Advances of exosome regulating‑FXR to repair inflammatory bowel disease (Review)
- Authors:
- Published online on: July 3, 2025 https://doi.org/10.3892/ijmm.2025.5576
- Article Number: 135
-
Copyright: © Muro et al. This is an open access article distributed under the terms of Creative Commons Attribution License.
Abstract
Introduction
Inflammatory bowel disease (IBD), a chronic, recurrent disorder of the gastrointestinal tract that includes ulcerative colitis (UC) and Crohn's disease (CD), is characterized by inflammation and impairment of the mucosal barrier (1). There has been a notable increase in the incidence of IBD in recent years. Over 1 million individuals in the USA and 2.5 million in Europe are considered to have IBD, with significant costs for healthcare (2). In Southeast Asia, the prevalence of IBD has risen alongside its economic development and urbanization during the last two decades (3). Although the exact cause of IBD remains unknown, it is considered to be the result of a complex interaction of genetic, environmental and immunological factors (4). IBD treatment continually develops, with studies exploring new medications to enhance patient results. Numerous medications and treatment approaches have been developed, including targeted therapies (5), personalized medicine (6), stem cell therapies (7) and fecal microbiota transplantation (8). Conventional treatments, such as anti-inflammatory medications, immunosuppressants and biologics, typically aim to control symptoms rather than target the root causes of the disease, and they can also involve considerable side effects (9).
In recent years, innovative biological therapeutics targeting molecular pathways associated with intestinal homeostasis have emerged as feasible alternatives. Among these pathways, the farnesoid X receptor (FXR), a nuclear receptor known for regulating bile acid (BA) homeostasis, has attracted considerable attention due to its role in modulating inflammation and maintaining intestinal health (10). FXR plays a crucial role in BA metabolism, and its activation has been shown to defend and lessen inflammation in the intestinal lining. Impaired FXR signaling is associated with the onset of IBD, indicating that targeting this pathway may offer a potential treatment approach (10). Notwithstanding this potential, the direct activation of FXR with synthetic agonists has encountered obstacles, including inadequate efficacy and off-target consequences (11). Therefore, alternative methods for modulating FXR, primarily through endogenous biological agents, are currently under investigation.
Exosomes, nanosized extracellular vesicles released by nearly all cell types, have emerged as essential mediators in intercellular communication and possess significant potential for therapeutic applications (12). Exosomes contain a variety of bioactive molecules, including proteins, lipids and nucleic acids, which facilitate the modulation of specific signaling pathways in target cells (13). Exosomes are primarily derived from the multi-vesicles formed by the invagination of lysosomal particles in cells, and they are released into the extracellular matrix following fusion between the multi-vesicle outer membrane and the cell membrane (14). Exosomes are naturally present in multiple bodily fluids, such as blood, saliva, urine, cerebrospinal fluid and milk. The exact molecular mechanisms governing their secretion, absorption, composition and associated functions have emerged as a novel area of study interest (15). Exosomes are recognized as membrane vesicles specially secreted and involved in intercellular communication.
There is increasing interest in investigating exosomes, both to understand their functions and to explore their potential uses in developing less invasive diagnostic methods in treating and diagnosing diseases. Research has shown that exosomes obtained from certain cell types possess therapeutic benefits in regulating FXR and mitigating intestinal inflammation in IBD. Specifically, a recent study revealed that exosomes from well-defined cells, such as mesenchymal stem cells (MSCs), can influence FXR signaling to reduce IBD-related symptoms, positioning them as a promising treatment option for the disease (16). In the same way, a recent study has further demonstrated that exosomes derived from human umbilical cord MSCs (hucMSC-Ex) can mitigate IBD by triggering the SIRT1-FXR pathway in macrophages. This process decreases FXR acetylation and suppresses NLRP3 inflammasome activation (17). The distinctive ability of exosomes to transport specific therapeutic agents to inflamed tissues presents an effective approach to addressing the difficulties linked to direct FXR modulation. The present review examines the molecular mechanisms underlying FXR regulation through exosomes, their therapeutic potential, and the increasing preclinical and clinical evidence supporting their use in IBD treatment. Leveraging exosome-driven FXR modulation could lead to novel, targeted therapies that enhance outcomes for patients with IBD, providing a safer and more effective option compared with conventional treatments.
Exosomes: Biogenesis, function and composition
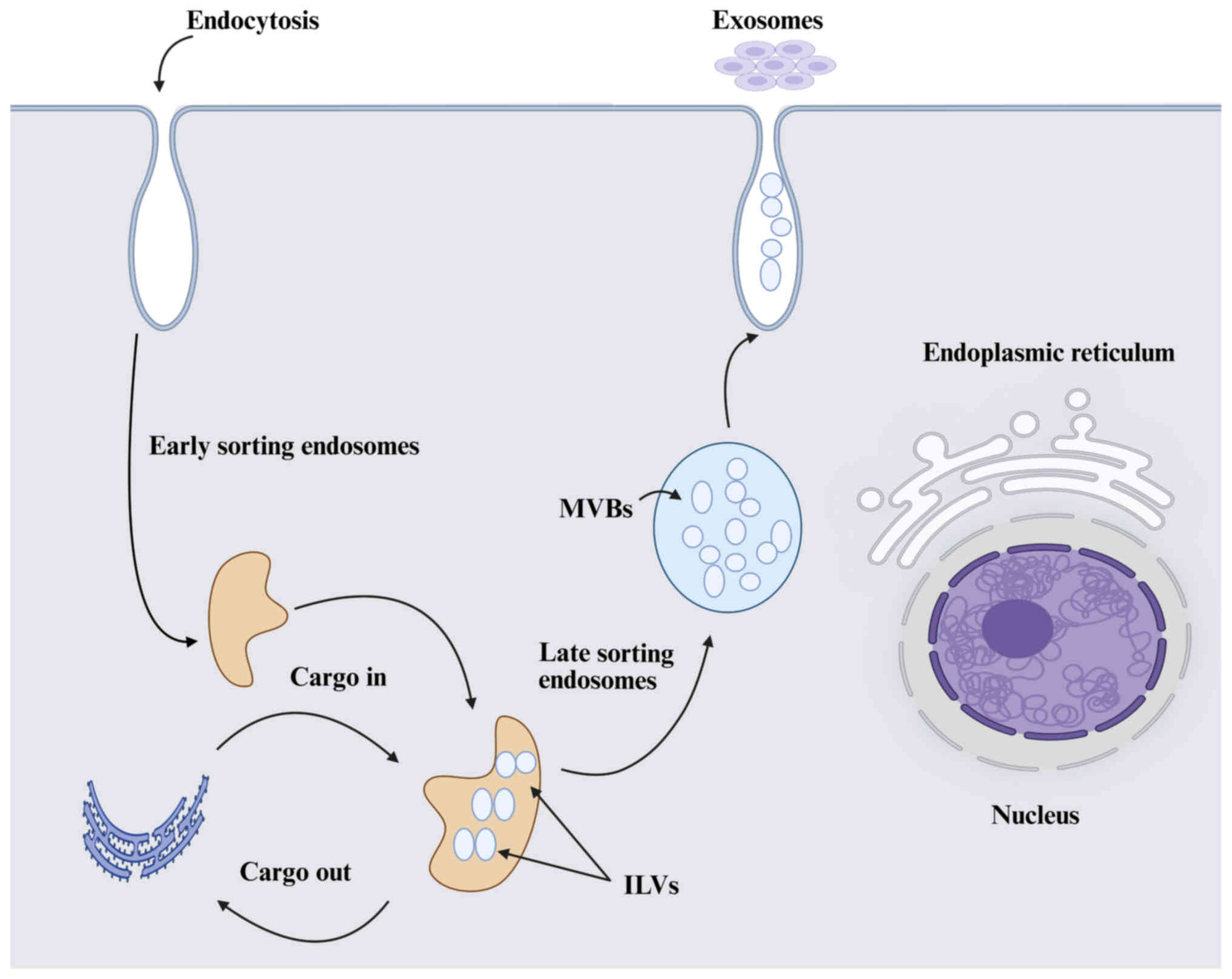
Exosomes are nanoscale extracellular vesicles (EVs) (30-150 nm) formed by the plasma membrane's inward budding, which also produces early endosomes (18). Exosomes, the smallest category of EVs, are generated within the endosomal system through the maturation process from early to late endosomes (Fig. 1). The early endosome is formed by the inward budding of the cell membrane, which progresses to form multivesicular bodies (MVBs), which are characterized by the presence of intraluminal vesicles (ILVs). MVBs release ILVs into the extracellular space as exosomes upon fusion with the plasma membrane (19). The MVB may either merge with lysosomes or autophagosomes for degradation or fuse with the plasma membrane to release the internal ILVs as exosomes (20). The key components involved in exosome biogenesis consist of ceramide and various lipid-associated proteins, the endosomal sorting complex required for transport (ESCRT) machinery that facilitates the sorting of proteins into ILVs, and tetraspanins including CD63, CD9 and CD81, which are present on the surfaces of exosomes and play a significant role in their formation and release (21). The inhibition of ALIX proteins also modulates the release of exosomes (22). The mechanism of cargo sorting is not fully understood; still, it has been revealed that the ESCRT-dependent endo-lysosomal pathway plays a crucial role in exosome formation and cargo sorting involving the syndecan-syntenin-ALIX axis (23). The released exosomes are taken up by the recipient cells via receptor-mediated endocytosis or the receptor-ligand fusion process (24). It is worth mentioning that exosomes can be released by various cell types, including B cells (25), T cells (26), mast cells (27), MSCs (28), reticulocytes (29) and neurons (30), in both normal and pathological states. They are also widespread in body fluids, food, IECs, tumors, immune cells, and stem cells (31).
Exosomes primarily function as vehicles for intercellular communication, influencing recipient cells through the transfer of their cargo (32). In a disease context, exosomes can have both beneficial and detrimental effects. Tumor-derived exosomes can promote metastasis in cancer by preparing pre-metastatic niches and suppressing immune responses, thereby facilitating tumor growth and distribution (33). In neurodegenerative diseases such as Alzheimer's and Parkinson's, exosomes are considered to contribute to the spread of pathogenic proteins across neural networks and worsen the disease progression (34). By contrast, exosomes also possess therapeutic potential due to their ability to carry and deliver drugs or genetic material directly to specific cells, thereby reducing off-target effects and improving treatment efficacy. The cardiovascular system depends on exosomes for proper functioning. In addition, exosomes are involved in cardiovascular diseases by influencing processes such as angiogenesis and inflammation, as previously documented (35). Interestingly, exosome functional effects have been extensively explored in a variety of clinical and non-pathological conditions, including stroke (36), rheumatoid arthritis (37), diabetic cardiomyopathy (38), cardiac diseases (39), respiratory viral diseases (40), skin wound healing (41) and embryo implantation (42), among others. These findings highlight the increasing interest in utilizing exosomes for therapeutic applications, especially in the development of non-invasive diagnostic tools and novel treatment strategies.
Furthermore, exosomes are composed of diverse biological substances, such as proteins, nucleic acids and lipids. Research on exosomes derived from various sources suggests they possess shared exosomal components. Currently, there are 41,860 reported exosomal proteins, over 7,540 exosomal RNA molecules and 1,116 exosomal lipid molecules derived from more than 286 exosome-based studies, all annotated according to the International Society for EVs minimal experimental requirements for defining EVs (43). The standard proteins found in exosomes include tetraspanins (CD9, CD63 and CD81), heat shock proteins (HSP70 and HSP90), and proteins involved in membrane transport and fusion (44). These proteins are integral to exosome formation and functions. Exosomes also carry various RNA species, including mRNA, microRNA (miRNA or miR), and long non-coding RNA (45). These nucleic acids can modulate gene expression in recipient cells, influencing various biological processes. Moreover, apart from protein and nucleic acid, the lipid composition of exosomes is distinct and includes phosphatidylserine, sphingomyelin, cholesterol, arachidonic acid and prostaglandins, which contribute to their structural integrity and facilitate membrane fusion events of exosomes (46). Importantly, these compounds are associated with the physiological role or pathological alterations in the original cells and also reflect the original cell types.
Exosomes and their role in IBD
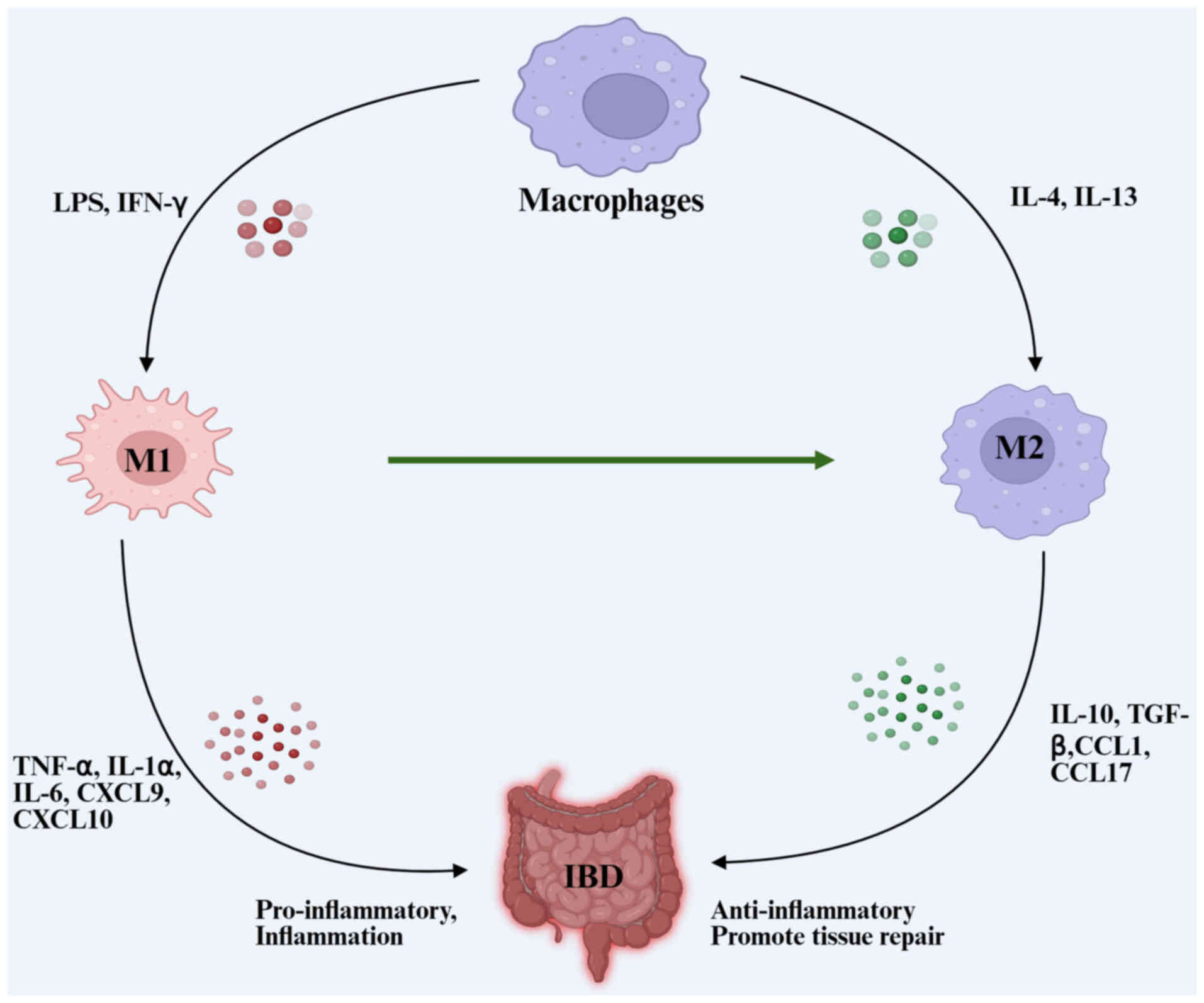
IBD is a chronic, non-specific intestinal inflammatory condition encompassing UC, CD and unidentified colitis. The exact etiology and pathophysiology remain unclear, but studies suggest that IBD arises from a combination of genetic predisposition, environmental factors, immune dysregulation, intestinal barrier dysfunction and gut microbiota imbalance, leading to symptoms such as chronic abdominal pain, diarrhea and recurrent mucus-filled pus discharge (47). Treatments generally depend on anti-inflammatory medications, immunosuppressive medicines, purine analogs and anti-TNF monoclonal antibodies; nonetheless, the therapeutic efficacy remains suboptimal. Several studies indicate that the pathogenesis of IBD is associated with intestinal mucosal innate immunity and acquired immunodeficiency or atypical responses (48,49). The imbalance of intestinal anti-inflammatory and inflammatory substances is a major cause of IBD pathogenesis. Research has shown that exosomes derived or released from macrophages, hucMSCs, dendritic cells (DCs), bone marrow MSCs (bmMSCs), human mast cells-1 (HMCs-1) and adipose-derived MSCs, among others, have a role in both the pathogenesis and diagnosis of IBD. A summary of the studies on IBD exosomes using various cells or tissues is presented in Table I. Macrophages play a substantial role in the inflammatory response. Macrophages are categorized into M1 and M2 subtypes based on their activation status (Fig. 2). M1 macrophages release proinflammatory mediators including TNF-α, IL-1α, IL-1β, IL-6, CXCL9 and CXCL10. M2 macrophages synthesize anti-inflammatory mediators such as IL-10, TGF-β, C-C motif chemokine ligand 1 (CCL1), CCL17, CCL18 and CCL22 (50,51). Macrophage-derived exosome activation is critical for IBD pathogenesis (52). M2 macrophage-derived exosomes enhance regulatory T (Treg) cell levels and IL-4 production while decreasing the synthesis of pro-inflammatory cytokines IL-1β, IL-6 and IL-17A. These exosomes mitigate the severity of dextran sodium sulphate (DSS)-induced colitis in mice and provide a protective effect in colitis (53). Copper chaperone antioxidant-1 (Atox1) promotes M1 polarization and enhances the production of pro-inflammatory cytokines through the reactive oxygen species-NOD-like receptor protein 3 (NLRP3) inflammasome pathway, suggesting its potential as a therapeutic target in IBD treatments. This indicates a crucial link between Atox1 activity and the exacerbation of inflammatory responses in IBD through macrophage-derived exosomes influencing M1 polarization. Similarly, tofacitinib, a Janus kinase inhibitor, modulates the production of pro-inflammatory cytokines in M1-like macrophages. It significantly hinders the development of the M2-like phenotype, as evidenced by decreased IL-10 levels and reduced CD206 expression. Consequently, these effects influence the study outcomes concerning macrophage function in inflammation and immune response modulation (54).
Moreover, exosomes released by hucMSCs have been shown to alleviate DSS-induced IBD by modulating enzyme 15-LOX-1 gene expression in macrophages, reducing inflammatory responses, and inhibiting STAT3 activation in affected tissues, thereby relieving the inflammatory response (55). Correspondingly, it was previously revealed that hucMSCs-exosome reduced the expression of the pro-inflammatory cytokines TNF-α, IL-1β and IL-6 in the colon in IBD mice, increasing the expression of the anti-inflammatory cytokine IL-10 and attenuating the inflammatory response, thus lessening DSS-induced colitis in mice (56). In addition, a recent study revealed that hucMSCs-exosome also alleviates IBD in mice by enhancing M2-type macrophage polarization via the METTL3-Slc37a2-YTHDF1 axis (57). Besides, hucMSCs-exosome specifically shuttle miR-129-5p, which plays a key role in IBD by targeting the key enzyme, ACSL4, to inhibit ferroptosis and lipid peroxidation in intestinal epithelial cells (58). This finding suggests a novel therapeutic pathway for IBD treatment through the modulation of ferroptosis.
Exosomes derived from DCs (DCs-exosomes) regulate the immune response and inhibit the onset of autoimmune disorders (59). DCs-exosomes have been used to encapsulate triptolide (TP), resulting in reduced systemic toxicity and enhanced immunosuppressive effects in models of UC and rheumatoid arthritis (60). Combining TP with DCs-exosomes enhanced targeted delivery to DCs while transforming the immune landscape by influencing T-cell differentiation and suppressing the activation of DCs. These findings highlight the capability of DCs-exosomes-TP to offer a targeted therapeutic strategy that reduces side effects and encourages immune control. Notably, DCs-exosomes treated with staphylococcus enterotoxin A were found to lower the levels of pro-inflammatory cytokines, including TNF-α, IFN-γ, IL-17A, IL-12 and IL-22, in mice with acute DSS-induced colitis (61). In the same way, the increased expression of the anti-inflammatory cytokine TGF-β not only reduced intestinal inflammation but also contributed to the unique role of DCs-exosomes in reprogramming immune cells responsible for alveolar bone loss (62). Several subtypes of DCs-exosomes were investigated, including immuno-regulatory (regDC-EXO), immune stimulatory and immune 'null' immature. The regDC EXO, which contained the TGFB1 gene and IL-10, had a high affinity for inflamed areas and was effective in inhibiting the maturation of recipient DCs and Th17 effectors while increasing Treg cell recruitment. This resulted in a decrease in bone resorption cytokines and osteoclastic bone loss, presenting a unique therapy option for degenerative alveolar bone disease. In addition, numerous studies have shown the considerate effects of exosomes derived from bone marrow MSCs (bmMSCs-exosomes) in IBD treatment. The administration of bmMSCs-exosomes in patients with CD can aid in curing perianal fistula in CD (63). This reveals that bmMSC-exosomes therapy is a safe option, with sustained fistula closure observed in a significant proportion of patients with CD. Another study reported that bmMSCs-exosomes reveal higher efficacy in reducing leukocyte infiltration and neuronal loss in the myenteric plexus compared with adipose tissue-derived MSCs (64). Although both cell types ameliorated general symptoms of IBD, such as body weight loss and morphological damage to the colon, bmMSCs-exosomes were particularly effective in targeting enteric neuropathy, highlighting their potential as a more targeted treatment option for the neural aspects of IBD. In addition, a recent animal study also revealed that NLRP3 plays a critical role in modulating the function of bmMSCs by promoting glycolysis and increasing IL-10 production (65). Notably, the study found that bmMSCs-exosome lacking NLRP3 exhibited decreased IL-10 levels and diminished protective effects against colitis. Conversely, overexpression of NLRP3 in bmMSCs-exosomes significantly improved therapeutic outcomes, highlighting the crucial role of NLRP3 in enhancing the efficacy of bmMSCs-exosomes.
Furthermore, it is worth mentioning that exosomes derived from HMCs-1 significantly disrupt intestinal barrier function by transferring functional miRNAs to intestinal epithelial cells, leading to decreased expression of tight junction proteins (TJP) (66). Notably, exosomal miR-223 from HMCs-1 was shown to inhibit the expression of claudin 8 (CLDN8), thereby impairing intestinal barrier integrity. This suggests potential new therapeutic targets for IBD treatment. The HMCs-1 enhances intestinal epithelial permeability by delivering miRNAs that downregulate TJP, notably through miR-223 targeting CLDN8. The inhibition of CLDN8 disrupts intestinal barrier integrity, suggesting potential therapeutic targets for IBD management. In the same way, HMCs-1 derived from exosomes also upregulate intestinal epithelial permeability and destroy intestinal barrier function, which is attributed to the exosome-mediated transfer of functional miRNAs from HMCs-1 (67). These transferred miRNAs also decrease the expression of TJP, including CLDN8, TJP1 and occludin (OCLN). By contrast, adipose-derived MSCs release exosomes (adMSC-exosomes), which influence the pathogenic alterations of IBD. For example, a previous study showed that intraperitoneal administration of adMSC-exosomes on acute DSS-induced colitis in mice reduces levels of TNF-α, IFN-γ, IL-12 and IL-17 while enhancing levels of IL-4, TGF-β and IL-10 in the lymph nodes and spleen (68). Intraperitoneal administration of AdMSCs and MSC-CM improves symptoms and histopathology in a mouse model of chronic colitis (69). Yet again, intraperitoneal administration of adMSC-exosomes elevates the proportions of CD4+ CD25+ Foxp3+ Treg cells in the lymph nodes and spleen of mice (70).
Exosomes play a role not only in disease development but also in diagnosis and prognosis. In IBD, numerous studies highlight the role of exosomes in the diagnosis and prognosis of the disease. For instance, serum exosomal microRNA-144-3p, a non-invasive biomarker for diagnosing and monitoring CD, proves its reliability in detecting mucosal inflammation and predicting postoperative recurrence with greater accuracy than conventional CRP testing (71). Serum exosomes from patients with CD also exacerbate colitis by activating macrophages and disrupting epithelial barrier function, with altered exosomal microRNA profiles, particularly the let-7b-5p/TLR4 pathway, playing a critical role in these processes and suggesting potential diagnostic and therapeutic applications (72). MiRNAs exhibit differential expression patterns in UC and contribute to the regulation of inflammatory processes. Research has revealed multiple miRNAs with significantly altered expression levels in UC tissues when compared with healthy controls (73). A study conducted miRNA microarray analysis on peripheral blood samples from patients with CD, UC and healthy controls, comparing the miRNA expression patterns of exosomes among these three groups, and discovered that miRNA expression varied between patients with IBD and healthy controls (74). Notably, miRNA expression varied between patients with CD and UC (75). This finding suggests that miRNAs in blood could be valuable diagnostic tools for IBD. Further research is needed to distinguish between different types of IBD. On the other hand, DSS-induced colitis in mice models revealed that proteomics analysis identified 56 differentially expressed proteins, most of which were acute phase proteins and immunoglobulins, suggesting that specific exosomal proteins are potential inflammatory markers of IBD (76). According to a recent review (77), MSC-derived EVs reveal their diagnostic potential in IBD, highlighting their capacity to mimic parental cell functions, which could enhance diagnostic precision by evaluating their bioactive cargoes through transcriptomic, proteomic and lipidomic analyses. Also, exosomes derived from the saliva of patients with active IBD may exacerbate colitis by influencing macrophage activity, disrupting intestinal epithelial function, and altering the gut microbiome, potentially highlighting a diagnostic role of salivary exosomes in reflecting IBD activity. Similarly, salivary exosomes from both patients with IBD and healthy controls were examined, showing that PSMA7 expression was higher in the oral epithelium of the IBD group, consistent with its expression in the colonic tissues of the same cohort (78). The findings indicate that salivary exosomal PSMA7 serves as a significant protein biomarker for IBD, potentially functioning as a diagnostic instrument for this condition.
Farnesoid X receptor
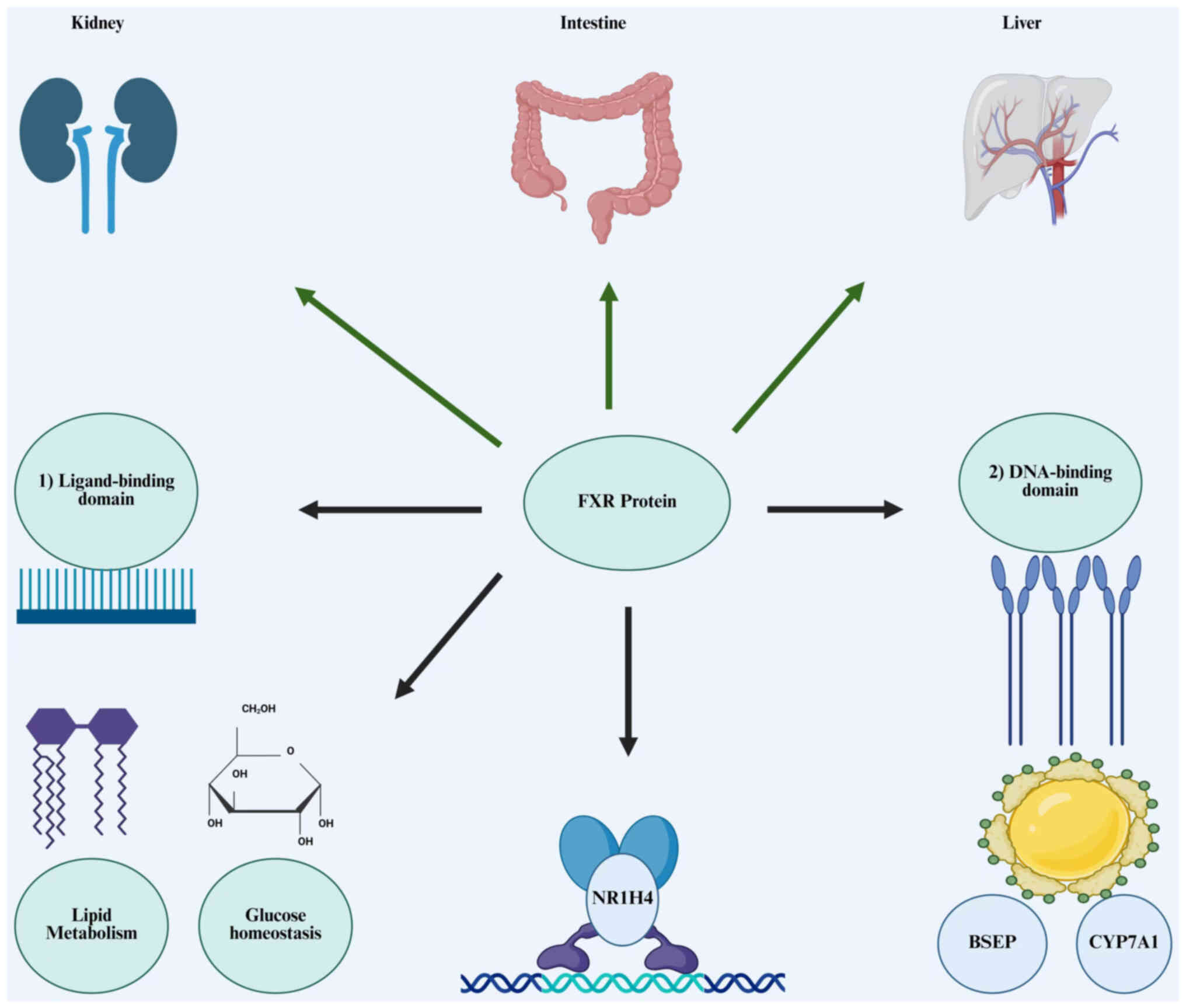
FXR is a ligand-activated transcription factor that regulates BA production and enterohepatic circulation. It is predominantly expressed in the liver, intestines, kidneys and adipose tissue (79). FXR is encoded by the NR1H4 gene and acts as a transcription factor, regulating the expression of numerous genes involved in various metabolic processes (Fig. 3) (80). FXR is composed of two members: FXRα and FXRβ. FXRβ is a pseudogene in humans and primates; however, it encodes a functioning receptor in other animals. The FXRα gene encodes four isoforms, FXRα1-α4 (81). The four isoforms (α1, α2, α3, α4) exhibit distinct tissue distribution and roles. FXRα1 and FXRα2 are mainly found in the liver, kidney and intestine, whereas FXRα3 and FXRα4 are more highly expressed in the ileum (82). The overall structure of FXR comprises an n-terminal DNA binding domain, a distinctive ligand binding domain facilitating receptor dimerization, AF1, and a c-terminal Activation domain (AF2) for interactions with co-regulators (83). AF1 and AF2 are the domains of FXR molecules that engage with regulatory proteins. Ligand-activated FXR binds to FXR response elements in target genes and can function as heterodimers with retinoid X receptor (RXR) or as monomers to control metabolism (84). Recent explorations of FXR agonists have produced positive results attributable to their role as a ligand-activated transcription factor (85).
The key physiological functions of FXR include BA metabolism, lipid and glucose homeostasis, and inflammatory responses. FXR is crucial for regulating BA metabolism and sustaining metabolic homeostasis. BA is synthesized by the liver and reabsorbed in the small intestine via enterohepatic circulation, with ~95% of BA being reabsorbed in the small intestine (86). FXR is primarily stimulated by BA, especially chenodeoxycholic acid (CDCA), recognized as the most effective natural activator of FXR (87). Other BA, such as lithocholic acid (LCA) and deoxycholic acid (DCA), can activate FXR but with less affinity relative to CDCA (88). In response to BA stimulation, FXR functions as an essential regulator of numerous physiological processes. It regulates the production of BA by inhibiting key enzymes such as cholesterol 7α-hydroxylase (CYP7A1) and sterol 12α-hydroxylase (CYP8B1), which are essential for the conversion of cholesterol into BA (89). FXR regulates BA metabolism mainly through the hepatic FXR/small heterodimer partner (SHP) axis and the ileal fibroblast growth factor 15/19 (FGF15/19)/hepatic FGF receptor 4 axis, hence it may reduce CYP7A1 activity, therefore inhibiting BA production (90). This regulation is essential for the maintenance of BA homeostasis. FXR also affects BA transport and secretion by promoting the expression of proteins such as the bile salt export pump (BSEP) and the intestinal BA binding protein (IBABP), which aid in the transfer of BA into bile and their later reabsorption in the intestine (91). A previous study revealed that the protein level of BSEP in hyperoside-treated rats is 1.44-fold greater than that in the model group, indicating that BSEP overexpression in mice diminishes hepatic steatosis when subjected to a lithogenic or methionine choline-deficient diet, likely due to FXR activation (92). Numerous studies indicate that FXR modulates a network of genes involved in hepatic BA synthesis, biliary BA secretion, intestinal BA absorption and hepatic BA uptake, thus playing a crucial role in the regulation of BA homeostasis (93-95).
The FXR activation influences not only BA metabolism but also the regulation of lipid and glucose metabolism. Targeted modulation of FXR may effectively address obesity-related metabolic disorders. Research using mice with FXR gene deletion or the administration of FXR agonists revealed critical insights into the pivotal role of FXR in lipid homeostasis. FXR regulates the production and secretion of lipoproteins and triglycerides (96). The activation of FXR can lead to a decrease in the production and secretion of very low-density lipoprotein (VLDL) from the liver. This effect is mediated by the regulation of genes involved in triglyceride synthesis, such as phosphatidate phosphohydrolase, resulting in a marked reduction in triglyceride levels. For instance, FXR regulates serum triglyceride (TG) levels, with FXR activation through CDCA or agonist (GW4064) significantly reducing the expression of PLA2G12B in HepG2 cells. By inhibiting the expression of hepatic phospholipase PLA2G12B, the secretion of TG-rich VLDL is decreased subsequently (97). FXR also positively affects high-density lipoprotein cholesterol (HDL-C) levels by enhancing the expression of apolipoprotein C-II and reducing the expression of apolipoprotein C-III, thus improving overall lipid profiles (98). A previous study revealed that FXR activation with the agonist GSK2324 regulates hepatic lipids through both reduced absorption and selective decreases in fatty acid synthesis, with distinct pathways controlled by hepatic and intestinal FXR; hence, its activation protects against non-alcoholic fatty liver disease (NAFLD) via BA-dependent reductions in lipid absorption (99). Notably, calsyntenin-3 (Clstn3), a transmembrane protein, also improves lipid metabolism disorders in NAFLD models, with its effects potentially mediated through the FXR, offering a novel therapeutic approach for NAFLD treatment (100). Interestingly, a recent study on the humanized dyslipidemia mice model revealed that fecal microbiota transplantation from donors with dyslipidemia reshapes the gut microbiota in mice, leading to alterations in BA synthesis and cholesterol accumulation through the hepatic FXR-SHP axis, while a high-fat diet reduces Muribaculum, affecting lipid absorption via the intestinal FXR-FGF19 axis, highlighting the role of intestinal FXR in regulating lipid metabolism in diet-induced dyslipidemia mediated by gut microbiota BA crosstalk (101).
Moreover, FXR also plays a significant role in glucose homeostasis, which aids in the management of type 2 diabetes (102). FXR can inhibit gluconeogenesis, the process of producing glucose from non-carbohydrate sources in the liver, by decreasing the expression of essential gluconeogenic enzymes such as phosphoenolpyruvate carboxy-kinase and glucose-6-phosphatase (103). This action reduces fasting blood glucose levels. The generation and phenotypic analysis of Fxr−/− mice validate this crucial function in the regulation of lipid metabolism and glucose homeostasis. In early studies, Fxr−/− mice have increased blood concentrations of free fatty acids, triglycerides and HDL-C (104,105); a previous study further showed that PPARα activation can mitigate some of these effects, by providing a potential therapeutic strategy for conditions such as NAFLD and atherosclerosis in FXR-deficient states (106). Moreover, although previous research (107,108) has indicated insulin resistance marked by hyperglycemia, impaired glucose tolerance, and significantly reduced insulin signaling in the liver and muscle, a more recent study revealed that Mebhydrolin, an FXR antagonist, enhances glucose regulation in type 2 diabetic mice. This improvement occurs by inhibiting hepatic gluconeogenesis through the FXR/miR-22-3p/PI3K/AKT/FoxO1 pathway and stimulating glycogen synthesis via the FXR/miR-22-3p/PI3K/AKT/GSK3β pathway (109).
Numerous studies show that FXR contributes to enhanced insulin sensitivity through its impact on lipid and glucose metabolism, thereby reinforcing its role in the regulation of glucose homeostasis (96,103). According to a recent study, BA-activated FXR in glucose homeostasis and inflammation in white adipose tissue (WAT) was investigated using both whole-body and adipocyte-specific FXR-deficient mice (FXR−/− and Ad-FXR−/−) (110). The results revealed that FXR deficiency in adipocytes reduced WAT pro-inflammatory macrophage infiltration and inflammation, leading to improved systemic glucose tolerance, insulin sensitivity and oxidative stress. Transcriptomic analysis identified the antioxidant enzyme Gsta4 as the most upregulated gene in Ad-FXR−/− adipocytes, suggesting that FXR regulates glucose metabolism through the modulation of oxidative stress and inflammation (110). Also, notoginsenoside Ft1 (Ft1), a potent agonist of the BA receptors membrane-bound G protein-coupled receptor (TGR5) and FXR, boosts hepatic BA production and serum BA levels, which in turn activates TGR5 in adipose tissues to alleviate high-fat diet-induced obesity and insulin resistance in mice (111). The study utilized diet-induced obese mice to investigate these effects. These findings indicate that Ft1 could be a dual-target compound for treating obesity and insulin resistance. A similar study also revealed that activation of intestinal FXR by the agonist fexaramine (FEX) modulates the gut microbiota to induce LCA-producing bacteria, which activate TGR5 and stimulate GLP-1 secretion. This signaling pathway improves hepatic glucose and insulin sensitivity, enhances lipid metabolism, and promotes white adipose tissue browning in mice, suggesting that FXR plays a crucial role in regulating glucose homeostasis and metabolic diseases through its interaction with the gut microbiota (112).
On the other hand, FXR possesses anti-inflammatory properties that may diminish the expression of pro-inflammatory cytokines. It has emerged as a significant modulator of inflammatory responses in various tissues, notably the liver and intestines. It has been documented in both animal models (MDR2−/− mice and diethoxycarbonyl-1,4-dihydrocollidine-induced SC) and patient-derived mononuclear cells that systemic activation of FXR reduces BA synthesis and suppressed pro-inflammatory cytokines, including IL1β and TNFα, through the inhibition of hepatic macrophage activity and TH1/TH17 lymphocyte polarization (113). The result indicates that the deletion of FXR in myeloid cells led to resistance to the anti-inflammatory and liver-protective effects of FXR agonists, highlighting the importance of FXR in regulating immune responses and disease progression in disease such as sclerosing cholangitis (SC). Yet again, in the Mdr2/Abcb4 knockout (-/-) mouse model of SC, cilofexor (also known as GS-9674), a non-steroidal FXR agonist, improved liver function by normalizing serum biomarkers such as aspartate aminotransferase and alkaline phosphatase, reducing hepatic fibrosis, and lowering intrahepatic BA concentrations (114). The aforementioned study suggests that cilofexor's action through FXR activation may attenuate liver inflammation and fibrosis by modulating BA synthesis and signaling, mainly through the FGF15 pathway. In addition, FXR influences inflammatory responses by maintaining the integrity of the intestinal barrier. For example, activation of the FXR in the intestine has been shown to play a protective role in inflammation-driven models of IBD by regulating the intestinal barrier and inflammatory responses, mainly through the modulation of innate lymphoid cells (ILCs) and the reduction of pro-inflammatory cytokines such as IL17, highlighting FXR as a potential therapeutic target for inflammatory intestinal diseases (115). In the same way, a study using a mouse CAC model showed that inflammation-induced epithelial damage disrupts FXR signaling, altering BA profiles, while FXR activation in gut macrophages detects abnormal BA, releasing pro-inflammatory cytokines, promoting intestinal stem cell proliferation, and reducing intestinal inflammation and tumor growth by modulating macrophage recruitment, polarization and Th17 cell interactions (116).
Furthermore, FXR also interacts with significant inflammatory signaling pathways. NF-κB activation reduces FXR activity, likely through NF-κB-dependent tethering, highlighting a potential vicious cycle where reduced FXR activation contributes to the development of chronic intestinal inflammation (117). It is worth mentioning that FXR also has significant effects on cardiovascular inflammation. Atherosclerosis has a potential therapeutic target via the intestinal FXR/SMPD3 axis (118). The aforementioned study highlights the relationship between intestinal FXR, ceramide metabolism and atherosclerosis, and reveals that inhibition of FXR or SMPD3 reduces atherosclerosis and ceramide levels in ApoE−/− mice. Hence, FXR serves as a key relationship between metabolic processes and immunological responses. Its activation exhibits significant anti-inflammatory effects across multiple organs, establishing it as a prospective therapeutic target for inflammatory diseases associated with metabolic dysfunction.
FXR and its role in IBD
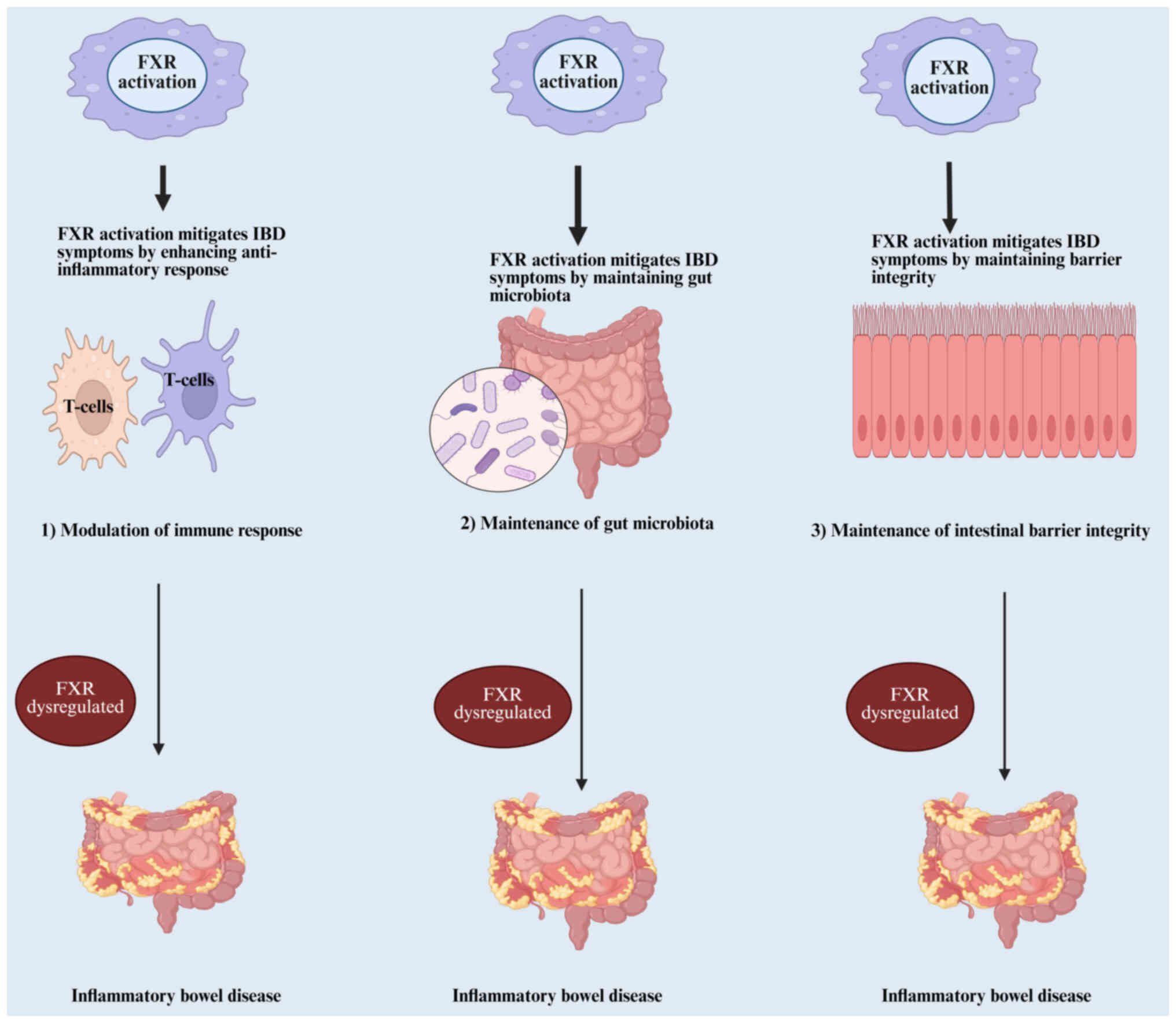
IBD, which includes UC and CD, are chronic diseases that cause inflammation in the gastrointestinal system (119). Despite extensive investigation into IBD, the precise pathogenetic pathways remain inadequately clarified. Currently, IBD is generally considered to arise from disruptions in gut microbiota balance, impaired intestinal barrier function, and an irregular immune response in the mucosal lining (120). Research shows that FXR activation helps modulate immune responses, promote healthy gut microbiota, decrease inflammatory processes, and strengthen the intestinal epithelial barrier. These effects are achieved through multiple pathways, such as controlling inflammatory signals, maintaining the structure and function of the gut barrier, and preventing the movement of bacteria within the digestive system (Fig. 4) (121).
FXR maintains inflammatory responses in IBD
Maintaining inflammatory responses is a key function of FXR, which significantly contributes to the mucosal immune response and impacts immunoregulation (122). FXR activation in the intestine has shown protective effects in inflammation-driven models of IBD by regulating inflammatory responses, mainly through the modulation of ILCs and cytokine production, including IL-17, highlighting its therapeutic potential in IBD (115). However, the hyperactivation of NF-κB suppresses FXR activation and its downstream anti-inflammatory actions, likely through NF-κB-dependent tethering of FXR, creating a detrimental cycle of chronic intestinal inflammation (117). The core molecular mechanism clarified in the present study is the direct physical interaction between activated NF-κB (p50/p65) and FXR, leading to suppression of FXR transcriptional activity via tethering/cofactor competition. This results in reduced expression of key anti-inflammatory and homeostatic genes (SHP, FGF15/19 and IBABP). The loss of this protective FXR signaling creates a vicious cycle where inflammation suppresses its own negative regulator, driving chronicity as in IBD. Targeting the FXR/NF-κB axis, potentially including exosomal communication pathways, holds significant therapeutic promise for IBD. FXR's impairment in patients with IBD and CAC influences gut macrophages' response to microbiota and metabolite-induced disruptions, exacerbating epithelial abnormalities and promoting intestinal stem cell proliferation (116). FXR activation also mitigates intestinal inflammation and colon cancer progression by modulating macrophage recruitment, polarization and interaction with Th17 cells, whereas FXR deletion intensifies inflammation (123).
Numerous studies further clarify the interaction between diet, microbiota-derived signals, and FXR in modulating inflammation (124-126). Dietary fiber, such as inulin, influences microbial metabolites, affecting inflammatory responses via FXR in a murine model, suggesting the relevance of precision nutrition in IBD pathogenesis (127). Another study shows that high-fat diets increase BA secretion and downregulate FXR target genes, exacerbating UC symptoms. In addition, FXR agonists such as FexD alleviate these symptoms through the TGFB signaling pathway in both in vivo and in vitro models (128). These studies have shown that Fxr−/− mice exhibit elevated expression of pro-inflammatory cytokines in support of these outcomes. Furthermore, the synthetic FXR ligand (6-ECDCA) has been found to reduce inflammation in wild-type mice effectively, but this anti-inflammatory effect is absent in FXR-deficient mice (129). Notably, previous studies (130,131) revealed that FXR activation through agonists (INT-747) alleviates colitis symptoms, reduces epithelial permeability, and preserves goblet cells, highlighting FXR's counter-regulatory effects on innate immune cells. In addition, a recent study indicated that agonists INT-747 and obeticholic acid help lessen colitis symptoms, improve epithelial tightness, and prevent the depletion of goblet cells (132). Collectively, these findings establish FXR as a critical regulator of intestinal inflammation and a promising therapeutic target for IBD and CAC.
FXR maintenance of intestinal barrier function in IBD
Maintaining the intestinal barrier is essential for both mechanical protection and gut homeostasis. Disruption of this barrier causes protein loss, electrolyte imbalances, bacterial translocation, gut-origin sepsis and mortality. Research highlights that FXR is key in regulating gut barrier stability, both under normal conditions and during pathophysiological challenges. The activation of FXR in the setting of pathological conditions can alleviate detrimental histological damage to the intestines observed in various barrier injury models, including LPS, ischemia-reperfusion injury (IRI), DSS-induced colitis and trinitro-benzene-sulfonic acid (TNBS) exposure. In LPS-induced injury, FXR activation with tauro-DCA (TDCA), conjugated BA and natural FXR ligand mitigates intestinal mucosal damage while preserving villus height and architecture, restoring histology to normal values (133). The same histological recovery with TDCA therapy was not evident in LPS-treated FXR knockout mice. Importantly, FXR KO animals do not appear to have baseline intestinal architectural changes; however, they show increased villus necrosis and inflammatory cell infiltration after LPS treatment (134). A recent study using GW4064, an FXR agonist, on FXR-knockout mice showed that GW4064 effectively reduces LPS-induced intestinal barrier disruption and colon carcinogenesis. This highlights the importance of the FXR/αKlotho/βKlotho/FGFs pathway and the interplay between BA and gut microbiota (135). In IRI models, pretreatment with the FXR agonist (OCA) prevents the shortening of villus length, reduces the decrease in trans-epithelial electrical resistance, and alleviates the rise in Park/Chiu scores, which are used to evaluate barrier damage during ischemia (136). In the same way, FXR inactivation in tubular epithelial cells following IRI damage induces apoptosis in renal tubular epithelial cells by blocking PI3k/Akt-mediated Bad phosphorylation to cause renal I/R damage, hence contributing to renal IRI (137).
Numerous studies have shown the effects of FXR in the modulation of intestinal barrier function in IBD models (138,139) FXR activation reduces chemically induced intestinal inflammation in DSS-induced colitis and TNBS mice of both wild-type (WT) and Fxr-null mice, revealing that FXR agonists, particularly INT-747, significantly mitigated symptoms and pathophysiological markers of colitis in treated mice, such as reduced body weight loss, lower epithelial permeability, and decreased pro-inflammatory cytokine production in various immune cells (131). The aforementioned study supports the potential of FXR agonists as a novel therapeutic approach for IBD. Similarly, in a pre-clinical study, non-BA FXR agonists, particularly compound 33, have shown promising effects for IBD treatment (11). The study uses a structure-based drug design strategy, with compound 33 demonstrating potent FXR agonistic activity, favorable intestinal distribution, anti-inflammatory effects, and efficacy in repairing the colon epithelium in a DSS-induced acute enteritis model. Interestingly, ginsenoside Rc, a significant component of Panax ginseng, ameliorates DSS-induced IBD by reducing inflammation and restoring intestinal barrier function, with its effects mediated through the activation of FXR signaling, as demonstrated in both in vivo and in vitro FXR-knockout model (140).
Moreover, the activation of FXR enhances the intestinal epithelial barrier by promoting the expression of TJPs, which are crucial for maintaining epithelial integrity and preventing the translocation of luminal contents into the underlying tissue. Research indicates that FXR knockout (KO) mice demonstrate increased protection against acute intestinal inflammation, as reflected by preserved tight junction integrity and diminished inflammatory cytokine expression in various murine models, including LPS injection, dithizone/Klebsiella, and cecal ligation/puncture, potentially providing novel insights into therapeutic targets for IBD and other associated disorders (139). Yet again, GW4064 also affects the regulation of TJP and inflammation (134). The study indicates that GW4064 alleviates mucosal injury and tight junction dysfunction in the ileum of WT mice but not in FXR KO mice and modulates NALP3 inflammasome activity and inflammatory cytokine production in both WT and KO mice. Besides, in exploring the impact of lubiprostone on intestinal permeability and related pathologies in a bile duct ligation (BDL) model, the study highlights the significant role of FXR and TJP, including claudin-1, OCLN and claudin-2, in maintaining intestinal barrier integrity (141). Specifically, administering lubiprostone post-BDL effectively mitigated the increased intestinal permeability and systemic inflammation, evidenced by reduced serum LPS levels and improved expression of key barrier proteins and FXR genes (141). The aforementioned study highlights the therapeutic potential of targeting FXR and tight junction regulation in managing intestinal barrier failure in illnesses such as IBD and other associated diseases.
FXR modulation of gut microbiota in IBD
FXR has been associated with barrier function by regulating gut antibacterial proliferation. The gut microbiota is crucial for pathogen protection, immune function and nutritional absorption. Recent research indicates a regulatory link between the development of IBD and changed gut flora (142-145). BA and gut flora are intricately interconnected. The gut microbiota participates in the biotransformation of BA by deconjugation, dehydroxylation and conjugation of BA (146). BA exhibits antibacterial properties by compromising the bacterial cell membrane, impeding bacterial proliferation (147). It has been revealed that the variety of intestinal microbiota in patients with IBD is weakened, characterized by a notable decrease in firmicutes and an increase in proteobacteria (148). Firmicutes are the primary cause of BA modification; hence, their considerable reduction in patients with IBD has an impact on the BA pattern. In a bioinformatics analysis of secondary BA metabolites, a previous study discovered that the abundance of firmicutes and their BA biotransformation genes were dominant in normal human and IBD samples and the reduction of Firmicutes in IBD was associated with a reduced capacity of biliary salt transformation (149). Furthermore, the ileum predominantly facilitates the reabsorption of conjugated BA; thus, patients with CD frequently experience BA malabsorption due to ileal dysfunction, resulting in a diminished BA pool (150). It is worth mentioning that the imbalance of BA metabolism impairs intestinal mucosa and exacerbates the inflammatory response.
FXR functions as a key receptor governing BA production and is significant in IBD. It has been well-documented that intestinal FXR plays a vital role in decreasing bacterial overgrowth, thereby safeguarding the intestine from damage caused by bacteria (151). It has been shown that mice lacking FXR had bacterial overgrowth, increased intestinal permeability, and high levels of bacteria in mesenteric lymph nodes, as well as inflammation of the intestinal walls. Interestingly, it was reported that activation of intestinal FXR by GW4064 not only leads to the identification of several novel intestinal FXR target genes, including those encoding angiogenin, carbonic anhydrase 12, and inducible nitric oxide synthase, which have been reported to possess antibacterial properties but also induces the cytokine IL-18, which stimulates resistance to a variety of pathogens such as intracellular and extracellular bacteria and mycobacteria and appears to play a protective role during the early, acute phase of the mucosal immune response (152). Similarly, the administration of bile or conjugated BA to ascitic cirrhotic rats or rats with obstructive jaundice eradicates intestinal bacterial overgrowth and reduces bacterial translocation and endotoxemia (153,154). These findings support the concept that FXR plays a vital role in regulating intestinal bacterial proliferation, which is crucial for sustaining an effective barrier and thus helps prevent intestinal inflammation. Consistent with these previous findings, the modulation of FXR and its effects on microbiota and IBD were explored. For example, a recent study revealed the downregulation of FXR expression in both patients with IBD and a DSS-induced IBD mouse model, which was associated with modified primary BA production (155). These results indicate that administering exosomes derived from MSCs to the animal model significantly restored colonic FXR levels, improved gut microbiota metagenomics and metabolomics, and resulted in the reduction of both macroscopic and microscopic symptoms of IBD.
Moreover, another study investigating Bai Tou Weng Tang, a traditional Chinese herbal medicine, for the treatment of UC showed that it not only restored normal BA levels but also improved the diversity of gut microbiota, remarkably increasing Firmicutes and normalizing Bacteroidetes levels while increasing the expression of FXR and TGR5 in the liver (156). In the same way, moxibustion, another traditional Chinese medicine therapy that uses heat from burning moxa (dried mugwort), significantly improved inflammation control and mucosal barrier repair through the upregulation of FXR and inhibition of the TLR4/MyD88 pathway in rats with CD (157). The aforementioned study elucidates that gut-liver axis exosomes, carrying dysbiosis-induced cargo (for example, LPS, miR-144/451 and hydrophobic BAs), act as central communicators linking FXR suppression to TLR4/NF-κB hyperactivation in CD pathogenesis by simultaneously delivering FXR-antagonizing miRNAs, TLR4 agonists and inflammatory mediators to target cells; critically, herb-partitioned moxibustion repairs this axis by restoring exosomal composition by normalizing BA enterohepatic circulation to enhance FXR/RXR-SHP signaling which represses CYP7A1 gene and blocks MyD88 recruitment, enriching barrier-protective mucins/claudins, and promoting anti-inflammatory exosomal miRNAs (for example, miR-194) that target TLR4, thereby suppressing inflammation and re-establishing mucosal homeostasis. In addition, subsequent research has examined the role of changes in BA composition in the development of IBD. For instance, in CD, changes in plasma BA composition led to diminished activation of FXR and PXR. The study found reductions in glycol-CDCA, taurocholic acid and lithocholic acid alongside increases in glyco-DCA and glycocholic acid in the BA profile of patients with CD. This dysregulation corresponded to decreased plasma concentrations of 4β-hydroxycholesterol and fibroblast growth factor 19, markers of PXR and FXR activation, respectively (158). These findings highlight microbial dysbiosis's potential influence on IBD's BA profiles and FXR signaling. In another study, the activation of FXR reduced liver enzyme (CYP8B1) expression and improved colitis in mice (154). The aforementioned study reveals a connection between the hepatic CYP8B1-CA axis and colitis through the regulation of intestinal epithelial regeneration, indicating that BA-based strategies may be advantageous in IBD (159). Besides, the present review highlights that FXR deficiency contributes to intestinal inflammation by facilitating communication between the gut microbiota and the immune system (160). This emphasizes the potential of BA signaling as a therapeutic target for IBD.
Exosome-mediated FXR regulation on repairing IBD
Exosomes are EVs carrying nucleic acids, lipids and proteins, playing pivotal roles in IBD pathogenesis and repair. They modulate immune responses, gut barrier integrity, and microbiota homeostasis, influencing key IBD-associated pathways (161). Their therapeutic potential stems from their ability to shift macrophages toward the anti-inflammatory M2 phenotype, suppress pro-inflammatory cytokines, and deliver regenerative cargo to damaged tissues (162). Their ability to regulate immune cells, gut microbiota and barrier function emphasizes their potential as diagnostic tools, drug carriers and therapeutics.
The interplay between exosomes and FXR is crucial for intestinal repair. FXR activation is impaired in IBD, exacerbating inflammation and dysbiosis (163). Exosomes derived from MSCs, or other sources enhance FXR activity by delivering anti-inflammatory cargo, modulating miRNAs, and targeting gut microbiota. For example, MSC-exosomes reduce pro-inflammatory cytokines (for example, IL-1β and IL-6) and elevate IL-10, rebalancing gut immunity (50). The aforementioned study revealed that MSC exosomes act as natural anti-inflammatory delivery vehicles, reprogramming immune cells in the gut to suppress harmful inflammation while promoting healing. Their ability to target specific pathways (IL-10 induction) makes them a promising therapeutic strategy for inflammatory gut diseases. Interestingly, research has shown that exosomes modulate and facilitate cell-to-cell communication by transporting miRNAs, which regulate various IBD-associated processes, including barrier function and immune responses (19). These exosomal miRNAs modulate oxidative stress pathways in colitis-associated cancer and interact with the gut microbiome and metabolites to mitigate colitis (164). MiRNAs play a vital role in regulating intestinal tight junction barrier function, with defects in this barrier implicated in IBD pathogenesis (165). Besides, exosomes also play a crucial role in mediating communication between the gut microbiota and host cells, potentially FXR signaling and metabolic homeostasis. MSC-derived exosomes mitigate colitis by altering gut metagenomics and metabolomics profiles, increasing colonic FXR expression, and restoring microbial diversity (16). In the same way, exosomal microRNA-181a also plays a crucial role in these protective effects, influencing gut microbiota composition and immune responses (166). Yet again, a recent study demonstrated that exosomes influence gut microbiota composition and promote intestinal barrier integrity, which is essential for maintaining microbiota and preventing exacerbations of IBD (167). This interaction between exosomes, gut microbiota and FXR is crucial, as it suggests exosomes as mediators of the microbiota-FXR axis in IBD. As aforementioned, exosomes also play a key role in barrier repair of IBD by modulating FXR signaling, as explored in numerous studies. FXR activation protects the intestinal barrier, reduces inflammation, and regulates immune cell function (138). Peroxiredoxin 3 deficiency exacerbates colitis by increasing exosomal miR-1260b, which disrupts the intestinal barrier and activates pro-inflammatory signaling, highlighting the interplay between mitochondrial dysfunction, exosome-mediated miRNA transfer and IBD pathogenesis, a mechanism potentially modulated by FXR, given its role in gut barrier integrity and inflammation regulation (168). Macrophage-derived exosomal miR-223 also contributes to intestinal barrier dysfunction in IBD by inhibiting TMIGD1 (169).
Furthermore, the mechanistic interaction between exosomes and FXR is characterized by bidirectional regulation, where FXR activation induces the release of anti-inflammatory exosomes, demonstrating their intertwined roles in modulating inflammation. For example, FXR activation in epithelial cells triggers the release of miRNA-enriched exosomes, creating a feedback loop that amplifies anti-inflammatory effects (10). A recent study further elucidated this interplay, demonstrating that hucMSC-Ex alleviates IBD by activating the SIRT1-FXR pathway in macrophages, leading to reduced FXR acetylation and subsequent suppression of NLRP3 inflammasome activation (17). The hucMSC-Ex administration upregulates SIRT1 in macrophages, leading to FXR deacetylation. Deacetylated FXR becomes more stable, suppressing pro-inflammatory pathways. Normally, acetylated FXR promotes NLRP3 inflammasome activation, worsening IBD inflammation. The hucMSC-Ex-induced FXR deacetylation blocks NLRP3, reducing IL-1β and IL-18. FXR regulates BA metabolism and gut immunity; its dysfunction in IBD disrupts barrier integrity. By activating SIRT1-FXR, hucMSC-Ex restores anti-inflammatory macrophages and gut homeostasis. Inhibiting SIRT1 (EX 527) reversed these benefits, confirming the pathway's role. The treatment of hucMSC-Ex in mice showed higher SIRT1/FXR, lower FXR acetylation, reduced NLRP3 activity and improved colitis symptoms. Thus, hucMSC-Ex modulates SIRT1-FXR to suppress NLRP3, offering a novel IBD therapy. In the same way, a recent study also highlighted the potential of miRNA-mediated mechanisms, such as the NEAT1/miR-34a-5p/PEA15 axis, as critical therapeutic targets for reducing IBD-associated CRC progression (170). Macrophage-derived exosomes enriched with NEAT1 promote CRC development by acting as a sponge for miR-34a-5p, which subsequently restores PEA15 expression and enhances tumor stemness. Given that FXR, a key regulator of BA metabolism and inflammation, is known to modulate miRNA expression in IBD and CRC, these findings highlight the importance of targeting such pathways to mitigate CRC progression in IBD.
Moreover, research has shown that exosome-mediated delivery enables the targeted transport of FXR modulators to inflamed tissues, enhancing therapeutic precision (171). For instance, studies revealed that exosomes can deliver miRNAs such as miR-155 and miR-146a to regulate inflammatory responses (172,173). Targeting the FXR-exosome axis offers promising therapeutic strategies, including combinatorial FXR agonist-exosome therapies and engineered exosomes for enhanced mucosal repair (98). It has been well-documented that a modified exosome approach carrying FXR-regulating miRNAs or proteins (for example, hucMSC-Ex) may accelerate mucosal healing (174). This exosome's regenerative ability, along with its anti-inflammatory effects, makes it a dual-action treatment option for IBD. Besides, the therapeutic potential of exosomes is reinforced by their capacity to target specific tissues, such as the inflamed gut. Administering exosomes can migrate to the intestinal mucosa, improving their effectiveness in delivering therapeutic agents directly to the site of inflammation (175). This targeted delivery is particularly advantageous in the treatment of IBD, as it helps reduce systemic side effects and enhance therapeutic results by focusing treatment on the affected area.
In summary, targeting the FXR-exosome axis shows promise for IBD therapy through potential strategies such as FXR agonists to enhance FXR activation and promote therapeutic exosome release, exosome-based therapies using engineered exosomes to deliver FXR-regulated miRNAs or proteins for targeted anti-inflammatory and regenerative effects, and combination therapies that integrate FXR agonists with exosome-based treatments to improve mucosal healing and reduce inflammation in IBD. While preclinical evidence is compelling, clinical trials are needed to validate these mechanisms and optimize delivery systems for human applications.
Recent advances in exosome-based therapies for IBD
Recent developments in exosome-based therapies for IBD have attracted considerable interest because of their potential to transform treatment strategies. Exosomes, tiny EVs, are key players in intercellular communication and have emerged as promising carriers for targeted drug delivery, especially in the case of IBD.
Exosome isolation and modification for targeted delivery
Isolating exosomes from different biological sources is an essential process for their therapeutic use. Methods including ultracentrifugation, size exclusion chromatography and microfluidic approaches are commonly used to extract exosomes from cell cultures, plasma, and other bodily fluids and tissues (176,177). These techniques maintain the purity and integrity of exosomes, which is crucial for their later use in therapeutic treatments. A previous study emphasized the need to refine isolation protocols to improve the yield and functionality of exosomes from MSCs, which possess immunomodulatory properties that are advantageous for treating IBD (178). Moreover, modifying the surface of exosomes has become a strategy to improve their targeting ability. Approaches such as post-insertion techniques enable the attachment of specific ligands or peptides to exosomal surfaces, promoting targeted delivery to inflamed tissues (179). The engineered exosomes can be altered to display homing peptides that guide them to the inflamed intestinal mucosa, enhancing therapeutic effectiveness while reducing off-target side effects (180). This method is fundamental in IBD, where localized therapy is key in controlling inflammation and supporting mucosal healing.
Pre-clinical and clinical trials involving exosome-based therapies in IBD
The therapeutic potential of exosomes in IBD is currently being investigated in both preclinical and clinical trials. Previous research has shown that exosomes from MSCs can notably decrease colonic inflammation in mouse models of IBD, mainly by activating macrophage-dependent pathways (181). These findings highlight the immunoregulatory abilities of exosomes derived from MSCs, which can influence inflammatory reactions and promote tissue repair (178). Recent pre-clinical studies of exosome-based therapies in IBD is included in Table II.
By contrast, clinical trials are currently exploring the effectiveness of exosome-based therapies in treating human subjects with IBD. For example, exosomes have been found to contain microRNAs and proteins that can impact the inflammatory pathways associated with IBD, providing a new potential for therapeutic intervention (182). The exosome-FXR pathway has been identified as a potential target for repairing IBD, with research suggesting that exosomes can boost FXR signaling, which helps improve intestinal barrier function and decrease inflammation (161). Also, the safety of exosome-based therapies is being thoroughly assessed in clinical trials. A previous study using MSC-derived exosomes showed no major adverse effects, emphasizing their potential as a safe treatment option for patients with IBD (178). As the field advances, the emphasis is moving toward comprehending the pharmacokinetics and biodistribution of exosomes in vivo, which is crucial for enhancing their therapeutic uses (183). Recent clinical studies of exosome-based therapies in IBD are shown in Table III.
Challenges and risks in exosome therapeutics
Exosome-based treatments offer significant potential in regenerative medicine, targeted drug delivery, and immunotherapy owing to their natural ability to facilitate intercellular signaling. However, their broad clinical use faces hurdles due to several challenges and risks such as exosome heterogeneity, pro-tumorigenic effects, immune-related side effects, and stability issues.
Exosomes exhibit significant heterogeneity in their biophysical properties, molecular composition and biological functions (184). This heterogeneity arises from variations in cellular origin, biogenesis pathways and selective cargo enrichment (185). Different types of exosomes have been discovered, each with unique protein and RNA compositions, and they influence target cells in varying ways. The variety of molecules within exosomes, such as proteins, lipids and different types of RNA, enables their multifaceted functions in cell-to-cell signaling and the development of diseases (186). In cancer, exosomes released by tumors affect the spread of cancer cells and the development of pre-metastatic environments by delivering cancer-promoting molecules (187). Understanding exosome heterogeneity is vital for developing effective exosome-based therapeutics and diagnostics.
Moreover, tumor-derived exosomes (TDEs) significantly influence cancer growth and metastasis. They aid in establishing pre-metastatic conditions by modifying stromal cells and encouraging the recruitment of macrophages that suppress immune responses (188,189). TDEs support cancer progression by aiding cell dispersal, blood vessel growth, and dormant states via communication between cells and the transfer of biological materials. They regulate every stage of tumor metastasis, including the initial mechanisms, pre-metastatic niche formation, immunosuppression and angiogenesis (190). TDEs can also suppress the immune system by affecting various tumor-associated immune cells, such as natural killer cells, DCs, T and B lymphocytes, and myeloid-derived suppressor cells (191). Comprehending the function of TDEs in cancer development is crucial for developing targeted therapies and diagnostic tools.
Interestingly, exosomes have superior biocompatibility, but they also face challenges compared with synthetic nanoparticles (192). Despite their strengths in targeting and biocompatibility, exosomes have drawbacks, including low drug-loading efficiency, scalability issues and batch variability (193). Clinical use is also hindered by stability challenges, such as the need for specific storage conditions and quick removal from the bloodstream (194). To address these limitations, researchers are exploring hybrid exosomes, combining natural and synthetic components.
Furthermore, while EVs, including exosomes, hold potential as therapeutic tools and drug delivery vehicles, they may also cause immune-related adverse effects (195). Factors influencing EV or exosome immunogenicity include surface proteins, cargo and cell source. A previous study revealed minimal toxicity and immunogenicity of HEK293T-derived EVs in mice (196). Dendritic cell-derived exosomes can stimulate immune responses and have been explored for cancer immunotherapy (197). Strategies to enhance or mitigate immune responses involve optimizing antigen loading, exosome composition, and in vivo trafficking. For mRNA delivery, exosomes offer advantages over synthetic carriers due to reduced immunogenicity (198). Despite progress, engineering exosomes for optimal delivery and minimizing immune activation remains challenging. Further investigation is essential to fully understand and control the immunogenicity of EVs in therapy. It should be noted that exosomes also present challenges in terms of long-term stability and preservation (199). These challenges include sensitivity to temperature fluctuations, proteolytic degradation and oxidative stress (200). Temperature fluctuations between freezing and thawing can promote particle aggregation and decreased potency, though cryoprotective agents such as sucrose and trehalose enhance stability (201). The aforementioned recent study shows that freeze-drying has proven effective for extended storage, with research indicating that lyophilized cow's milk exosomes maintained stability for up to 3 months at 50°C and up to 2 years when stored at 2-8°C (202). However, the effectiveness of lyophilization can vary based on the origin of the exosomes and the procedures used. Existing storage techniques typically demand extremely low temperatures, which reduces their feasibility in clinical settings. Ongoing research aims to improve methods for isolating, characterizing, and storing exosomes to address these challenges and enhance their use in clinical settings.
Conclusion and future perspective
The complex relationship between exosomes and FXR in the regulation of IBD presents a promising frontier for novel therapeutic interventions. The evidence discussed in the present review highlights exosomes as key mediators of intercellular communication, capable of modulating FXR signaling to alleviate intestinal inflammation, restore gut microbiota balance, and enhance intestinal barrier function. By serving as natural carriers of bioactive molecules, exosomes offer a unique strategy for targeted drug delivery, minimizing systemic side effects while maximizing therapeutic efficacy in IBD treatment. Moreover, the potential of exosome-based therapies extends beyond FXR regulation, opening avenues for personalized and precision medicine in managing chronic gastrointestinal diseases.
Notwithstanding the hopeful findings, several challenges must be addressed before exosome-based FXR therapies can be widely adopted in clinical settings. One of the primary limitations is the standardization of exosome isolation, purification and characterization methods. Current techniques often yield heterogeneous exosome populations, leading to variability in therapeutic outcomes. Additionally, the stability and scalability of exosome production for large-scale clinical applications remain significant hurdles. Further research is needed to refine engineering approaches that enhance exosomal targeting, cargo loading and delivery efficiency to inflamed intestinal tissues. Another critical challenge lies in the complexity of FXR signaling. While FXR activation has demonstrated protective effects against intestinal inflammation, its role is context-dependent, and excessive FXR stimulation may have unintended metabolic consequences. Understanding the precise mechanisms by which exosomes influence FXR in different pathological states of IBD is essential for optimizing therapeutic strategies. Moreover, the immunomodulatory effects of exosomes necessitate rigorous safety evaluations to ensure they do not trigger adverse immune responses or contribute to disease exacerbation. Future research should focus on conducting well-designed preclinical and clinical studies to validate the efficacy and safety of exosome-based FXR modulation in IBD. The integration of cutting-edge technologies, such as single-cell RNA sequencing and proteomics, will provide deeper insights into exosomal cargo composition and its impact on FXR signaling.
In summary, exosome-mediated FXR regulation represents a promising therapeutic avenue for IBD, offering targeted, minimally invasive and potentially long-lasting benefits. Addressing the current limitations through innovative research and technological advancements will be crucial in translating these findings into viable clinical applications. As the field continues to evolve, exosome-based therapies hold the potential to revolutionize IBD treatment, improving patient outcomes and quality of life.
Availability of data and materials
Not applicable.
Authors' contributions
PM and FM conceptualized the study. CHJ and FM conducted project administration and acquired funding. WBW performed visualization. PM prepared the original draft. BW and YRQ wrote, reviewed and edited the manuscript. All authors read and approved the final version of the manuscript. Data authentication is not applicable.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Acknowledgments
Not applicable.
Funding
The present study was supported by the Zhenjiang 2024 Plan-Basic Research Project (grant no. JC2024035), the Henan 2024 Science and Technology Development Plan (grant no. 242102310081), the open topic at the university level of Shangqiu Medical College in 2023 (grant no. KFKT23005), and the Jiangsu Provincial Medical Key Discipline Cultivation Unit (grant no. JSDW202241).
References
|
Muro P, Zhang L, Li S, Zhao Z, Jin T, Mao F and Mao Z: The emerging role of oxidative stress in inflammatory bowel disease. Front Endocrinol (Lausanne). 15:13903512024. View Article : Google Scholar : PubMed/NCBI | |
|
Al-Amrah H, Saadah OI, Mosli M, Edris S, Alhindi R and Bahieldin A: Alteration of the gut microbiome for patients with inflammatory bowel disease: A review. Appl Ecol Environ Res. 18:7379–7392. 2020. View Article : Google Scholar | |
|
Chew DCH, Khoo XH, Lee TS, Chin KY, Raja Ali RA, Muhammad Nawawi KN, Wan Ibrahim NR and Hilmi I: A systematic review on the increasing incidence of inflammatory bowel disease in southeast asia: Looking beyond the urbanization phenomenon. Inflamm Bowel Dis. 30:1566–1578. 2024. View Article : Google Scholar | |
|
Saleem H, Muhammad Jaffry SHB, Zia U, Marwat ZI, Shah N and Alamzeb J: Examining the autoimmune, genetic, environmental, and microbial aspects of the complex etiology of inflammatory bowel diseases: A comprehensive review and comparative analysis. J Women Med Dent Coll. 2:2024 View Article : Google Scholar | |
|
Bastaki SMA, Amir N, Adeghate E and Ojha S: Lycopodium mitigates oxidative stress and inflammation in the colonic mucosa of acetic Acid-induced colitis in rats. Molecules. 27:27742022. View Article : Google Scholar : PubMed/NCBI | |
|
Gao C, Zhou Y, Chen Z, Li H, Xiao Y, Hao W, Zhu Y, Vong CT, Farag MA, Wang Y and Wang S: Turmeric-derived nanovesicles as novel nanobiologics for targeted therapy of ulcerative colitis. Theranostics. 12:5596–5614. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Liao HX, Mao X, Wang L, Wang N, Ocansey DKW, Wang B and Mao F: The role of mesenchymal stem cells in attenuating inflammatory bowel disease through ubiquitination. Front Immunol. 15:14230692014. View Article : Google Scholar | |
|
Noguera-Fernández N, Candela-González J and Orenes-Piñero E: Probiotics, prebiotics, fecal microbiota transplantation, and dietary patterns in inflammatory bowel disease. Mol Nutr Food Res. 68:e24004292024. View Article : Google Scholar : PubMed/NCBI | |
|
Higashiyama M and Hokari R: New and emerging treatments for inflammatory bowel disease. Digestion. 104:74–81. 2023. View Article : Google Scholar | |
|
Zhou M, Wang D, Li X, Cao Y, Yi C, Wiredu Ocansey DK, Zhou Y and Mao F: Farnesoid-X receptor as a therapeutic target for inflammatory bowel disease and colorectal cancer. Front Pharmacol. 13:10168362022. View Article : Google Scholar : PubMed/NCBI | |
|
Li Y, Xu T, Zhao Y, Zhang H, Liu Z, Wang H, Huang C, Shu Z, Gao L, Xie R, et al: Discovery and optimization of novel nonbile acid FXR agonists as preclinical candidates for the treatment of inflammatory bowel disease. J Med Chem. 67:5642–5661. 2024. View Article : Google Scholar : PubMed/NCBI | |
|
Kim HI, Park J, Zhu Y, Wang X, Han Y and Zhang D: Recent advances in extracellular vesicles for therapeutic cargo delivery. Exp Mol Med. 56:836–849. 2024. View Article : Google Scholar : PubMed/NCBI | |
|
Li J, Zhang Y, Dong PY, Yang GM and Gurunathan S: A comprehensive review on the composition, biogenesis, purification, and multifunctional role of exosome as delivery vehicles for cancer therapy. Biomed Pharmacother. 165:1150872023. View Article : Google Scholar : PubMed/NCBI | |
|
Miao C, Wang X, Zhou W and Huang J: The emerging roles of exosomes in autoimmune diseases, with special emphasis on microRNAs in exosomes. Pharmacol Res. 169:1056802021. View Article : Google Scholar : PubMed/NCBI | |
|
Ludwig N, Whiteside TL and Reichert TE: Challenges in exosome isolation and analysis in health and disease. Int J Mol Sci. 20:46842019. View Article : Google Scholar : PubMed/NCBI | |
|
Ocansey DKW, Zhang Z, Xu X, Liu L, Amoah S, Chen X, Wang B, Zhang X and Mao F: Mesenchymal stem cell-derived exosome mitigates colitis via the modulation of the gut metagenomics-metabolomics-farnesoid X receptor axis. Biomater Sci. 10:4822–4836. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Zhou M, Pei B, Cai P, Yi C, Akanyibah FA, Lyu C and Mao F: Human umbilical cord mesenchymal stem cell-derived exosomes repair IBD by activating the SIRT1-FXR pathway in macrophages. Stem Cell Res Ther. 16:2332025. View Article : Google Scholar : PubMed/NCBI | |
|
Jella KK, Nasti TH, Li Z, Malla SR, Buchwald ZS and Khan MK: Exosomes, their biogenesis and role in inter-cellular communication, tumor microenvironment and cancer immunotherapy. Vaccines (Basel). 6:692018. View Article : Google Scholar : PubMed/NCBI | |
|
Wani S, Man Law IK and Pothoulakis C: Role and mechanisms of exosomal miRNAs in IBD pathophysiology. Am J Physiol Liver Physiol. 319:G646–G654. 2020. | |
|
van Niel G, D'Angelo G and Raposo G: Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol. 19:213–228. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Gurung S, Perocheau D, Touramanidou L and Baruteau J: The exosome journey: From biogenesis to uptake and intracellular signalling. Cell Commun Signal. 19:472021. View Article : Google Scholar : PubMed/NCBI | |
|
Matsui T, Osaki F, Hiragi S, Sakamaki Y and Fukuda M: ALIX and ceramide differentially control polarized small extracellular vesicle release from epithelial cells. EMBO Rep. 22:e514752021. View Article : Google Scholar : PubMed/NCBI | |
|
Lee KM, Seo EC, Lee JH, Kim HJ and Hwangbo C: The multifunctional protein Syntenin-1: Regulator of exosome biogenesis, cellular function, and tumor progression. Int J Mol Sci. 24:94182023. View Article : Google Scholar : PubMed/NCBI | |
|
Kim G, Zhu R, Zhang Y, Jeon H, Shirinichi F and Wang Y: Fluorescent chiral quantum dots to unveil Origin-dependent exosome uptake and cargo release. ACS Appl Bio Mater. 7:3358–3374. 2024. View Article : Google Scholar : PubMed/NCBI | |
|
Kang M, Yadav MK, Mbanefo EC, Yu CR and Egwuagu CE: IL-27-containing exosomes secreted by innate B-1a cells suppress and ameliorate uveitis. Front Immunol. 14:10711622023. View Article : Google Scholar : PubMed/NCBI | |
|
Fu W, Lei C, Liu S, Cui Y, Wang C, Qian K, Li T, Shen Y, Fan X, Lin F, et al: CAR exosomes derived from effector CAR-T cells have potent antitumour effects and low toxicity. Nat Commun. 10:43552019. View Article : Google Scholar : PubMed/NCBI | |
|
Yang H, Chen J, Liu S, Xue Y, Li Z, Wang T, Jiao L, An Q, Liu B, Wang J and Zhao H: Exosomes From IgE-stimulated mast cells aggravate asthma-mediated atherosclerosis through circRNA CDR1as-mediated endothelial cell dysfunction in mice. Arterioscler Thromb Vasc Biol. 44:e99–e115. 2024. View Article : Google Scholar : PubMed/NCBI | |
|
Liu H, Zhang X, Zhang M, Zhang S, Li J, Zhang Y, Wang Q, Cai JP, Cheng K and Wang S: Mesenchymal stem cell derived exosomes repair uterine injury by targeting transforming growth factor-β signaling. ACS Nano. 18:3509–3519. 2024. View Article : Google Scholar : PubMed/NCBI | |
|
Lin Y, Anderson JD, Rahnama LMA, Gu SV and Knowlton AA: Exosomes in disease and regeneration: Biological functions, diagnostics, and beneficial effects. Am J Physiol Circ Physiol. 319:H1162–H1180. 2020. View Article : Google Scholar | |
|
Huang KY, Upadhyay G, Ahn Y, Sakakura M, Pagan-Diaz GJ, Cho Y, Weiss AC, Huang C, Mitchell JW, Li J, et al: Neuronal innervation regulates the secretion of neurotrophic myokines and exosomes from skeletal muscle. Proc Natl Acad Sci USA. 121:e23135901212024. View Article : Google Scholar : PubMed/NCBI | |
|
Ocansey DKW, Zhang L, Wang Y, Yan Y, Qian H, Zhang X, Xu W and Mao F: Exosome-mediated effects and applications in inflammatory bowel disease. Biol Rev. 95:1287–1307. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Kourembanas S: Exosomes: Vehicles of intercellular signaling, biomarkers, and vectors of cell therapy. Annu Rev Physiol. 77:13–27. 2015. View Article : Google Scholar | |
|
Chen H, Chengalvala V, Hu H and Sun D: Tumor-derived exosomes: Nanovesicles made by cancer cells to promote cancer metastasis. Acta Pharm Sin B. 11:2136–2149. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Huber CC and Wang H: Pathogenic and therapeutic role of exosomes in neurodegenerative disorders. Neural Regen Res. 19:75–79. 2024. View Article : Google Scholar | |
|
Zhang Z, Zou Y, Song C, Cao K, Cai K, Chen S, Wu Y, Geng D, Sun G, Zhang N, et al: Advances in the study of exosomes in cardiovascular diseases. J Adv Res. 66:133–153. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Larson A, Natera-Rodriguez DE, Crane A, Larocca D, Low WC, Grande AW and Lee J: Emerging roles of exosomes in stroke therapy. Int J Mol Sci. 25:65072024. View Article : Google Scholar : PubMed/NCBI | |
|
Singh A, Behl T, Sehgal A, Singh S, Sharma N, Naqwi M, Mavi A and Singh R: Exploring the role of exosomes in rheumatoid arthritis. Inflammopharmacology. 31:119–128. 2023. View Article : Google Scholar | |
|
Gao S, Dong Y, Yan C, Yu T and Cao H: The role of exosomes and exosomal microRNA in diabetic cardiomyopathy. Front Endocrinol (Lausanne). 14:13274952024. View Article : Google Scholar : PubMed/NCBI | |
|
Hassanzadeh A, Shomali N, Kamrani A, Nasiri H, Ahmadian Heris J, Pashaiasl M, Sadeghi M, Sadeghvand S, Valedkarimi Z and Akbari M: Detailed role of mesenchymal stem cell (MSC)-derived exosome therapy in cardiac diseases. EXCLI J. 23:401–420. 2024.PubMed/NCBI | |
|
Gheitasi H, Sabbaghian M, Shekarchi AA, Mirmazhary AA and Poortahmasebi V: Exosome-mediated regulation of inflammatory pathway during respiratory viral disease. Virol J. 21:302024. View Article : Google Scholar : PubMed/NCBI | |
|
Sun Y, Zhang S, Shen Y, Lu H, Zhao X, Wang X, Wang Y, Wang T, Liu B, Yao L and Wen J: Therapeutic application of mesenchymal stem cell-derived exosomes in skin wound healing. Front Bioeng Biotechnol. 12:14287932024. View Article : Google Scholar : PubMed/NCBI | |
|
Dehghan Z, Rezaee D, Noori E, Pilehchi T, Saberi F, Taheri Z, Darya G and Mehdinejadiani S: Exosomes as modulators of embryo implantation. Mol Biol Rep. 51:2842024. View Article : Google Scholar : PubMed/NCBI | |
|
Keerthikumar S, Chisanga D, Ariyaratne D, Al Saffar H, Anand S, Zhao K, Samuel M, Pathan M, Jois M, Chilamkurti N, et al: ExoCarta: A Web-Based compendium of exosomal cargo. J Mol Biol. 428:688–692. 2016. View Article : Google Scholar : | |
|
Huang D, Chen J, Hu D, Xie F, Yang T, Li Z, Wang X, Xiao Y, Zhong J, Jiang Y, et al: Advances in biological function and clinical application of small extracellular vesicle membrane proteins. Front Oncol. 11:6759402021. View Article : Google Scholar : PubMed/NCBI | |
|
Zheng D, Huo M, Li B, Wang W, Piao H, Wang Y, Zhu Z, Li D, Wang T and Liu K: The role of exosomes and exosomal MicroRNA in cardiovascular disease. Front Cell Dev Biol. 8:161612021. View Article : Google Scholar | |
|
Wu X, Xu X, Xiang Y, Fan D, An Q, Yue G, Jin Z, Ding J, Hu Y, Du Q, et al: Exosome-mediated effects and applications in inflammatory diseases of the digestive system. Eur J Med Res. 27:1632022. View Article : Google Scholar : PubMed/NCBI | |
|
Guan Q: A comprehensive review and update on the pathogenesis of inflammatory bowel disease. J Immunol Res. 2019:72472382019. View Article : Google Scholar : PubMed/NCBI | |
|
Lv X, Gao X, Liu J, Deng Y, Nie Q, Fan X, Ye Z, Liu P and Wen J: Immune-mediated inflammatory diseases and risk of venous thromboembolism: A Mendelian randomization study. Front Immunol. 13:10427512022. View Article : Google Scholar : PubMed/NCBI | |
|
Kaplan GG and Ng SC: Globalisation of inflammatory bowel disease: Perspectives from the evolution of inflammatory bowel disease in the UK and China. Lancet Gastroenterol Hepatol. 1:307–316. 2016. View Article : Google Scholar | |
|
Arabpour M, Saghazadeh A and Rezaei N: Anti-inflammatory and M2 macrophage polarization-promoting effect of mesenchymal stem cell-derived exosomes. Int Immunopharmacol. 97:1078232021. View Article : Google Scholar : PubMed/NCBI | |
|
Eissa N, Hussein H and Ghia JE: A Gene expression analysis of M1 and M2 polarized macrophages. Methods Mol Biol. 2184:131–144. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang K, Guo J, Yan W and Xu L: Macrophage polarization in inflammatory bowel disease. Cell Commun Signal. 21:3672023. View Article : Google Scholar : PubMed/NCBI | |
|
Yang R, Liao Y, Wang L, He P, Hu Y, Yuan D, Wu Z and Sun X: Exosomes derived from M2b macrophages attenuate DSS-induced colitis. Front Immunol. 10:23462019. View Article : Google Scholar : PubMed/NCBI | |
|
Lethen I, Lechner-Grimm K, Gabel M, Knauss A, Atreya R, Neurath MF and Weigmann B: Tofacitinib affects M1-like and M2-like polarization and tissue factor expression in macrophages of healthy donors and IBD patients. Inflamm Bowel Dis. 30:1151–1163. 2024. View Article : Google Scholar | |
|
Mao F, Wu Y, Tang X, Wang J, Pan Z, Zhang P, Zhang B, Yan Y, Zhang X, Qian H and Xu W: Human umbilical cord mesenchymal stem cells alleviate inflammatory bowel disease through the regulation of 15-LOX-1 in macrophages. Biotechnol Lett. 39:929–938. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Mao F, Wu Y, Tang X, Kang J, Zhang B, Yan Y, Qian H, Zhang X and Xu W: Exosomes derived from human umbilical cord mesenchymal stem cells relieve inflammatory bowel disease in mice. Biomed Res Int. 2017:53567602017. View Article : Google Scholar : PubMed/NCBI | |
|
Xu X, Peng J, Wang N, Ocansey DKW, Zhang X and Mao F: hucMSC-Ex alleviates inflammatory bowel disease in mice by enhancing M2-type macrophage polarization via the METTL3-Slc37a2-YTHDF1 axis. Cell Biol Toxicol. 40:742024. View Article : Google Scholar : PubMed/NCBI | |
|
Wei Z, Hang S, Wiredu Ocansey DK, Zhang Z, Wang B, Zhang X and Mao F: Human umbilical cord mesenchymal stem cells derived exosome shuttling mir-129-5p attenuates inflammatory bowel disease by inhibiting ferroptosis. J Nanobiotechnology. 21:1882023. View Article : Google Scholar : PubMed/NCBI | |
|
Kowal J and Tkach M: Dendritic cell extracellular vesicles. Int Rev Cell Mol Biol. 349:213–249. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Rao Q, Ma G, Li M, Wu H, Zhang Y, Zhang C, Ma Z and Huang L: Targeted delivery of triptolide by dendritic cell-derived exosomes for colitis and rheumatoid arthritis therapy in murine models. Br J Pharmacol. 180:330–346. 2023. View Article : Google Scholar | |
|
Wang L, Yu Z, Wan S, Wu F, Chen W, Zhang B, Lin D, Liu J, Xie H, Sun X and Wu Z: Exosomes derived from dendritic cells treated with schistosoma japonicum soluble egg antigen attenuate DSS-induced colitis. Front Pharmacol. 8:6512017. View Article : Google Scholar : PubMed/NCBI | |
|
Elashiry M, Elashiry MM, Elsayed R, Rajendran M, Auersvald C, Zeitoun R, Rashid MH, Ara R, Meghil MM, Liu Y, et al: Dendritic cell derived exosomes loaded with immunoregulatory cargo reprogram local immune responses and inhibit degenerative bone disease in vivo. J Extracell Vesicles. 9:17953622020. View Article : Google Scholar : PubMed/NCBI | |
|
Barnhoorn MC, Wasser MNJM, Roelofs H, Maljaars PWJ, Molendijk I, Bonsing BA, Oosten LEM, Dijkstra G, van der Woude CJ, Roelen DL, et al: Long-term evaluation of allogeneic bone marrow-derived mesenchymal stromal cell therapy for Crohn's disease perianal fistulas. J Crohns Colitis. 14:64–70. 2020. View Article : Google Scholar | |
|
Stavely R, Robinson AM, Miller S, Boyd R, Sakkal S and Nurgali K: Human adult stem cells derived from adipose tissue and bone marrow attenuate enteric neuropathy in the guinea-pig model of acute colitis. Stem Cell Res Ther. 6:2442015. View Article : Google Scholar : PubMed/NCBI | |
|
Chen J, Xie S, Qiu D, Xie M, Wu M, Li X, Zhang X, Wu Q, Xiong Y, Wu C, et al: The NLRP3 molecule influences the therapeutic effects of mesenchymal stem cells through Glut1-mediated energy metabolic reprogramming. J Adv Res. 65:125–136. 2024. View Article : Google Scholar : | |
|
Li M, Zhao J, Cao M, Liu R, Chen G, Li S, Xie Y, Xie J, Cheng Y, Huang L, et al: Mast cells-derived MiR-223 destroys intestinal barrier function by inhibition of CLDN8 expression in intestinal epithelial cells. Biol Res. 53:122020. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang S, Xu W, Wang H, Cao M, Li M, Zhao J, Hu Y, Wang Y, Li S, Xie Y, et al: Inhibition of CREB-mediated ZO-1 and activation of NF-κB-induced IL-6 by colonic epithelial MCT4 destroys intestinal barrier function. Cell Prolif. 52:e126732019. View Article : Google Scholar | |
|
Chojnacki C, Wisniewska-Jarosinska M, Walecka-Kapica E, Klupinska G, Jaworek J and Chojnacki J: Evaluation of melatonin effectiveness in the adjuvant treatment of ulcerative colitis. J Physiol Pharmacol. 62:327–334. 2011.PubMed/NCBI | |
|
Heidari M, Pouya S, Baghaei K, Aghdaei HA, Namaki S, Zali MR and Hashemi SM: The immunomodulatory effects of adipose-derived mesenchymal stem cells and mesenchymal stem cells-conditioned medium in chronic colitis. J Cell Physiol. 233:8754–8766. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Heidari N, Abbasi-Kenarsari H, Namaki S, Baghaei K, Zali MR, Ghaffari Khaligh S and Hashemi SM: Adipose-derived mesenchymal stem cell-secreted exosome alleviates dextran sulfate sodium-induced acute colitis by Treg cell induction and inflammatory cytokine reduction. J Cell Physiol. 236:5906–5920. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Chen P, Huang S, Yu Q, Chao K, Wang Y, Zhou G, Zhuang X, Zeng Z, Chen M and Zhang S: Serum exosomal microRNA-144-3p: A promising biomarker for monitoring Crohn's disease. Gastroenterol Rep (Oxf). 10:goab0562021. View Article : Google Scholar | |
|
Gong L, Xiao J, Yi J, Xiao J, Lu F and Liu X: Immunomodulatory effect of serum exosomes from crohn disease on macrophages via Let-7b-5p/TLR4 signaling. Inflamm Bowel Dis. 28:96–108. 2022. View Article : Google Scholar | |
|
Yarani R, Shojaeian A, Palasca O, Doncheva NT, Jensen LJ, Gorodkin J and Pociot F: Differentially expressed miRNAs in ulcerative colitis and Crohn's disease. Front Immunol. 13:8657772022. View Article : Google Scholar : PubMed/NCBI | |
|
Wu F, Zhang S, Dassopoulos T, Harris ML, Bayless TM, Meltzer SJ, Brant SR and Kwon JH: Identification of microRNAs associated with ileal and colonic Crohnʼs disease. Inflamm Bowel Dis. 16:1729–1738. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Quaglio AEV, Santaella FJ, Rodrigues MAM, Sassaki LY and Di Stasi LC: MicroRNAs expression influence in ulcerative colitis and Crohn's disease: A pilot study for the identification of diagnostic biomarkers. World J Gastroenterol. 27:7801–7812. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Wong W, Lee MM, Chan BD, Kam RK, Zhang G, Lu AP and Tai WC: Proteomic profiling of dextran sulfate sodium induced acute ulcerative colitis mice serum exosomes and their immunomodulatory impact on macrophages. Proteomics. 16:1131–1145. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Clua-Ferré L, Suau R, Vañó-Segarra I, Ginés I, Serena C and Manyé J: Therapeutic potential of mesenchymal stem cellderived extracellular vesicles: A focus on inflammatory bowel disease. Clin Transl Med. 14:e700752024. View Article : Google Scholar | |
|
Zheng X, Chen F, Zhang Q, Liu Y, You P, Sun S, Lin J and Chen N: Salivary exosomal PSMA7: A promising biomarker of inflammatory bowel disease. Protein Cell. 8:686–695. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Zhou W and Anakk S: Enterohepatic and non-canonical roles of farnesoid X receptor in controlling lipid and glucose metabolism. Mol Cell Endocrinol. 549:1116162022. View Article : Google Scholar : PubMed/NCBI | |
|
Ramos Pittol JM, Milona A, Morris I, Willemsen ECL, van der Veen SW, Kalkhoven E and van Mil SWC: FXR isoforms control different metabolic functions in liver cells via binding to specific DNA motifs. Gastroenterology. 159:1853–1865.e10. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Jiang L, Zhang H, Xiao D, Wei H and Chen Y: Farnesoid X receptor (FXR): Structures and ligands. Comput Struct Biotechnol J. 19:2148–2159. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Vaquero J, Monte MJ, Dominguez M, Muntané J and Marin JJG: Differential activation of the human farnesoid X receptor depends on the pattern of expressed isoforms and the bile acid pool composition. Biochem Pharmacol. 86:926–939. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Tian SY, Chen SM, Pan CX and Li Y: FXR: Structures, biology, and drug development for NASH and fibrosis diseases. Acta Pharmacol Sin. 43:1120–1132. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Stojancevic M, Stankov K and Mikov M: The impact of Farnesoid X receptor activation on intestinal permeability in inflammatory bowel disease. Can J Gastroenterol. 26:631–637. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Gioiello A, Rosatelli E and Cerra B: Patented farnesoid X receptor modulators: A review (2019-present). Expert Opin Ther Pat. 34:547–564. 2024. View Article : Google Scholar : PubMed/NCBI | |
|
Ocvirk S and O'Keefe SJD: Dietary fat, bile acid metabolism and colorectal cancer. Semin Cancer Biol. 73:347–355. 2021. View Article : Google Scholar | |
|
Stofan M and Guo GL: Bile Acids and FXR: Novel targets for liver diseases. Front Med (Lausanne). 7:5442020. View Article : Google Scholar : PubMed/NCBI | |
|
Xiang J, Zhang Z, Xie H, Zhang C, Bai Y, Cao H, Che Q, Guo J and Su Z: Effect of different bile acids on the intestine through enterohepatic circulation based on FXR. Gut Microbes. 13:19490952021. View Article : Google Scholar : PubMed/NCBI | |
|
Chiang JYL and Ferrell JM: Up to date on cholesterol 7 alpha-hydroxylase (CYP7A1) in bile acid synthesis. Liver Res. 4:47–63. 2020. View Article : Google Scholar | |
|
Fiorucci S, Baldoni M, Ricci P, Zampella A, Distrutti E and Biagioli M: Bile acid-activated receptors and the regulation of macrophages function in metabolic disorders. Curr Opin Pharmacol. 53:45–54. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Cui JY, Aleksunes LM, Tanaka Y, Fu ZD, Guo Y, Guo GL, Guo Y, Guo GL, Lu H, Zhong XB and Klaassen CD: Bile acids via FXR initiate the expression of major transporters involved in the enterohepatic circulation of bile acids in newborn mice. Am J Physiol Liver Physiol. 302:G979–G996. 2012. | |
|
Fuchs CD, Krivanec S, Steinacher D, Mlitz V, Wahlström A, Stahlman M, Claudel T, Scharnagl H, Stojakovic T, Marschall HU and Trauner M: Absence of Bsep/Abcb11 attenuates MCD dietinduced hepatic steatosis but aggravates inflammation in mice. Liver Int. 40:1366–1377. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Fuchs CD and Trauner M: Role of bile acids and their receptors in gastrointestinal and hepatic pathophysiology. Nat Rev Gastroenterol Hepatol. 19:432–450. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Jiang M, Li F, Liu Y, Gu Z, Zhang L, Lee J, He L, Vatsalya V, Zhang HG, Deng Z, et al: Probiotic-derived nanoparticles inhibit ALD through intestinal miR194 suppression and subsequent FXR activation. Hepatology. 77:1164–1180. 2023. View Article : Google Scholar | |
|
Fleishman JS and Kumar S: Bile acid metabolism and signaling in health and disease: Molecular mechanisms and therapeutic targets. Signal Transduct Target Ther. 9:972024. View Article : Google Scholar : PubMed/NCBI | |
|
Panzitt K and Wagner M: FXR in liver physiology: Multiple faces to regulate liver metabolism. Biochim Biophys Acta Mol Basis Dis. 1867:1661332021. View Article : Google Scholar : PubMed/NCBI | |
|
Liu Q, Yang M, Fu X, Liu R, Sun C, Pan H, Wong CW and Guan M: Activation of farnesoid X receptor promotes triglycerides lowering by suppressing phospholipase A2 G12B expression. Mol Cell Endocrinol. 436:93–101. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Han C: Update on FXR Biology: Promising therapeutic Target? Int J Mol Sci. 19:20692018. View Article : Google Scholar : PubMed/NCBI | |
|
Clifford BL, Sedgeman LR, Williams KJ, Morand P, Cheng A, Jarrett KE, Chan AP, Brearley-Sholto MC, Wahlström A, Ashby JW, et al: FXR activation protects against NAFLD via bile-acid-dependent reductions in lipid absorption. Cell Metab. 33:1671–1684.e4. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Guo J, Huang S, Yi Q, Liu N, Cui T, Duan S, Chen J, Li J, Li J, Wang L, et al: Hepatic Clstn3 ameliorates lipid metabolism disorders in high Fat Diet-Induced NAFLD through activation of FXR. ACS Omega. 8:26158–26169. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Xu H, Fang F, Wu K, Song J, Li Y, Lu X, Liu J, Zhou L, Yu W, Yu F and Gao J: Gut microbiota-bile acid crosstalk regulates murine lipid metabolism via the intestinal FXR-FGF19 axis in diet-induced humanized dyslipidemia. Microbiome. 11:2622023. View Article : Google Scholar : PubMed/NCBI | |
|
Sonne DP: Mechanisms in endocrinology: FXR signalling: A novel target in metabolic diseases. Eur J Endocrinol. 184:R193–R205. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Hou Y, Fan W, Yang W, Samdani AQ, Jackson AO and Qu S: Farnesoid X receptor: An important factor in blood glucose regulation. Clin Chim Acta. 495:29–34. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Sinal CJ, Tohkin M, Miyata M, Ward JM, Lambert G and Gonzalez FJ: Targeted disruption of the nuclear receptor FXR/BAR impairs bile acid and lipid homeostasis. Cell. 102:731–744. 2000. View Article : Google Scholar : PubMed/NCBI | |
|
Lambert G, Amar MJA, Guo G, Brewer HB, Gonzalez FJ and Sinal CJ: The Farnesoid X-receptor is an essential regulator of cholesterol homeostasis. J Biol Chem. 278:2563–2570. 2003. View Article : Google Scholar | |
|
Lee Y, Kim BR, Kang GH, Lee GJ, Park YJ, Kim H, Jang HC and Choi SH: The effects of PPAR agonists on atherosclerosis and nonalcoholic fatty liver disease in ApoE-/-FXR-/-mice. Endocrinol Metab (Seoul). 36:1243–1253. 2021. View Article : Google Scholar | |
|
Zhang Y, Lee FY, Barrera G, Lee H, Vales C, Gonzalez FJ, Willson TM and Edwards PA: Activation of the nuclear receptor FXR improves hyperglycemia and hyperlipidemia in diabetic mice. Proc Natl Acad Sci. 103:1006–1011. 2006. View Article : Google Scholar : PubMed/NCBI | |
|
Stayrook KR, Bramlett KS, Savkur RS, Ficorilli J, Cook T, Christe ME, Michael LF and Burris TP: Regulation of carbohydrate metabolism by the farnesoid X receptor. Endocrinology. 146:984–991. 2005. View Article : Google Scholar | |
|
Zhao T, Wang J, He A, Wang S, Chen Y, Lu J, Lv J, Li S, Wang J, Qian M, et al: Mebhydrolin ameliorates glucose homeostasis in type 2 diabetic mice by functioning as a selective FXR antagonist. Metabolism. 119:1547712021. View Article : Google Scholar : PubMed/NCBI | |
|
Dehondt H, Marino A, Butruille L, Mogilenko DA, Nzoussi Loubota AC, Chávez-Talavera O, Dorchies E, Vallez E, Haas J, Derudas B, et al: Adipocyte-specific FXR-deficiency protects adipose tissue from oxidative stress and insulin resistance and improves glucose homeostasis. Mol Metab. 69:1016862023. View Article : Google Scholar : PubMed/NCBI | |
|
Ding L, Yang Q, Zhang E, Wang Y, Sun S, Yang Y, Tian T, Ju Z, Jiang L, Wang X, et al: Notoginsenoside Ft1 acts as a TGR5 agonist but FXR antagonist to alleviate high fat diet-induced obesity and insulin resistance in mice. Acta Pharm Sin B. 11:1541–1554. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Pathak P, Xie C, Nichols RG, Ferrell JM, Boehme S, Krausz KW, Patterson AD, Gonzalez FJ and Chiang JYL: Intestine farnesoid X receptor agonist and the gut microbiota activate G-protein bile acid receptor-1 signaling to improve metabolism. Hepatology. 68:1574–1588. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Shi T, Malik A, Yang vom Hofe A, Matuschek L, Mullen M, Lages CS, Kudira R, Singh R, Zhang W, Setchell KDR, et al: Farnesoid X receptor antagonizes macrophage-dependent licensing of effector T lymphocytes and progression of sclerosing cholangitis. Sci Transl Med. 14:eabi43542022. View Article : Google Scholar : PubMed/NCBI | |
|
Fuchs CD, Sroda N, Scharnagl H, Gupta R, Minto W, Stojakovic T, Liles JT, Budas G, Hollenback D and Trauner M: Non-steroidal FXR agonist cilofexor improves cholestatic liver injury in the Mdr2-/-mouse model of sclerosing cholangitis. JHEP Rep. 5:1008742023. View Article : Google Scholar | |
|
Fu T, Li Y, Oh TG, Cayabyab F, He N, Tang Q, Coulter S, Truitt M, Medina P, He M, et al: FXR mediates ILC-intrinsic responses to intestinal inflammation. Proc Natl Acad Sci. 119:e22130411192022. View Article : Google Scholar : PubMed/NCBI | |
|
Dong X, Qi M, Cai C, Zhu Y, Li Y, Coulter S, Sun F, Liddle C, Uboha NV and Halberg R: Farnesoid X receptor mediates macrophage-intrinsic responses to suppress colitis-induced colon cancer progression. JCI Insight. 9:e1704282024. View Article : Google Scholar : PubMed/NCBI | |
|
Gadaleta RM, Oldenburg B, Willemsen ECL, Spit M, Murzilli S, Salvatore L, Klomp LW, Siersema PD, van Erpecum KJ and van Mil SW: Activation of bile salt nuclear receptor FXR is repressed by pro-inflammatory cytokines activating NF-κB signaling in the intestine. Biochim Biophys Acta. 1812:851–858. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
Wu Q, Sun L, Hu X, Wang X, Xu F, Chen B, Liang X, Xia J, Wang P, Aibara D, et al: Suppressing the intestinal farnesoid X receptor/sphingomyelin phosphodiesterase 3 axis decreases atherosclerosis. J Clin Invest. 131:e1428652021. View Article : Google Scholar : PubMed/NCBI | |
|
McDowell C, Farooq U and Haseeb M: Inflammatory Bowel Disease StatPearls. 2024, Available from: http://www.ncbi.nlm.nih.gov/pubmed/30137275. | |
|
Little RD, Jayawardana T, Koentgen S, Zhang F, Connor SJ, Boussioutas A, Ward MG, Gibson PR, Sparrow MP and Hold GL: Pathogenesis and precision medicine for predicting response in inflammatory bowel disease: Advances and future directions. eGastroenterology. 2:e1000062024. View Article : Google Scholar | |
|
Ding L, Yang L, Wang Z and Huang W: Bile acid nuclear receptor FXR and digestive system diseases. Acta Pharm Sin B. 5:135–144. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Fiorucci S, Zampella A, Ricci P, Distrutti E and Biagioli M: Immunomodulatory functions of FXR. Mol Cell Endocrinol. 551:1116502022. View Article : Google Scholar : PubMed/NCBI | |
|
Fu T and Dong X: Abstract 3442: FXR mediates macrophage intrinsic responses to suppress colon cancer progression. Cancer Res. 83(7_Suppl): S34422023. View Article : Google Scholar | |
|
Zhang L, Xie C, Nichols RG, Chan SHJ, Jiang C, Hao R, Smith PB, Cai J, Simons MN, Hatzakis E, et al: Farnesoid X receptor signaling shapes the gut microbiota and controls hepatic lipid metabolism. mSystems. 1:e00070–16. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Jena PK, Sheng L, Liu HX, Kalanetra KM, Mirsoian A, Murphy WJ, French SW, Krishnan VV, Mills DA and Wan YY: Western Diet-induced dysbiosis in farnesoid X receptor knockout mice causes persistent hepatic inflammation after antibiotic treatment. Am J Pathol. 187:1800–1813. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Xu M, Shen Y, Cen M, Zhu Y, Cheng F, Tang L, Zheng X, Kim JJ, Dai N and Hu W: Modulation of the gut Microbiota-farnesoid X receptor axis improves deoxycholic Acid-induced intestinal inflammation in mice. J Crohns Colitis. 15:1197–1210. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Arifuzzaman M, Won TH, Yano H, Uddin J, Emanuel ER, Hu E, Zhang W, Li TT, Jin WB, Grier A, et al: Dietary fiber is a critical determinant of pathologic ILC2 responses and intestinal inflammation. J Exp Med. 221:e202321482024. View Article : Google Scholar : PubMed/NCBI | |
|
Zhao D, Cai C, Chen Q, Jin S, Yang B and Li N: High-fat diet promotes DSS-induced ulcerative colitis by downregulated FXR expression through the TGFB pathway. Biomed Res Int. 2020:35161282020. View Article : Google Scholar : PubMed/NCBI | |
|
Vavassori P, Mencarelli A, Renga B, Distrutti E and Fiorucci S: The bile acid receptor FXR is a modulator of intestinal innate immunity. J Immunol. 183:6251–6261. 2009. View Article : Google Scholar : PubMed/NCBI | |
|
Raybould HE: Gut microbiota, epithelial function and derangements in obesity. J Physiol. 590:441–446. 2012. View Article : Google Scholar : | |
|
Gadaleta RM, van Erpecum KJ, Oldenburg B, Willemsen ECL, Renooij W, Murzilli S, Klomp LW, Siersema PD, Schipper ME, Danese S, et al: Farnesoid X receptor activation inhibits inflammation and preserves the intestinal barrier in inflammatory bowel disease. Gut. 60:463–472. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
Curley C, Lajczak-McGinley N, Tambuwala M, Adorini L, Fallon C, Smyth J and Keely S: Farnesoid X receptor activation attenuates intestinal inflammation and preserves epithelial barrier function in vivo and in vitro. Physiology. 38:2023. View Article : Google Scholar | |
|
Abraham BP, Ahmed T and Ali T: Inflammatory bowel disease: Pathophysiology and current therapeutic approaches. Handb Exp Pharmacol. 239:115–146. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Liu HM, Liao JF and Lee TY: Farnesoid X receptor agonist GW4064 ameliorates lipopolysaccharide-induced ileocolitis through TLR4/MyD88 pathway related mitochondrial dysfunction in mice. Biochem Biophys Res Commun. 490:841–848. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Liu HM, Chang ZY, Yang CW, Chang HH and Lee TY: Farnesoid X receptor agonist GW4064 protects lipopolysaccharide-induced intestinal epithelial barrier function and colorectal tumorigenesis signaling through the αKlotho/βKlotho/FGFs pathways in mice. Int J Mol Sci. 24:169322023. View Article : Google Scholar | |
|
Ceulemans LJ, Verbeke L, Decuypere JP, Farré R, De Hertogh G, Lenaerts K, Jochmans I, Monbaliu D, Nevens F, Tack J, et al: Farnesoid X receptor activation attenuates intestinal ischemia reperfusion injury in rats. PLoS One. 12:e01693312017. View Article : Google Scholar : PubMed/NCBI | |
|
Xu Y, Li D, Wu J, Zhang M, Shao X, Xu L, Tang L, Zhu M, Ni Z, Zhang M, et al: Farnesoid X receptor promotes renal ischaemiareperfusion injury by inducing tubular epithelial cell apoptosis. Cell Prolif. 54:e130052021. View Article : Google Scholar | |
|
Anderson KM and Gayer CP: The pathophysiology of farnesoid X receptor (FXR) in the GI Tract: Inflammation, barrier function and innate immunity. Cells. 10:32062021. View Article : Google Scholar : PubMed/NCBI | |
|
O'Guinn ML, Handler DA, Hsieh JJ, Mallicote MU, Feliciano K and Gayer CP: FXR deletion attenuates intestinal barrier dysfunction in murine acute intestinal inflammation. Am J Physiol Liver Physiol. 327:G175–G187. 2024. | |
|
Tang K, Kong D, Peng Y, Guo J, Zhong Y, Yu H, Mai Z, Chen Y, Chen Y, Cui T, et al: Ginsenoside Rc attenuates DSS-induced ulcerative colitis, intestinal inflammatory, and barrier function by activating the farnesoid X receptor. Front Pharmacol. 13:10004442022. View Article : Google Scholar : PubMed/NCBI | |
|
Safari F, Sharifi M, Talebi A, Mehranfard N and Ghasemi M: Alleviation of cholestatic liver injury and intestinal permeability by lubiprostone treatment in bile duct ligated rats: Role of intestinal FXR and tight junction proteins claudin-1, claudin-2, and occludin. Naunyn Schmiedebergs Arch Pharmacol. 396:2009–2022. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Comito D and Romano C: Dysbiosis in the pathogenesis of pediatric inflammatory bowel diseases. Int J Inflam. 2012:6871432012.PubMed/NCBI | |
|
Caenepeel C, Falony G, Machiels K, Verstockt B, Goncalves PJ, Ferrante M, Sabino J, Raes J, Vieira-Silva S and Vermeire S: Dysbiosis and associated stool features improve prediction of response to biological therapy in inflammatory bowel disease. Gastroenterology. 166:483–495 | |
|
Lan J, Zhang Y, Jin C, Chen H, Su Z, Wu J, Ma N, Zhang X, Lu Y, Chen Y, et al: Gut dysbiosis drives inflammatory bowel disease through the CCL4L2-VSIR axis in glycogen storage disease. Adv Sci (Weinh). 11:e23094712024. View Article : Google Scholar : PubMed/NCBI | |
|
Cao Z, Fan D, Sun Y, Huang Z, Li Y, Su R, Zhang F, Li Q, Yang H, Zhang F, et al: The gut ileal mucosal virome is disturbed in patients with Crohn's disease and exacerbates intestinal inflammation in mice. Nat Commun. 15:16382024. View Article : Google Scholar : PubMed/NCBI | |
|
Collins SL, Stine JG, Bisanz JE, Okafor CD and Patterson AD: Bile acids and the gut microbiota: Metabolic interactions and impacts on disease. Nat Rev Microbiol. 21:236–247. 2023. View Article : Google Scholar | |
|
Tian Y, Gui W, Koo I, Smith PB, Allman EL, Nichols RG, Rimal B, Cai J, Liu Q and Patterson AD: The microbiome modulating activity of bile acids. Gut Microbes. 11:979–996. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Dong LN, Wang M, Guo J and Wang JP: Role of intestinal microbiota and metabolites in inflammatory bowel disease. Chin Med J (Engl). 132:1610–1614. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Das P, Marcišauskas S, Ji B and Nielsen J: Metagenomic analysis of bile salt biotransformation in the human gut microbiome. BMC Genomics. 20:5172019. View Article : Google Scholar : PubMed/NCBI | |
|
Fitzpatrick LR and Jenabzadeh P: IBD and bile acid absorption: Focus on Pre-clinical and clinical observations. Front Physiol. 11:5642020. View Article : Google Scholar : PubMed/NCBI | |
|
Ceulemans LJ, Canovai E, Verbeke L, Pirenne J and Farré R: The expanding role of the bile acid receptor farnesoid X in the intestine and its potential clinical implications. Acta Chir Belg. 116:156–163. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Biet F, Locht C and Kremer L: Immunoregulatory functions of interleukin 18 and its role in defense against bacterial pathogens. J Mol Med. 80:147–162. 2002. View Article : Google Scholar : PubMed/NCBI | |
|
Ding JW, Andersson R, Soltesz V, Willén R and Bengmark S: The role of bile and bile acids in bacterial translocation in obstructive jaundice in rats. Eur Surg Res. 25:11–19. 1993. View Article : Google Scholar : PubMed/NCBI | |
|
Lorenzo-Zúñiga V, Bartolí R, Planas R, Hofmann AF, Viñado B, Hagey LR, Hernández JM, Mañé J, Alvarez MA, Ausina V, et al: Oral bile acids reduce bacterial overgrowth, bacterial translocation, and endotoxemia in cirrhotic rats. Hepatology. 37:551–557. 2003. View Article : Google Scholar : PubMed/NCBI | |
|
Xu X, Ocansey DKW, Hang S, Wang B, Amoah S, Yi C, Zhang X, Liu L and Mao F: The gut metagenomics and metabolomics signature in patients with inflammatory bowel disease. Gut Pathog. 14:262022. View Article : Google Scholar : PubMed/NCBI | |
|
Hua Y, Jia Y, Zhang X, Yuan Z, Ji P, Hu J and Wei YM: Baitouweng tang ameliorates DSS-induced ulcerative colitis through the regulation of the gut microbiota and bile acids via pathways involving FXR and TGR5. Biomed Pharmacother. 137:1113202021. View Article : Google Scholar : PubMed/NCBI | |
|
Shen J, Qi Q, Han D, Lu Y, Huang R, Zhu Y, Zhang LS, Qin XD, Zhang F, Wu HG and Liu HR: Moxibustion improves experimental colitis in rats with Crohn's disease by regulating bile acid enterohepatic circulation and intestinal farnesoid X receptor. J Integr Med. 21:194–204. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Wilson A, Almousa A, Teft WA and Kim RB: Attenuation of bile acid-mediated FXR and PXR activation in patients with Crohn's disease. Sci Rep. 10:18662020. View Article : Google Scholar : PubMed/NCBI | |
|
Chen L, Jiao T, Liu W, Luo Y, Wang J, Guo X, Tong X, Lin Z, Sun C, Wang K, et al: Hepatic cytochrome P450 8B1 and cholic acid potentiate intestinal epithelial injury in colitis by suppressing intestinal stem cell renewal. Cell Stem Cell. 29:1366–1381.e9. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Fiorucci S, Carino A, Baldoni M, Santucci L, Costanzi E, Graziosi L, Distrutti E and Biagioli M: Bile acid signaling in inflammatory bowel diseases. Dig Dis Sci. 66:674–693. 2021. View Article : Google Scholar : | |
|
Zhang H, Wang L, Li C, Yu Y, Yi Y, Wang J and Chen D: Exosome-induced regulation in inflammatory bowel disease. Front Immunol. 10:14642019. View Article : Google Scholar : PubMed/NCBI | |
|
Ma F, Zhang S, Akanyibah FA, Zhang W, Chen K, Ocansey DKW, Lyu C and Mao F: Exosome-mediated macrophage regulation for inflammatory bowel disease repair: A potential target of gut inflammation. Am J Transl Res. 15:6970–6987. 2023. | |
|
Lin S, Wang S, Wang P, Tang C, Wang Z, Chen L, Luo G, Chen H, Liu Y, Feng B, et al: Bile acids and their receptors in regulation of gut health and diseases. Prog Lipid Res. 89:1012102023. View Article : Google Scholar | |
|
Li Y, Li H, Cui M, Zhou Y and Zhang M and Zhang M: Interaction of exosomal MicroRNA and oxidative stress in the pathogenesis of Colitis-associated cancer. Front Biosci (Landmark Ed). 29:2762024. View Article : Google Scholar : PubMed/NCBI | |
|
Al-Sadi R, Engers J and Abdulqadir R: Talk about micromanaging! Role of microRNAs in intestinal barrier function. Am J Physiol Liver Physiol. 319:G170–G14. 2020. | |
|
Gu L, Ren F, Fang X, Yuan L, Liu G and Wang S: Exosomal MicroRNA-181a derived from mesenchymal stem cells improves gut microbiota composition, barrier function, and inflammatory status in an experimental colitis model. Front Med (Lausanne). 8:6606142021. View Article : Google Scholar : PubMed/NCBI | |
|
Gao F, Wu S, Zhang K, Xu Z, Zhang X, Zhu Z and Quan F: Goat milk exosomes ameliorate ulcerative colitis in mice through modulation of the intestinal barrier, gut microbiota, and metabolites. J Agric Food Chem. 72:23196–23210. 2024. View Article : Google Scholar : PubMed/NCBI | |
|
Jin J, Jung M, Sonn SK, Seo S, Suh J, Kweon HY, Moon SH, Jo H, Yoon NH and Oh GT: Peroxiredoxin 3 deficiency exacerbates DSS-induced acute colitis via exosomal miR-1260b-Mediated barrier disruption and proinflammatory signaling. Antioxid Redox Signal. 42:133–149. 2025. View Article : Google Scholar | |
|
Chang X, Song Y, Xia T, He Z, Zhao S, Wang ZJ, Gu L, Li ZS, Xu C, Wang SL and Bai Y: Macrophage-derived exosomes promote intestinal mucosal barrier dysfunction in inflammatory bowel disease by regulating TMIGD1 via mircroRNA-223. Int Immunopharmacol. 121:1104472023. View Article : Google Scholar : PubMed/NCBI | |
|
Liu F, Ai F, Tang A, Yang Z, Li Z and Liu S: Macrophage-derived exosomes promoted the development and stemness of inflammatory bowel Disease-related colorectal cancer via nuclear paraspeckle assembly transcript 1-Mediated miRNA-34a-5p/phosphoprotein enriched in astrocytes 15 Axis. Inflamm Bowel Dis. 31:524–538. 2025. View Article : Google Scholar | |
|
Liang Y, Duan L, Lu J and Xia J: Engineering exosomes for targeted drug delivery. Theranostics. 11:3183–3195. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Alexander M and O'Connell R: Exosomal miRNAs regulate inflammatory responses (IRM11P.624). J Immunol. 194(1_Suppl): S132.32015. View Article : Google Scholar | |
|
Alexander M, Hu R, Runtsch MC, Kagele DA, Mosbruger TL, Tolmachova T, Seabra MC, Round JL, Ward DM and O'Connell RM: Exosome-delivered microRNAs modulate the inflammatory response to endotoxin. Nat Commun. 6:73212015. View Article : Google Scholar : PubMed/NCBI | |
|
Liang X, Li C, Song J, Liu A, Wang C, Wang W, Kang Y, Sun D, Qian J and Zhang X: HucMSC-exo promote mucosal healing in experimental colitis by accelerating intestinal stem cells and epithelium regeneration via wnt signaling pathway. Int J Nanomedicine. 18:2799–2818. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Yu H, Yang X, Xiao X, Xu M, Yang Y, Xue C, Li X, Wang S and Zhao RC: Human adipose mesenchymal stem Cell-derived exosomes protect mice from DSS-Induced inflammatory bowel disease by promoting Intestinal-stem-cell and epithelial regeneration. Aging Dis. 12:14232021. View Article : Google Scholar : PubMed/NCBI | |
|
Al-Madhagi H: The landscape of exosomes biogenesis to clinical applications. Int J Nanomedicine. 19:3657–3675. 2024. View Article : Google Scholar : PubMed/NCBI | |
|
Kurian TK, Banik S, Gopal D, Chakrabarti S and Mazumder N: Elucidating methods for isolation and quantification of exosomes: A review. Mol Biotechnol. 63:249–266. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Lopez-Santalla M and Garin MI: Improving the efficacy of mesenchymal Stem/Stromal-based therapy for treatment of inflammatory bowel diseases. Biomedicines. 9:15072021. View Article : Google Scholar : PubMed/NCBI | |
|
Jang H, Kim H, Kim EH, Han G, Jang Y, Kim Y, Lee JW, Shin SC, Kim EE, Kim SH and Yang Y: Post-insertion technique to introduce targeting moieties in milk exosomes for targeted drug delivery. Biomater Res. 27:1242023. View Article : Google Scholar : PubMed/NCBI | |
|
Choi H, Choi Y, Yim HY, Mirzaaghasi A, Yoo JK and Choi C: Biodistribution of exosomes and engineering strategies for targeted delivery of therapeutic exosomes. Tissue Eng Regen Med. 18:499–511. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Liu H, Liang Z, Wang F, Zhou C, Zheng X, Hu T, He X, Wu X and Lan P: Exosomes from mesenchymal stromal cells reduce murine colonic inflammation via a macrophage-dependent mechanism. JCI Insight. 4:e1312732019. View Article : Google Scholar : PubMed/NCBI | |
|
Baghaei K, Tokhanbigli S, Asadzadeh H, Nmaki S, Reza Zali M and Hashemi SM: Exosomes as a novel cell-free therapeutic approach in gastrointestinal diseases. J Cell Physiol. 234:9910–9926. 2019. View Article : Google Scholar | |
|
Kim DH, Kothandan VK, Kim HW, Kim KS, Kim JY, Cho HJ, Lee YK, Lee DE and Hwang SR: Noninvasive assessment of exosome pharmacokinetics in vivo: A review. Pharmaceutics. 11:6492019. View Article : Google Scholar : PubMed/NCBI | |
|
Willms E, Johansson HJ, Mäger I, Lee Y, Blomberg KEM, Sadik M, Alaarg A, Smith CI, Lehtiö J, El Andaloussi S, et al: Cells release subpopulations of exosomes with distinct molecular and biological properties. Sci Rep. 6:225192016. View Article : Google Scholar : PubMed/NCBI | |
|
Shukla S, Currim F and Singh R: Do different exosome biogenesis pathways and selective cargo enrichment contribute to exosomal heterogeneity? Biol Cell. 115:e22001162023. View Article : Google Scholar : PubMed/NCBI | |
|
Ferguson SW and Nguyen J: Exosomes as therapeutics: The implications of molecular composition and exosomal heterogeneity. J Control Release. 228:179–190. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Willms E, Cabañas C, Mäger I, Wood MJA and Vader P: Extracellular vesicle heterogeneity: Subpopulations, isolation techniques, and diverse functions in cancer progression. Front Immunol. 9:7382018. View Article : Google Scholar : PubMed/NCBI | |
|
Morrissey SM, Zhang F, Ding C, Montoya-Durango DE, Hu X, Yang C, Wang Z, Yuan F, Fox M, Zhang HG, et al: Tumor-derived exosomes drive immunosuppressive macrophages in a pre-metastatic niche through glycolytic dominant metabolic reprogramming. Cell Metab. 33:2040–2058.e10. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Bhatia R, Chang J, Munoz JL and Walker ND: Forging new therapeutic targets: Efforts of tumor derived exosomes to prepare the Pre-metastatic niche for cancer cell dissemination and dormancy. Biomedicines. 11:16142023. View Article : Google Scholar : PubMed/NCBI | |
|
Bai S, Wei Y, Liu R, Xu R, Xiang L and Du J: Role of tumour-derived exosomes in metastasis. Biomed Pharmacother. 147:1126572022. View Article : Google Scholar : PubMed/NCBI | |
|
Truong NC, Huynh TN, Pham KD and Pham P: The role of tumor-derived exosomes in tumor immune escape: A concise review. Biomed Res Ther. 7:4132–4137. 2020. View Article : Google Scholar | |
|
Witwer KW and Wolfram J: Extracellular vesicles versus synthetic nanoparticles for drug delivery. Nat Rev Mater. 6:103–106. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Kimiz-Gebologlu I and Oncel SS: Exosomes: Large-scale production, isolation, drug loading efficiency, and biodistribution and uptake. J Control Release. 347:533–543. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Palakurthi SS, Shah B, Kapre S, Charbe N, Immanuel S, Pasham S, Thalla M, Jain A and Palakurthi S: A comprehensive review of challenges and advances in exosome-based drug delivery systems. Nanoscale Adv. 6:5803–5826. 2024. View Article : Google Scholar : PubMed/NCBI | |
|
Xia Y, Zhang J, Liu G and Wolfram J: Immunogenicity of extracellular vesicles. Adv Mater. 36:e24031992024. View Article : Google Scholar : PubMed/NCBI | |
|
Zhu X, Badawi M, Pomeroy S, Sutaria DS, Xie Z, Baek A, Jiang J, Elgamal OA, Mo X, Perle K, et al: Comprehensive toxicity and immunogenicity studies reveal minimal effects in mice following sustained dosing of extracellular vesicles derived from HEK293T cells. J Extracell Vesicles. 6:13247302017. View Article : Google Scholar : PubMed/NCBI | |
|
Gehrmann U, Näslund TI, Hiltbrunner S, Larssen P and Gabrielsson S: Harnessing the exosome-induced immune response for cancer immunotherapy. Semin Cancer Biol. 28:58–67. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Aslan C, Kiaie SH, Zolbanin NM, Lotfinejad P, Ramezani R, Kashanchi F and Jafari R: Exosomes for mRNA delivery: A novel biotherapeutic strategy with hurdles and hope. BMC Biotechnol. 21:202021. View Article : Google Scholar : PubMed/NCBI | |
|
Wang J, Chen D and Ho EA: Challenges in the development and establishment of exosome-based drug delivery systems. J Control Release. 329:894–906. 2021. View Article : Google Scholar | |
|
Zhang Y, Bi J, Huang J, Tang Y, Du S and Li P: Exosome: A review of its classification, isolation techniques, storage, diagnostic and targeted therapy applications. Int J Nanomedicine. 15:6917–6934. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Ball R, Bajaj P and Whitehead K: Achieving long-term stability of lipid nanoparticles: Examining the effect of pH, temperature, and lyophilization. Int J Nanomedicine. 12:305–315. 2016. View Article : Google Scholar | |
|
Lu L, Han C, Wang M, Du H, Chen N, Gao M, Wang N, Qi D, Bai W, Yin J, et al: Assessment of bovine milk exosome preparation and lyophilized powder stability. J Extracell Biol. 3:e700092024. View Article : Google Scholar : PubMed/NCBI | |
|
Kheradmand F, Yasaman Rahimzadeh SF, Esmaeili SA, Negah SS, Farkhad NK, Nazari SE, Nazari SE, Hajinejad M, Khodadoust MA, Fadaee A, et al: Efficacy of umbilical cord-derived mesenchymal stem cells and exosomes in conjunction with standard IBD drug on immune responses in an IBD mouse model. Stem Cell Res Ther. 16:52025. View Article : Google Scholar : PubMed/NCBI | |
|
Yang S, Liang X, Song J, Li C, Liu A, Luo Y, Ma H, Tan Y and Zhang X: A novel therapeutic approach for inflammatory bowel disease by exosomes derived from human umbilical cord mesenchymal stem cells to repair intestinal barrier via TSG-6. Stem Cell Res Ther. 12:3152021. View Article : Google Scholar : PubMed/NCBI | |
|
Yang X, Meng S, Jiang H, Chen T and Wu W: Exosomes derived from interleukin-10-treated dendritic cells can inhibit trinitrobenzene sulfonic acid-induced rat colitis. Scand J Gastroenterol. 45:1168–1177. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Kou Y, Li J, Zhu Y, Liu J, Ren R, Jiang Y, Wang Y, Qiu C, Zhou J, Yang Z, et al: Human amniotic epithelial stem cells promote colonic recovery in experimental colitis via exosomal MiR-23a-TNFR1-NF-κB signaling. Adv Sci (Weinh). 11:e24014292024. View Article : Google Scholar | |
|
Park N, Kim KS, Park CG, Jung HD, Park W and Na K: Adipose-derived stem cell-based anti-inflammatory paracrine factor regulation for the treatment of inflammatory bowel disease. J Control Release. 374:384–399. 2024. View Article : Google Scholar : PubMed/NCBI | |
|
Guo J, Wang F, Hu Y, Luo Y, Wei Y, Xu K, Zhang H, Liu H, Bo L, Lv S, et al: Exosome-based bone-targeting drug delivery alleviates impaired osteoblastic bone formation and bone loss in inflammatory bowel diseases. Cell Reports Med. 4:1008812023. View Article : Google Scholar | |
|
Liu C, Yan X, Zhang Y, Yang M, Ma Y, Zhang Y, Xu Q, Tu K and Zhang M: Oral administration of turmeric-derived exosome-like nanovesicles with anti-inflammatory and pro-resolving bioactions for murine colitis therapy. J Nanobiotechnology. 20:2062022. View Article : Google Scholar : PubMed/NCBI | |
|
Swaroop S, Vuyyuru SK, Kante B, Kumar P, Mundhra SK, Arora U, Goyal A, Kandasamy D, Sharma R, Kabilan K, et al: A phase I/II clinical trial of ex-vivo expanded human bone marrow derived allogeneic mesenchymal stromal cells in adult patients with perianal fistulizing Crohn's Disease. Stem Cell Res Ther. 15:1402024. View Article : Google Scholar : PubMed/NCBI | |
|
Lightner AL, Reese J, Ream J, Nachand D, Jia X, Dadgar N, Steele SR and Hull T: A Phase IB/IIA study of ex vivo expanded allogeneic bone marrow derived mesenchymal stem cells for the treatment of perianal fistulizing Crohn's disease. Dis Colon Rectum. 66:1359–1372. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Lightner AL, Otero-Pineiro A, Reese J, Ream J, Nachand D, Adams AC, VanDenBossche A and Kurowski JA: A Phase I study of ex vivo expanded allogeneic bone marrow-derived mesenchymal stem cells for the treatment of pediatric perianal Fistulizing Crohn's disease. Inflamm Bowel Dis. 29:1912–1919. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Hadizadeh A, Akbari Asbagh R, Heirani-Tabasi A, Soleimani M, Gorovanchi P, Ebrahimi Daryani N, Vahedi A, Nazari H, Banikarimi SP, Abbaszade Dibavar M, et al: Localized administration of mesenchymal stem cell-derived exosomes for the treatment of refractory perianal fistula in patients with Crohn's disease: A Phase II clinical trial. Dis Colon Rectum. 67:1564–1575. 2024. View Article : Google Scholar : PubMed/NCBI | |
|
Keung C, Nguyen TC, Lim R, Gerstenmaier A, Sievert W and Moore GT: Local fistula injection of allogeneic human amnion epithelial cells is safe and well tolerated in patients with refractory complex perianal Crohn's disease: A phase I open label study with long-term follow up. EBioMedicine. 98:1048792023. View Article : Google Scholar : PubMed/NCBI | |
|
Nazari H, Alborzi F, Heirani-Tabasi A, Hadizadeh A, Asbagh RA, Behboudi B, Fazeli MS, Rahimi M, Keramati MR, Keshvari A, et al: Evaluating the safety and efficacy of mesenchymal stem cell-derived exosomes for treatment of refractory perianal fistula in IBD patients: Clinical trial phase I. Gastroenterol Rep (Oxf). 10:goac0752022. View Article : Google Scholar : PubMed/NCBI |