosis (181). A significant increase of MIF levels was found in patients with PsA, while the-173C allele and the CG genotype were revealed to be associated with both an increased susceptibility to PsA and elevated levels of MIF in circulation, in several populations (72). It is worth noting that the increase in serum levels of MIF was observed in early stages of PsA, thus suggesting a crucial role of MIF in PsA onset by elevating its levels at the beginning of the inflammatory process (72). Moreover, a previous study by Donn et al (176) demonstrated that the presence of ‘C’ allele of rs755622 SNP creates a binding site for the activating enhancer binding protein 4 (AP-4) transcription factor, and as a consequence, this allele significantly increases the affinity of the MIF promoter for AP-4 binding.
The protein tyrosine phosphatase non-receptor type 22 (PTPN22) gene, located on the 1p13.3-13.1 region, encodes the lymphoid-specific phosphatase Lyp that prevents spontaneous T-cell activation (182). The Lyp protein physically binds to the Csk protein, thus functioning as a downregulator of T-cell activation (183). The PTPN22 functional polymorphism rs2476601 (R620W, C1885T), leads to the production of a gain-of-function Lyp protein that is unable to bind to the Csk efficiently and, therefore, T-cell activation cannot be suppressed (182). This SNP has been reported to be associated with an increased susceptibility to both endometriosis and PsA (68,95,96). Regarding endometriosis, the minor ‘T’ allele has been associated with an increased risk of the disease. Moreover, this allele appears to be associated with moderate/severe (III/IV) stages of endometriosis, rather than with minimal/mild (I/II) forms (95). In the same context, the minor ‘T’ allele has been found at an increased frequency in patients with PsA compared with the control individuals (68,96). The finding that wild-type Lyp is among the most potent inhibitors of T-cell activation provides a potential explanation for the association between the presence of ‘T’ allele and increased risk of PsA (96). Furthermore, patients with PsA carrying the ‘T’ allele appear to have a significantly higher number of joints with deformities, suggesting that this variant may be associated with a more aggressive disease phenotype (184).
5. Conclusions and future perspectives
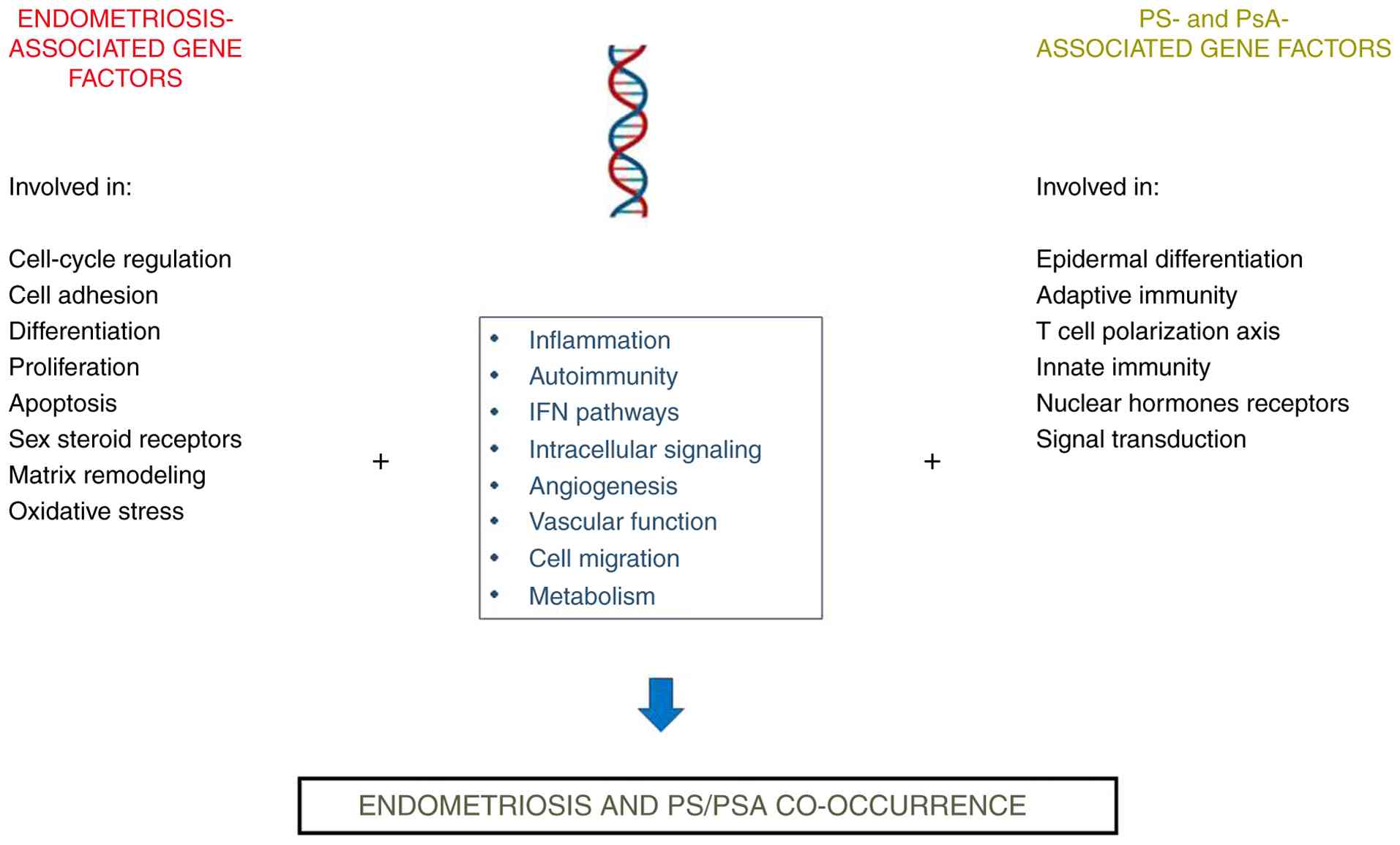
Recent data have highlighted the bidirectional associations between laparoscopically confirmed endometriosis and physician-diagnosed PS and PsA (5). Although the underlying molecular mechanisms involved in the development of these diseases remain elusive, a number of shared gene polymorphisms associated with an increased risk of the diseases under examination were identified and cross-linked through common pathways (Fig. 2). To the best of our knowledge, the current study represents the first attempt in the literature to address this issue. The findings indicate that certain genes may be considered as potential risk factors for the development of these disorders, thus suggesting a partially shared genetic predisposition. Moreover, in the present review, the functional effect of some genetic factors on this co-occurrence was analyzed. Despite the endometriosis-associated loci identified to date either by independent gene association studies or the hypothesis-free approach of GWAS as well as the high-throughput NGS techniques, numerous SNPs associated with an increased risk for developing PS have been also discovered. However, only a small number of these genetic variants have had their functional significance investigated, highlighting the need for both in-depth analyses of the role of additional gene polymorphisms and the elucidation of the underlying molecular mechanisms. Such efforts are essential for translating these genetic findings into novel therapeutic interventions.
As detailed in the Introduction, endometriosis has been shown to fulfill most of the classification criteria for an autoimmune disease (14,19-22). Moreover, it has been demonstrated that endometriosis may co-occur with a large number of different autoimmune diseases. Consistently, the current investigation revealed that many of the genetic polymorphisms that are associated with an increased risk of PS or PsA were also found to be associated with at least one other autoimmune disease previously reported to co-exist with endometriosis (18,41,139). Over the last years, researchers have attempted to use strategies for immunomodulation as a potential treatment for endometriosis, in addition to the formal surgical approach and hormone therapies that include oral contraceptives, progestins, and gonadotropin-releasing hormone agonists (185,186). Components of inflammatory or immune-cell dysfunction are challenging therapeutic targets for improved management of both endometriosis and PS (with or without PsA). In this context, the investigation of shared genetic factors underlying these diseases may substantially contribute to the generation of valuable insights with potential applications in translational medicine. Thus, the upregulation of the JAK/STAT3 pathway in endometriosis (185), provides a rationale for drug repurposing, considering that several relevant inhibitors have already been developed. In this context, inhibition of JAK/STAT signaling, specifically through the use of tofacitinib to disrupt the transduction of this pathway, may represent a promising novel therapeutic option for the treatment of endometriosis (41,185,187), since it has been shown to effectively reduce STAT3 phosphorylation in human stromal and epithelial cells derived from the eutopic endometrium of women with endometriosis (185). Notably, tofacitinib, which targets JAK1 and JAK3 and regulates the immune response, has been used for the treatment of both chronic plaques in PS (188) as well as PsA (189). Furthermore, as presented in Table I, the rs7574865 SNP of STAT4 gene, which is associated with chronic inflammation and plays a crucial role in the JAK/STAT pathway-mediated IFN signaling (190), has been reported to be associated with both endometriosis and PS.
In addition, as shown in Table I, the rs1800629 SNP of the TNF-α gene is associated with an increased susceptibility to endometriosis, PS and PsA. Notably, elevated levels of TNF-α protein have been detected in both the serum and peritoneal fluid of patients with endometriosis (103,107,191), while increased cytokine production has also been observed in both psoriatic lesional skin (109) and the serum (111) of patients with PS. Thus, anti-TNF-α agents targeting TNF-α have been considered beneficial alternative therapeutics for women with endometriosis. Anti-TNF-α agents, etanercept and adalimumab, have been found to be active and effective in the treatment of both PS and PsA (192). Etanercept, a TNF-α blocker, is a soluble receptor fusion protein, that functions by directly binding to TNF molecules in both the blood and affected tissue. It represents an effective treatment for patients with moderate to severe PS, improving psoriatic skin lesions and clearance rates (193). Notably, etanercept has been reported to block the ability of the peritoneal fluid to increase the proliferation levels of eutopic or ectopic endometrial cells in endometriosis (194). Adalimumab, another TNF-α blocker, is a recombinant monoclonal antibody that binds with high affinity and specificity to TNF-α, and has demonstrated significant efficacy for the treatment of patients with PsA (195) as well as those sufferring from moderate to severe PS, improving both skin and joint manifestations of the disease (196,197). In the case of endometriosis, adalimumab has been suggested to play a role in the regression of endometrial implants, since it has shown antioxidant and anti-inflammatory effects on histopathological damage and fibrosis (198).
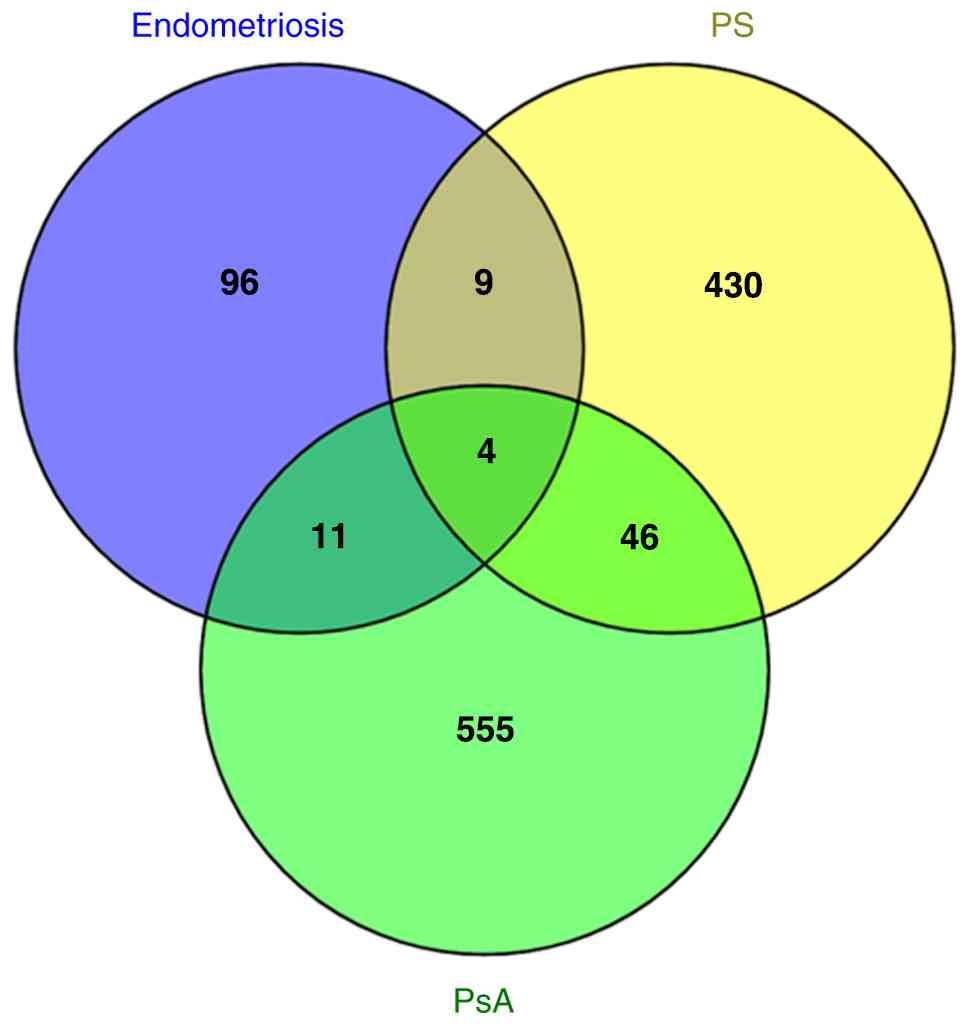
The genetic data (Table I, Figs. 1 and 2) suggest that shared pathways may underlie the co-occurrence of endometriosis, PS and/or PsA. Therefore, an attempt was made to further explore and validate this hypothesis through transcriptomic analyses, aiming to strengthen the conclusions regarding the potential therapeutic targets that have been presented above. These analyses revealed 13 genes that were found to be upregulated in both endometriosis and PS, namely CXCL1, CXCL10, CXCL9, CXCR2, FABP4, IL18R1, IL18RAP, IL6R, S100A8, SCGB2A1, SERPINE1, STAT1 and SYK (199-208). Moreover, 15 genes were found to be expressed at elevated levels in endometriosis and PsA, namely C1QB, C6, C7, CCR2, CD27, CLU, CXCL9, CXCL10, CXCL12, GREM1, IL-17, SERPINE2, S100A8, SIGIRR and STAT1 (209-212). Among these genes, four of them were found to be upregulated in the three diseases under study, namely CXCL10, CXCL9, S100A8 and STAT1. Collectively this data is presented in Fig. 3. Of note, in addition to the aforementioned therapeutic targets identified through genetic data, transcriptomic analyses from the literature have also provided some valuable insights regarding challenging therapeutic targets. For example, tocilizumab (TCZ), a humanized antibody against the α-chain of the IL-6 receptor (IL6R) commonly used for the treatment of inflammatory diseases, has been considered a promising option for the treatment of endometriosis (213-215). However, TCZ has the potential to induce adverse drug events including PS-like eruptions, as described when TCZ was used for the treatment of patients with RA (216,217). Notably, the STAT1 gene was found to be upregulated in endometriosis, PS and PsA (Fig. 3). STAT1 is a member of the JAK/STAT pathway and can enable the transcription of genes which inhibit cell division and stimulate inflammation (190). As previously reported, tofacitinib, a JAK/STAT signaling pathway inhibitor, has been used for the treatment of endometriosis (185,215), PS (188) and PsA (189). A limitation of the transcriptomic analyses across different diseases is the wide variety of tissue types investigated for each disease in the research conducted thus far, including ectopic and eutopic endometrium, fibrotic endometrium, endometriotic or peritoneal lesions, peritoneal fluid, psoriatic skin, skin fibroblasts, PS plaques, whole blood, epithelium, dermis and synovial fluid. However, transcriptome analysis has significantly contributed to the development of novel biomarkers as well as diagnostic and prognostic tools.
A limitation to be considered is the lack of replication studies in the literature to date, regarding the co-occurrence of endometriosis and PS or PsA in patients of different racial and/or ethnic backgrounds. This is a significant issue, given the differential role of the various polymorphisms involved in the development of these disorders depending on the ancestry of the populations under examination (218).
In conclusion, the genetic risk factors shared between endometriosis, PS, and/or PsA, combined with insights into the underlying cellular and biochemical pathogenetic pathways presented in this review, may significantly aid in identifying novel therapeutic targets and developing new treatment protocols. This, in turn, could offer substantial benefits for patients suffering from endometriosis and PS or PsA. Moreover, further elucidation and replication of the results presented by Harris et al (5) in patients of diverse ancestries, would reinforce the need for gynecologists and dermatologists to be vigilant and acknowledge the potential association between endometriosis and PS and/or PsA. This awareness would encourage collaborative efforts to coordinate the management and treatment of these co-existing conditions.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
MIZ and GNG designed the current study and drafted the manuscript. GNG, TBN, TBT, BCT, GFG, GB and MIZ searched the literature. MIZ, GNG, GB and DAS analyzed and organized the data. DAS, TBT, BCT, GFG, GB and TBN critically revised the manuscript. All authors have read and approved the final manuscript. Data authentication is not applicable.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
MIZ, BCT, GFG, GB, TBN and GNG (all authors other than DAS) declare that they have no competing interests related to this study. DAS is the Editor-in-chief for the journal, but had no personal involvement in the reviewing process, or any influence in terms of adjudicating on the final decision, for this article.
References
|
Sinaii N, Cleary SD, Ballweg ML, Nieman LK and Stratton P: High rates of autoimmune and endocrine disorders, fibromyalgia, chronic fatigue syndrome and atopic diseases among women with endometriosis: A survey analysis. Hum Reprod. 17:2715–2724. 2002.PubMed/NCBI View Article : Google Scholar | |
|
Kvaskoff M, Mu F, Terry KL, Harris HR, Poole EM, Farland L and Missmer SA: Endometriosis: A high-risk population for major chronic diseases? Hum Reprod Update. 21:500–516. 2015.PubMed/NCBI View Article : Google Scholar | |
|
Rafi U, Ahmad S, Bokhari SS, Iqbal MA, Zia A, Khan MA and Roohi N: Association of inflammatory markers/cytokines with cardiovascular risk manifestation in patients with endometriosis. Mediators Inflamm. 2021(3425560)2021.PubMed/NCBI View Article : Google Scholar | |
|
Surrey ES, Soliman AM, Johnson SJ, Davis M, Castelli-Haley J and Snabes MC: Risk of developing comorbidities among women with endometriosis: A retrospective matched cohort study. J Womens Health (Larchmt). 27:1114–1123. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Harris HR, Korkes KMN, Li T, Kvaskoff M, Cho E, Carvalho LF, Qureshi AA, Abrao M and Missmer SA: Endometriosis, psoriasis and psoriatic arthritis: A prospective cohort study. Am J Epidemiol. 191:1050–1060. 2022.PubMed/NCBI View Article : Google Scholar | |
|
Lebovic DI, Mueller MD and Taylor RN: Immunobiology of endometriosis. Fertil Steril. 75:1–10. 2001.PubMed/NCBI View Article : Google Scholar | |
|
Treloar SA, O'Connor DT, O'Connor VM and Martin NG: Genetic influences on endometriosis in an Australian twin sample. sueT@qimr.edu.au. Fertil Steril. 71:701–710. 1999.PubMed/NCBI View Article : Google Scholar | |
|
Giudice LC and Kao LC: Endometriosis. Lancet. 364:1789–1799. 2004.PubMed/NCBI View Article : Google Scholar | |
|
Bulun SE: Endometriosis. N Engl J Med. 360:268–279. 2009.PubMed/NCBI View Article : Google Scholar | |
|
Symons LK, Miller JE, Kay VR, Marks RM, Liblik K, Koti M and Tayade C: The immunopathophysiology of endometriosis. Trends Mol Med. 24:748–762. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Zondervan KT, Becker CM, Koga K, Missmer SA, Taylor RN and Viganò P: Endometriosis. Nat Rev Dis Primers. 4(9)2018.PubMed/NCBI View Article : Google Scholar | |
|
Sourial S, Tempest N and Hapangama DK: Theories on the pathogenesis of endometriosis. Int J Reprod Med. 2014(179515)2014.PubMed/NCBI View Article : Google Scholar | |
|
Sampson JA: Peritoneal endometriosis due to menstrual dissemination of endometrial tissue into the peritoneal cavity. Am J Obstet Gynecol. 14:422–469. 1927. | |
|
Vercellini P, Viganò P, Somigliana E and Fedele L: Endometriosis: Pathogenesis and treatment. Nat Rev Endocrinol. 10:261–275. 2014.PubMed/NCBI View Article : Google Scholar | |
|
Szymanowski K: Apoptosis pattern in human endometrium in women with pelvic endometriosis. Eur J Obstet Gynecol Reprod Biol. 132:107–110. 2007.PubMed/NCBI View Article : Google Scholar | |
|
Dmowski WP, Gebel HM and Braun DP: The role of cell-mediated immunity in pathogenesis of endometriosis. Acta Obstet Gynecol Scand Suppl. 159:7–14. 1994.PubMed/NCBI | |
|
Siristatidis C, Nissotakis C, Chrelias C, Iacovidou H and Salamalekis E: Immunological factors and their role in the genesis and development of endometriosis. J Obstet Gynaecol Res. 32:162–170. 2006.PubMed/NCBI View Article : Google Scholar | |
|
Zervou MI, Tarlatzis BC, Grimbizis GF, Spandidos DA, Niewold TB and Goulielmos GN: Association of endometriosis with Sjögren's syndrome: Genetic insights (review). Int J Mol Med. 53(20)2024.PubMed/NCBI View Article : Google Scholar | |
|
Eisenberg VH, Zolti M and Soriano D: Is there an association between autoimmunity and endometriosis? Autoimmun Rev. 11:806–814. 2012.PubMed/NCBI View Article : Google Scholar | |
|
Matarese G, De Placido G, Nikas Y and Alviggi C: Pathogenesis of endometriosis: Natural immunity dysfunction or autoimmune disease? Trends Mol Med. 9:223–228. 2003.PubMed/NCBI View Article : Google Scholar | |
|
Iborra A, Palacio JR, Ulcova-Gallova Z and Martínez P: Autoimmune response in women with endometriosis. Am J Reprod Immunol. 44:236–241. 2000.PubMed/NCBI View Article : Google Scholar | |
|
Nothnick WB: Treating endometriosis as an autoimmune disease. Fertil Steril. 76:223–230. 2001.PubMed/NCBI View Article : Google Scholar | |
|
Singh R, Koppu S, Perche PO and Feldman SR: The cytokine mediated molecular pathophysiology of psoriasis and its clinical implications. Int J Mol Sci. 22(12793)2021.PubMed/NCBI View Article : Google Scholar | |
|
Grän F, Kerstan A, Serfling E, Goebeler M and Muhammad K: Current developments in the immunology of psoriasis. Yale J Biol Med. 93(97)2020.PubMed/NCBI | |
|
Elder JT, Bruce AT, Gudjonsson JE, Johnston A, Stuart PE, Tejasvi T, Voorhees JJ, Abecasis GR and Nair RP: Molecular dissection of psoriasis: Integrating genetics and biology. J Investig Dermatol. 130:1213–1226. 2010.PubMed/NCBI View Article : Google Scholar | |
|
Lebwohl M: Psoriasis. Lancet. 361:1197–1204. 2003.PubMed/NCBI View Article : Google Scholar | |
|
Bronckers IM, Paller AS, van Geel MJ, van de Kerkhof PC and Seyger MM: Psoriasis in children and adolescents: Diagnosis, management and comorbidities. Paediatr Drugs. 17:373–384. 2015.PubMed/NCBI View Article : Google Scholar | |
|
Raychaudhuri SK, Maverakis E and Raychaudhuri SP: Diagnosis and classification of psoriasis. Autoimmun Rev. 13:490–495. 2014.PubMed/NCBI View Article : Google Scholar | |
|
Al-Mazeedi K, El-Shazly M and Al-Ajmi HS: Impact of psoriasis on quality of life in Kuwait. Int J Dermatol. 45:418–424. 2006.PubMed/NCBI View Article : Google Scholar | |
|
Campalani E and Barker JNWN: The clinical genetics of psoriasis. Curr Genomics. 6:51–60. 2005.PubMed/NCBI View Article : Google Scholar | |
|
Langier S, Sade K and Kivity S: Regulatory T cells: The suppressor arm of the immune system. Autoimmun Rev. 10:112–115. 2010.PubMed/NCBI View Article : Google Scholar | |
|
Owczarczyk-Saczonek A, Czerwińska J and Placek W: The role of regulatory T cells and anti-inflammatory cytokines in psoriasis. Acta Dermatovenerol Alp Pannonica Adriat. 27:17–23. 2018.PubMed/NCBI | |
|
Nussbaum L, Chen YL and Ogg GS: Role of regulatory T cells in psoriasis pathogenesis and treatment. Br J Dermatol. 184:14–24. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Deng Y, Chang C and Lu Q: The inflammatory response in psoriasis: A comprehensive review. Clin Rev Allergy Immunol. 50:377–389. 2016.PubMed/NCBI View Article : Google Scholar | |
|
Nedoszytko B, Lange M, Sokołowska-Wojdyło M, Renke J, Trzonkowski P, Sobjanek M, Szczerkowska-Dobosz A, Niedoszytko M, Górska A, Romantowski J, et al: The role of regulatory T cells and genes involved in their differentiation in pathogenesis of selected inflammatory and neoplastic skin diseases. Part II: The Treg role in skin diseases pathogenesis. Postepy Dermatol Alergol. 34:405–417. 2017.PubMed/NCBI View Article : Google Scholar | |
|
Dasgupta A and Saxena R: Regulatory T cells: A review. Natl Med J India. 25:341–351. 2012.PubMed/NCBI | |
|
Lowes MA, Bowcock AM and Krueger JG: Pathogenesis and therapy of psoriasis. Nature. 445:866–873. 2007.PubMed/NCBI View Article : Google Scholar | |
|
Gladman DD, Antoni C, Mease P, Clegg DO and Nash P: Psoriatic arthritis: Epidemiology, clinical features, course, and outcome. Ann Rheum Dis. 64 (Suppl 2):ii14–ii7. 2005.PubMed/NCBI View Article : Google Scholar | |
|
Ocampo DV and Gladman D: Psoriatic arthritis. F1000Res. 8(1665)2019.PubMed/NCBI View Article : Google Scholar | |
|
Rahman P and Elder JT: Genetic epidemiology of psoriasis and psoriatic arthritis. Ann Rheum Dis. 64 (Suppl 2):S37–S41. 2005.PubMed/NCBI View Article : Google Scholar | |
|
Zervou MI, Vlachakis D, Papageorgiou L, Eliopoulos E and Goulielmos GN: Increased risk of rheumatoid arthritis in patients with endometriosis: Genetic aspects. Rheumatology (Oxford). 61:4252–4262. 2022.PubMed/NCBI View Article : Google Scholar | |
|
Rahmioglu N, Nyholt DR, Morris AP, Missmer SA, Montgomery GW and Zondervan KT: Genetic variants underlying risk of endometriosis: Insights from meta-analysis of eight genome-wide association and replication datasets. Hum Reprod Update. 20:702–716. 2014.PubMed/NCBI View Article : Google Scholar | |
|
Sapkota Y, Steinthorsdottir V, Morris AP, Fassbender A, Rahmioglu N, De Vivo I, Buring JE, Zhang F, Edwards TL, Jones S, et al: Meta-analysis identifies five novel loci associated with endometriosis highlighting key genes involved in hormone metabolism. Nat Commun. 8(15539)2017.PubMed/NCBI View Article : Google Scholar | |
|
Rahmioglu N, Mortlock S, Ghiasi M, Møller PL, Stefansdottir L, Galarneau G, Turman C, Danning R, Law MH, Sapkota Y, et al: The genetic basis of endometriosis and comorbidity with other pain and inflammatory conditions. Nat Genet. 55:423–436. 2023.PubMed/NCBI View Article : Google Scholar | |
|
Saha R, Pettersson HJ, Svedberg P, Olovsson M, Bergqvist A, Marions L, Tornvall P and Kuja-Halkola R: Heritability of endometriosis. Fertil Steril. 104:947–952. 2015.PubMed/NCBI View Article : Google Scholar | |
|
Lee SH, Harold D and Nyholt DR: ANZGene Consortium; International Endogene Consortium; Genetic and Environmental Risk for Alzheimer's disease Consortium. Goddard ME, Zondervan KT, Williams J, Montgomery GW, et al: Estimation and partitioning of polygenic variation captured by common SNPs for Alzheimer's disease, multiple sclerosis and endometriosis. Hum Mol Genet. 22:832–841. 2013.PubMed/NCBI View Article : Google Scholar | |
|
Nyholt DR, Low SK, Anderson CA, Painter JN, Uno S, Morris AP, MacGregor S, Gordon SD, Henders AK, Martin NG, et al: Genome-wide association meta-analysis identifies new endometriosis risk loci. Nat Genet. 44:1355–1359. 2012.PubMed/NCBI View Article : Google Scholar | |
|
Kobayashi H, Imanaka S, Nakamura H and Tsuji A: Understanding the role of epigenomic, genomic and genetic alterations in the development of endometriosis (review). Mol Med Rep. 9:1483–1505. 2014.PubMed/NCBI View Article : Google Scholar | |
|
Vassilopoulou L, Matalliotakis M, Zervou MI, Matalliotaki C, Krithinakis K, Matalliotakis I, Spandidos DA and Goulielmos GN: Defining the genetic profile of endometriosis. Exp Therap Med. 17:3267–3281. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Albertsen HM, Matalliotaki C, Matalliotakis M, Zervou MI, Matalliotakis I, Spandidos DA, Chettier R, Ward K and Goulielmos GN: Whole exome sequencing identifies hemizygous deletions in the UGT2B28 and USP17L2 genes in a three-generation family with endometriosis. Mol Med Rep. 19:1716–1720. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Goulielmos GN, Matalliotakis M, Matalliotaki C, Eliopoulos E, Matalliotakis I and Zervou MI: Endometriosis research in the-omics era. Gene. 741(144545)2020.PubMed/NCBI View Article : Google Scholar | |
|
Vazgiourakis VM, Zervou MI, Papageorgiou L, Chaniotis D, Spandidos DA, Vlachakis D, Eliopoulos E and Goulielmos GN: Association of endometriosis with cardiovascular disease: Genetic aspects (review). Int J Mol Med. 51(29)2023.PubMed/NCBI View Article : Google Scholar | |
|
Elder JT, Nair RP, Guo SW, Henseler T, Christophers E and Voorhees JJ: The genetics of psoriasis. Arch Dermatol. 130:216–224. 1994.PubMed/NCBI | |
|
Brandrup F, Hauge M, Henningen K and Eriksen B: Psoriasis in an unselected series of twins. Arch Dermatol. 114:874–878. 1978.PubMed/NCBI | |
|
Tagami H: Triggering factors. Clin Dermatol. 15:677–685. 1997.PubMed/NCBI View Article : Google Scholar | |
|
Farber EM and Nall ML: The natural history of psoriasis in 5,600 patients. Dermatologica. 148:1–18. 1974.PubMed/NCBI View Article : Google Scholar | |
|
Stuart PE, Nair RP, Tsoi LC, Tejasvi T, Das S, Kang HM, Ellinghaus E, Chandran V, Callis-Duffin K, Ike R, et al: Genome-wide association analysis of psoriatic arthritis and cutaneous psoriasis reveals differences in their genetic architecture. Am J Hum Genet. 97:816–836. 2015.PubMed/NCBI View Article : Google Scholar | |
|
Osmola-Mańkowska A, Teresiak-Mikołajczak E, Skrzypczak-Zielińska M and Adamski Z: Genetic polymorphism in psoriasis and its meaning for the treatment efficacy in the future. Postepy Dermatol Alergol. 35:331–337. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Singh S, Pradhan D, Puri P, Ramesh V, Aggarwal S, Nayek A and Jain AK: Genomic alterations driving psoriasis pathogenesis. Gene. 683:61–71. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Prinz JC: Autoimmune aspects of psoriasis: Heritability and autoantigens. Autoimmun Rev. 16:970–979. 2017.PubMed/NCBI View Article : Google Scholar | |
|
Sun LD, Cheng H, Wang ZX, Zhang AP, Wang PG, Xu JH, Zhu QX, Zhou HS, Ellinghaus E, Zhang FR, et al: Association analyses identify six new psoriasis susceptibility loci in the Chinese population. Nat Genet. 42:1005–1009. 2010.PubMed/NCBI View Article : Google Scholar | |
|
Bieber T and Nestle F (eds): Personalized treatment options in dermatology. Springer-Verlag, Berlin Heidelberg, pp86-87, 2015. | |
|
Liu Y, Helms C, Liao W, Zaba LC, Duan S, Gardner J, Wise C, Miner A, Malloy MJ, Pullinger CR, et al: A genome-wide association study of psoriasis and psoriatic arthritis identifies new disease loci. PLoS Genet. 4(e1000041)2008.PubMed/NCBI View Article : Google Scholar | |
|
Patel HA, Revankar RR, Pedroza ST, Graham S and Feldman SR: The genetic susceptibility to psoriasis and the relationship of linked genes to our treatment options. Int J Mol Sci. 24(12310)2023.PubMed/NCBI View Article : Google Scholar | |
|
Karason A, Love TJ and Gudbjornsson B: A strong heritability of psoriatic arthritis over four generations-the Reykjavik psoriatic arthritis study. Rheumatology (Oxford). 48:1424–1428. 2009.PubMed/NCBI View Article : Google Scholar | |
|
Eder L, Chandran V, Pellet F, Shanmugarajah S, Rosen CF, Bull SB and Gladman DD: Human leucocyte antigen risk alleles for psoriatic arthritis among patients with psoriasis. Ann Rheum Dis. 71:50–55. 2012.PubMed/NCBI View Article : Google Scholar | |
|
Bowes J, Budu-Aggrey A, Huffmeier U, Uebe S, Steel K, Hebert HL, Wallace C, Massey J, Bruce IN, Bluett J, et al: Dense genotyping of immune-related susceptibility loci reveals new insights into the genetics of psoriatic arthritis. Nat Commun. 6(6046)2015.PubMed/NCBI View Article : Google Scholar | |
|
Bowes J, Loehr S, Budu-Aggrey A, Uebe S, Bruce IN, Feletar M, Marzo-Ortega H, Helliwell P, Ryan AW, Kane D, et al: PTPN22 is associated with susceptibility to psoriatic arthritis but not psoriasis: Evidence for a further PsA-specific risk locus. Ann Rheum Dis. 74:1882–1885. 2015.PubMed/NCBI View Article : Google Scholar | |
|
Chandran V, Bull SB, Pellett FJ, Ayearst R, Pollock RA and Gladman DD: Killer-cell immunoglobulin-like receptor gene polymorphisms and susceptibility to psoriatic arthritis. Rheumatology (Oxford). 53:233–239. 2014.PubMed/NCBI View Article : Google Scholar | |
|
Bowes J, Eyre S, Flynn E, Ho P, Salah S, Warren RB, Marzo-Ortega H, Coates L, McManus R, Ryan AW, et al: Evidence to support IL-13 as a risk locus for psoriatic arthritis but not psoriasis vulgaris. Ann Rheum Dis. 70:1016–1019. 2011.PubMed/NCBI View Article : Google Scholar | |
|
Yang Q, Liu H, Qu L, Fu X, Yu Y, Yu G, Tian H, Yu Y, Sun D, Peng J, et al: Investigation of 20 non-HLA (human leucocyte antigen) psoriasis susceptibility loci in Chinese patients with psoriatic arthritis and psoriasis vulgaris. Br J Dermatol. 168:1060–1065. 2013.PubMed/NCBI View Article : Google Scholar | |
|
Morales-Zambrano R, Bautista-Herrera LA, De la Cruz-Mosso U, Villanueva-Quintero GD, Padilla-Gutiérrez JR, Valle Y, Parra-Rojas I, Rangel-Villalobos H, Gutiérrez-Ureña SR and Muñoz-Valle JF: Macrophage migration inhibitory factor (MIF) promoter polymorphisms (-794 CATT5-8 and -173 G>C): Association with MIF and TNFα in psoriatic arthritis. Int J Clin Exp Med. 7:2605–2614. 2014.PubMed/NCBI | |
|
Taylor HS, Kotlyar AM and Flores VA: Endometriosis is a chronic systemic disease: Clinical challenges and novel innovations. Lancet. 397:839–852. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Mier-Cabrera J, Cruz-Orozco O, de la Jara-Díaz J, Galicia-Castillo O, Buenrostro-Jáuregui M, Parra-Carriedo A and Hernández-Guerrero C: Polymorphisms of TNF-alpha (-308), IL-1beta (+ 3954) and IL1-Ra (VNTR) are associated to severe stage of endometriosis in Mexican women: A case control study. BMC Womens Health. 22(356)2022.PubMed/NCBI View Article : Google Scholar | |
|
Höhler T, Kruger A, Schneider PM, Schopf RE, Knop J, Rittner C, Meyer zum Büschenfelde KH and Märker-Hermann E: A TNF-alpha promoter polymorphism is associated with juvenile onset psoriasis and psoriatic arthritis. J Invest Dermatol. 109:562–565. 1997.PubMed/NCBI View Article : Google Scholar | |
|
André GM, Barbosa CP, Teles JS, Vilarino FL, Christofolini DM and Bianco B: Analysis of FOXP3 polymorphisms in infertile women with and without endometriosis. Fertil Steril. 95:2223–2227. 2011.PubMed/NCBI View Article : Google Scholar | |
|
Song QH, Shen Z, Xing XJ, Yin R, Wu YZ, You Y, Guo H, Chen L, Hao F and Bai Y: An association study of single nucleotide polymorphisms of the FOXP3 intron-1 and the risk of psoriasis vulgaris. Indian J Biochem Biophys. 49:25–35. 2012.PubMed/NCBI | |
|
Sundqvist J, Falconer H, Seddighzadeh M, Vodolazkaia A, Fassbender A, Kyama C, Bokor A, Stephansson O, Padyukov L, Gemzell-Danielsson K and D'Hooghe TM: Endometriosis and autoimmune disease: Association of susceptibility to moderate/severe endometriosis with CCL21 and HLA-DRB1. Fertil Steril. 95:437–440. 2011.PubMed/NCBI View Article : Google Scholar | |
|
Juo SHH, Wu R, Lin CS, Wu MT, Lee JN and Tsai EM: A functional promoter polymorphism in interleukin-10 gene influences susceptibility to endometriosis. Fertil Steril. 92:1228–1233. 2009.PubMed/NCBI View Article : Google Scholar | |
|
Indhumathi S, Rajappa M, Chandrashekar L, Ananthanarayanan PH, Thappa DM and Negi VS: T helper-2 cytokine/regulatory T-cell gene polymorphisms and their relation with risk of psoriasis in a South Indian Tamil cohort. Hum Immunol. 78:209–215. 2017.PubMed/NCBI View Article : Google Scholar | |
|
Delli Carpini G, Giannella L, Di Giuseppe J, Montik N, Montanari M, Fichera M, Crescenzi D, Marzocchini C, Meccariello ML, Di Biase D, et al: Homozygous C677T methylenetetrahydrofolate reductase (MTHFR) polymorphism as a risk factor for endometriosis: A retrospective case-control study. Int J Mol Sci. 24(15404)2023.PubMed/NCBI View Article : Google Scholar | |
|
Asefi M, Vaisi-Raygani A, Khodarahmi R, Nemati H, Rahimi Z, Vaisi-Raygani H, Tavilani H and Pourmotabbed T: Methylentetrahydrofolatereductase (rs1801133) polymorphism and psoriasis: Contribution to oxidative stress, lipid peroxidation and correlation with vascular adhesion protein 1, preliminary report. J Eur Acad Dermatol Venereol. 28:1192–1198. 2014.PubMed/NCBI View Article : Google Scholar | |
|
Zhu J, Wang Z, Tao L, Han L, Huang Q, Fang X, Yang K, Huang G, Zheng Z, Yawalkar N, et al: MTHFR gene polymorphism association with psoriatic arthritis risk and the efficacy and hepatotoxicity of methotrexate in psoriasis. Front Med (Lausanne). 9(869912)2022.PubMed/NCBI View Article : Google Scholar | |
|
Bianco B, Fernandes RFM, Trevisan CM, Christofolini DM, Sanz-Lomana CM, de Bernabe JV and Barbosa CP: Influence of STAT4 gene polymorphisms in the pathogenesis of endometriosis. Ann Hum Genet. 83:249–255. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Zervou MI, Goulielmos GN, Castro-Giner F, Tosca AD and Krueger-Krasagakis S: STAT4 gene polymorphism is associated with psoriasis in the genetically homogeneous population of Crete, Greece. Hum Immunol. 70:738–741. 2009.PubMed/NCBI View Article : Google Scholar | |
|
Xu S, Wu W, Sun H, Lu J, Yuan B, Xia Y, De Moor B, Marchal K, Wang X, Xu P and Cheng W: Association of the vascular endothelial growth factor gene polymorphisms (-460C/T, +405G/C and +936T/C) with endometriosis: A meta-analysis. Ann Hum Genet. 76:464–471. 2012.PubMed/NCBI View Article : Google Scholar | |
|
Sudhesan A, Rajappa M, Chandrashekar L, Ananthanarayanan PH, Thappa DM, Satheesh S and Chandrasekaran A: Vascular endothelial growth factor (VEGF) gene polymorphisms (rs699947, rs833061, and rs2010963) and psoriatic risk in South Indian Tamils. Hum Immunol. 78:657–663. 2017.PubMed/NCBI View Article : Google Scholar | |
|
Zablotna M, Sobjanek M, Nedoszytko B, Lange M, Kozicka D, Glen J and Roszkiewicz J: Association of psoriasis with the VEGF gene polymorphism in the northern Polish population. J Eur Acad Dermatol Venereol. 27:319–323. 2013.PubMed/NCBI View Article : Google Scholar | |
|
Lee YH and Song GG: Vascular endothelial growth factor gene polymorphisms and psoriasis susceptibility: A meta-analysis. Genet Mol Res. 14:14396–14405. 2015.PubMed/NCBI View Article : Google Scholar | |
|
Perini JA, Cardoso JV, Berardo PT, Vianna-Jorge R, Nasciutti LE, Bellodi-Privato M, Machado DE and Abrão MS: Role of vascular endothelial growth factor polymorphisms (-2578C>A, -460 T>C, -1154G>A, +405G>C and +936C>T) in endometriosis: A case-control study with Brazilians. BMC Womens Health. 14(117)2014.PubMed/NCBI View Article : Google Scholar | |
|
Qi M, Huang X, Zhou L and Zhang J: Four polymorphisms of VEGF (+405C>G, -460T>C, -2578C>A, and -1154G>A) in susceptibility to psoriasis: a meta-analysis. DNA Cell Biol. 33:234–244. 2014.PubMed/NCBI View Article : Google Scholar | |
|
Hata Y, Nakaoka H, Yoshihara K, Adachi S, Haino K, Yamaguchi M, Nishikawa N, Kashima K, Yahata T, Tajima A, et al: A nonsynonymous variant of IL1A is associated with endometriosis in Japanese population. J Hum Genet. 58:517–520. 2013.PubMed/NCBI View Article : Google Scholar | |
|
Sapkota Y, Low SK, Attia J, Gordon SD, Henders AK, Holliday EG, MacGregor S, Martin NG, McEvoy M, Morris AP, et al: Association between endometriosis and the interleukin 1A (IL1A) locus. Hum Reprod. 30:239–248. 2015.PubMed/NCBI View Article : Google Scholar | |
|
Chekini Z, Shahhoseini M, Aflatoonian R and Afsharian P: The relationship between functional promoter variants of macrophage migration inhibitory factor and endometriosis. Cell J. 22:450–456. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Gomes FMCS, Bianco B, Teles JS, Christofolini DM, de Souza AMB, Guedes AD and Barbosa CP: PTPN22 C1858T polymorphism in women with endometriosis. Am J Reprod Immunol. 63:227–232. 2010.PubMed/NCBI View Article : Google Scholar | |
|
Butt C, Peddle L, Greenwood C, Hamilton S, Gladman D and Rahman P: Association of functional variants of PTPN22 and tp53 in psoriatic arthritis: A case-control study. Arthritis Res Ther. 8(R27)2006.PubMed/NCBI View Article : Google Scholar | |
|
Dunham I, Sargent CA, Trowsdale J and Campbell RD: Molecular mapping of the human major histocompatibility complex by pulsed-field gel electrophoresis. Proc Natl Acad Sci USA. 84:7237–7241. 1987.PubMed/NCBI View Article : Google Scholar | |
|
Kalliolias GD and Ivashkiv LB: TNF biology, pathogenic mechanisms and emerging therapeutic strategies. Nat Rev Rheumatol. 12:49–62. 2016.PubMed/NCBI View Article : Google Scholar | |
|
Abutorabi R, Baradaran A, Mostafavi F, Zarrin Y and Mardanian F: Evaluation of tumor necrosis factor alpha polymorphism frequencies in endometriosis. Int J Fertil Steril. 9:329–337. 2015.PubMed/NCBI View Article : Google Scholar | |
|
Queiro R, Tejón P, Alonso S and Coto P: Age at disease onset: A key factor for understanding psoriatic disease. Rheumatology (Oxford). 53:1178–1185. 2014.PubMed/NCBI View Article : Google Scholar | |
|
Herrera F, Gutiérrez L, Salazar Alcalá E, Balbas O and Fernández Mestre M: Role of TNF-Alpha and IL10 genes in the development and clinical manifestations of psoriatic arthritis. Rev Colomb Reumatol. 25:9–15. 2018. | |
|
Wilson AG, Symons JA, McDowell TL, McDevitt HO and Duff GW: Effects of a polymorphism in the human tumor necrosis factor alpha promoter on transcriptional activation. Proc Natl Acad Sci USA. 94:3195–3199. 1997.PubMed/NCBI View Article : Google Scholar | |
|
Gupta V, Gupta A, Jafar T, Gupta V, Agrawal S, Srivastava N, Kumar S, Singh AK, Natu SM, Agarwal CG and Agarwal GG: Association of TNF-α promoter gene G-308A polymorphism with metabolic syndrome, insulin resistance, serum TNF-α and leptin levels in Indian adult women. Cytokine. 57:32–36. 2012.PubMed/NCBI View Article : Google Scholar | |
|
Yang ZC, Xu F, Tang M and Xiong X: Association between TNF-α promoter-308 A/G polymorphism and systemic lupus erythematosus susceptibility: A case-control study and meta-analysis. Scand J Immunol. 85:197–210. 2017.PubMed/NCBI View Article : Google Scholar | |
|
Keenan JA, Chen TT, Chadwell NL, Torry DS and Caudle MR: IL-1 beta, TNF-alpha, and IL-2 in peritoneal fluid and macrophage-conditioned media of women with endometriosis. Am J Reprod Immunol. 34:381–385. 1995.PubMed/NCBI View Article : Google Scholar | |
|
Braun DP, Gebel H, House R, Rana N and Dmowski NP: Spontaneous and induced synthesis of cytokines by peripheral blood monocytes in patients with endometriosis. Fertil Steril. 65:1125–1129. 1996.PubMed/NCBI | |
|
Eisermann J, Gast M, Pineda J, Odem RR and Collins JL: Tumor necrosis factor in peritoneal fluid of women undergoing laparoscopic surgery. Fertil Steril. 50:573–579. 1988.PubMed/NCBI View Article : Google Scholar | |
|
Bischoff F and Simpson JL: Genetic basis of endometriosis. Ann N Y Acad Sci. 1034:284–299. 2004.PubMed/NCBI View Article : Google Scholar | |
|
Ettehadi P, Greaves MW, Wallach D, Aderka D and Camp RD: Elevated tumour necrosis factor-alpha (TNF-alpha) biological activity in psoriatic skin lesions. Clin Exp Immunol. 96:146–151. 1994.PubMed/NCBI View Article : Google Scholar | |
|
Kristensen M, Chu CQ, Eedy DJ, Feldmann M, Brennan FM and Breathnach SM: Localization of tumour necrosis factor-alpha (TNF-alpha) and its receptors in normal and psoriatic skin: Epidermal cells express the 55-kD but not the 75-kD TNF receptor. Clin Exp Immunol. 94:354–362. 1993.PubMed/NCBI View Article : Google Scholar | |
|
Bonifati C, Carducci M, Cordiali Fei P, Trento E, Sacerdoti G, Fazio M and Ameglio F: Correlated increases of tumour necrosis factor-alpha, interleukin-6 and granulocyte monocyte-colony stimulating factor levels in suction blister fluids and sera of psoriatic patients-relationships with disease severity. Clin Exp Dermatol. 19:383–387. 1994.PubMed/NCBI View Article : Google Scholar | |
|
Heydarinejad P, Gholijani N, Habibagahi Z, Malekmakan MR and Amirghofran Z: FOXP3 gene variants in patients with systemic lupus erythematosus: Association with disease susceptibility in men and relationship with abortion in women. Iran J Immunol. 19:172–183. 2022.PubMed/NCBI View Article : Google Scholar | |
|
Yagi H, Nomura T, Nakamura K, Yamazaki S, Kitawaki T, Hori S, Maeda M, Onodera M, Uchiyama T, Fujii S, et al: Crucial role of FOXP3 in the development and function of human CD25+CD4+ regulatory T cells. Int Immunol. 16:1643–1656. 2004.PubMed/NCBI View Article : Google Scholar | |
|
Pereira LMS, Gomes STM, Ishak R and Vallinoto ACR: Regulatory T Cell and forkhead box protein 3 as modulators of immune homeostasis. Front Immunol. 8(605)2017.PubMed/NCBI View Article : Google Scholar | |
|
Gao L, Li K, Li F, Li H, Liu L, Wang L, Zhang Z, Gao T and Liu Y: Polymorphisms in the FOXP3 gene in Han Chinese psoriasis patients. J Dermatol Sci. 57:51–56. 2010.PubMed/NCBI View Article : Google Scholar | |
|
Oda JMm, Hirata BKb, Guembarovski RL and Watanabe MAe: Genetic polymorphism in FOXP3 gene: Imbalance in regulatory T-cell role and development of human diseases. J Genet. 92:163–171. 2013.PubMed/NCBI View Article : Google Scholar | |
|
Goronzy JJ, Zettl A and Weyand CM: T cell receptor repertoire in rheumatoid arthritis. Int Rev Immunol. 17:339–363. 1998.PubMed/NCBI View Article : Google Scholar | |
|
Nordquist H and Jamil RT: Biochemistry, HLA antigens. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing, 2025. | |
|
Wysocki T, Olesińska M and Paradowska-Gorycka A: Current understanding of an emerging role of HLA-DRB1 gene in rheumatoid arthritis-from research to clinical practice. Cells. 9(1127)2020.PubMed/NCBI View Article : Google Scholar | |
|
Ota H and Igarashi S: Expression of major histocompatibility complex class II antigen in endometriotic tissue in patients with endometriosis and adenomyosis. Fertil Steril. 60:834–838. 1993.PubMed/NCBI View Article : Google Scholar | |
|
Capon F: The genetic basis of psoriasis. Int J Mol Sci. 18(2526)2017.PubMed/NCBI View Article : Google Scholar | |
|
Griffiths CE and Barker JN: Pathogenesis and clinical features of psoriasis. Lancet. 370:263–271. 2007.PubMed/NCBI View Article : Google Scholar | |
|
Zong W, Ge Y, Han Y, Yang X, Li Q and Chen M: Hypomethylation of HLA-DRB1 and its clinical significance in psoriasis. Oncotarget. 8:12323–12332. 2017.PubMed/NCBI View Article : Google Scholar | |
|
Chong WP, Ip WK, Wong WHS, Lau CS, Chan TM and Lau YL: Association of interleukin-10 promoter polymorphisms with systemic lupus erythematosus. Genes Immun. 5:484–492. 2004.PubMed/NCBI View Article : Google Scholar | |
|
Döcke WD, Asadullah K, Belbe G, Ebeling M, Höflich C, Friedrich M, Sterry W and Volk HD: Comprehensive biomarker monitoring in cytokine therapy: Heterogeneous, time-dependent, and persisting immune effects of interleukin-10 application in psoriasis. J Leukoc Biol. 85:582–593. 2009.PubMed/NCBI View Article : Google Scholar | |
|
Tedgui A and Mallat Z: Interleukin-10: An anti-atherogenic cytokine. Pathol Biol (Paris). 49:107–108. 2001.PubMed/NCBI View Article : Google Scholar : (In French). | |
|
de Waal Malefyt R, Abrams J, Bennett B, Figdor CG and de Vries JE: Interleukin 10(IL-10) inhibits cytokine synthesis by human monocytes: An autoregulatory role of IL-10 produced by monocytes. J Exp Med. 174:1209–1220. 1991.PubMed/NCBI View Article : Google Scholar | |
|
Groux H and Cottrez F: The complex role of interleukin-10 in autoimmunity. J Autoimmun. 20:281–285. 2003.PubMed/NCBI View Article : Google Scholar | |
|
Liaqat I, Jahan N, Lone KP, Pakstis A and Taylor HS: Genetic polymorphisms associated with endometriosis in Pakistani women. J Endometr Pelvic Pain Disord. 5:134–143. 2013. | |
|
Edwards-Smith CJ, Jonsson JR, Purdie DM, Bansal A, Shorthouse C and Powell EE: Interleukin-10 promoter polymorphism predicts initial response of chronic hepatitis C to interferon alfa. Hepatology. 30:526–530. 1999.PubMed/NCBI View Article : Google Scholar | |
|
Zhang X, Hei P, Deng L and Lin J: Interleukin-10 gene promoter polymorphisms and their protein production in peritoneal fluid in patients with endometriosis. Mol Hum Reprod. 13:135–140. 2007.PubMed/NCBI View Article : Google Scholar | |
|
Goyette P, Pai A, Milos R, Frosst P, Tran P, Chen Z, Chan M and Rozen R: Gene structure of human and mouse methylenetetrahydrofolate reductase (MTHFR). Mamm Genome. 9:652–656. 1998.PubMed/NCBI View Article : Google Scholar | |
|
Watkins D and Rosenblatt DS: Inherited disorders of folate and cobalamin transport and Metabolism. In: The Online Metabolic and Molecular Bases of Inherited Disease. Valle DL, Antonarakis S, Ballabio A, Beaudet AL and Mitchell GA (eds). McGraw-Hill Education, New York, NY, 2019. | |
|
Qi J, Zhang Y, Zhang L and Nie G: Association between the MTHFR 677C/T polymorphism and susceptibility to psoriasis: An updated meta-analysis. Res Sq, 2022. | |
|
Frosst P, Blom HJ, Milos R, Goyette P, Sheppard CA, Matthews RG, Boers GJ, den Heijer M, Kluijtmans LA, van den Heuvel LP, et al: A candidate genetic risk factor for vascular disease: A common mutation in methylenetetrahydrofolate reductase. Nat Genet. 10:111–113. 1995.PubMed/NCBI View Article : Google Scholar | |
|
van der Put NM, Gabreëls F, Stevens EM, Smeitink JA, Trijbels FJ, Eskes TK, van den Heuvel LP and Blom HJ: A second common mutation in the methylenetetrahydrofolate reductase gene: an additional risk factor for neural-tube defects? Am J Hum Genet. 62:1044–1051. 1998.PubMed/NCBI View Article : Google Scholar | |
|
Sharp L and Little J: Polymorphisms in genes involved in folate metabolism and colorectal neoplasia: A HuGE review. Am J Epidemiol. 159:423–443. 2004.PubMed/NCBI View Article : Google Scholar | |
|
Lu M, Peng K, Song L, Luo L, Liang P and Liang Y: Association between genetic polymorphisms in methylenetetrahydrofolate reductase and risk of autoimmune diseases: A systematic review and meta-analysis. Dis Markers. 2022(4568145)2022.PubMed/NCBI View Article : Google Scholar | |
|
Zervou MI, Papageorgiou L, Vlachakis D, Spandidos DA, Eliopoulos E and Goulielmos GN: Genetic factors involved in the co-occurrence of endometriosis with ankylosing spondylitis (review). Mol Med Rep. 27(96)2023.PubMed/NCBI View Article : Google Scholar | |
|
Luo Q, Zeng J, Li W, Lin L, Zhou X, Tian X, Liu W, Zhang L and Zhang X: Interaction of MTHFR gene with smoking and alcohol use and haplotype combination susceptibility to psoriasis in Chinese population. Immunol Res. 66:543–547. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Huraib GB, Harthi FA, Arfin M, Khlaiwi AA, Rizvi S and Al-Asmari A: Methylenetetrahydrofolate reductase C677T gene polymorphism as risk factor for psoriasis in Saudis. Biomark Insights. 14(1177271919830973)2019.PubMed/NCBI View Article : Google Scholar | |
|
Karabacak E, Aydin E, Ozcan O, Dogan B, Gultepe M, Cosar A and Muftuoglu T: Methylenetetrahydrofolate reductase (MTHFR) 677C>T gene polymorphism as a possible factor for reducing clinical severity of psoriasis. Int J Clin Exp Med. 7:697–702. 2014.PubMed/NCBI | |
|
Wu D, Shi D, Yang L and Zhu X: Association between methylenetetrahydrofolate reductase C677T polymorphism and psoriasis: A meta-analysis. J Dermatol. 43:162–169. 2016.PubMed/NCBI View Article : Google Scholar | |
|
Zamani MR, Salmaninejad A, Akbari Asbagh F, Masoud A and Rezaei N: STAT4 single nucleotide gene polymorphisms and susceptibility to endometriosis-related infertility. Eur J Obstet Gynecol Reprod Biol. 203:20–24. 2016.PubMed/NCBI View Article : Google Scholar | |
|
Frucht DM, Aringer M, Galon J, Danning C, Brown M, Fan S, Centola M, Wu CY, Yamada N, El Gabalawy H and O'Shea JJ: Stat4 is expressed in activated peripheral blood monocytes, dendritic cells, and macrophages at sites of Th1-mediated inflammation. J Immunol. 164:4659–4664. 2000.PubMed/NCBI View Article : Google Scholar | |
|
Mathur AN, Chang HC, Zisoulis DG, Stritesky GL, Yu Q, O'Malley JT, Kapur R, Levy DE, Kansas GS and Kaplan MH: Stat3 and Stat4 direct development of IL-17-secreting Th cells. J Immunol. 178:4901–4907. 2007.PubMed/NCBI View Article : Google Scholar | |
|
Thierfelder WE, van Deursen JM, Yamamoto K, Tripp RA, Sarawar SR, Carson RT, Sangster MY, Vignali DA, Doherty PC, Grosveld GC and Ihle JN: Requirement for Stat4 in interleukin-12-mediated responses of natural killer and T cells. Nature. 382:171–174. 1996.PubMed/NCBI View Article : Google Scholar | |
|
Wun CM, Piao Z, Hong KT, Choi JY, Hong CR, Park JD, Park KD, Shin HY and Kang HJ: Effect of donor STAT4 polymorphism rs7574865 on clinical outcomes of pediatric acute leukemia patients after hematopoietic stem cell transplant. Int Immunopharmacol. 43:62–69. 2017.PubMed/NCBI View Article : Google Scholar | |
|
Zervou MI, Mamoulakis D, Panierakis C, Boumpas DT and Goulielmos GN: STAT4: A risk factor for type 1 diabetes? Hum Immunol. 69:647–650. 2008.PubMed/NCBI View Article : Google Scholar | |
|
Podgaec S, Dias Junior JA, Chapron C, de Oliveira RM, Baracat EC and Abrão MS: Th1 and Th2 immune responses related to pelvic endometriosis. Rev Assoc Med Bras (1992). 56:92–98. 2010.PubMed/NCBI View Article : Google Scholar | |
|
Remmers EF, Plenge RM, Lee AT, Graham RR, Hom G, Behrens TW, de Bakker PIW, Le JM, Lee HS, Batliwalla F, et al: STAT4 and the risk of rheumatoid arthritis and systemic lupus erythematosus. N Engl J Med. 357:977–986. 2007.PubMed/NCBI View Article : Google Scholar | |
|
Li B, Huang L, Lv P, Li X, Liu G, Chen Y, Wang Z, Qian X, Shen Y, Li Y and Fang W: The role of Th17 cells in psoriasis. Immunol Res. 68:296–309. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Ferrara N, Gerber HP and LeCouter J: The biology of VEGF and its receptors. Nat Med. 9:669–676. 2003.PubMed/NCBI View Article : Google Scholar | |
|
Girling JE and Rogers PA: Recent advances in endometrial angiogenesis research. Angiogenesis. 8:89–99. 2005.PubMed/NCBI View Article : Google Scholar | |
|
Pandey AK, Singhi EK, Arroyo JP, Ikizler TA, Gould ER, Brown J, Beckman JA, Harrison DG and Moslehi J: Mechanisms of VEGF (vascular endothelial growth factor) inhibitor-associated hypertension and vascular disease. Hypertension. 71:e1–e8. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Fukumura D, Xavier R, Sugiura T, Chen Y, Park EC, Lu N, Selig M, Nielsen G, Taksir T, Jain RK and Seed JB: Tumor induction of VEGF promoter activity in stromal cells. Cell. 94:715–725. 1998.PubMed/NCBI View Article : Google Scholar | |
|
Bhanoori M, Arvind Babu K, Pavankumar Reddy NG, Lakshmi Rao K, Zondervan K, Deenadayal M, Kennedy S and Shivaji S: The vascular endothelial growth factor (VEGF) +405G>C 5'-untranslated region polymorphism and increased risk of endometriosis in South Indian women: A case control study. Hum. Reprod. 20:1844–1849. 2005.PubMed/NCBI View Article : Google Scholar | |
|
Li YZ, Wang LJ, Li X, Li SL, Wang JL, Wu ZH, Gong L and Zhang XD: Vascular endothelial growth factor gene polymorphisms contribute to the risk of endometriosis: An updated systematic review and meta-analysis of 14 case-control studies. Genet Mol Res. 12:1035–1044. 2013.PubMed/NCBI View Article : Google Scholar | |
|
Buroker NE: SNP (rs1570360) in transcriptional factor binding sites of the VEGFA promoter is associated with hypertensive nephropathy and diabetic retinopathy. Austin J Endocrinol Diabetes. 2(1035)2015. | |
|
Tan XJ, Lang JH, Liu DY, Shen K, Leng JH and Zhu L: Expression of vascular endothelial growth factor and thrombospondin-1 mRNA in patients with endometriosis. Fertil Steril. 78:148–153. 2002.PubMed/NCBI View Article : Google Scholar | |
|
Cosín R, Gilabert-Estellés J, Ramón LA, Gómez-Lechón MJ, Gilabert J, Chirivella M, Braza-Boïls A, España F and Estellés A: Influence of peritoneal fluid on the expression of angiogenic and proteolytic factors in cultures of endometrial cells from women with endometriosis. Hum Reprod. 25:398–405. 2010.PubMed/NCBI View Article : Google Scholar | |
|
Donnez J, Smoes P, Gillerot S, Casanas-Roux F and Nisolle M: Vascular endothelial growth factor (VEGF) in endometriosis. Hum Reprod. 13:1686–1690. 1998.PubMed/NCBI View Article : Google Scholar | |
|
McLaren J: Vascular endothelial growth factor and endometriotic angiogenesis. Hum Reprod Update. 6:45–55. 2000.PubMed/NCBI View Article : Google Scholar | |
|
Viganò P, Parazzini F, Somigliana E and Vercellini P: Endometriosis: Epidemiology and aetiological factors. Best Pract Res Clin Obstet Gynaecol. 18:177–200. 2004.PubMed/NCBI View Article : Google Scholar | |
|
Cox A, Camp NJ, Nicklin MJ, di Giovine FS and Duff GW: An analysis of linkage disequilibrium in the interleukin-1 gene cluster, using a novel grouping method for multiallelic markers. Am J Hum Genet. 62:1180–1188. 1998.PubMed/NCBI View Article : Google Scholar | |
|
Johnson K, Hashimoto S, Lotz M, Pritzker K and Terkeltaub R: Interleukin-1 induces pro-mineralizing activity of cartilage tissue transglutaminase and factor XIIIa. Am J Pathol. 159:149–163. 2001.PubMed/NCBI View Article : Google Scholar | |
|
Badie A, Saliminejad K, Salahshourifar I and Khorram Khorshid HR: Interleukin 1 alpha (IL1A) polymorphisms and risk of endometriosis in Iranian population: a case-control study. Gynecol Endocrinol. 36:135–138. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Sikora J, Ferrero S, Mielczarek-Palacz A and Kondera-Anasz Z: The delicate balance between the good and the bad IL-1 proinflammatory effects in endometriosis. Curr Med Chem. 25:2105–2121. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Kondera-Anasz Z, Sikora J, Mielczarek-Palacz A and Jońca M: Concentrations of interleukin (IL)-1alpha, IL-1 soluble receptor type II (IL-1 sRII) and IL-1 receptor antagonist (IL-1 Ra) in the peritoneal fluid and serum of infertile women with endometriosis. Eur J Obstet Gynecol Reprod Biol. 123:198–203. 2005.PubMed/NCBI View Article : Google Scholar | |
|
Koumantakis E, Matalliotakis I, Neonaki M, Froudarakis G and Georgoulias V: Soluble serum interleukin-2 receptor, interleukin-6 and interleukin-1a in patients with endometriosis and in controls. Arch Gynecol Obstet. 255:107–112. 1994.PubMed/NCBI View Article : Google Scholar | |
|
Nackley AG, Shabalina SA, Tchivileva IE, Satterfield K, Korchynskyi O, Makarov SS, Maixner W and Diatchenko L: Human catechol-O-methyltransferase haplotypes modulate protein expression by altering mRNA secondary structure. Science. 314:1930–1933. 2006.PubMed/NCBI View Article : Google Scholar | |
|
Graves BJ, Hatada MH, Hendrickson WA, Miller JK, Madison VS and Satow Y: Structure of interleukin 1 alpha at 2.7-A resolution. Biochemistry. 29:2679–2684. 1990.PubMed/NCBI View Article : Google Scholar | |
|
Adzhubei IA, Schmidt S, Peshkin L, Ramensky VE, Gerasimova A, Bork P, Kondrashov AS and Sunyaev SR: A method and server for predicting damaging missense mutations. Nat Methods. 7:248–249. 2010.PubMed/NCBI View Article : Google Scholar | |
|
Partsch G, Steiner G, Leeb BF, Dunky A, Bröll H and Smolen JS: Highly increased levels of tumor necrosis factor-alpha and other proinflammatory cytokines in psoriatic arthritis synovial fluid. J Rheumatol. 24:518–523. 1997.PubMed/NCBI | |
|
Ritchlin C, Haas-Smith SA, Hicks D, Cappuccio J, Osterland CK and Looney RJ: Patterns of cytokine production in psoriatic synovium. J Rheumatol. 25:1544–1552. 1998.PubMed/NCBI | |
|
Donn R, Alourfi Z, De Benedetti F, Meazza C, Zeggini E, Lunt M, Stevens A, Shelley E, Lamb R, Ollier WER, et al: Mutation screening of the macrophage migration inhibitory factor gene: positive association of a functional polymorphism of macrophage migration inhibitory factor with juvenile idiopathic arthritis. Arthritis Rheum. 46:2402–2409. 2002.PubMed/NCBI View Article : Google Scholar | |
|
Calandra T and Roger T: Macrophage migration inhibitory factor: A regulator of innate immunity. Nat Rev Immunol. 3:791–800. 2003.PubMed/NCBI View Article : Google Scholar | |
|
Bacher M, Metz CN, Calandra T, Mayer K, Chesney J, Lohoff M, Gemsa D, Donnelly T and Bucala R: An essential regulatory role for macrophage migration inhibitory factor in T-cell activation. Proc Natl Acad Sci USA. 93:7849–7854. 1996.PubMed/NCBI View Article : Google Scholar | |
|
Denkinger CM, Metz C, Fingerle-Rowson G, Denkinger MD and Forsthuber T: Macrophage migration inhibitory factor and its role in autoimmune diseases. Arch Immunol Ther Exp (Warsz). 52:389–400. 2004.PubMed/NCBI | |
|
Kats R, Metz CN and Akoum A: Macrophage migration inhibitory factoris markedly expressed in active and early-stage endometriotic lesions. J Clin Endocrinol Metab. 87:883–889. 2002.PubMed/NCBI View Article : Google Scholar | |
|
Veillat V, Sengers V, Metz CN, Roger T, Leboeuf M, Mailloux J and Akoum A: Macrophage migration inhibitory factor is involved in a positive feedback loop increasing aromatase expression in endometriosis. Am J Pathol. 181:917–927. 2012.PubMed/NCBI View Article : Google Scholar | |
|
Cloutier JF and Veillette A: Cooperative inhibition of T-cell antigen receptor signaling by a complex between a kinase and a phosphatase. J Exp Med. 189:111–121. 1999.PubMed/NCBI View Article : Google Scholar | |
|
Wu J, Katrekar A, Honigberg LA, Smith AM, Conn MT, Tang J, Jeffery D, Mortara K, Sampang J, Williams SR, et al: Identification of substrates of human protein-tyrosine phosphatase PTPN22. J Biol Chem. 281:11002–11010. 2006.PubMed/NCBI View Article : Google Scholar | |
|
Juneblad K, Johansson M, Rantapää-Dahlqvist S and Alenius GM: Association between the PTPN22 +1858 C/T polymorphism and psoriatic arthritis. Arthritis Res Ther. 13(R45)2011.PubMed/NCBI View Article : Google Scholar | |
|
Kotlyar AM, Mamillapalli R, Flores VA and Taylor HS: Tofacitinib alters STAT3 signaling and leads to endometriosis lesion regression. Mol Hum Reprod. 27(gaab016)2021.PubMed/NCBI View Article : Google Scholar | |
|
Abisror N, Kolanska K, Cheloufi M, Selleret L, d'Argent E, Kayem G and Mekinian A: Endometriosis and autoimmunity. Explor Immunol. 2:25–31. 2022. | |
|
Gaffen SL, Jain R, Garg AV and Cua DJ: The IL-23-IL-17 immune axis: From mechanisms to therapeutic testing. Nat Rev Immunol. 14:585–600. 2014.PubMed/NCBI View Article : Google Scholar | |
|
Tian F, Chen Z and Xu T: Efficacy and safety of tofacitinib for the treatment of chronic plaque psoriasis: A systematic review and meta-analysis. J Int Med Res. 47:2342–2350. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Ighani A, Georgakopoulos JR and Yeung J: Tofacitinib for the treatment of psoriasis and psoriatic arthritis. G Ital Dermatol Venereol. 155:400–410. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Hu X, Li J, Fu M, Zhao X and Wang W: The JAK/STAT signaling pathway: From bench to clinic. Signal Transduct Target Ther. 6(402)2021.PubMed/NCBI View Article : Google Scholar | |
|
Falconer H, Sundqvist J, Gemzell-Danielsson K, von Schoultz B, D'Hooghe TM and Fried G: IVF outcome in women with endometriosis in relation to tumour necrosis factor and anti-Müllerian hormone. Reprod Biomed Online. 18:582–588. 2009.PubMed/NCBI View Article : Google Scholar | |
|
Gisondi P and Girolomoni G: Biologic therapies in psoriasis: A new therapeutic approach. Autoimmun Rev. 6:515–519. 2007.PubMed/NCBI View Article : Google Scholar | |
|
Mease PJ, Goffe BS, Metz J, Van der Stoep A, Finck B and Burge DJ: Etanercept in the treatment of psoriatic arthritis and psoriasis: A randomised trial. Lancet. 356:385–390. 2000.PubMed/NCBI View Article : Google Scholar | |
|
Braun DP, Ding J and Dmowski WP: Peritoneal fluid-mediated enhancement of eutopic and ectopic endometrial cell proliferation is dependent on tumor necrosis factor-alpha in women with endometriosis. Fertil Steril. 78:727–732. 2002.PubMed/NCBI View Article : Google Scholar | |
|
D'Angelo S, Cantini F, Ramonda R, Cantarini L, Carletto A, Chimenti MS, Delle Sedie A, Foti R, Gerli R, Lomater C, et al: Effectiveness of adalimumab for the treatment of psoriatic arthritis: An Italian real-life retrospective study. Front Pharmacol. 10(1497)2019.PubMed/NCBI View Article : Google Scholar | |
|
Calabrese LH: Molecular differences in anticytokine therapies. Clin Exp Rheumatol. 21:241–248. 2003.PubMed/NCBI | |
|
Alwawi EA, Mehlis SL and Gordon KB: Treating psoriasis with adalimumab. Ther Clin Risk Manag. 4:345–351. 2008.PubMed/NCBI View Article : Google Scholar | |
|
Kaplan S, Kırıcı P and Türk A: The effects of adalimumab on the rat autotransplantation endometriosis model: A placebo-controlled randomized study. Adv Clin Exp Med. 31:417–426. 2022.PubMed/NCBI View Article : Google Scholar | |
|
Ahn SH, Khalaj K, Young SL, Lessey BA, Koti M and Tayade C: Immune-inflammation gene signatures in endometriosis patients. Fertil Steril. 106:1420–1431.e7. 2016.PubMed/NCBI View Article : Google Scholar | |
|
Szóstek-Mioduchowska A, Wójtowicz A, Sadowska A, Moza Jalali B, Słyszewska M, Łukasik K, Gurgul A, Szmatoła T, Bugno-Poniewierska M, Ferreira-Dias G and Skarzynski DJ: Transcriptomic profiling of mare endometrium at different stages of endometrosis. Sci Rep. 13(16263)2023.PubMed/NCBI View Article : Google Scholar | |
|
Liu S, Li X, Gu Z, Wu J, Jia S, Shi J, Dai Y, Wu Y, Yan H, Zhang J, et al: Single-cell and spatial transcriptomic profiling revealed niche interactions sustaining growth of endometriotic lesions. Cell Genom. 5(100737)2025.PubMed/NCBI View Article : Google Scholar | |
|
Burns GW, Fu Z, Vegter EL, Madaj ZB, Greaves E, Flores I and Fazleabas AT: Spatial transcriptomic analysis identifies epithelium-macrophage crosstalk in endometriotic lesions. iScience. 28(111790)2025.PubMed/NCBI View Article : Google Scholar | |
|
Swindell WR, Johnston A, Voorhees JJ, Elder JT and Gudjonsson JE: Dissecting the psoriasis transcriptome: Inflammatory- and cytokine-driven gene expression in lesions from 163 patients. BMC Genomics. 14(527)2013.PubMed/NCBI View Article : Google Scholar | |
|
Choudhary S, Anand R, Pradhan D, Bastia B, Kumar SN, Singh H, Puri P, Thomas G and Jain AK: Transcriptomic landscaping of core genes and pathways of mild and severe psoriasis vulgaris. Int J Mol Med. 47:219–231. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Rawat A, Rinchai D, Toufiq M, Marr AK, Kino T, Garand M, Tatari-Calderone Z, Kabeer BSA, Krishnamoorthy N, Bedognetti D, et al: A Neutrophil-driven inflammatory signature characterizes the blood transcriptome fingerprint of psoriasis. Front Immunol. 11(587946)2020.PubMed/NCBI View Article : Google Scholar | |
|
Garshick MS, Barrett TJ, Cornwell MG, Drenkova K, Garelik J, Weber BN, Schlamp F, Rockman C, Ruggles KV, Reynolds HR and Berger JS: An inflammatory transcriptomic signature in psoriasis associates with future cardiovascular events. J Eur Acad Dermatol Venereol. 37:1361–1365. 2023.PubMed/NCBI View Article : Google Scholar | |
|
Krishnan VS and Kõks S: Transcriptional basis of psoriasis from large scale gene expression studies: The importance of moving towards a precision medicine approach. Int J Mol Sci. 23(6130)2022.PubMed/NCBI View Article : Google Scholar | |
|
Rioux G, Ridha Z, Simard M, Turgeon F, Guérin SL and Pouliot R: Transcriptome profiling analyses in psoriasis: A dynamic contribution of keratinocytes to the pathogenesis. Genes (Basel). 11(1155)2020.PubMed/NCBI View Article : Google Scholar | |
|
Laborde CM, Larzabal L, González-Cantero Á, Castro-Santos P and Díaz-Peña R: Advances of genomic medicine in psoriatic arthritis. J Pers Med. 12(35)2022.PubMed/NCBI View Article : Google Scholar | |
|
Johnsson H, Cole J, McInnes IB, Graham G and Siebert S: Differences in transcriptional changes in psoriasis and psoriatic arthritis skin with immunoglobulin gene enrichment in psoriatic arthritis. Rheumatology (Oxford). 63:218–225. 2024.PubMed/NCBI View Article : Google Scholar | |
|
Grivas A, Grigoriou M, Malissovas N, Sentis G, Filia A, Flouda S, Katsimpri P, Verginis P and Boumpas DT: Combined-whole blood and skin fibroblasts-transcriptomic analysis in psoriatic arthritis reveals molecular signatures of activity, resistance and early response to treatment. Front Immunol. 13(964274)2022.PubMed/NCBI View Article : Google Scholar | |
|
Grivas A, Fragoulis G, Garantziotis P, Banos A, Nikiphorou E and Boumpas D: Unraveling the complexities of psoriatic arthritis by the use of-Omics and their relevance for clinical care. Autoimmun Rev. 20(102949)2021.PubMed/NCBI View Article : Google Scholar | |
|
Ding J, Su S, You T, Xia T, Lin X, Chen Z and Zhang L: Serum interleukin-6 level is correlated with the disease activity of systemic lupus erythematosus: A meta-analysis. Clinics (Sao Paulo). 75(e1801)2020.PubMed/NCBI View Article : Google Scholar | |
|
Atzeni F, Ventura D, Batticciotto A, Boccassini L and Sarzi-Puttini P: Interleukin 6 blockade: tocilizumab in psoriatic arthritis. J Rheumatol Suppl. 89:97–99. 2012.PubMed/NCBI View Article : Google Scholar | |
|
Costa L, Caso F, Cantarini L, Del Puente A, Scarpa R and Atteno M: Efficacy of tocilizumab in a patient with refractory psoriatic arthritis. Clin Rheumatol. 33:1355–1357. 2014.PubMed/NCBI View Article : Google Scholar | |
|
Hayakawa M, Izumi K, Higashida-Konishi M, Ushikubo M, Tsukamoto M, Akiya K, Araki K and Oshima H: Tocilizumab-induced psoriasis-like eruption resolved by shortening the dose interval in a patient with rheumatoid arthritis: A case-based review. Rheumatol Int. 39:161–166. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Nielly H, Bialé L, Gilardin L, Carmoi T, Éon A, Vanquaethem H and Fougerousse AC: Tocilizumab-induced psoriatic eruption: A case report and a case-based review. Rheumatol Int. 44:2205–2212. 2024.PubMed/NCBI View Article : Google Scholar | |
|
Papageorgiou L, Andreou A, Zervou M, Vlachakis D, Goulielmos GN and Eliopoulos E: A global population genomic analysis shows novel insights into the genetic characteristics of endometriosis. World Acad Sci J. 5(12)2023. | |
|
Oliveros JC: Venny. An interactive tool for comparing lists with Venn's diagrams, 2007-2015. https://bioinfogp.cnb.csic.es/tools/venny/index.html. |