Advances of exosome regulating‑FXR to repair inflammatory bowel disease (Review)
- Authors:
- Peter Muro
- Caihong Jing
- Yaru Qiao
- Wenbing Wang
- Bo Wang
- Fei Mao
-
Affiliations: Key Laboratory of Medical Science and Laboratory Medicine of Jiangsu, School of Medicine, Jiangsu University, Zhenjiang, Jiangsu 212013, P.R. China, The People's Hospital of Danyang, Affiliated Danyang Hospital of Nantong University, Zhenjiang, Jiangsu 212300, P.R. China - Published online on: July 3, 2025 https://doi.org/10.3892/ijmm.2025.5576
- Article Number: 135
-
Copyright: © Muro et al. This is an open access article distributed under the terms of Creative Commons Attribution License.
This article is mentioned in:
Abstract
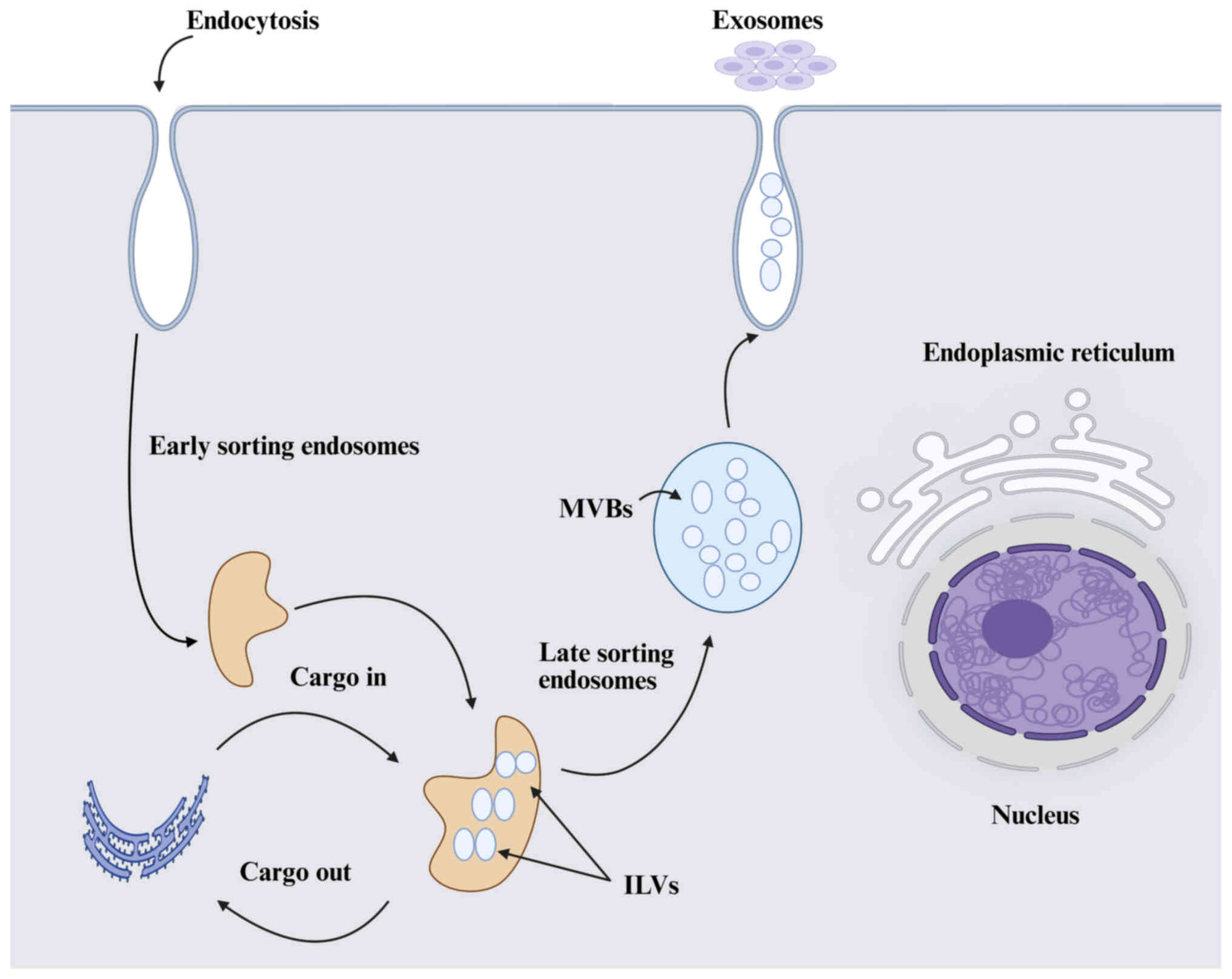 |
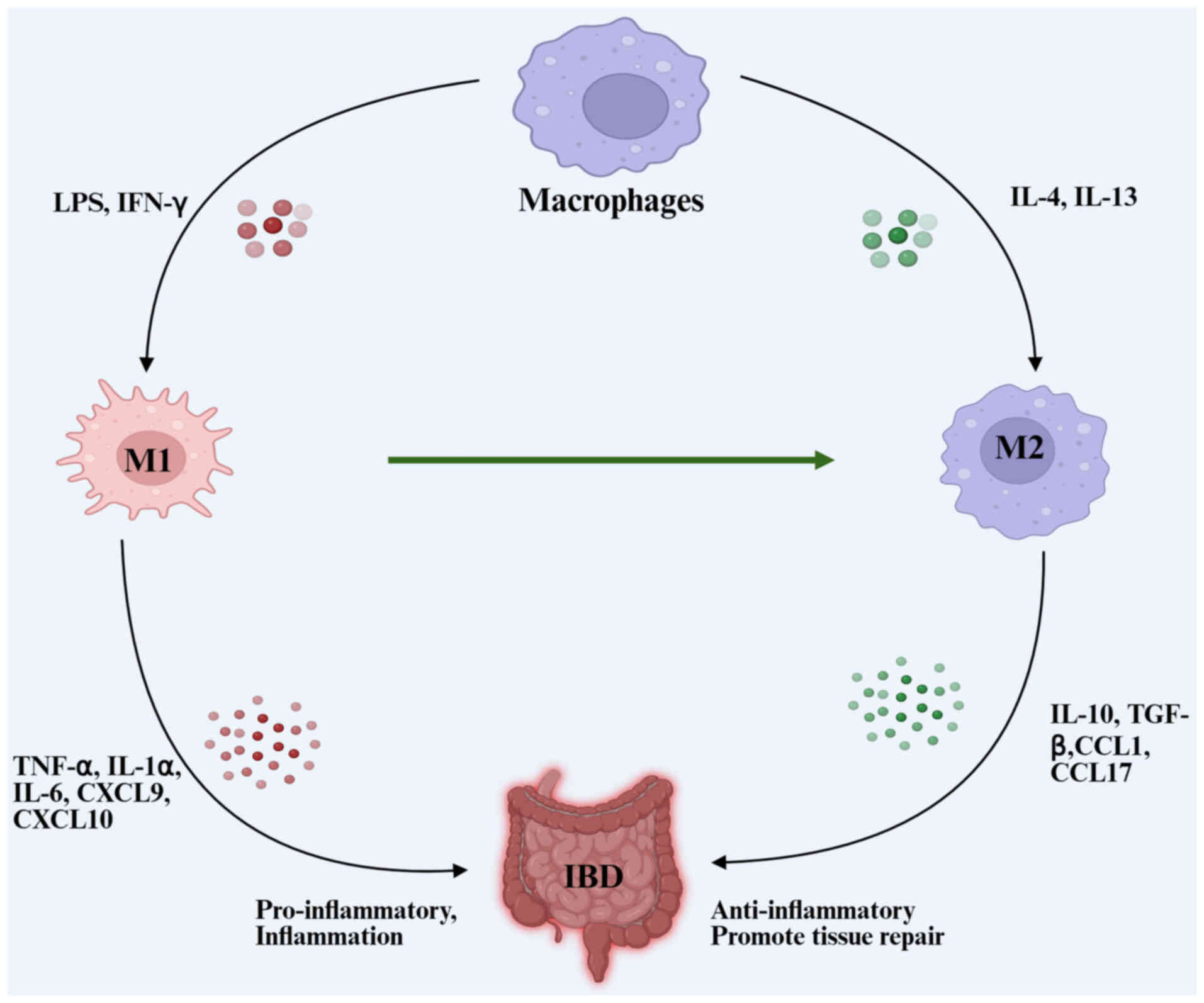 |
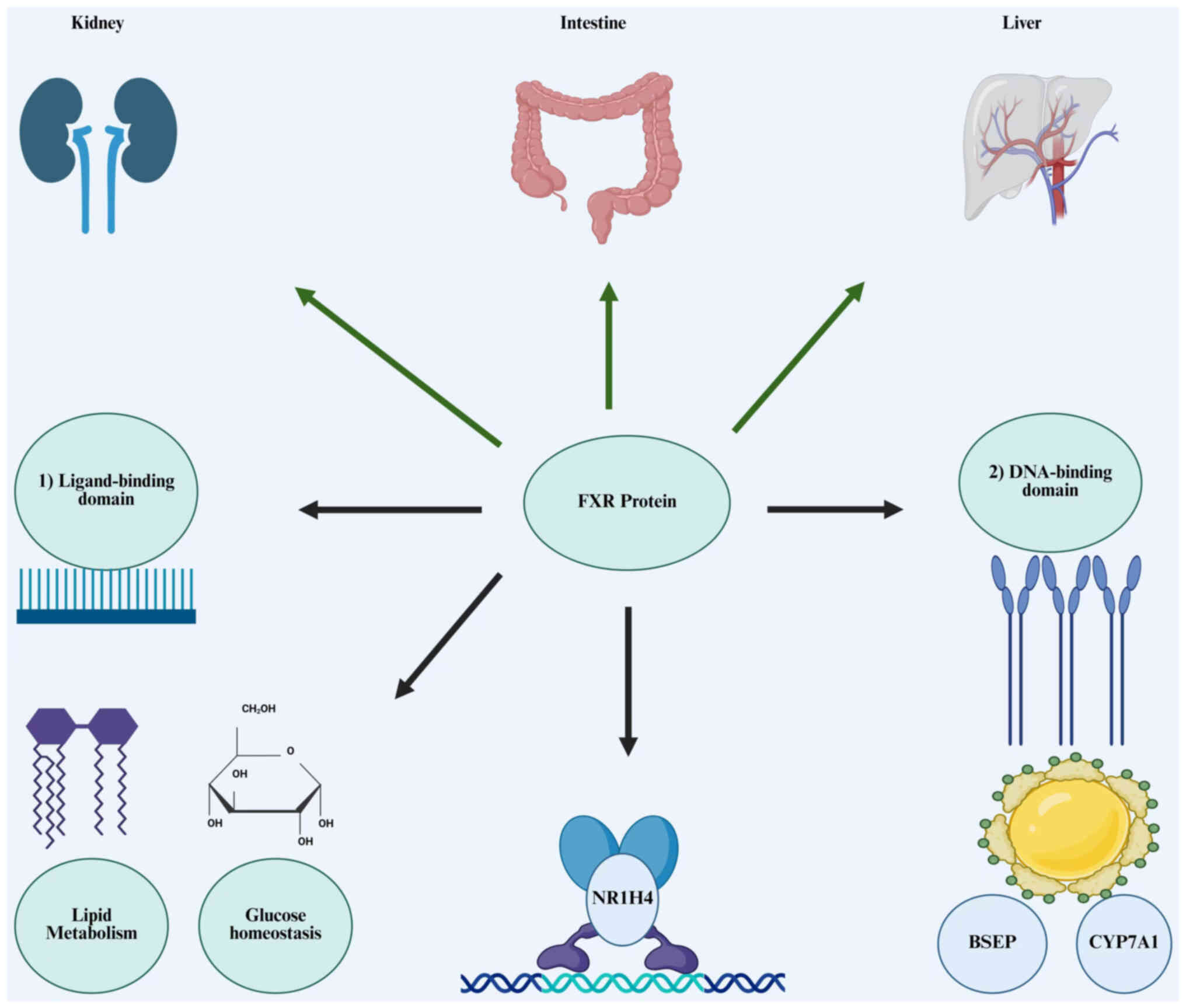 |
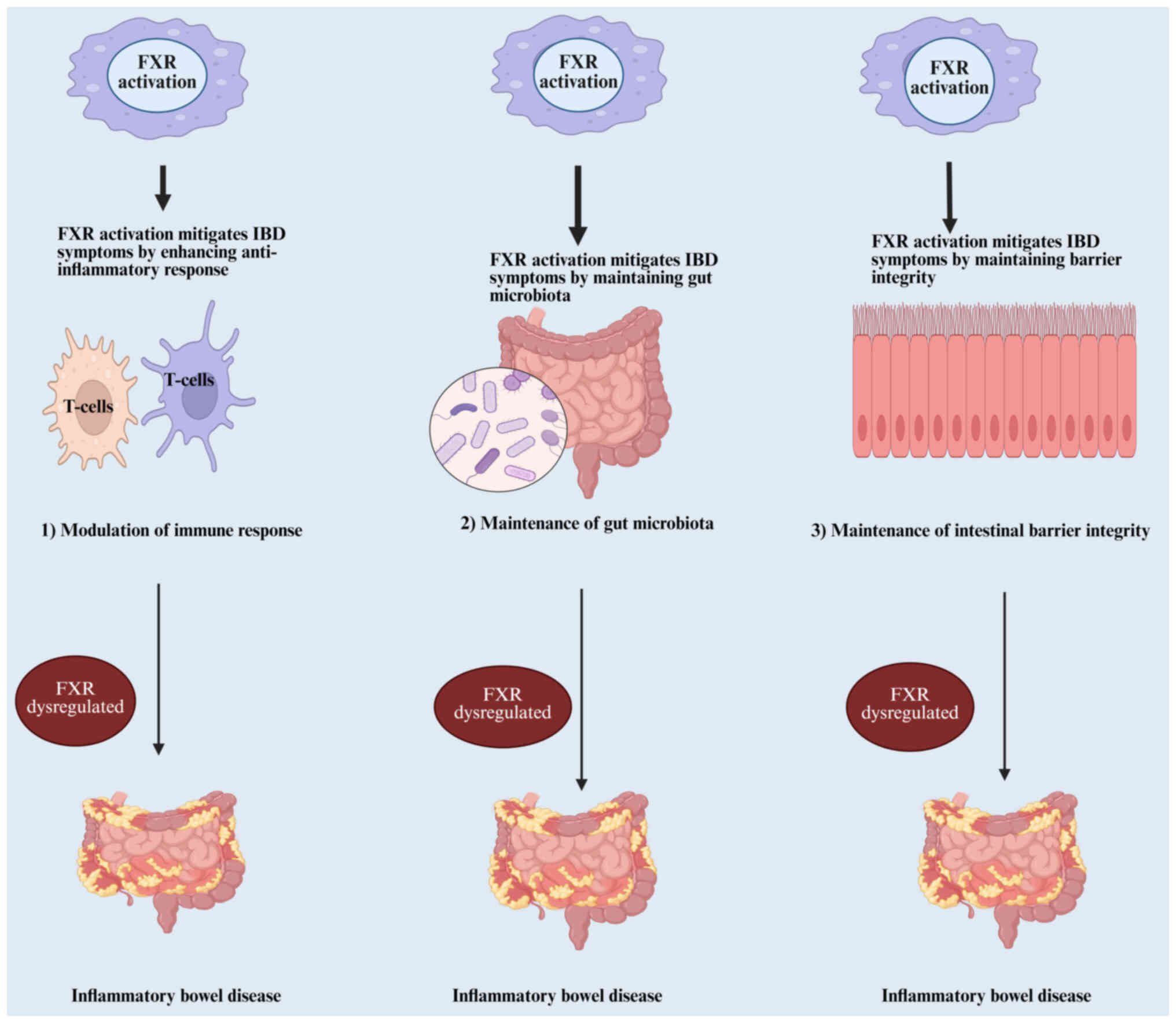 |
|
Muro P, Zhang L, Li S, Zhao Z, Jin T, Mao F and Mao Z: The emerging role of oxidative stress in inflammatory bowel disease. Front Endocrinol (Lausanne). 15:13903512024. View Article : Google Scholar : PubMed/NCBI | |
|
Al-Amrah H, Saadah OI, Mosli M, Edris S, Alhindi R and Bahieldin A: Alteration of the gut microbiome for patients with inflammatory bowel disease: A review. Appl Ecol Environ Res. 18:7379–7392. 2020. View Article : Google Scholar | |
|
Chew DCH, Khoo XH, Lee TS, Chin KY, Raja Ali RA, Muhammad Nawawi KN, Wan Ibrahim NR and Hilmi I: A systematic review on the increasing incidence of inflammatory bowel disease in southeast asia: Looking beyond the urbanization phenomenon. Inflamm Bowel Dis. 30:1566–1578. 2024. View Article : Google Scholar | |
|
Saleem H, Muhammad Jaffry SHB, Zia U, Marwat ZI, Shah N and Alamzeb J: Examining the autoimmune, genetic, environmental, and microbial aspects of the complex etiology of inflammatory bowel diseases: A comprehensive review and comparative analysis. J Women Med Dent Coll. 2:2024 View Article : Google Scholar | |
|
Bastaki SMA, Amir N, Adeghate E and Ojha S: Lycopodium mitigates oxidative stress and inflammation in the colonic mucosa of acetic Acid-induced colitis in rats. Molecules. 27:27742022. View Article : Google Scholar : PubMed/NCBI | |
|
Gao C, Zhou Y, Chen Z, Li H, Xiao Y, Hao W, Zhu Y, Vong CT, Farag MA, Wang Y and Wang S: Turmeric-derived nanovesicles as novel nanobiologics for targeted therapy of ulcerative colitis. Theranostics. 12:5596–5614. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Liao HX, Mao X, Wang L, Wang N, Ocansey DKW, Wang B and Mao F: The role of mesenchymal stem cells in attenuating inflammatory bowel disease through ubiquitination. Front Immunol. 15:14230692014. View Article : Google Scholar | |
|
Noguera-Fernández N, Candela-González J and Orenes-Piñero E: Probiotics, prebiotics, fecal microbiota transplantation, and dietary patterns in inflammatory bowel disease. Mol Nutr Food Res. 68:e24004292024. View Article : Google Scholar : PubMed/NCBI | |
|
Higashiyama M and Hokari R: New and emerging treatments for inflammatory bowel disease. Digestion. 104:74–81. 2023. View Article : Google Scholar | |
|
Zhou M, Wang D, Li X, Cao Y, Yi C, Wiredu Ocansey DK, Zhou Y and Mao F: Farnesoid-X receptor as a therapeutic target for inflammatory bowel disease and colorectal cancer. Front Pharmacol. 13:10168362022. View Article : Google Scholar : PubMed/NCBI | |
|
Li Y, Xu T, Zhao Y, Zhang H, Liu Z, Wang H, Huang C, Shu Z, Gao L, Xie R, et al: Discovery and optimization of novel nonbile acid FXR agonists as preclinical candidates for the treatment of inflammatory bowel disease. J Med Chem. 67:5642–5661. 2024. View Article : Google Scholar : PubMed/NCBI | |
|
Kim HI, Park J, Zhu Y, Wang X, Han Y and Zhang D: Recent advances in extracellular vesicles for therapeutic cargo delivery. Exp Mol Med. 56:836–849. 2024. View Article : Google Scholar : PubMed/NCBI | |
|
Li J, Zhang Y, Dong PY, Yang GM and Gurunathan S: A comprehensive review on the composition, biogenesis, purification, and multifunctional role of exosome as delivery vehicles for cancer therapy. Biomed Pharmacother. 165:1150872023. View Article : Google Scholar : PubMed/NCBI | |
|
Miao C, Wang X, Zhou W and Huang J: The emerging roles of exosomes in autoimmune diseases, with special emphasis on microRNAs in exosomes. Pharmacol Res. 169:1056802021. View Article : Google Scholar : PubMed/NCBI | |
|
Ludwig N, Whiteside TL and Reichert TE: Challenges in exosome isolation and analysis in health and disease. Int J Mol Sci. 20:46842019. View Article : Google Scholar : PubMed/NCBI | |
|
Ocansey DKW, Zhang Z, Xu X, Liu L, Amoah S, Chen X, Wang B, Zhang X and Mao F: Mesenchymal stem cell-derived exosome mitigates colitis via the modulation of the gut metagenomics-metabolomics-farnesoid X receptor axis. Biomater Sci. 10:4822–4836. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Zhou M, Pei B, Cai P, Yi C, Akanyibah FA, Lyu C and Mao F: Human umbilical cord mesenchymal stem cell-derived exosomes repair IBD by activating the SIRT1-FXR pathway in macrophages. Stem Cell Res Ther. 16:2332025. View Article : Google Scholar : PubMed/NCBI | |
|
Jella KK, Nasti TH, Li Z, Malla SR, Buchwald ZS and Khan MK: Exosomes, their biogenesis and role in inter-cellular communication, tumor microenvironment and cancer immunotherapy. Vaccines (Basel). 6:692018. View Article : Google Scholar : PubMed/NCBI | |
|
Wani S, Man Law IK and Pothoulakis C: Role and mechanisms of exosomal miRNAs in IBD pathophysiology. Am J Physiol Liver Physiol. 319:G646–G654. 2020. | |
|
van Niel G, D'Angelo G and Raposo G: Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol. 19:213–228. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Gurung S, Perocheau D, Touramanidou L and Baruteau J: The exosome journey: From biogenesis to uptake and intracellular signalling. Cell Commun Signal. 19:472021. View Article : Google Scholar : PubMed/NCBI | |
|
Matsui T, Osaki F, Hiragi S, Sakamaki Y and Fukuda M: ALIX and ceramide differentially control polarized small extracellular vesicle release from epithelial cells. EMBO Rep. 22:e514752021. View Article : Google Scholar : PubMed/NCBI | |
|
Lee KM, Seo EC, Lee JH, Kim HJ and Hwangbo C: The multifunctional protein Syntenin-1: Regulator of exosome biogenesis, cellular function, and tumor progression. Int J Mol Sci. 24:94182023. View Article : Google Scholar : PubMed/NCBI | |
|
Kim G, Zhu R, Zhang Y, Jeon H, Shirinichi F and Wang Y: Fluorescent chiral quantum dots to unveil Origin-dependent exosome uptake and cargo release. ACS Appl Bio Mater. 7:3358–3374. 2024. View Article : Google Scholar : PubMed/NCBI | |
|
Kang M, Yadav MK, Mbanefo EC, Yu CR and Egwuagu CE: IL-27-containing exosomes secreted by innate B-1a cells suppress and ameliorate uveitis. Front Immunol. 14:10711622023. View Article : Google Scholar : PubMed/NCBI | |
|
Fu W, Lei C, Liu S, Cui Y, Wang C, Qian K, Li T, Shen Y, Fan X, Lin F, et al: CAR exosomes derived from effector CAR-T cells have potent antitumour effects and low toxicity. Nat Commun. 10:43552019. View Article : Google Scholar : PubMed/NCBI | |
|
Yang H, Chen J, Liu S, Xue Y, Li Z, Wang T, Jiao L, An Q, Liu B, Wang J and Zhao H: Exosomes From IgE-stimulated mast cells aggravate asthma-mediated atherosclerosis through circRNA CDR1as-mediated endothelial cell dysfunction in mice. Arterioscler Thromb Vasc Biol. 44:e99–e115. 2024. View Article : Google Scholar : PubMed/NCBI | |
|
Liu H, Zhang X, Zhang M, Zhang S, Li J, Zhang Y, Wang Q, Cai JP, Cheng K and Wang S: Mesenchymal stem cell derived exosomes repair uterine injury by targeting transforming growth factor-β signaling. ACS Nano. 18:3509–3519. 2024. View Article : Google Scholar : PubMed/NCBI | |
|
Lin Y, Anderson JD, Rahnama LMA, Gu SV and Knowlton AA: Exosomes in disease and regeneration: Biological functions, diagnostics, and beneficial effects. Am J Physiol Circ Physiol. 319:H1162–H1180. 2020. View Article : Google Scholar | |
|
Huang KY, Upadhyay G, Ahn Y, Sakakura M, Pagan-Diaz GJ, Cho Y, Weiss AC, Huang C, Mitchell JW, Li J, et al: Neuronal innervation regulates the secretion of neurotrophic myokines and exosomes from skeletal muscle. Proc Natl Acad Sci USA. 121:e23135901212024. View Article : Google Scholar : PubMed/NCBI | |
|
Ocansey DKW, Zhang L, Wang Y, Yan Y, Qian H, Zhang X, Xu W and Mao F: Exosome-mediated effects and applications in inflammatory bowel disease. Biol Rev. 95:1287–1307. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Kourembanas S: Exosomes: Vehicles of intercellular signaling, biomarkers, and vectors of cell therapy. Annu Rev Physiol. 77:13–27. 2015. View Article : Google Scholar | |
|
Chen H, Chengalvala V, Hu H and Sun D: Tumor-derived exosomes: Nanovesicles made by cancer cells to promote cancer metastasis. Acta Pharm Sin B. 11:2136–2149. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Huber CC and Wang H: Pathogenic and therapeutic role of exosomes in neurodegenerative disorders. Neural Regen Res. 19:75–79. 2024. View Article : Google Scholar | |
|
Zhang Z, Zou Y, Song C, Cao K, Cai K, Chen S, Wu Y, Geng D, Sun G, Zhang N, et al: Advances in the study of exosomes in cardiovascular diseases. J Adv Res. 66:133–153. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Larson A, Natera-Rodriguez DE, Crane A, Larocca D, Low WC, Grande AW and Lee J: Emerging roles of exosomes in stroke therapy. Int J Mol Sci. 25:65072024. View Article : Google Scholar : PubMed/NCBI | |
|
Singh A, Behl T, Sehgal A, Singh S, Sharma N, Naqwi M, Mavi A and Singh R: Exploring the role of exosomes in rheumatoid arthritis. Inflammopharmacology. 31:119–128. 2023. View Article : Google Scholar | |
|
Gao S, Dong Y, Yan C, Yu T and Cao H: The role of exosomes and exosomal microRNA in diabetic cardiomyopathy. Front Endocrinol (Lausanne). 14:13274952024. View Article : Google Scholar : PubMed/NCBI | |
|
Hassanzadeh A, Shomali N, Kamrani A, Nasiri H, Ahmadian Heris J, Pashaiasl M, Sadeghi M, Sadeghvand S, Valedkarimi Z and Akbari M: Detailed role of mesenchymal stem cell (MSC)-derived exosome therapy in cardiac diseases. EXCLI J. 23:401–420. 2024.PubMed/NCBI | |
|
Gheitasi H, Sabbaghian M, Shekarchi AA, Mirmazhary AA and Poortahmasebi V: Exosome-mediated regulation of inflammatory pathway during respiratory viral disease. Virol J. 21:302024. View Article : Google Scholar : PubMed/NCBI | |
|
Sun Y, Zhang S, Shen Y, Lu H, Zhao X, Wang X, Wang Y, Wang T, Liu B, Yao L and Wen J: Therapeutic application of mesenchymal stem cell-derived exosomes in skin wound healing. Front Bioeng Biotechnol. 12:14287932024. View Article : Google Scholar : PubMed/NCBI | |
|
Dehghan Z, Rezaee D, Noori E, Pilehchi T, Saberi F, Taheri Z, Darya G and Mehdinejadiani S: Exosomes as modulators of embryo implantation. Mol Biol Rep. 51:2842024. View Article : Google Scholar : PubMed/NCBI | |
|
Keerthikumar S, Chisanga D, Ariyaratne D, Al Saffar H, Anand S, Zhao K, Samuel M, Pathan M, Jois M, Chilamkurti N, et al: ExoCarta: A Web-Based compendium of exosomal cargo. J Mol Biol. 428:688–692. 2016. View Article : Google Scholar : | |
|
Huang D, Chen J, Hu D, Xie F, Yang T, Li Z, Wang X, Xiao Y, Zhong J, Jiang Y, et al: Advances in biological function and clinical application of small extracellular vesicle membrane proteins. Front Oncol. 11:6759402021. View Article : Google Scholar : PubMed/NCBI | |
|
Zheng D, Huo M, Li B, Wang W, Piao H, Wang Y, Zhu Z, Li D, Wang T and Liu K: The role of exosomes and exosomal MicroRNA in cardiovascular disease. Front Cell Dev Biol. 8:161612021. View Article : Google Scholar | |
|
Wu X, Xu X, Xiang Y, Fan D, An Q, Yue G, Jin Z, Ding J, Hu Y, Du Q, et al: Exosome-mediated effects and applications in inflammatory diseases of the digestive system. Eur J Med Res. 27:1632022. View Article : Google Scholar : PubMed/NCBI | |
|
Guan Q: A comprehensive review and update on the pathogenesis of inflammatory bowel disease. J Immunol Res. 2019:72472382019. View Article : Google Scholar : PubMed/NCBI | |
|
Lv X, Gao X, Liu J, Deng Y, Nie Q, Fan X, Ye Z, Liu P and Wen J: Immune-mediated inflammatory diseases and risk of venous thromboembolism: A Mendelian randomization study. Front Immunol. 13:10427512022. View Article : Google Scholar : PubMed/NCBI | |
|
Kaplan GG and Ng SC: Globalisation of inflammatory bowel disease: Perspectives from the evolution of inflammatory bowel disease in the UK and China. Lancet Gastroenterol Hepatol. 1:307–316. 2016. View Article : Google Scholar | |
|
Arabpour M, Saghazadeh A and Rezaei N: Anti-inflammatory and M2 macrophage polarization-promoting effect of mesenchymal stem cell-derived exosomes. Int Immunopharmacol. 97:1078232021. View Article : Google Scholar : PubMed/NCBI | |
|
Eissa N, Hussein H and Ghia JE: A Gene expression analysis of M1 and M2 polarized macrophages. Methods Mol Biol. 2184:131–144. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang K, Guo J, Yan W and Xu L: Macrophage polarization in inflammatory bowel disease. Cell Commun Signal. 21:3672023. View Article : Google Scholar : PubMed/NCBI | |
|
Yang R, Liao Y, Wang L, He P, Hu Y, Yuan D, Wu Z and Sun X: Exosomes derived from M2b macrophages attenuate DSS-induced colitis. Front Immunol. 10:23462019. View Article : Google Scholar : PubMed/NCBI | |
|
Lethen I, Lechner-Grimm K, Gabel M, Knauss A, Atreya R, Neurath MF and Weigmann B: Tofacitinib affects M1-like and M2-like polarization and tissue factor expression in macrophages of healthy donors and IBD patients. Inflamm Bowel Dis. 30:1151–1163. 2024. View Article : Google Scholar | |
|
Mao F, Wu Y, Tang X, Wang J, Pan Z, Zhang P, Zhang B, Yan Y, Zhang X, Qian H and Xu W: Human umbilical cord mesenchymal stem cells alleviate inflammatory bowel disease through the regulation of 15-LOX-1 in macrophages. Biotechnol Lett. 39:929–938. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Mao F, Wu Y, Tang X, Kang J, Zhang B, Yan Y, Qian H, Zhang X and Xu W: Exosomes derived from human umbilical cord mesenchymal stem cells relieve inflammatory bowel disease in mice. Biomed Res Int. 2017:53567602017. View Article : Google Scholar : PubMed/NCBI | |
|
Xu X, Peng J, Wang N, Ocansey DKW, Zhang X and Mao F: hucMSC-Ex alleviates inflammatory bowel disease in mice by enhancing M2-type macrophage polarization via the METTL3-Slc37a2-YTHDF1 axis. Cell Biol Toxicol. 40:742024. View Article : Google Scholar : PubMed/NCBI | |
|
Wei Z, Hang S, Wiredu Ocansey DK, Zhang Z, Wang B, Zhang X and Mao F: Human umbilical cord mesenchymal stem cells derived exosome shuttling mir-129-5p attenuates inflammatory bowel disease by inhibiting ferroptosis. J Nanobiotechnology. 21:1882023. View Article : Google Scholar : PubMed/NCBI | |
|
Kowal J and Tkach M: Dendritic cell extracellular vesicles. Int Rev Cell Mol Biol. 349:213–249. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Rao Q, Ma G, Li M, Wu H, Zhang Y, Zhang C, Ma Z and Huang L: Targeted delivery of triptolide by dendritic cell-derived exosomes for colitis and rheumatoid arthritis therapy in murine models. Br J Pharmacol. 180:330–346. 2023. View Article : Google Scholar | |
|
Wang L, Yu Z, Wan S, Wu F, Chen W, Zhang B, Lin D, Liu J, Xie H, Sun X and Wu Z: Exosomes derived from dendritic cells treated with schistosoma japonicum soluble egg antigen attenuate DSS-induced colitis. Front Pharmacol. 8:6512017. View Article : Google Scholar : PubMed/NCBI | |
|
Elashiry M, Elashiry MM, Elsayed R, Rajendran M, Auersvald C, Zeitoun R, Rashid MH, Ara R, Meghil MM, Liu Y, et al: Dendritic cell derived exosomes loaded with immunoregulatory cargo reprogram local immune responses and inhibit degenerative bone disease in vivo. J Extracell Vesicles. 9:17953622020. View Article : Google Scholar : PubMed/NCBI | |
|
Barnhoorn MC, Wasser MNJM, Roelofs H, Maljaars PWJ, Molendijk I, Bonsing BA, Oosten LEM, Dijkstra G, van der Woude CJ, Roelen DL, et al: Long-term evaluation of allogeneic bone marrow-derived mesenchymal stromal cell therapy for Crohn's disease perianal fistulas. J Crohns Colitis. 14:64–70. 2020. View Article : Google Scholar | |
|
Stavely R, Robinson AM, Miller S, Boyd R, Sakkal S and Nurgali K: Human adult stem cells derived from adipose tissue and bone marrow attenuate enteric neuropathy in the guinea-pig model of acute colitis. Stem Cell Res Ther. 6:2442015. View Article : Google Scholar : PubMed/NCBI | |
|
Chen J, Xie S, Qiu D, Xie M, Wu M, Li X, Zhang X, Wu Q, Xiong Y, Wu C, et al: The NLRP3 molecule influences the therapeutic effects of mesenchymal stem cells through Glut1-mediated energy metabolic reprogramming. J Adv Res. 65:125–136. 2024. View Article : Google Scholar : | |
|
Li M, Zhao J, Cao M, Liu R, Chen G, Li S, Xie Y, Xie J, Cheng Y, Huang L, et al: Mast cells-derived MiR-223 destroys intestinal barrier function by inhibition of CLDN8 expression in intestinal epithelial cells. Biol Res. 53:122020. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang S, Xu W, Wang H, Cao M, Li M, Zhao J, Hu Y, Wang Y, Li S, Xie Y, et al: Inhibition of CREB-mediated ZO-1 and activation of NF-κB-induced IL-6 by colonic epithelial MCT4 destroys intestinal barrier function. Cell Prolif. 52:e126732019. View Article : Google Scholar | |
|
Chojnacki C, Wisniewska-Jarosinska M, Walecka-Kapica E, Klupinska G, Jaworek J and Chojnacki J: Evaluation of melatonin effectiveness in the adjuvant treatment of ulcerative colitis. J Physiol Pharmacol. 62:327–334. 2011.PubMed/NCBI | |
|
Heidari M, Pouya S, Baghaei K, Aghdaei HA, Namaki S, Zali MR and Hashemi SM: The immunomodulatory effects of adipose-derived mesenchymal stem cells and mesenchymal stem cells-conditioned medium in chronic colitis. J Cell Physiol. 233:8754–8766. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Heidari N, Abbasi-Kenarsari H, Namaki S, Baghaei K, Zali MR, Ghaffari Khaligh S and Hashemi SM: Adipose-derived mesenchymal stem cell-secreted exosome alleviates dextran sulfate sodium-induced acute colitis by Treg cell induction and inflammatory cytokine reduction. J Cell Physiol. 236:5906–5920. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Chen P, Huang S, Yu Q, Chao K, Wang Y, Zhou G, Zhuang X, Zeng Z, Chen M and Zhang S: Serum exosomal microRNA-144-3p: A promising biomarker for monitoring Crohn's disease. Gastroenterol Rep (Oxf). 10:goab0562021. View Article : Google Scholar | |
|
Gong L, Xiao J, Yi J, Xiao J, Lu F and Liu X: Immunomodulatory effect of serum exosomes from crohn disease on macrophages via Let-7b-5p/TLR4 signaling. Inflamm Bowel Dis. 28:96–108. 2022. View Article : Google Scholar | |
|
Yarani R, Shojaeian A, Palasca O, Doncheva NT, Jensen LJ, Gorodkin J and Pociot F: Differentially expressed miRNAs in ulcerative colitis and Crohn's disease. Front Immunol. 13:8657772022. View Article : Google Scholar : PubMed/NCBI | |
|
Wu F, Zhang S, Dassopoulos T, Harris ML, Bayless TM, Meltzer SJ, Brant SR and Kwon JH: Identification of microRNAs associated with ileal and colonic Crohnʼs disease. Inflamm Bowel Dis. 16:1729–1738. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Quaglio AEV, Santaella FJ, Rodrigues MAM, Sassaki LY and Di Stasi LC: MicroRNAs expression influence in ulcerative colitis and Crohn's disease: A pilot study for the identification of diagnostic biomarkers. World J Gastroenterol. 27:7801–7812. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Wong W, Lee MM, Chan BD, Kam RK, Zhang G, Lu AP and Tai WC: Proteomic profiling of dextran sulfate sodium induced acute ulcerative colitis mice serum exosomes and their immunomodulatory impact on macrophages. Proteomics. 16:1131–1145. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Clua-Ferré L, Suau R, Vañó-Segarra I, Ginés I, Serena C and Manyé J: Therapeutic potential of mesenchymal stem cellderived extracellular vesicles: A focus on inflammatory bowel disease. Clin Transl Med. 14:e700752024. View Article : Google Scholar | |
|
Zheng X, Chen F, Zhang Q, Liu Y, You P, Sun S, Lin J and Chen N: Salivary exosomal PSMA7: A promising biomarker of inflammatory bowel disease. Protein Cell. 8:686–695. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Zhou W and Anakk S: Enterohepatic and non-canonical roles of farnesoid X receptor in controlling lipid and glucose metabolism. Mol Cell Endocrinol. 549:1116162022. View Article : Google Scholar : PubMed/NCBI | |
|
Ramos Pittol JM, Milona A, Morris I, Willemsen ECL, van der Veen SW, Kalkhoven E and van Mil SWC: FXR isoforms control different metabolic functions in liver cells via binding to specific DNA motifs. Gastroenterology. 159:1853–1865.e10. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Jiang L, Zhang H, Xiao D, Wei H and Chen Y: Farnesoid X receptor (FXR): Structures and ligands. Comput Struct Biotechnol J. 19:2148–2159. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Vaquero J, Monte MJ, Dominguez M, Muntané J and Marin JJG: Differential activation of the human farnesoid X receptor depends on the pattern of expressed isoforms and the bile acid pool composition. Biochem Pharmacol. 86:926–939. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Tian SY, Chen SM, Pan CX and Li Y: FXR: Structures, biology, and drug development for NASH and fibrosis diseases. Acta Pharmacol Sin. 43:1120–1132. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Stojancevic M, Stankov K and Mikov M: The impact of Farnesoid X receptor activation on intestinal permeability in inflammatory bowel disease. Can J Gastroenterol. 26:631–637. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Gioiello A, Rosatelli E and Cerra B: Patented farnesoid X receptor modulators: A review (2019-present). Expert Opin Ther Pat. 34:547–564. 2024. View Article : Google Scholar : PubMed/NCBI | |
|
Ocvirk S and O'Keefe SJD: Dietary fat, bile acid metabolism and colorectal cancer. Semin Cancer Biol. 73:347–355. 2021. View Article : Google Scholar | |
|
Stofan M and Guo GL: Bile Acids and FXR: Novel targets for liver diseases. Front Med (Lausanne). 7:5442020. View Article : Google Scholar : PubMed/NCBI | |
|
Xiang J, Zhang Z, Xie H, Zhang C, Bai Y, Cao H, Che Q, Guo J and Su Z: Effect of different bile acids on the intestine through enterohepatic circulation based on FXR. Gut Microbes. 13:19490952021. View Article : Google Scholar : PubMed/NCBI | |
|
Chiang JYL and Ferrell JM: Up to date on cholesterol 7 alpha-hydroxylase (CYP7A1) in bile acid synthesis. Liver Res. 4:47–63. 2020. View Article : Google Scholar | |
|
Fiorucci S, Baldoni M, Ricci P, Zampella A, Distrutti E and Biagioli M: Bile acid-activated receptors and the regulation of macrophages function in metabolic disorders. Curr Opin Pharmacol. 53:45–54. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Cui JY, Aleksunes LM, Tanaka Y, Fu ZD, Guo Y, Guo GL, Guo Y, Guo GL, Lu H, Zhong XB and Klaassen CD: Bile acids via FXR initiate the expression of major transporters involved in the enterohepatic circulation of bile acids in newborn mice. Am J Physiol Liver Physiol. 302:G979–G996. 2012. | |
|
Fuchs CD, Krivanec S, Steinacher D, Mlitz V, Wahlström A, Stahlman M, Claudel T, Scharnagl H, Stojakovic T, Marschall HU and Trauner M: Absence of Bsep/Abcb11 attenuates MCD dietinduced hepatic steatosis but aggravates inflammation in mice. Liver Int. 40:1366–1377. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Fuchs CD and Trauner M: Role of bile acids and their receptors in gastrointestinal and hepatic pathophysiology. Nat Rev Gastroenterol Hepatol. 19:432–450. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Jiang M, Li F, Liu Y, Gu Z, Zhang L, Lee J, He L, Vatsalya V, Zhang HG, Deng Z, et al: Probiotic-derived nanoparticles inhibit ALD through intestinal miR194 suppression and subsequent FXR activation. Hepatology. 77:1164–1180. 2023. View Article : Google Scholar | |
|
Fleishman JS and Kumar S: Bile acid metabolism and signaling in health and disease: Molecular mechanisms and therapeutic targets. Signal Transduct Target Ther. 9:972024. View Article : Google Scholar : PubMed/NCBI | |
|
Panzitt K and Wagner M: FXR in liver physiology: Multiple faces to regulate liver metabolism. Biochim Biophys Acta Mol Basis Dis. 1867:1661332021. View Article : Google Scholar : PubMed/NCBI | |
|
Liu Q, Yang M, Fu X, Liu R, Sun C, Pan H, Wong CW and Guan M: Activation of farnesoid X receptor promotes triglycerides lowering by suppressing phospholipase A2 G12B expression. Mol Cell Endocrinol. 436:93–101. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Han C: Update on FXR Biology: Promising therapeutic Target? Int J Mol Sci. 19:20692018. View Article : Google Scholar : PubMed/NCBI | |
|
Clifford BL, Sedgeman LR, Williams KJ, Morand P, Cheng A, Jarrett KE, Chan AP, Brearley-Sholto MC, Wahlström A, Ashby JW, et al: FXR activation protects against NAFLD via bile-acid-dependent reductions in lipid absorption. Cell Metab. 33:1671–1684.e4. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Guo J, Huang S, Yi Q, Liu N, Cui T, Duan S, Chen J, Li J, Li J, Wang L, et al: Hepatic Clstn3 ameliorates lipid metabolism disorders in high Fat Diet-Induced NAFLD through activation of FXR. ACS Omega. 8:26158–26169. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Xu H, Fang F, Wu K, Song J, Li Y, Lu X, Liu J, Zhou L, Yu W, Yu F and Gao J: Gut microbiota-bile acid crosstalk regulates murine lipid metabolism via the intestinal FXR-FGF19 axis in diet-induced humanized dyslipidemia. Microbiome. 11:2622023. View Article : Google Scholar : PubMed/NCBI | |
|
Sonne DP: Mechanisms in endocrinology: FXR signalling: A novel target in metabolic diseases. Eur J Endocrinol. 184:R193–R205. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Hou Y, Fan W, Yang W, Samdani AQ, Jackson AO and Qu S: Farnesoid X receptor: An important factor in blood glucose regulation. Clin Chim Acta. 495:29–34. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Sinal CJ, Tohkin M, Miyata M, Ward JM, Lambert G and Gonzalez FJ: Targeted disruption of the nuclear receptor FXR/BAR impairs bile acid and lipid homeostasis. Cell. 102:731–744. 2000. View Article : Google Scholar : PubMed/NCBI | |
|
Lambert G, Amar MJA, Guo G, Brewer HB, Gonzalez FJ and Sinal CJ: The Farnesoid X-receptor is an essential regulator of cholesterol homeostasis. J Biol Chem. 278:2563–2570. 2003. View Article : Google Scholar | |
|
Lee Y, Kim BR, Kang GH, Lee GJ, Park YJ, Kim H, Jang HC and Choi SH: The effects of PPAR agonists on atherosclerosis and nonalcoholic fatty liver disease in ApoE-/-FXR-/-mice. Endocrinol Metab (Seoul). 36:1243–1253. 2021. View Article : Google Scholar | |
|
Zhang Y, Lee FY, Barrera G, Lee H, Vales C, Gonzalez FJ, Willson TM and Edwards PA: Activation of the nuclear receptor FXR improves hyperglycemia and hyperlipidemia in diabetic mice. Proc Natl Acad Sci. 103:1006–1011. 2006. View Article : Google Scholar : PubMed/NCBI | |
|
Stayrook KR, Bramlett KS, Savkur RS, Ficorilli J, Cook T, Christe ME, Michael LF and Burris TP: Regulation of carbohydrate metabolism by the farnesoid X receptor. Endocrinology. 146:984–991. 2005. View Article : Google Scholar | |
|
Zhao T, Wang J, He A, Wang S, Chen Y, Lu J, Lv J, Li S, Wang J, Qian M, et al: Mebhydrolin ameliorates glucose homeostasis in type 2 diabetic mice by functioning as a selective FXR antagonist. Metabolism. 119:1547712021. View Article : Google Scholar : PubMed/NCBI | |
|
Dehondt H, Marino A, Butruille L, Mogilenko DA, Nzoussi Loubota AC, Chávez-Talavera O, Dorchies E, Vallez E, Haas J, Derudas B, et al: Adipocyte-specific FXR-deficiency protects adipose tissue from oxidative stress and insulin resistance and improves glucose homeostasis. Mol Metab. 69:1016862023. View Article : Google Scholar : PubMed/NCBI | |
|
Ding L, Yang Q, Zhang E, Wang Y, Sun S, Yang Y, Tian T, Ju Z, Jiang L, Wang X, et al: Notoginsenoside Ft1 acts as a TGR5 agonist but FXR antagonist to alleviate high fat diet-induced obesity and insulin resistance in mice. Acta Pharm Sin B. 11:1541–1554. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Pathak P, Xie C, Nichols RG, Ferrell JM, Boehme S, Krausz KW, Patterson AD, Gonzalez FJ and Chiang JYL: Intestine farnesoid X receptor agonist and the gut microbiota activate G-protein bile acid receptor-1 signaling to improve metabolism. Hepatology. 68:1574–1588. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Shi T, Malik A, Yang vom Hofe A, Matuschek L, Mullen M, Lages CS, Kudira R, Singh R, Zhang W, Setchell KDR, et al: Farnesoid X receptor antagonizes macrophage-dependent licensing of effector T lymphocytes and progression of sclerosing cholangitis. Sci Transl Med. 14:eabi43542022. View Article : Google Scholar : PubMed/NCBI | |
|
Fuchs CD, Sroda N, Scharnagl H, Gupta R, Minto W, Stojakovic T, Liles JT, Budas G, Hollenback D and Trauner M: Non-steroidal FXR agonist cilofexor improves cholestatic liver injury in the Mdr2-/-mouse model of sclerosing cholangitis. JHEP Rep. 5:1008742023. View Article : Google Scholar | |
|
Fu T, Li Y, Oh TG, Cayabyab F, He N, Tang Q, Coulter S, Truitt M, Medina P, He M, et al: FXR mediates ILC-intrinsic responses to intestinal inflammation. Proc Natl Acad Sci. 119:e22130411192022. View Article : Google Scholar : PubMed/NCBI | |
|
Dong X, Qi M, Cai C, Zhu Y, Li Y, Coulter S, Sun F, Liddle C, Uboha NV and Halberg R: Farnesoid X receptor mediates macrophage-intrinsic responses to suppress colitis-induced colon cancer progression. JCI Insight. 9:e1704282024. View Article : Google Scholar : PubMed/NCBI | |
|
Gadaleta RM, Oldenburg B, Willemsen ECL, Spit M, Murzilli S, Salvatore L, Klomp LW, Siersema PD, van Erpecum KJ and van Mil SW: Activation of bile salt nuclear receptor FXR is repressed by pro-inflammatory cytokines activating NF-κB signaling in the intestine. Biochim Biophys Acta. 1812:851–858. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
Wu Q, Sun L, Hu X, Wang X, Xu F, Chen B, Liang X, Xia J, Wang P, Aibara D, et al: Suppressing the intestinal farnesoid X receptor/sphingomyelin phosphodiesterase 3 axis decreases atherosclerosis. J Clin Invest. 131:e1428652021. View Article : Google Scholar : PubMed/NCBI | |
|
McDowell C, Farooq U and Haseeb M: Inflammatory Bowel Disease StatPearls. 2024, Available from: http://www.ncbi.nlm.nih.gov/pubmed/30137275. | |
|
Little RD, Jayawardana T, Koentgen S, Zhang F, Connor SJ, Boussioutas A, Ward MG, Gibson PR, Sparrow MP and Hold GL: Pathogenesis and precision medicine for predicting response in inflammatory bowel disease: Advances and future directions. eGastroenterology. 2:e1000062024. View Article : Google Scholar | |
|
Ding L, Yang L, Wang Z and Huang W: Bile acid nuclear receptor FXR and digestive system diseases. Acta Pharm Sin B. 5:135–144. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Fiorucci S, Zampella A, Ricci P, Distrutti E and Biagioli M: Immunomodulatory functions of FXR. Mol Cell Endocrinol. 551:1116502022. View Article : Google Scholar : PubMed/NCBI | |
|
Fu T and Dong X: Abstract 3442: FXR mediates macrophage intrinsic responses to suppress colon cancer progression. Cancer Res. 83(7_Suppl): S34422023. View Article : Google Scholar | |
|
Zhang L, Xie C, Nichols RG, Chan SHJ, Jiang C, Hao R, Smith PB, Cai J, Simons MN, Hatzakis E, et al: Farnesoid X receptor signaling shapes the gut microbiota and controls hepatic lipid metabolism. mSystems. 1:e00070–16. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Jena PK, Sheng L, Liu HX, Kalanetra KM, Mirsoian A, Murphy WJ, French SW, Krishnan VV, Mills DA and Wan YY: Western Diet-induced dysbiosis in farnesoid X receptor knockout mice causes persistent hepatic inflammation after antibiotic treatment. Am J Pathol. 187:1800–1813. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Xu M, Shen Y, Cen M, Zhu Y, Cheng F, Tang L, Zheng X, Kim JJ, Dai N and Hu W: Modulation of the gut Microbiota-farnesoid X receptor axis improves deoxycholic Acid-induced intestinal inflammation in mice. J Crohns Colitis. 15:1197–1210. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Arifuzzaman M, Won TH, Yano H, Uddin J, Emanuel ER, Hu E, Zhang W, Li TT, Jin WB, Grier A, et al: Dietary fiber is a critical determinant of pathologic ILC2 responses and intestinal inflammation. J Exp Med. 221:e202321482024. View Article : Google Scholar : PubMed/NCBI | |
|
Zhao D, Cai C, Chen Q, Jin S, Yang B and Li N: High-fat diet promotes DSS-induced ulcerative colitis by downregulated FXR expression through the TGFB pathway. Biomed Res Int. 2020:35161282020. View Article : Google Scholar : PubMed/NCBI | |
|
Vavassori P, Mencarelli A, Renga B, Distrutti E and Fiorucci S: The bile acid receptor FXR is a modulator of intestinal innate immunity. J Immunol. 183:6251–6261. 2009. View Article : Google Scholar : PubMed/NCBI | |
|
Raybould HE: Gut microbiota, epithelial function and derangements in obesity. J Physiol. 590:441–446. 2012. View Article : Google Scholar : | |
|
Gadaleta RM, van Erpecum KJ, Oldenburg B, Willemsen ECL, Renooij W, Murzilli S, Klomp LW, Siersema PD, Schipper ME, Danese S, et al: Farnesoid X receptor activation inhibits inflammation and preserves the intestinal barrier in inflammatory bowel disease. Gut. 60:463–472. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
Curley C, Lajczak-McGinley N, Tambuwala M, Adorini L, Fallon C, Smyth J and Keely S: Farnesoid X receptor activation attenuates intestinal inflammation and preserves epithelial barrier function in vivo and in vitro. Physiology. 38:2023. View Article : Google Scholar | |
|
Abraham BP, Ahmed T and Ali T: Inflammatory bowel disease: Pathophysiology and current therapeutic approaches. Handb Exp Pharmacol. 239:115–146. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Liu HM, Liao JF and Lee TY: Farnesoid X receptor agonist GW4064 ameliorates lipopolysaccharide-induced ileocolitis through TLR4/MyD88 pathway related mitochondrial dysfunction in mice. Biochem Biophys Res Commun. 490:841–848. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Liu HM, Chang ZY, Yang CW, Chang HH and Lee TY: Farnesoid X receptor agonist GW4064 protects lipopolysaccharide-induced intestinal epithelial barrier function and colorectal tumorigenesis signaling through the αKlotho/βKlotho/FGFs pathways in mice. Int J Mol Sci. 24:169322023. View Article : Google Scholar | |
|
Ceulemans LJ, Verbeke L, Decuypere JP, Farré R, De Hertogh G, Lenaerts K, Jochmans I, Monbaliu D, Nevens F, Tack J, et al: Farnesoid X receptor activation attenuates intestinal ischemia reperfusion injury in rats. PLoS One. 12:e01693312017. View Article : Google Scholar : PubMed/NCBI | |
|
Xu Y, Li D, Wu J, Zhang M, Shao X, Xu L, Tang L, Zhu M, Ni Z, Zhang M, et al: Farnesoid X receptor promotes renal ischaemiareperfusion injury by inducing tubular epithelial cell apoptosis. Cell Prolif. 54:e130052021. View Article : Google Scholar | |
|
Anderson KM and Gayer CP: The pathophysiology of farnesoid X receptor (FXR) in the GI Tract: Inflammation, barrier function and innate immunity. Cells. 10:32062021. View Article : Google Scholar : PubMed/NCBI | |
|
O'Guinn ML, Handler DA, Hsieh JJ, Mallicote MU, Feliciano K and Gayer CP: FXR deletion attenuates intestinal barrier dysfunction in murine acute intestinal inflammation. Am J Physiol Liver Physiol. 327:G175–G187. 2024. | |
|
Tang K, Kong D, Peng Y, Guo J, Zhong Y, Yu H, Mai Z, Chen Y, Chen Y, Cui T, et al: Ginsenoside Rc attenuates DSS-induced ulcerative colitis, intestinal inflammatory, and barrier function by activating the farnesoid X receptor. Front Pharmacol. 13:10004442022. View Article : Google Scholar : PubMed/NCBI | |
|
Safari F, Sharifi M, Talebi A, Mehranfard N and Ghasemi M: Alleviation of cholestatic liver injury and intestinal permeability by lubiprostone treatment in bile duct ligated rats: Role of intestinal FXR and tight junction proteins claudin-1, claudin-2, and occludin. Naunyn Schmiedebergs Arch Pharmacol. 396:2009–2022. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Comito D and Romano C: Dysbiosis in the pathogenesis of pediatric inflammatory bowel diseases. Int J Inflam. 2012:6871432012.PubMed/NCBI | |
|
Caenepeel C, Falony G, Machiels K, Verstockt B, Goncalves PJ, Ferrante M, Sabino J, Raes J, Vieira-Silva S and Vermeire S: Dysbiosis and associated stool features improve prediction of response to biological therapy in inflammatory bowel disease. Gastroenterology. 166:483–495 | |
|
Lan J, Zhang Y, Jin C, Chen H, Su Z, Wu J, Ma N, Zhang X, Lu Y, Chen Y, et al: Gut dysbiosis drives inflammatory bowel disease through the CCL4L2-VSIR axis in glycogen storage disease. Adv Sci (Weinh). 11:e23094712024. View Article : Google Scholar : PubMed/NCBI | |
|
Cao Z, Fan D, Sun Y, Huang Z, Li Y, Su R, Zhang F, Li Q, Yang H, Zhang F, et al: The gut ileal mucosal virome is disturbed in patients with Crohn's disease and exacerbates intestinal inflammation in mice. Nat Commun. 15:16382024. View Article : Google Scholar : PubMed/NCBI | |
|
Collins SL, Stine JG, Bisanz JE, Okafor CD and Patterson AD: Bile acids and the gut microbiota: Metabolic interactions and impacts on disease. Nat Rev Microbiol. 21:236–247. 2023. View Article : Google Scholar | |
|
Tian Y, Gui W, Koo I, Smith PB, Allman EL, Nichols RG, Rimal B, Cai J, Liu Q and Patterson AD: The microbiome modulating activity of bile acids. Gut Microbes. 11:979–996. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Dong LN, Wang M, Guo J and Wang JP: Role of intestinal microbiota and metabolites in inflammatory bowel disease. Chin Med J (Engl). 132:1610–1614. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Das P, Marcišauskas S, Ji B and Nielsen J: Metagenomic analysis of bile salt biotransformation in the human gut microbiome. BMC Genomics. 20:5172019. View Article : Google Scholar : PubMed/NCBI | |
|
Fitzpatrick LR and Jenabzadeh P: IBD and bile acid absorption: Focus on Pre-clinical and clinical observations. Front Physiol. 11:5642020. View Article : Google Scholar : PubMed/NCBI | |
|
Ceulemans LJ, Canovai E, Verbeke L, Pirenne J and Farré R: The expanding role of the bile acid receptor farnesoid X in the intestine and its potential clinical implications. Acta Chir Belg. 116:156–163. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Biet F, Locht C and Kremer L: Immunoregulatory functions of interleukin 18 and its role in defense against bacterial pathogens. J Mol Med. 80:147–162. 2002. View Article : Google Scholar : PubMed/NCBI | |
|
Ding JW, Andersson R, Soltesz V, Willén R and Bengmark S: The role of bile and bile acids in bacterial translocation in obstructive jaundice in rats. Eur Surg Res. 25:11–19. 1993. View Article : Google Scholar : PubMed/NCBI | |
|
Lorenzo-Zúñiga V, Bartolí R, Planas R, Hofmann AF, Viñado B, Hagey LR, Hernández JM, Mañé J, Alvarez MA, Ausina V, et al: Oral bile acids reduce bacterial overgrowth, bacterial translocation, and endotoxemia in cirrhotic rats. Hepatology. 37:551–557. 2003. View Article : Google Scholar : PubMed/NCBI | |
|
Xu X, Ocansey DKW, Hang S, Wang B, Amoah S, Yi C, Zhang X, Liu L and Mao F: The gut metagenomics and metabolomics signature in patients with inflammatory bowel disease. Gut Pathog. 14:262022. View Article : Google Scholar : PubMed/NCBI | |
|
Hua Y, Jia Y, Zhang X, Yuan Z, Ji P, Hu J and Wei YM: Baitouweng tang ameliorates DSS-induced ulcerative colitis through the regulation of the gut microbiota and bile acids via pathways involving FXR and TGR5. Biomed Pharmacother. 137:1113202021. View Article : Google Scholar : PubMed/NCBI | |
|
Shen J, Qi Q, Han D, Lu Y, Huang R, Zhu Y, Zhang LS, Qin XD, Zhang F, Wu HG and Liu HR: Moxibustion improves experimental colitis in rats with Crohn's disease by regulating bile acid enterohepatic circulation and intestinal farnesoid X receptor. J Integr Med. 21:194–204. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Wilson A, Almousa A, Teft WA and Kim RB: Attenuation of bile acid-mediated FXR and PXR activation in patients with Crohn's disease. Sci Rep. 10:18662020. View Article : Google Scholar : PubMed/NCBI | |
|
Chen L, Jiao T, Liu W, Luo Y, Wang J, Guo X, Tong X, Lin Z, Sun C, Wang K, et al: Hepatic cytochrome P450 8B1 and cholic acid potentiate intestinal epithelial injury in colitis by suppressing intestinal stem cell renewal. Cell Stem Cell. 29:1366–1381.e9. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Fiorucci S, Carino A, Baldoni M, Santucci L, Costanzi E, Graziosi L, Distrutti E and Biagioli M: Bile acid signaling in inflammatory bowel diseases. Dig Dis Sci. 66:674–693. 2021. View Article : Google Scholar : | |
|
Zhang H, Wang L, Li C, Yu Y, Yi Y, Wang J and Chen D: Exosome-induced regulation in inflammatory bowel disease. Front Immunol. 10:14642019. View Article : Google Scholar : PubMed/NCBI | |
|
Ma F, Zhang S, Akanyibah FA, Zhang W, Chen K, Ocansey DKW, Lyu C and Mao F: Exosome-mediated macrophage regulation for inflammatory bowel disease repair: A potential target of gut inflammation. Am J Transl Res. 15:6970–6987. 2023. | |
|
Lin S, Wang S, Wang P, Tang C, Wang Z, Chen L, Luo G, Chen H, Liu Y, Feng B, et al: Bile acids and their receptors in regulation of gut health and diseases. Prog Lipid Res. 89:1012102023. View Article : Google Scholar | |
|
Li Y, Li H, Cui M, Zhou Y and Zhang M and Zhang M: Interaction of exosomal MicroRNA and oxidative stress in the pathogenesis of Colitis-associated cancer. Front Biosci (Landmark Ed). 29:2762024. View Article : Google Scholar : PubMed/NCBI | |
|
Al-Sadi R, Engers J and Abdulqadir R: Talk about micromanaging! Role of microRNAs in intestinal barrier function. Am J Physiol Liver Physiol. 319:G170–G14. 2020. | |
|
Gu L, Ren F, Fang X, Yuan L, Liu G and Wang S: Exosomal MicroRNA-181a derived from mesenchymal stem cells improves gut microbiota composition, barrier function, and inflammatory status in an experimental colitis model. Front Med (Lausanne). 8:6606142021. View Article : Google Scholar : PubMed/NCBI | |
|
Gao F, Wu S, Zhang K, Xu Z, Zhang X, Zhu Z and Quan F: Goat milk exosomes ameliorate ulcerative colitis in mice through modulation of the intestinal barrier, gut microbiota, and metabolites. J Agric Food Chem. 72:23196–23210. 2024. View Article : Google Scholar : PubMed/NCBI | |
|
Jin J, Jung M, Sonn SK, Seo S, Suh J, Kweon HY, Moon SH, Jo H, Yoon NH and Oh GT: Peroxiredoxin 3 deficiency exacerbates DSS-induced acute colitis via exosomal miR-1260b-Mediated barrier disruption and proinflammatory signaling. Antioxid Redox Signal. 42:133–149. 2025. View Article : Google Scholar | |
|
Chang X, Song Y, Xia T, He Z, Zhao S, Wang ZJ, Gu L, Li ZS, Xu C, Wang SL and Bai Y: Macrophage-derived exosomes promote intestinal mucosal barrier dysfunction in inflammatory bowel disease by regulating TMIGD1 via mircroRNA-223. Int Immunopharmacol. 121:1104472023. View Article : Google Scholar : PubMed/NCBI | |
|
Liu F, Ai F, Tang A, Yang Z, Li Z and Liu S: Macrophage-derived exosomes promoted the development and stemness of inflammatory bowel Disease-related colorectal cancer via nuclear paraspeckle assembly transcript 1-Mediated miRNA-34a-5p/phosphoprotein enriched in astrocytes 15 Axis. Inflamm Bowel Dis. 31:524–538. 2025. View Article : Google Scholar | |
|
Liang Y, Duan L, Lu J and Xia J: Engineering exosomes for targeted drug delivery. Theranostics. 11:3183–3195. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Alexander M and O'Connell R: Exosomal miRNAs regulate inflammatory responses (IRM11P.624). J Immunol. 194(1_Suppl): S132.32015. View Article : Google Scholar | |
|
Alexander M, Hu R, Runtsch MC, Kagele DA, Mosbruger TL, Tolmachova T, Seabra MC, Round JL, Ward DM and O'Connell RM: Exosome-delivered microRNAs modulate the inflammatory response to endotoxin. Nat Commun. 6:73212015. View Article : Google Scholar : PubMed/NCBI | |
|
Liang X, Li C, Song J, Liu A, Wang C, Wang W, Kang Y, Sun D, Qian J and Zhang X: HucMSC-exo promote mucosal healing in experimental colitis by accelerating intestinal stem cells and epithelium regeneration via wnt signaling pathway. Int J Nanomedicine. 18:2799–2818. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Yu H, Yang X, Xiao X, Xu M, Yang Y, Xue C, Li X, Wang S and Zhao RC: Human adipose mesenchymal stem Cell-derived exosomes protect mice from DSS-Induced inflammatory bowel disease by promoting Intestinal-stem-cell and epithelial regeneration. Aging Dis. 12:14232021. View Article : Google Scholar : PubMed/NCBI | |
|
Al-Madhagi H: The landscape of exosomes biogenesis to clinical applications. Int J Nanomedicine. 19:3657–3675. 2024. View Article : Google Scholar : PubMed/NCBI | |
|
Kurian TK, Banik S, Gopal D, Chakrabarti S and Mazumder N: Elucidating methods for isolation and quantification of exosomes: A review. Mol Biotechnol. 63:249–266. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Lopez-Santalla M and Garin MI: Improving the efficacy of mesenchymal Stem/Stromal-based therapy for treatment of inflammatory bowel diseases. Biomedicines. 9:15072021. View Article : Google Scholar : PubMed/NCBI | |
|
Jang H, Kim H, Kim EH, Han G, Jang Y, Kim Y, Lee JW, Shin SC, Kim EE, Kim SH and Yang Y: Post-insertion technique to introduce targeting moieties in milk exosomes for targeted drug delivery. Biomater Res. 27:1242023. View Article : Google Scholar : PubMed/NCBI | |
|
Choi H, Choi Y, Yim HY, Mirzaaghasi A, Yoo JK and Choi C: Biodistribution of exosomes and engineering strategies for targeted delivery of therapeutic exosomes. Tissue Eng Regen Med. 18:499–511. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Liu H, Liang Z, Wang F, Zhou C, Zheng X, Hu T, He X, Wu X and Lan P: Exosomes from mesenchymal stromal cells reduce murine colonic inflammation via a macrophage-dependent mechanism. JCI Insight. 4:e1312732019. View Article : Google Scholar : PubMed/NCBI | |
|
Baghaei K, Tokhanbigli S, Asadzadeh H, Nmaki S, Reza Zali M and Hashemi SM: Exosomes as a novel cell-free therapeutic approach in gastrointestinal diseases. J Cell Physiol. 234:9910–9926. 2019. View Article : Google Scholar | |
|
Kim DH, Kothandan VK, Kim HW, Kim KS, Kim JY, Cho HJ, Lee YK, Lee DE and Hwang SR: Noninvasive assessment of exosome pharmacokinetics in vivo: A review. Pharmaceutics. 11:6492019. View Article : Google Scholar : PubMed/NCBI | |
|
Willms E, Johansson HJ, Mäger I, Lee Y, Blomberg KEM, Sadik M, Alaarg A, Smith CI, Lehtiö J, El Andaloussi S, et al: Cells release subpopulations of exosomes with distinct molecular and biological properties. Sci Rep. 6:225192016. View Article : Google Scholar : PubMed/NCBI | |
|
Shukla S, Currim F and Singh R: Do different exosome biogenesis pathways and selective cargo enrichment contribute to exosomal heterogeneity? Biol Cell. 115:e22001162023. View Article : Google Scholar : PubMed/NCBI | |
|
Ferguson SW and Nguyen J: Exosomes as therapeutics: The implications of molecular composition and exosomal heterogeneity. J Control Release. 228:179–190. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Willms E, Cabañas C, Mäger I, Wood MJA and Vader P: Extracellular vesicle heterogeneity: Subpopulations, isolation techniques, and diverse functions in cancer progression. Front Immunol. 9:7382018. View Article : Google Scholar : PubMed/NCBI | |
|
Morrissey SM, Zhang F, Ding C, Montoya-Durango DE, Hu X, Yang C, Wang Z, Yuan F, Fox M, Zhang HG, et al: Tumor-derived exosomes drive immunosuppressive macrophages in a pre-metastatic niche through glycolytic dominant metabolic reprogramming. Cell Metab. 33:2040–2058.e10. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Bhatia R, Chang J, Munoz JL and Walker ND: Forging new therapeutic targets: Efforts of tumor derived exosomes to prepare the Pre-metastatic niche for cancer cell dissemination and dormancy. Biomedicines. 11:16142023. View Article : Google Scholar : PubMed/NCBI | |
|
Bai S, Wei Y, Liu R, Xu R, Xiang L and Du J: Role of tumour-derived exosomes in metastasis. Biomed Pharmacother. 147:1126572022. View Article : Google Scholar : PubMed/NCBI | |
|
Truong NC, Huynh TN, Pham KD and Pham P: The role of tumor-derived exosomes in tumor immune escape: A concise review. Biomed Res Ther. 7:4132–4137. 2020. View Article : Google Scholar | |
|
Witwer KW and Wolfram J: Extracellular vesicles versus synthetic nanoparticles for drug delivery. Nat Rev Mater. 6:103–106. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Kimiz-Gebologlu I and Oncel SS: Exosomes: Large-scale production, isolation, drug loading efficiency, and biodistribution and uptake. J Control Release. 347:533–543. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Palakurthi SS, Shah B, Kapre S, Charbe N, Immanuel S, Pasham S, Thalla M, Jain A and Palakurthi S: A comprehensive review of challenges and advances in exosome-based drug delivery systems. Nanoscale Adv. 6:5803–5826. 2024. View Article : Google Scholar : PubMed/NCBI | |
|
Xia Y, Zhang J, Liu G and Wolfram J: Immunogenicity of extracellular vesicles. Adv Mater. 36:e24031992024. View Article : Google Scholar : PubMed/NCBI | |
|
Zhu X, Badawi M, Pomeroy S, Sutaria DS, Xie Z, Baek A, Jiang J, Elgamal OA, Mo X, Perle K, et al: Comprehensive toxicity and immunogenicity studies reveal minimal effects in mice following sustained dosing of extracellular vesicles derived from HEK293T cells. J Extracell Vesicles. 6:13247302017. View Article : Google Scholar : PubMed/NCBI | |
|
Gehrmann U, Näslund TI, Hiltbrunner S, Larssen P and Gabrielsson S: Harnessing the exosome-induced immune response for cancer immunotherapy. Semin Cancer Biol. 28:58–67. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Aslan C, Kiaie SH, Zolbanin NM, Lotfinejad P, Ramezani R, Kashanchi F and Jafari R: Exosomes for mRNA delivery: A novel biotherapeutic strategy with hurdles and hope. BMC Biotechnol. 21:202021. View Article : Google Scholar : PubMed/NCBI | |
|
Wang J, Chen D and Ho EA: Challenges in the development and establishment of exosome-based drug delivery systems. J Control Release. 329:894–906. 2021. View Article : Google Scholar | |
|
Zhang Y, Bi J, Huang J, Tang Y, Du S and Li P: Exosome: A review of its classification, isolation techniques, storage, diagnostic and targeted therapy applications. Int J Nanomedicine. 15:6917–6934. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Ball R, Bajaj P and Whitehead K: Achieving long-term stability of lipid nanoparticles: Examining the effect of pH, temperature, and lyophilization. Int J Nanomedicine. 12:305–315. 2016. View Article : Google Scholar | |
|
Lu L, Han C, Wang M, Du H, Chen N, Gao M, Wang N, Qi D, Bai W, Yin J, et al: Assessment of bovine milk exosome preparation and lyophilized powder stability. J Extracell Biol. 3:e700092024. View Article : Google Scholar : PubMed/NCBI | |
|
Kheradmand F, Yasaman Rahimzadeh SF, Esmaeili SA, Negah SS, Farkhad NK, Nazari SE, Nazari SE, Hajinejad M, Khodadoust MA, Fadaee A, et al: Efficacy of umbilical cord-derived mesenchymal stem cells and exosomes in conjunction with standard IBD drug on immune responses in an IBD mouse model. Stem Cell Res Ther. 16:52025. View Article : Google Scholar : PubMed/NCBI | |
|
Yang S, Liang X, Song J, Li C, Liu A, Luo Y, Ma H, Tan Y and Zhang X: A novel therapeutic approach for inflammatory bowel disease by exosomes derived from human umbilical cord mesenchymal stem cells to repair intestinal barrier via TSG-6. Stem Cell Res Ther. 12:3152021. View Article : Google Scholar : PubMed/NCBI | |
|
Yang X, Meng S, Jiang H, Chen T and Wu W: Exosomes derived from interleukin-10-treated dendritic cells can inhibit trinitrobenzene sulfonic acid-induced rat colitis. Scand J Gastroenterol. 45:1168–1177. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Kou Y, Li J, Zhu Y, Liu J, Ren R, Jiang Y, Wang Y, Qiu C, Zhou J, Yang Z, et al: Human amniotic epithelial stem cells promote colonic recovery in experimental colitis via exosomal MiR-23a-TNFR1-NF-κB signaling. Adv Sci (Weinh). 11:e24014292024. View Article : Google Scholar | |
|
Park N, Kim KS, Park CG, Jung HD, Park W and Na K: Adipose-derived stem cell-based anti-inflammatory paracrine factor regulation for the treatment of inflammatory bowel disease. J Control Release. 374:384–399. 2024. View Article : Google Scholar : PubMed/NCBI | |
|
Guo J, Wang F, Hu Y, Luo Y, Wei Y, Xu K, Zhang H, Liu H, Bo L, Lv S, et al: Exosome-based bone-targeting drug delivery alleviates impaired osteoblastic bone formation and bone loss in inflammatory bowel diseases. Cell Reports Med. 4:1008812023. View Article : Google Scholar | |
|
Liu C, Yan X, Zhang Y, Yang M, Ma Y, Zhang Y, Xu Q, Tu K and Zhang M: Oral administration of turmeric-derived exosome-like nanovesicles with anti-inflammatory and pro-resolving bioactions for murine colitis therapy. J Nanobiotechnology. 20:2062022. View Article : Google Scholar : PubMed/NCBI | |
|
Swaroop S, Vuyyuru SK, Kante B, Kumar P, Mundhra SK, Arora U, Goyal A, Kandasamy D, Sharma R, Kabilan K, et al: A phase I/II clinical trial of ex-vivo expanded human bone marrow derived allogeneic mesenchymal stromal cells in adult patients with perianal fistulizing Crohn's Disease. Stem Cell Res Ther. 15:1402024. View Article : Google Scholar : PubMed/NCBI | |
|
Lightner AL, Reese J, Ream J, Nachand D, Jia X, Dadgar N, Steele SR and Hull T: A Phase IB/IIA study of ex vivo expanded allogeneic bone marrow derived mesenchymal stem cells for the treatment of perianal fistulizing Crohn's disease. Dis Colon Rectum. 66:1359–1372. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Lightner AL, Otero-Pineiro A, Reese J, Ream J, Nachand D, Adams AC, VanDenBossche A and Kurowski JA: A Phase I study of ex vivo expanded allogeneic bone marrow-derived mesenchymal stem cells for the treatment of pediatric perianal Fistulizing Crohn's disease. Inflamm Bowel Dis. 29:1912–1919. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Hadizadeh A, Akbari Asbagh R, Heirani-Tabasi A, Soleimani M, Gorovanchi P, Ebrahimi Daryani N, Vahedi A, Nazari H, Banikarimi SP, Abbaszade Dibavar M, et al: Localized administration of mesenchymal stem cell-derived exosomes for the treatment of refractory perianal fistula in patients with Crohn's disease: A Phase II clinical trial. Dis Colon Rectum. 67:1564–1575. 2024. View Article : Google Scholar : PubMed/NCBI | |
|
Keung C, Nguyen TC, Lim R, Gerstenmaier A, Sievert W and Moore GT: Local fistula injection of allogeneic human amnion epithelial cells is safe and well tolerated in patients with refractory complex perianal Crohn's disease: A phase I open label study with long-term follow up. EBioMedicine. 98:1048792023. View Article : Google Scholar : PubMed/NCBI | |
|
Nazari H, Alborzi F, Heirani-Tabasi A, Hadizadeh A, Asbagh RA, Behboudi B, Fazeli MS, Rahimi M, Keramati MR, Keshvari A, et al: Evaluating the safety and efficacy of mesenchymal stem cell-derived exosomes for treatment of refractory perianal fistula in IBD patients: Clinical trial phase I. Gastroenterol Rep (Oxf). 10:goac0752022. View Article : Google Scholar : PubMed/NCBI |










