Blood‑brain barrier dysfunction in epilepsy: Mechanisms, therapeutic strategies and future orientation (Review)
- Authors:
- Published online on: July 3, 2025 https://doi.org/10.3892/ijmm.2025.5577
- Article Number: 136
-
Copyright: © Huang et al. This is an open access article distributed under the terms of Creative Commons Attribution License.
Abstract
Introduction
Epilepsy is one of the most prevalent and severe neurological disorders, and its global disease burden continues to pose substantial challenges. Epidemiological studies estimate that ~70 million individuals worldwide are affected by epilepsy (1). The mortality rate among individuals with epilepsy is three-fold higher than that of the general population, with a number also suffering from comorbidities such as depression and bone fractures, imposing significant burdens on families and society (2). The annual global burden of epilepsy is >13 million disability-adjusted life years, with combined direct medical expenditures and indirect productivity losses amounting to US$28 billion, underscoring its status as a critical public health priority (2). The primary mechanism underlying epileptic seizures is an imbalance between the excitatory and inhibitory processes within the central nervous system (CNS) (3). Clinically, it manifests as transient recurrent neurological dysfunction caused by abnormal hypersynchronous neuronal discharges. Symptom heterogeneity is strongly correlated with epileptogenic focus localisation: Focal seizures may involve motor manifestations (e.g. limb automatisms), sensory disturbances (e.g. olfactory/visual hallucinations) or autonomic dysfunction (e.g., tachycardia, facial flushing); and generalised seizures are characterised by impaired consciousness, generalised tonic-clonic movements or respiratory arrest (4). Approximately 30% of patients with epilepsy demonstrate resistance to existing anti-seizure drugs (ASDs) (5), and patients with drug-resistant epilepsy (DRE) experience significantly elevated risks of progressive brain damage as the frequency of seizures increases. Chronic recurrent seizure activity accelerates the progression of hippocampal sclerosis, leading to irreversible neuronal loss and abnormal neural network reorganisation (6). This results in a self-perpetuating 'seizure-damage-increased seizure susceptibility' cycle that ultimately drives disease progression toward the refractory stages (7). DRE is characterised by the failure to achieve seizure control with at least two established ASDs (administered alone or in combination) (8). Globally, DRE has an estimated prevalence of 13.7%, with a cumulative incidence of 25% in paediatric populations and 14.6% in adult and mixed cohorts (9). Compared with treatment-responsive patients, individuals with DRE demonstrate elevated risks of depression, epilepsy-related injuries and neurological deficits (10,11), coupled with increased mortality rates (12,13). Current neuromodulation approaches for DRE include deep brain stimulation (DBS), vagus nerve stimulation and responsive neurostimulation. A longitudinal follow-up study revealed that these interventions achieve >50% seizure frequency reduction in 50-60% of recipients (14). Nevertheless, most drug-resistant patients fail to derive substantial benefits, and a few are ineligible for neurostimulation due to incomplete evaluations or contraindications. This clinical reality underscores the critical need to explore novel therapeutic targets and intervention strategies to deliver safer and more effective options to patients with refractory epilepsy populations.
The blood-brain barrier (BBB) plays a vital role in regulating epileptogenesis by maintaining CNS homeostasis (15). Brain stability, particularly neurotransmitter balance, depends on the controlled exchange of substances between blood and brain tissue. As a dynamic interface, the BBB regulates the passage of ions, proteins and other molecules, and maintaining this balance is preserved. The structure of the BBB comprises capillary endothelial cells, tight junctions (TJs) connecting these cells, a basement membrane and surrounding glial cells. Under normal physiological conditions, the BBB tightly controls the entry of substances into the brain parenchyma through paracellular and transcellular pathways, thereby preventing the infiltration of harmful elements into the CNS and ensuring a stable internal environment (16,17). When the BBB is compromised, toxic substances and immune cells can infiltrate brain tissue, contributing to the pathogenesis of neurological disorders, such as traumatic brain injury, multiple sclerosis, epilepsy and brain tumours (18). BBB integrity is pivotal in disease progression, influencing both the onset of neurological conditions and the efficacy of therapeutic interventions. Research suggests that epilepsy can disrupt BBB function, with seizures often triggering an excessive release of the excitatory neurotransmitter glutamate (Glu), leading to increased BBB permeability (19). Additionally, alterations in angiogenesis, and changes in the expression of receptors, transporters and ion channels, further increase the risk of recurring seizures (19-21).
The present review is focused on the alterations in the structure and function of the BBB and presents recent advances in understanding its effect on epilepsy. This review examines the mechanisms by which BBB dysfunction contributes to epilepsy progression and discusses emerging therapeutic strategies aimed at targeting the BBB to enhance treatment outcomes for epilepsy.
Structure and function of the BBB
Approximately 140 years ago, the German scientist Paul Ehrlich firstly observed that a dye injected into the blood vessels of mice stained peripheral organs but not the brain or spinal cord, suggesting the existence of a barrier (22,23). This barrier, now known as the BBB, was microscopically confirmed in the 1960s (24).
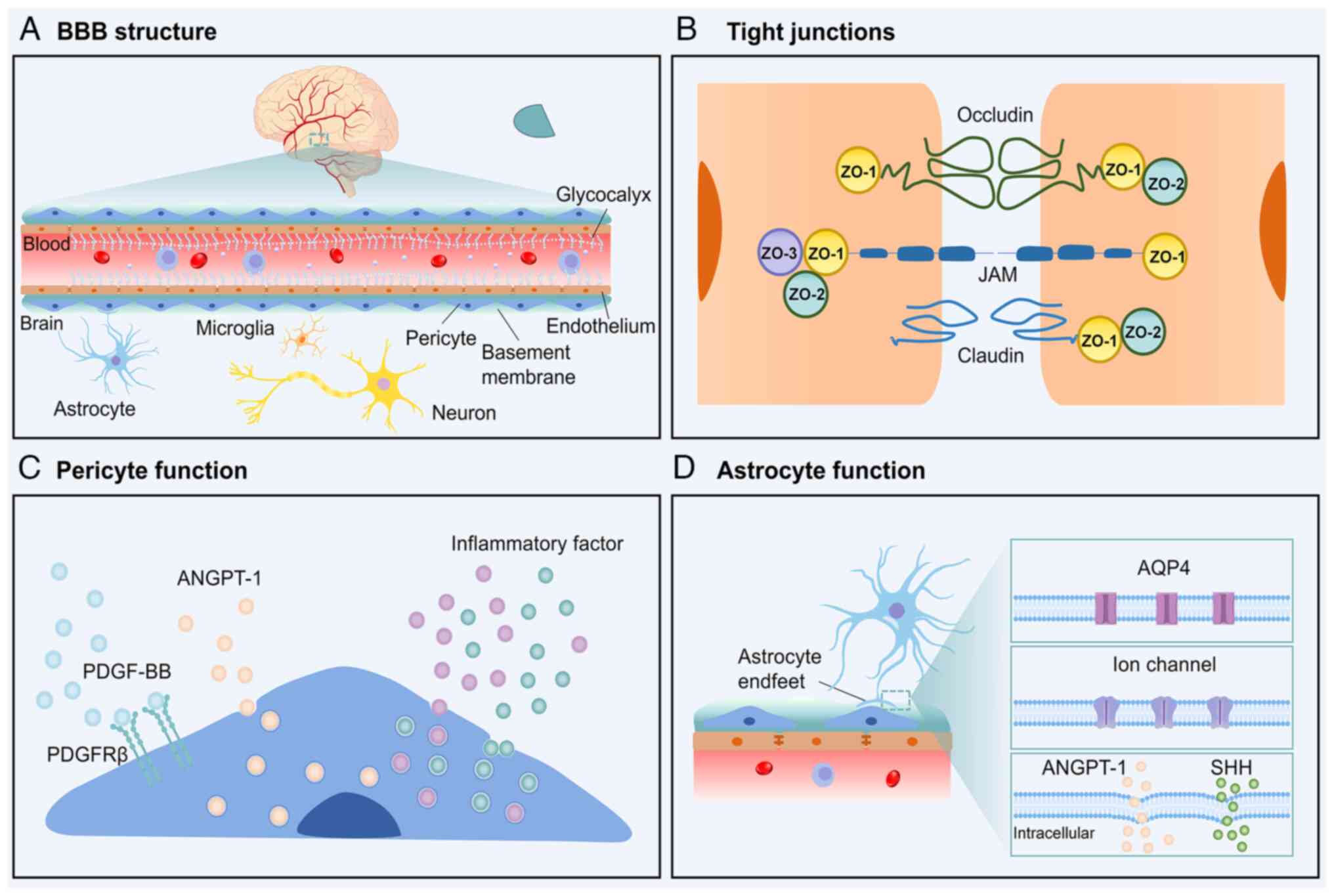
The BBB consists of three primary layers, with the first being formed by brain microvascular endothelial cells (BMECs) connected by TJs. BMECs are characterised by fewer pore structures and endocytic vesicles than peripheral endothelial cells, along with a high mitochondrial density, which is indicative of reduced permeability and increased metabolic activity. These cells feature transcellular transport pathways involving solute carriers, receptors and ion transporters (25,26). Additionally, BMECs produce a glycocalyx layer that prevents direct contact between BMECs and leukocytes, and plays a critical role in reducing inflammation and maintaining BBB integrity (27,28) (Fig. 1A). TJ complexes composed of proteins, such as claudins, occludin, zona occludens protein 1 (ZO-1), ZO-2 and ZO-3, further restrict paracellular diffusion, ensuring low BBB permeability (29) (Fig. 1B). The second layer of the BBB comprises a basement membrane and pericytes around the vascular endothelium. The basement membrane is composed of collagen IV, laminin, nidogen and heparan sulphate proteoglycans. This layer anchors cells and provides structural support, and its disruption leads to increased BBB permeability through altered expression of TJ proteins (30). Pericytes, a critical component of the BBB (31), maintain CNS homeostasis by promoting vascularisation via platelet-derived growth factor (PDGF) signalling and secreting factors such as angiopoietin-1 (ANGPT-1), which facilitates the development of BMECs and enhances BBB stability (32). Pericytes modulate inflammatory responses by releasing pro-inflammatory chemokines, such as CCL2 and CXCL1 (33) (Fig. 1C). The third layer consists of astrocyte end-feet that enwrap capillaries, forming a sheath-like structure. Astrocytes, which are the most abundant glial cells in the brain (34), regulate ion transport, blood flow and nutrient supply through ion channels and aquaporins (35,36); they secrete cytokines, including ANGPT-1 and sonic hedgehog (SHH), strengthening BBB integrity by upregulating TJ proteins and modulating BMEC transport properties (37,38). Furthermore, astrocytes contribute to neurotransmission by participating in 'tripartite synapses' with neurons, forming a link between nerve cells and blood vessels (39) (Fig. 1D). These three interconnected layers work synergistically to maintain brain homeostasis by tightly regulating the entry of substances into the brain and facilitating the clearance of harmful compounds.
BBB dysfunction in epilepsy
Maintaining the structural and functional integrity of the BBB is crucial to ensure a stable internal environment within the CNS. In 1986, Cornford and Oldendorf introduced the pivotal concept that seizures can affect BBB function (40). Subsequent studies have confirmed BBB disruption in seizure models induced by agents such as pilocarpine, fluoroethyl, kainic acid (KA) and bicuculline (41-44). Under normal physiological conditions, the BBB plays an essential role in stabilising the brain microenvironment. However, seizures compromise its integrity, accelerate epilepsy progression and potentially lead to drug-resistant forms of the disorder. This creates a detrimental feedback loop wherein further damage to the structural, molecular and immune regulatory mechanisms of the BBB exacerbates its dysfunction (45).
Epilepsy-induced BBB structural disruption
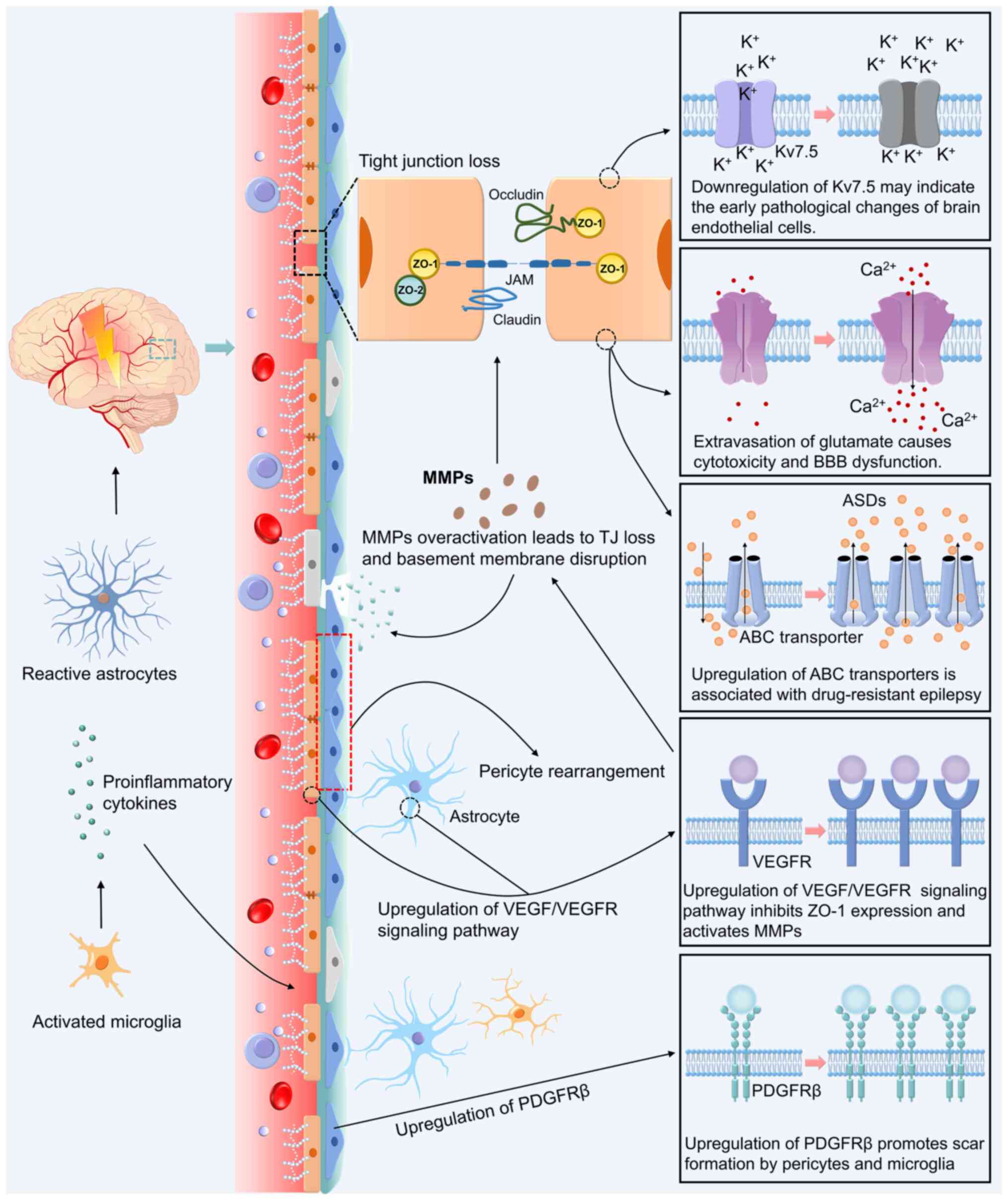
Epileptic activity significantly compromises BBB molecular integrity, primarily manifested by the downregulation of TJ proteins (occludin, ZO-1 and claudin-8), which culminates in elevated paracellular permeability (46,47). The activation of matrix metalloproteinase (MMP)-2/9 plays a pivotal role in mediating these alterations by degrading TJ components and remodelling the perivascular extracellular matrix architecture, further compromising barrier functionality (48). Clinical investigations have revealed persistent BBB compromise in epilepsy, as evidenced by elevated serum levels of MMP-2 and related biomarkers even during interictal periods (49). Tuberous sclerosis complex 1 emerges as a critical regulator of TJ assembly, with its deficiency being mechanistically linked to epileptogenesis via aberrant mechanistic target of rapamycin pathway activation that disrupts TJ protein expression and localization (50,51). This cascade of molecular events orchestrates structural and functional BBB destabilisation, highlighting the core vulnerability of the neurovascular interface (Fig. 2).
Epilepsy-induced neurovascular unit dysfunction
Epileptogenesis induces pathological alterations extending beyond cerebrovascular endothelial cells to encompass the entire neurovascular unit (NVU), which comprises BMECs, pericytes, astrocytes and microglia. In KA-induced epileptogenesis models, BMECs demonstrate significant Kv7.5 channel downregulation, potentially serving as early biomarkers for endothelial dysfunction (21). Concurrent seizure activity drives the marked activation of vascular endothelial growth factor (VEGF)/VEGF receptor signalling cascades, where sustained VEGF upregulation exerts dual inhibitory effects on ZO-1 expression while potentiating MMP activity, collectively diminishing BBB structural integrity (46,47,52,53). Pericytes undergo phenotypic reorganisation with concomitant PDGF receptor β (PDGFRβ) upregulation during epileptogenesis, synergising with activated microglia to form pro-fibrotic niches that exacerbate barrier impairment (54). Electrophysiological analyses have revealed seizure-induced inward currents in pericytes, indicating a functional compromise (55). Astrocyte activation enhances gap junction coupling and electrical synapse formation, amplifying neuronal hypersynchrony (56), whereas microglial activation propagates neuroinflammation through pro-inflammatory cytokine release, establishing a chronic inflammatory milieus that destabilises BBB permeability (57). This multifaceted cellular interplay ultimately drives NVU functional decoupling, thereby perpetuating epileptogenic cascades (Fig. 2).
Epilepsy-mediated BBB transport dysregulation
The selective barrier function of the BBB is maintained through the coordinated regulation of paracellular and transcellular transport. Epileptic seizures activate multiple signalling pathways that impair both routes, increasing the risk of entry of harmful substances into the brain. In the paracellular pathway, seizure-induced activation of the mitogen-activated protein kinase (MAPK) pathway leads to the degradation and redistribution of TJ proteins. This disrupts intercellular junctions and amplifies local inflammatory responses (58,59). Abnormal activation of the phosphoinositide 3-kinase/Akt pathway further reduces claudin-5 expression. This triggers cytoskeletal remodelling and compromises the barrier integrity (60,61). Oxidative stress enhances these effects by activating the nuclear factor-κB (NF-κB) signalling pathway. This promotes the expression of pro-inflammatory cytokines and MMPs, thereby accelerating TJ protein degradation (62). These alterations are particularly evident in the early stages of epilepsy, where changes in cerebral blood flow and increased mechanical stress further exacerbate BBB dysfunction (63,64).
In the transcellular pathway, efflux transporters, such as P-glycoprotein (P-gp), breast cancer resistance protein (BCRP) and multidrug resistance-associated proteins (MRPs) are upregulated. This limits the penetration of ASDs across the BBB (65-69). BCRP is upregulated in vascular endothelial cells and astrocytes, further restricting drug distribution within the brain (70,71). Members of the ATP-binding-cassette transporters sub-family C (ABCC) transporter family, including ABCC2, are overexpressed in endothelial and astroglial cells in the brains of patients with epilepsy. This expression profile contributes to the reduced efficacy of ASDs (72). Inhibition of these transporters enhances drug accumulation in the brain tissue and improves therapeutic outcomes (73,74). Monocarboxylate transporters (MCTs), including MCT1 and MCT2, regulate lactate and pH homeostasis in the brain. Decreased expression of these transporters in the hippocampus of patients with epilepsy leads to lactate accumulation and the formation of an acidic microenvironment. This contributes to neuronal damage, calcium dysregulation and oxidative stress, ultimately compromising BBB integrity (75,76).
Epilepsy disrupts the BBB via multiple mechanisms. These include degradation of TJ proteins, dysfunction of the NVU, impaired transporter systems and homeostatic imbalances within the brain microenvironment. Such changes increase brain vulnerability to harmful substances and may drive the progression of epilepsy and the development of pharmacoresistance. These alterations are considered to be key contributors to the pathophysiology of DRE. Furthermore, evidence from multimodal imaging techniques, including magnetic resonance imaging (MRI) and positron emission tomography, has revealed a significant spatial and temporal overlap between BBB dysfunction and epileptic activity. This provides imaging-based support for the causal relationship between BBB impairment and seizure generation (77-79).
Possible mechanisms underlying BBB dysfunction in epilepsy
The structure and function of the BBB are dynamic and influenced by various internal and external factors. In the pathogenesis of epilepsy, several mechanisms, either directly or indirectly, disrupt the BBB, contributing to the aggravation and persistence of epileptic conditions. The primary factors associated with epilepsy and the main pathways through which they affect the BBB are outlined below.
Glu and neurotransmitter imbalance in BBB dysfunction
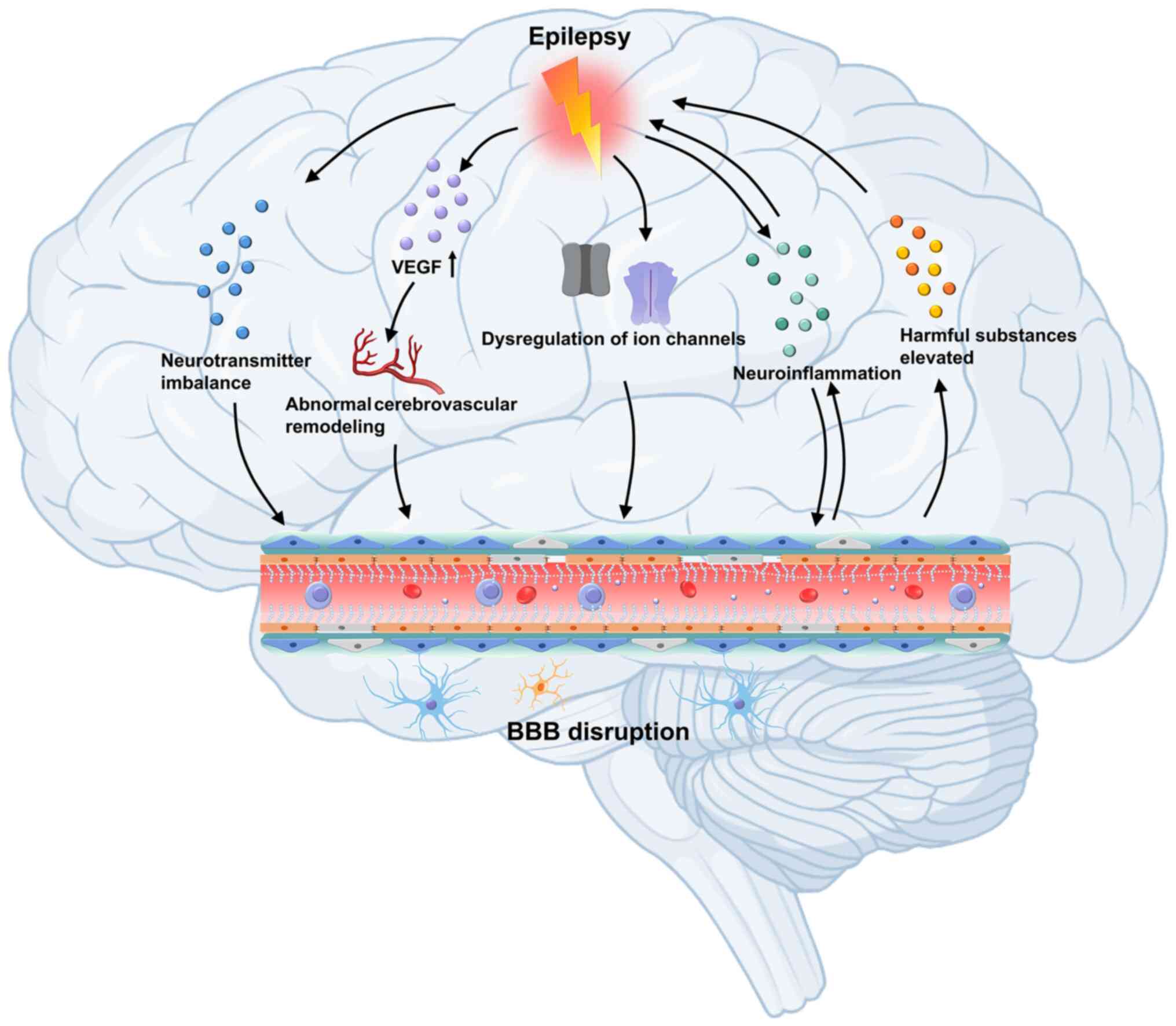
The BBB physiologically maintains cerebral Glu homeostasis through selective transport systems to prevent neurotoxic leakage (80). However, in epilepsy, excessive extracellular Glu accumulation triggers excitotoxicity, inducing neuronal damage and BBB structural-functional compromise (81). Glu exerts dual effects through ionotropic receptor [N-methyl-D-aspartate (NMDAR)/α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR)] and metabotropic Glu receptors (mGluRs) expressed in neurons, BMECs and astrocytes (82-84). In BMECs, activation of GluN1/GluN2B-containing NMDAR elevates intracellular Ca2+ levels, activates Ca2+/calmodulin (CaM)-dependent kinase II/protein kinase C (PKC) pathways to downregulate occludin/claudin-5, and induces cytoskeletal remodelling, ultimately impairing barrier integrity (19,85). Concurrent RhoA/Rho-associated protein kinase (ROCK) and MAPK kinase 1/2-Extracellular signal-regulated kinase (ERK)1/2 signalling exacerbates vascular permeability (19,85). Astrocytic AMPAR (GluA1/A2 subtypes) activation promotes Na+/Ca2+ influx, stimulating pro-inflammatory cytokines [interleukin (IL)-1β and tumor necrosis factor (TNF)-α] and MMP-2/9 secretion via paracrine mechanisms to amplify BBB disruption (19). Metabotropic mGluR5 demonstrates paradoxical BBB modulation: Genetic ablation reduces BBB leakage and neutrophil infiltration (86), whereas receptor activation enhances ZO-1 expression via phospholipase C/PKCµ/c-Jun signalling through α-actinin-1 protein complexes (87). In addition to Glu, epilepsy-associated γ-aminobutyric acid (GABA) depletion impairs astrocytic support to endothelia (88), whereas 5-hydroxytryptamine hyperactivation increases BBB permeability (89). Emerging evidence has implicated ATP/adenosine disequilibrium in BBB pathology. Seizure-elevated extracellular ATP activates microglial P2X7 receptors, triggering NLRP3 inflammasome-mediated IL-1β release (90), which induces MMP-9 production and ZO-1 downregulation. Purinergic P2X receptor 7 inhibition attenuates BBB damage in models of traumatic epilepsy models (91,92). These neurotransmitter disturbances collectively orchestrate BBB breakdown through receptor-specific signalling cascades, establishing a bidirectional interplay between epileptic hyperexcitability and barrier dysfunction (Fig. 3).
Abnormal cerebrovascular remodeling
During epileptic seizures, abnormal electrical activity in the brain affects neurons and induces pathological remodelling of blood vessels, thereby weakening the BBB. Studies using rat status epilepticus (SE) models have identified complex vascular networks in lesion areas, which are indicative of vascular remodelling (93,94). Similarly, increased vascular density observed in the hippocampus of patients with temporal lobe epilepsy is correlated with seizure frequency, suggesting that abnormal angiogenesis may contribute to epilepsy progression (95).
VEGF is a key pro-angiogenic factor that is significantly upregulated during seizures and promotes endothelial cell proliferation, migration and neovascularisation (96). Although neovascularisation enhances local blood supply, the newly formed vessels are often structurally unstable and exhibit increased permeability, leading to a compromised BBB (52). Seizure-induced local hypoxia activates hypoxia-inducible factor-1α (97), further accelerating angiogenesis by upregulating VEGF transcription (98). However, VEGF also downregulates TJ proteins, such as occludin and ZO-1, weakening BBB integrity and increasing vascular permeability (Fig. 2). This allows the infiltration of pro-inflammatory factors and immune cells across the BBB (47,99). Additionally, these infiltrating immune cells and inflammatory mediators exacerbate these effects by releasing proteases and pro-inflammatory factors that degrade the vascular structure, further aggravating BBB damage (99).
Taken together, the above findings indicate that abnormal vascular remodelling significantly contributes to BBB disruption through mechanisms involving angiogenesis, VEGF-mediated downregulation of TJ proteins and MMP-induced degradation, thereby playing a pivotal role in the pathogenesis of epilepsy (Fig. 3).
Dysregulation of ion channels
During seizures, ion channel dysfunction, particularly in the sodium, potassium and calcium channels, leads to excessive neuronal discharge and abnormal electrical activity. Dysregulated sodium and potassium channels cause prolonged depolarisation, maintaining neurons in a hyperexcited state and destabilising surrounding structures such as astrocytes, which impair BBB stability (100,101). Calcium ions (Ca2+) are pivotal in neuronal excitability, and intracellular Ca2+ imbalances frequently triggers seizures (102). Seizure-induced intracellular Ca2+ overload activates Ca2+/CaM complexes, triggering sequential activation of myosin light-chain kinase (MLCK), which phosphorylates MLC. This Ca2+-dependent cascade induces actomyosin contraction, drives cytoskeletal reorganisation and destabilises the intercellular TJ integrity (103,104). The pharmacological inhibition of MLCK using ML-7 has demonstrated therapeutic efficacy in ameliorating BBB dysfunction (104,105). Excessive Ca2+ influx activates PKC isoforms, with the PKCζ subtype directly phosphorylating occludin to disrupt its binding with ZO-1 scaffolding proteins, thereby destabilising intercellular TJs and compromising BBB integrity (103,104).
Under physiological conditions, coordinated aquaporin 4 (AQP4)-inwardly rectifying potassium channel 4.1 (Kir4.1) expression in astrocytic end-foot domains maintains cerebral water-potassium homeostasis through extracellular ion clearance and osmotic balance. Epileptic activity significantly downregulates AQP4 expression (106-108), induces mislocalisation with reduced polarised distribution (109,110) and impairs vascular structural support, thereby collectively destabilising TJ integrity. These pathological alterations increase the BBB permeability (111,112) and the risk of neurotoxic infiltration. Experimental validation using AQP4-knockout models demonstrates exacerbated BBB damage post-epileptogenesis (113). Additionally, AQP4 downregulation additionally hinders Kir4.1-mediated potassium reuptake, resulting in elevated extracellular K+ levels and increased intracellular osmotic pressure (114) (Fig. 3).
Thus, ion channel abnormalities during seizures initiate cellular damage and TJ disruption, thereby impairing BBB permeability. Cytoskeletal changes and PKC activation driven by Ca2+ influx weaken TJs, while AQP4 and Kir4.1 dysfunctions further disturb the water and potassium balance. These interconnected mechanisms contribute to BBB dysfunction and exacerbate the brain tissue damage during seizures.
BBB injury promotes epileptogenesis
An impaired BBB is increasingly recognized not only as a consequence of CNS disorders, such as epilepsy, but also as a pathogenic factor capable of promoting seizures. Early investigations demonstrated that BBB dysfunction could induce focal epilepsy, as shown in rat models, where bile salt-induced BBB disruption led to epileptiform activity within 4-49 days post-disruption (115). Notably, experimental disruption of the BBB in SE models using mannitol to induce osmotic opening has been shown to exacerbate seizure frequency in rats (116). Recent findings have highlighted the role of BBB disruption in seizure pathogenesis. In vitro introduction of thrombin has been shown to rapidly induce epileptiform discharge activity in hippocampal neurons, effectively replicating the consequences of BBB impairment (117). Additionally, the deletion of claudin-5, a crucial TJ protein, results in spontaneous seizures, severe neuroinflammation and mortality in mice. These effects can be mitigated by RepSox, which modulates claudin-5 expression, highlighting its critical role in maintaining BBB integrity and preventing seizures (118). Furthermore, in BMECs, selective knockout of cyclin-dependent kinase 5, an essential regulator of neuronal excitability, induces spontaneous epilepsy. This process involves astrocyte dysfunction mediated by the CXCL1/CXCR2 signalling pathway, linking BBB dysfunction to broader neuronal and astrocytic abnormalities (119).
Impaired BBB allows albumin to penetrate brain parenchyma
BBB dysfunction results in serum albumin leakage and significantly contributes to epileptogenesis (115,116). The leakage of albumin into the brain parenchyma, often in combination with antiepileptic drug exposure, may play a role in the development of drug resistance in patients (120). Albumin infiltration facilitates epilepsy by lowering the threshold for spreading depolarisations, enhancing neuronal hyperexcitability and impairing normal neuronal function (121). In normal rats, electroencephalographic (EEG) monitoring following intraventricular administration of albumin revealed rapid spiking activity in the hippocampus within 15-60 min, and a substantial reduction in seizure threshold was observed 3 months later (122).
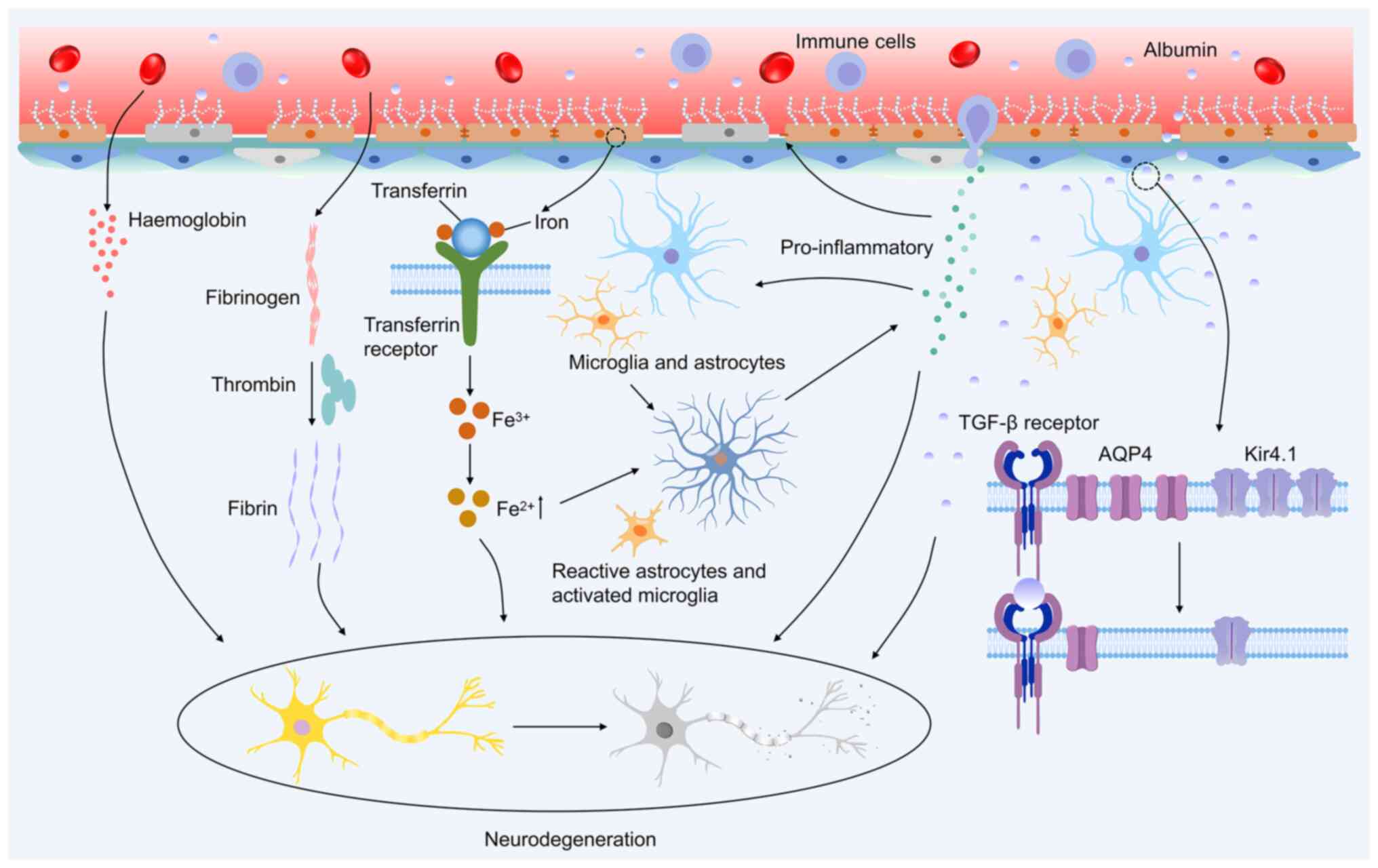
Additionally, albumin alters synaptic plasticity in the hippocampal CA1 region, potentially influencing progression of epilepsy (123). In guinea pig brain slices with KA-induced epileptiform activity, albumin application intensifies epileptiform discharges and triggers seizure recurrence upon subsequent KA exposure, highlighting its role in promoting acute seizures (124). When albumin infiltrates the brain parenchyma, it interacts with transforming growth factor β receptors (TGF-βRs) on astrocytes, resulting in reduced expression of Kir4.1 and AQP4 in these cells (Fig. 4). Decreased Kir4.1 disrupts intracellular potassium clearance, potentially contributing to neuronal hyperexcitability and synchronised neural activity (125). The downregulation of AQP4 further exacerbates this imbalance by affecting Kir4.1, altering cellular potassium and water homeostasis, and activating signaling pathways that may ultimately lead to seizures (106,126). Additionally, BBB dysfunction has been linked to reduced levels of the gap junction proteins connexin 30 and 43 in the cortical astrocytes. This reduction results in gap junction uncoupling, which impairs extracellular potassium clearance and creates favourable conditions for seizure generation (127,128).
These observations underscore the critical role of BBB dysfunction in allowing serum albumin to penetrate the brain parenchyma, triggering a cascade of molecular and cellular changes that contribute to the onset and progression of epilepsy (Fig. 4).
BBB damage facilitates inflammation to accelerate epilepsy progression
In physiological states, the BBB maintains its impermeability to peripheral immune cells and macromolecules (e.g. albumin) via a tripartite mechanism involving endothelial TJs (claudin-5 and occludin), astrocytic end-feet ensheathment and the supportive basement membrane. This structural and functional synergy safeguards the chemical and immunological homeostasis of the CNS parenchyma. A pathological breach of BBB integrity allows serum proteins (e.g., albumin) to penetrate the brain parenchyma, directly triggering TGFβRII-mediated SMAD phosphorylation in astrocytes (129). This signalling event drives the transcriptional upregulation of proinflammatory cytokines (IL-1β and TNF-α), thereby promoting neuroinflammation and lowering the seizure threshold (129). BBB dysfunction enables the translocation of serum proteins into the brain parenchyma while simultaneously creating a permissive pathway for peripheral immune cells (e.g. T cells and macrophages) to infiltrate through disrupted endothelial junctions. This dual invasion drives neuroinflammation and contributes to seizure susceptibility by destabilising the neurovascular niche (130-133). These infiltrating immune cells release large quantities of proinflammatory mediators, such as TNF-α, IL-1β and IL-6, which further activate microglia and astrocytes, initiating a neuroinflammatory cascade (Fig. 4). Progressive neuroinflammation drives marked upregulation of proinflammatory cytokines (e.g. IL-1β and TNF-α) and alarmins [e.g. high mobility group box 1 (HMGB1)], coupled with the activation of pattern recognition receptors, such as Toll-like receptor 4 (TLR4). Preclinical and clinical evidence has demonstrated that the magnitude of this inflammatory cascade directly correlates with seizure severity, likely through the amplification of excitotoxicity and synaptic dysfunction via glial-derived cytokine signalling (134). These factors exacerbate BBB permeability and act directly on neurons and synapses, promoting neuronal hyperexcitability and facilitating epileptogenesis.
TNF-α drives BBB disruption through NF-κB-dependent inhibition of claudin-5 transcription, ultimately decreasing claudin-5 expression (131,135). Moreover, TNF-α activates the RhoA/ROCK signalling pathway, inducing cytoskeletal remodelling and spatial redistribution of claudin-5 and occludin, ultimately increasing BBB permeability (136,137). IL-1β contributes to BBB dysfunction by downregulating ZO-1 expression via the MAPK signalling pathway (138), and promoting ZO-1 ubiquitination and degradation through the ERK pathway in cooperation with TNF-α (139). As BBB integrity continues to deteriorate, inflammatory factors, such as HMGB1, accumulate in the brain parenchyma, where they, together with IL-1β, activate neuronal NMDAR (GluN2B subunits), triggering an influx of Ca2+, leading to increased neuronal excitability and synaptic plasticity abnormalities (140,141). Moreover, IL-1β disrupts the excitatory-inhibitory balance by inhibiting astrocytic GABA reuptake while simultaneously increasing Glu release, further lowering the seizure threshold (141). Additionally, the inflammatory response resulting from BBB disruption exacerbates BBB degradation via MMPs, thereby contributing to epileptogenesis. IL-1β enhances MMP-9 activity via the ATP/P2X7R pathway (142), while HMGB1 upregulates MMP-9 through a TLR4-mediated, but TNF-α-independent, mechanism (143). This process further stimulates IL-1β release via the NLRP3 inflammasome, accelerating basement membrane degradation (144). Excessive MMP activation compromises BBB integrity and alters the neural network architecture by remodelling the perisynaptic extracellular matrix. This contributes to the formation of chronic epileptogenic foci (145).
Simultaneously, TNF-α upregulates AMPA receptor expression at neuronal synapses, enhances excitatory synaptic transmission and reduces astrocytic Glu uptake. These changes increase synaptic Glu concentrations, ultimately leading to excitotoxicity (146-148). Furthermore, elevated IL-1β levels downregulate GABA receptor expression, further weakening inhibitory neurotransmission. This disruption of excitation-inhibition homeostasis significantly increases the likelihood of epileptiform discharges (146,149). The breakdown of the BBB initiates a self-reinforcing cycle comprising inflammatory responses, neuronal hyperexcitability and seizure-promoting activities. As BBB permeability increases, more inflammatory mediators infiltrate the brain parenchyma, further activating the neurons and glial cells. This sustained activation leads to the excessive release of inflammatory factors, exacerbating neuronal damage and increasing the incidence of epileptiform discharges (150). Chronic neuroinflammation dysregulates synaptic homeostasis and glial-neuronal crosstalk, culminating in the destabilisation of neural circuits and the emergence of epileptogenic zones (151). Consequently, this pathological cycle significantly complicates epilepsy treatment, highlighting the critical need for therapeutic strategies targeting BBB protection and the attenuation of neuroinflammation (Fig. 4).
BBB damage-induced iron overload exacerbates seizure severity
The BBB plays a critical role in maintaining CNS iron homeostasis by regulating iron transport via transferrin (Tf) and its receptor, transferrin receptor protein 1, which are essential for myelin synthesis and catecholamine neurotransmitter metabolism, and contribute to neurodevelopment and protection under normal conditions (152). However, BBB damage disrupts this regulation, allowing excessive iron to enter the brain tissue from the bloodstream, leading to its accumulation in neurons and glial cells (153,154).
The studies by Willmore et al (155,156) were the first to demonstrate that cortical injections of FeCl2 or FeCl3 in rats and cats induced chronic seizures, establishing a connection between iron overload and epilepsy. In patients with focal epilepsy and in mouse hippocampal slices, significant iron deposition has been observed in seizure-prone regions, particularly in the hippocampus (157). A clinical study revealed elevated Tf saturation in individuals with epilepsy compared with controls, supporting the role of iron dysregulation in the pathogenesis of epilepsy (158).
Iron overload contributes to the epilepsy pathophysiology by triggering oxidative stress, which lowers the seizure threshold. The accumulated iron catalyses the Fenton reaction, generating free radicals that accelerate lipid peroxidation, disrupt neuronal membrane integrity and increase neurotoxicity (159,160). Elevated levels of reactive oxygen species and malondialdehyde, along with decreased GPX4 expression observed in mouse models of epilepsy, underscore the role of oxidative stress in epilepsy progression (161,162). Oxidative stress damages mitochondria, leading to neuronal apoptosis and necrosis, weakening neural networks, fostering epileptic foci and increasing the likelihood of spontaneous seizures (154,159).
Iron accumulation also activates microglia and astrocytes, driving the release of inflammatory mediators, such as TNF-α and IL-1β (163). These inflammatory factors increase neuronal excitability by reducing GABAergic inhibition and enhancing excitatory neurotransmitter release, thereby increasing seizure frequency. Oxidative stress and inflammation caused by iron overload exacerbate BBB dysfunction, facilitating further infiltration of iron and inflammatory mediators into the brain. This self-reinforcing cycle exacerbates neuronal damage, increases seizure frequency and complicates epilepsy management (153,164) (Fig. 4).
In conclusion, BBB-related iron overload plays a significant role in epilepsy pathophysiology. Oxidative stress and inflammation lower the seizure threshold, exacerbate neuronal damage and establish a cycle that drives epilepsy progression.
Harmful substances are elevated in the brain after BBB damage
The BBB plays a crucial role in regulating the movement of molecules in and out of the CNS and ensuring a stable chemical composition in the neuronal environment, which is essential for normal neural function. Under physiological conditions, the BBB acts as a protective barrier, preventing the entry of neurotoxic plasma components, blood cells and pathogens into the brain (165). However, in pathological states, BBB dysfunction manifests as cerebrovascular hyperpermeability, neurovascular decoupling or blood flow dysregulation, and is associated with disorders such as stroke, glioma, epilepsy, traumatic brain injury and neurodegenerative diseases (166,167). Disruption of the BBB integrity allows neurotoxic plasma components to leak into the brain, facilitates immune cell infiltration and disturbs the CNS environment, leading to neuronal death and worsening disease progression (53,166). Research using transgenic mouse models with chronic BBB disruption has revealed that the accumulation of neurotoxic proteins, including fibrinogen, thrombin, hemoglobin, iron-rich hemosiderin and plasmin, within neurons can initiate or aggravate neurodegeneration (31,168,169) (Fig. 4). A strong link exists between BBB dysfunction and epilepsy, suggesting that a compromised BBB may contribute to epileptogenesis or exacerbate seizure propagation. An experimental study demonstrated that artificial disruption of the BBB, such as through osmotic shock, can induce seizures (170). Furthermore, conditions that impair BBB integrity, including infection, inflammation, stroke and traumatic brain injury, are well-documented triggers of seizures and epilepsy development (116,171) (Fig. 4).
Disruption of the BBB plays a central role in both the initiation of epileptogenesis and the chronic progression of epilepsy. Emerging evidence suggests that BBB disruption facilitates albumin infiltration into the brain parenchyma, exacerbates neuroinflammation, induces iron overload and allows the entry of harmful substances into the CNS (172-174). These pathological changes collectively drive the chronic progression of epilepsy and seizure initiation. Given the integral role of BBB integrity in disease modulation, therapeutic strategies aimed at restoring BBB function are gaining increasing attention (175). Potential interventions include pharmacological agents that reinforce BBB integrity, anti-inflammatory therapies, iron chelators, advanced nanodelivery systems and cell-based therapies. The following section discusses these innovative approaches, highlighting their potential to optimise antiepileptic interventions, disrupt the pathological cycle and pave the way for precision medicine in epilepsy treatment.
Current novel therapeutic strategies targeting the BBB
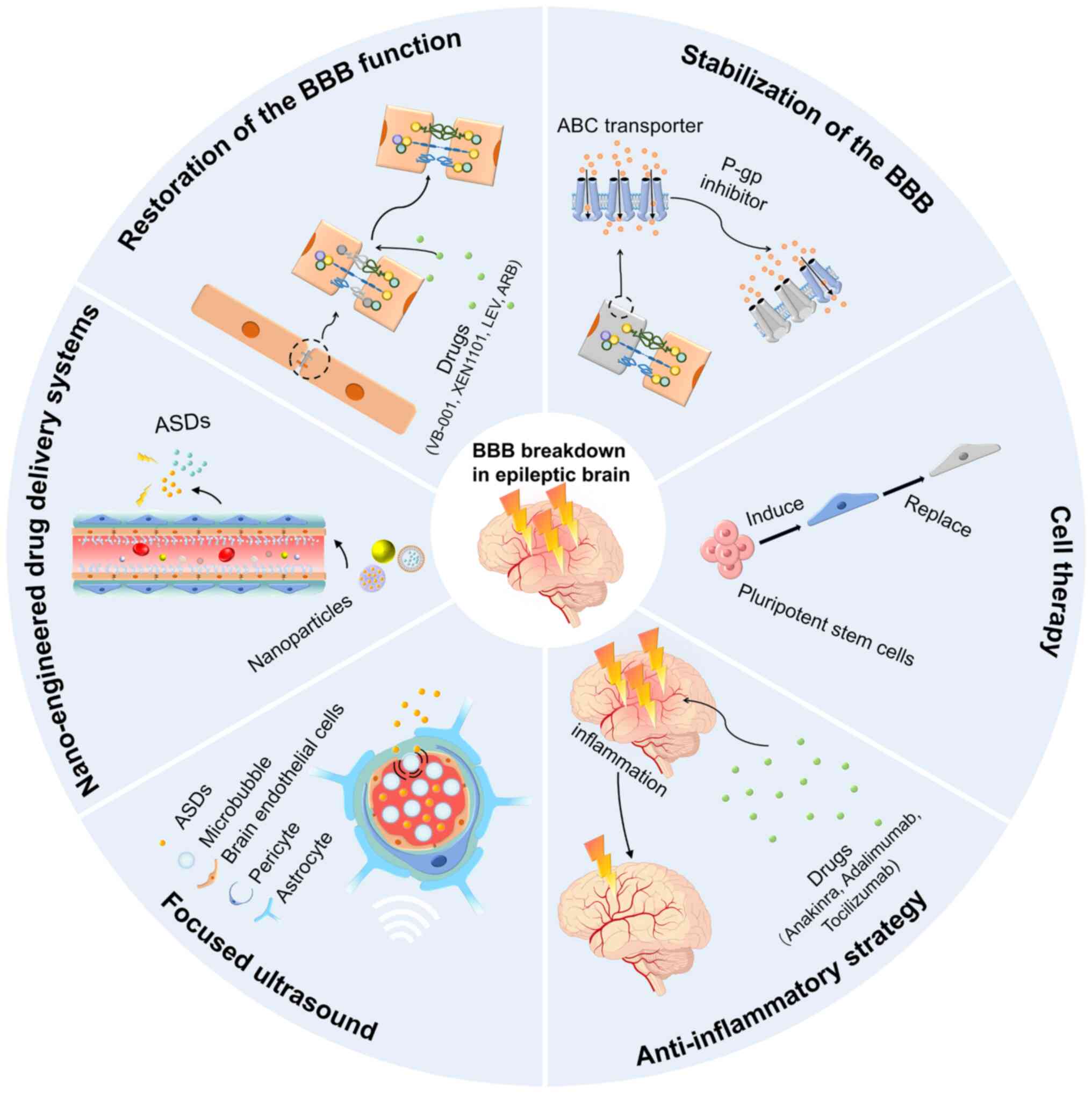
As outlined, BBB dysfunction plays a critical role in epileptogenesis, and the interplay between BBB disruption and epileptic activity forms a self-reinforcing cycle that exacerbates both conditions and accelerates disease progression (43,44). Given the pivotal role of the BBB in epilepsy, the development of therapeutic interventions targeting BBB dysfunction is increasingly prioritised. Strategies that focus on restoring BBB integrity, decreasing BBB permeability and blocking the infiltration of neurotoxic substances may help interrupt this detrimental cycle, slow disease progression and enhance patient outcomes.
BBB-targeted therapeutic strategies for epilepsy management
BBB dysfunction is a critical contributor to epilepsy initiation and progression. The pathological translocation of serum albumin and other blood-derived molecules into the CNS parenchyma disrupts the neurovascular niche, amplifying neuronal injury and seizure activity (176,177). This microenvironmental disruption amplifies oxidative stress and synaptic hyperexcitability, progressively lowering the seizure threshold and accelerating epileptogenesis. Given its pivotal role in epileptogenesis, restoration of BBB integrity has emerged as a novel and promising therapeutic strategy. Various approaches are being actively investigated to restore BBB integrity and function, with the goal of accelerating their translation into clinical use as effective therapies for epilepsy.
Among these strategies, natural compounds, such as the neuroprotective flavonoid vitexin, have demonstrated efficacy in promoting BBB integrity through the upregulation of TJ proteins, thus decreasing susceptibility to epilepsy (178). A derivative of vitexin, VB-001, has completed a phase I safety trial (CTR20190758) and may soon enter phase II clinical trials. Certain ASDs have been shown to exert protective effects on the BBB. For example, potassium channel modulators, such as retigabine, suppress neuronal hyperexcitability and upregulate TJs proteins, such as occludin and claudin-5, thereby decreasing BBB permeability (21). However, despite its initial approval by the U.S. Food and Drug Administration in 2011, retigabine was withdrawn due to adverse effects, such as skin and retinal pigmentation. The optimised variant, XEN1101, exhibited a ≥50% reduction in seizure frequency in 47% of participants with drug-resistant focal epilepsy during a phase II clinical trial (NCT03796962), with no reported retinal adverse effects. Another ASD, levetiracetam (LEV), has demonstrated the potential to maintain BBB integrity following febrile seizures by upregulating occludin and claudin-5 expression, and inhibiting BMEC pinocytosis (179). Angiotensin receptor blockers (ARBs) are attracting interest as adjunctive treatments for epilepsy, as they improve cerebral blood flow, prevent cerebral haemorrhage, preserve BBB function, reduce neuroinflammation and provide neuroprotection (180). Preclinical studies have shown that ARBs upregulate claudin-5 and ZO-1 expression, thereby facilitating structural restoration of the BBB in rodent models of senescence (181). Additionally, ARBs have been shown to significantly reduce seizure severity in experimental epilepsy models (182) (Fig. 5). An alternative therapeutic approach focuses on mitigating the detrimental consequences of BBB dysfunction. Iron chelation and antioxidant therapies have been proposed to counteract neurotoxic effects associated with BBB damage, such as intracerebral iron accumulation and oxidative stress (183).
Novel therapeutic strategies targeting BBB stabilisation for epilepsy
BBB-stabilising drugs are a promising strategy for epilepsy treatment. RepSox, a claudin-5 modulator, has been shown to inhibit seizure activity in a KA-induced mouse model of epilepsy (118). A promising therapeutic approach involves the administration of P-gp inhibitors to enhance the efficacy via BBB drug retention. P-gp is an efflux transporter that actively pumps drugs out of the brain, contributing to drug resistance in epilepsy. Inhibition of P-gp can increase intracranial ASD concentrations and improve therapeutic outcomes (184) (Fig. 5). Ferreira et al (73) reported that P-gp-inhibiting flavonoids significantly increased ASD brain penetration, underscoring the value of targeting P-gp to improve BBB drug delivery. Deng et al (185) further showed that miR-146a-5p, an anti-inflammatory microRNA, reduced P-gp expression in SE-model-derived cerebrovascular endothelial cells through NF-κB pathway inhibition, highlighting its potential as a novel therapy for drug resistance caused by SE. Recombinant human erythropoietin (rhEPO) inhibits P-gp activity and reverses P-gp-mediated drug resistance in refractory epilepsy (186). Although the inhibition of transporters can enhance drug accumulation in the brain, significant challenges remain. P-gp inhibition may cause systemic toxicity (e.g. liver damage) due to elevated drug levels (187), but trigger off-target effects that alter drug distribution in normal tissues. Therefore, transporter inhibition strategies require caution and precise control for clinical use. Critical factors include dosing timing and regimen selection, with a recommended low-dose initiation strategy combined with real-time blood drug monitoring and clinical effect evaluation to adjust inhibitor/anti-seizure medication doses and schedules (188). Close monitoring of liver/kidney function, infection markers and systemic toxicity risks is essential for minimising intervention-related hazards during the initial treatment.
Novel cell-based therapies for epilepsy
Cell-based therapies are promising strategies for BBB repair. Induced pluripotent stem cell (iPSC)-derived pericytes have been proposed to replace dysfunctional pericytes and restore BBB integrity, thereby increasing vascular stability, reducing neuroinflammation and improving selective BBB permeability (189). Chen (190) demonstrated that transplantation of iPSC-derived pericytes enhances BMEC interactions, upregulates the expression of TJ proteins, and suppresses the expression of inflammatory markers (TNF-α and IL-1β), ultimately decreasing BBB permeability and conferring neuroprotection. These findings suggest that iPSC-derived pericyte transplantation is a promising therapeutic approach for BBB restoration in patients with epilepsy. However, further studies are needed to refine transplantation protocols, increase cell survival rates, and evaluate the long-term safety and efficacy of this method (Fig. 5).
Novel anti-inflammatory strategies for epilepsy
A bidirectional pathological interaction exists between epileptic seizures and BBB disruption, both of which are accompanied by a pronounced neuroinflammatory response. Neuroinflammation increases BBB permeability and promotes seizure generation and chronicity. Therefore, targeting the inflammatory cascades has emerged as a promising strategy for epilepsy therapy. Recent studies have identified several critical cytokines and receptors as potential therapeutic targets, including IL-1R1, HMGB1 and TNF-α (191-193). In a KA-induced SE mouse model, conditional deletion of IL-1R1 in endothelial cells significantly suppressed hippocampal inflammation during KA-induced SE, reduced seizure susceptibility and improved behavioural outcomes (194), indicating the IL-1β/IL-1R1 axis as a viable target. HMGB1, a prototypical damage-associated molecular pattern, activates inflammatory signalling via TLR4 and the receptor for advanced glycation end products. Clinically, HMGB1 has been identified as a potential biomarker of refractory epilepsy (195). Experimental studies have suggested that HMGB1 antagonists or neutralising monoclonal antibodies can decrease seizure frequency and severity (196). TNF-α, a classical pro-inflammatory cytokine, regulates neuronal excitability and apoptosis; its inhibition in preclinical models has demonstrated neuroprotective effects and reduced seizure activity (197). However, systemic TNF-α suppression may increase the risk of infections and malignancies, thereby limiting its clinical application (198). Regarding efficacy, interventions targeting inflammatory mediators have shown the potential to reduce seizures and delay epileptogenesis in preclinical settings (199). Under certain conditions, such as SE or DRE, these strategies may offer advantages over conventional ASDs, which primarily control symptoms (200). Nevertheless, the risks associated with immune suppression necessitate precise regulation of the treatment dose, timing and duration to achieve therapeutic benefits while ensuring safety (201).
Several clinical trials have investigated the application of BBB-stabilising and anti-inflammatory agents in the treatment of epilepsy. For example, anakinra, an IL-1 receptor antagonist, has demonstrated efficacy in reducing seizure burden and recurrence in paediatric patients with febrile infection-related epilepsy syndrome (FIRES) (202). Humanised monoclonal antibodies targeting neuroinflammatory pathways have been used. Adalimumab, an anti-TNF-α agent, has shown seizure-reducing effects in clinical contexts (203). Tocilizumab, which blocks IL-6R, is currently under phase IV clinical trials for the treatment of paediatric FIRES (Chinese Clinical Trial Registry no. ChiCTR2400085185). Moreover, specific HMGB1 antagonists are under preclinical development and can potentially advance into clinical testing. In summary, IL-1R1, HMGB1 and TNF-α represent the most promising molecular targets in current anti-inflammatory approaches for epilepsy treatment. This strategy improves seizure control and delays disease progression, particularly in refractory cases. However, rigorous multicentre trials are essential to validate its clinical utility and define the optimal therapeutic windows and indications (Fig. 5).
Novel strategies for epilepsy treatment based on enhancing drug delivery to target brain regions
In addition to anti-inflammatory approaches, improving drug delivery to epileptogenic brain regions is another critical therapeutic strategy for epilepsy. Although epilepsy can alter the permeability of the BBB, elevated expression of efflux transporters such as BCRP, MRP1 and MRP2 continues to restrict the effective entry of drugs into the brain. Major challenges include the reduced bioavailability, solubility, stability and efficacy of therapeutic compounds before they reach their intended targets within the brain. To address these limitations, researchers have proposed various strategies to improve the efficiency of brain-targeted drug delivery. With the rapid growth of nanomedicine, nanoparticle-based drug delivery systems have become prominent solutions for increasing drug accumulation in the brain. Drug-loaded nanoparticles can cross the BBB and reach specific lesion sites in the brain without losing their activity, thereby improving drug utilization (204). Glucose transporter 1 (GLUT1) is abundantly expressed in the BBB, and several GLUT1-targeting nanocarriers have been developed to facilitate BBB penetration (205-207). Anraku et al (208) designed glucose-modified nanomaterials that could recognise and bind to GLUT1. This strategy takes advantage of the increased GLUT1 expression following fasting-induced hyperglycaemia, which promotes BBB penetration and brain accumulation of nanocarriers. Although this system has not yet been applied in epilepsy models, it may overcome the challenge of reduced GLUT1 expression in the epileptic brain and achieve effective drug delivery. Serum albumin, which leaks into the brain parenchyma following BBB disruption, has been considered a viable carrier for constructing brain-targeted nanocarriers. Although most albumin-based systems are currently used for anticancer drug delivery (209-211), their potential application in epilepsy treatment merits further investigation (Fig. 5).
Beyond optimising nanomaterials, the transient disruption of BBB integrity has been explored to enhance drug transport from the circulatory system into the CNS. Common BBB-opening approaches include focused ultrasound (FUS), static magnetic fields and near-infrared (NIR) laser stimulation, all of which are non-invasive. FUS, often used in combination with intravenously injected microbubbles (MBs), temporarily disrupts the BBB through acoustic cavitation, thereby improving drug penetration into brain tissues (212,213). A clinical study by Gasca-Salas et al (214) demonstrated that MRI-guided FUS combined with MBs successfully and reversibly opened the BBB at the temporoparietal junction in patients with Parkinson's disease and dementia, achieving success in 8 out of 10 treatments in 5 patients. In addition, Ozdas et al. developed FUS-responsive nanoassemblies that enabled non-invasive, targeted drug release in the brain with drug accumulation levels approximately 1300-fold higher than free drug, demonstrating significant potential for treating epilepsy and other neurological diseases (215).
Static magnetic fields can enhance the BBB penetration of magnetic nanoparticles, increasing the permeability rate from 3.36 to 8.47% (216). Gupta et al (217) reported that magnetic field-induced heating can temporarily loosen TJs in endothelial cells, facilitating drug release and delivery. Zhang et al (218) constructed a magnetoelectric core-shell nanostructure (Fe2O4@BaTiO3) using a static magnetic field-assisted interface co-assembly strategy. These nanostructures can be externally activated to produce electrical stimulation and modulate neuronal currents, with initial validation of their feasibility for the wireless modulation of epilepsy symptoms (219). Given the characteristic hypersynchronous discharges observed during epileptic seizures, electroresponsive nanomaterials containing Fc groups have been developed to enable on-demand drug release, increase local drug concentration and improve treatment outcomes (218).
NIR-activated photothermal nanomaterials can generate local heating, which facilitates BBB opening by enhancing interstitial fluid flow and increasing endothelial membrane permeability (220). Wu et al designed a dopamine-pyrrole hybrid system that combined receptor-mediated endocytosis with NIR-triggered photothermal conversion, thereby enhancing the brain delivery of ASDs (221). Yang et al (222) further demonstrated that under NIR stimulation, black phosphorous nanosheets modulate membrane currents in hippocampal neurons and suppress epileptiform discharges in mouse models, offering a new therapeutic direction for DRE. In addition, macrophage membrane-coated biomimetic nanoparticles (MA@RT-HMSNs) constructed by Geng et al showed excellent targeting ability. These nanoparticles successfully delivered the death-associated protein kinase (DAPK)1 inhibitor TC-DAPK6 across the BBB and selectively accumulated in inflammatory regions within epileptogenic zones, showing significant therapeutic benefits in both acute and chronic epilepsy models. Hou et al (223) developed a dual-target nanoparticle system using microfluidic techniques to encapsulate lamotrigine in polyethylene glycol for targeted delivery to epileptic neurons. This system significantly alleviated seizure symptoms. DBS, an established method for managing DRE, was recently integrated with FUS to achieve minimally invasive neuromodulation. Chen et al reported that low-intensity FUS pulses effectively suppress seizure-related EEG discharges in pentylenetetrazole-induced epileptic rats, optimising exposure parameters and improving the antiepileptic effect (224). Clinical research has confirmed the safety and feasibility of FUS in targeting epileptogenic brain areas without significant adverse effects, laying the groundwork for its clinical translation (225). A summary of studies investigating BBB-penetrating antiepileptic therapies is provided in Table I.
Despite encouraging progress in nanotechnology-based drug delivery systems for epilepsy, several significant challenges hinder their successful translation from laboratory studies to clinical applications. First, there are unresolved safety concerns in vivo. While some nanomaterials demonstrate satisfactory biocompatibility in vitro, their behavior in living organisms may differ considerably. The mechanisms of the metabolism, degradation and excretion of nanodrugs remain poorly understood, leading to concerns about their potential long-term toxicity. Nanoparticles may accumulate in vital organs, impairing physiological functions, and such accumulation over time could result in organ dysfunction or failure. Additionally, the degradation products of nanomaterials may be toxic, posing continuous risks to human health even after therapeutic effects have ended. To address these safety issues, comprehensive studies on the biocompatibility, immunogenicity and chronic toxicity of nanomaterials are essential. Second, the potential immunogenicity of different nanoparticle formulations remains a concern. Although various nanomaterials have been extensively studied for the diagnosis of CNS disorders, relatively few studies have focused on their cytotoxicity, genotoxicity and immunotoxicity (226-228). Factors such as surface properties, particle size, morphology and chemical composition can all influence immune responses. Immunomodulatory therapies are currently under investigation for autoimmune-related epilepsy, in which abnormal immune activity contributes to seizure generation. Third, translating pharmacokinetic data from rodent models to humans presents additional complexity. The pathogenesis of a number of CNS disorders remains unclear, and significant physiological differences exist between humans and animal models. As a result, most rodent models may fail to accurately replicate the pathophysiological features of human epilepsy. Furthermore, controlling drug release in vivo with high precision is still challenging. Variations in the rate, timing and quantity of drug release can significantly impact therapeutic efficacy. Rapid release may cause excessive local concentrations, while slow or incomplete release may prevent the drug from reaching therapeutic levels at the target site. For instance, Zhang et al (229) observed that 2 h after treatment with FUS combined with MBs, Evans blue dye was unable to penetrate the brain parenchyma, indicating that the effect of FUS is reversible. MBs have shown good biocompatibility and safety in multiple studies (230-232), and initial clinical trials applying this method to temporarily open the BBB have reported no adverse patient outcomes (231). It has also been reported that FUS combined with MBs is safe and effective in patients with Parkinson's disease, with the BBB returning to normal within 24 h post-treatment (214). Nevertheless, before this technique can be widely adopted in clinical settings, its complete safety must be validated. In particular, the optimal frequency range for ultrasound application requires further clarification, as frequencies that are too high may pose serious safety risks, whereas lower frequencies may not sufficiently increase BBB permeability. Magnetically guided brain-targeted delivery using ionic nanoparticles has also been validated by several studies (217,233). However, recent findings indicate that nanomaterials, especially metal-based ones, may induce oxidative stress, inflammatory responses and alterations in neurotransmitter expression (234). Xiong et al (235) found that increased BBB permeability induced by photothermal effects is reversible, with barrier function returning to baseline within 48 h after treatment, providing further evidence for the safety of this strategy. Despite these findings, the long-term use of nanodrugs in epilepsy treatment may pose health risks and requires thorough evaluation through extensive preclinical and clinical studies.
In conclusion, nanodrug delivery systems can improve the solubility, stability and BBB permeability of antiepileptic compounds, offering a promising approach for overcoming drug resistance and reducing adverse effects. Although their unique capacity to cross the BBB has shown considerable potential in neurological therapies, their safety and efficacy remain major barriers to clinical translation. Furthermore, the development of wireless and minimally invasive DBS platforms is urgently required. Early diagnosis of CNS diseases can improve patient outcomes (236), and understanding the prognostic factors of epilepsy can help guide personalised treatment strategies. Accurate and reliable survival predictions can support clinical decision-making for epilepsy and related neurological disorders (237,238).
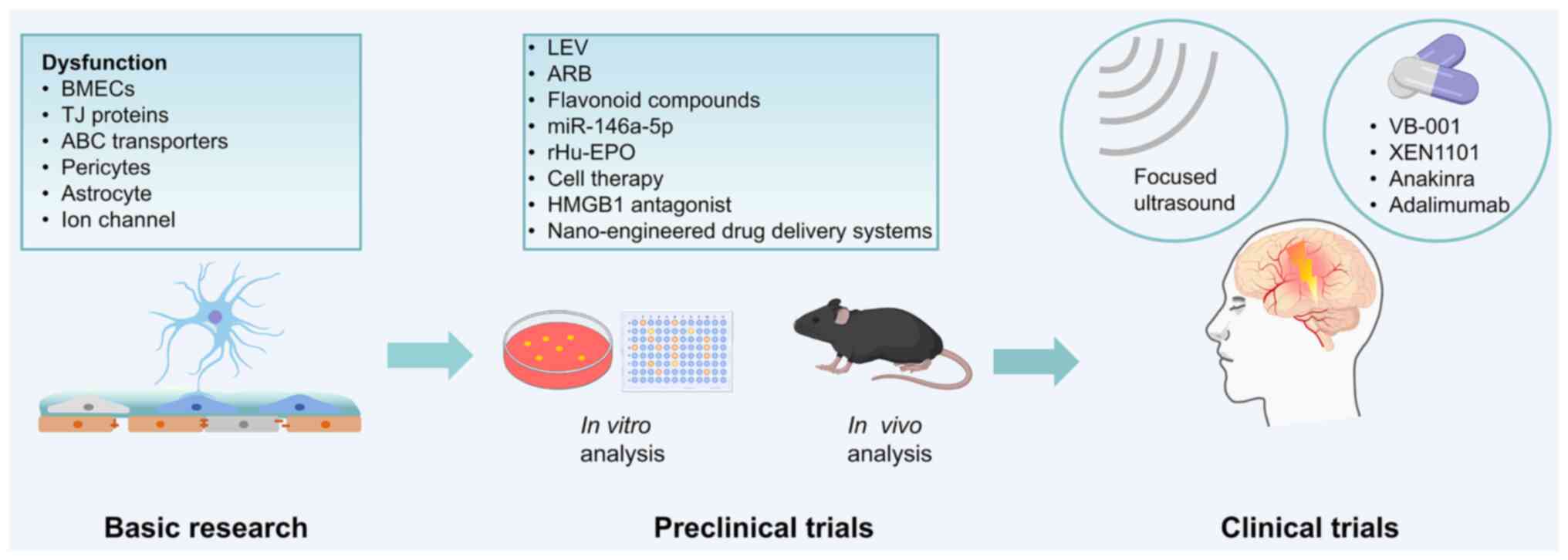
To illustrate the translational pathway of BBB-targeted therapies in epilepsy, a schematic overview of the developmental pipeline from basic research to clinical applications is presented in Fig. 6. Foundational studies have focused on BBB components such as endothelial cells, TJ proteins, astrocytes and transporters, which inform therapeutic target identification. Preclinical studies have used in vitro and in vivo models to assess candidate therapies, including LEV, ARBs, miR-146a-5p, rhEPO, flavonoids and nanocarrier systems, to control seizures and preserve BBB integrity. At the clinical stage, techniques such as FUS are being explored for BBB modulation, and agents such as VB-001, XEN1101, anakinra and adalimumab are being evaluated for their efficacy and safety. This framework highlights the stepwise process required to translate BBB-focused strategies from the laboratory to the clinic (Fig. 6).
Conclusion
The BBB is a complex and highly selective structure essential for maintaining a stable, extracellular environment in the CNS and ensuring optimal brain function. BBB integrity is closely associated with the progression of various neurological disorders, with BBB dysfunction serving as a significant pathological factor in CNS diseases. Evidence highlights a bidirectional relationship between epilepsy and BBB disruption. Epilepsy alters the molecular structure and functionality of the BBB through multiple mechanisms, whereas BBB damage can accelerate the onset and progression of epilepsy.
Seizure activity can compromise the BBB through mechanisms such as the release of excitatory neurotransmitters, abnormal vascular remodelling and altered ion channel function, resulting in increased permeability. These changes may increase brain excitability, facilitate seizure progression and elicit inflammatory responses. Simultaneously, BBB disruption allows plasma proteins such as albumin to enter the brain parenchyma, leading to inflammatory reactions, iron accumulation and infiltration of neurotoxic substances, which further aggravate seizure activity. This reciprocal relationship underscores the intricate connection between epilepsy and BBB integrity, with each condition exacerbating the other. Understanding these dynamics is vital for the development of novel therapeutic strategies.
Advancements in research have significantly enhanced the understanding of BBB molecular and cellular functions, as well as the pathological consequences of its dysfunction in CNS diseases. Disruptions in the brain microenvironment can impair neuronal function, promote cellular damage and trigger apoptosis, accelerating the pathological progression of epilepsy. Restoring BBB integrity is increasingly recognised as a critical strategy in treating epilepsy.
Given the unpredictability of epileptic seizures and the necessity for long-term drug therapy, the development of innovative treatment strategies to effectively manage seizures remains a priority. Future research should focus on several key areas: i) BBB repair and protection. Advancing therapeutic interventions to restore BBB integrity to mitigate the heightened neuronal excitability and inflammatory responses resulting from BBB injury. ii) Application of nanotechnology. Nanotechnology can be utilised to enhance drug penetration across the BBB and develop more efficient brain-targeted drug delivery systems, thereby achieving significant reductions in seizure frequency. iii) Ultrasound-mediated BBB opening. Exploring ultrasound technology to temporarily open the BBB, facilitating improved drug delivery through a non-invasive approach that offers potential for epilepsy treatment. iv) Comprehensive treatment strategies. Integrating BBB repair, cutting-edge drug delivery techniques and antiepileptic therapies to create a holistic treatment model for superior therapeutic outcomes.
Nanomaterial-based drug delivery systems have demonstrated great potential due to their favourable bioavailability and low toxicity, demonstrating efficacy in inhibiting or reducing seizure activity. However, additional clinical trials are required to validate their safety and efficacy. BBB-centred interventions represent a promising breakthrough in epilepsy treatment, particularly for mitigating disease progression and enabling secondary prevention. When integrated with other therapeutic approaches, these interventions could pave the way for novel strategies to prevent, control and reverse epilepsy progression. Comprehensive research on the chemical mediators and receptors involved in neuroinflammation may provide critical insights into the neurobiological mechanisms of epileptogenesis. This understanding could serve as a basis for identifying new biomarkers and therapeutic targets, facilitating the screening of individuals at high risk for epilepsy, and advancing both preventative and therapeutic strategies. A deeper understanding of the pathophysiological mechanisms underlying epilepsy is necessary to facilitate the development of novel drugs and therapeutic approaches. Currently, several new diagnostic and treatment strategies are being progressively implemented in clinical practice, including wearable devices for automatic seizure detection, minimally invasive surgical techniques for selective ablation of epileptogenic zones and precision medicine based on the genetic causes of epilepsy. Gene therapy for epilepsy has advanced significantly over the past decade, with some approaches entering clinical trials. In the future, clinical research should adopt more precise methods for regulating neural networks, such as optogenetics, to specifically target hyperactive neurons with abnormal gene expression. These efforts are expected to provide safer and more effective treatment options for epilepsy patients, improve their quality of life and contribute to major advances in epilepsy therapy.
Availability of data and materials
Not applicable.
Authors' contributions
NH, YWH and ZYD reviewed the literature and drafted the manuscript. SYQ and WZ were involved in reviewing and editing the manuscript. GHT and YYL revised and approved the final manuscript. All authors have read and approved the manuscript. Data authentication is not applicable.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Abbreviations:
|
ABCC |
ATP-binding-cassette transporters sub-family C |
|
ANGPT-1 |
angiopoietin-1 |
|
AQP4 |
aquaporin 4 |
|
ARB |
angiotensin receptor blocker |
|
ASD |
anti-seizure drugs |
|
BBB |
blood-brain barrier |
|
BCRP |
breast cancer resistance protein |
|
BMEC |
brain microvessel endothelial cell |
|
CNS |
central nervous system |
|
DBS |
deep brain stimulation |
|
DRE |
drug-resistant epilepsy |
|
EEG |
electroencephalographic |
|
FUS |
focused ultrasound |
|
GABA |
γ-aminobutyric acid |
|
GLUT1 |
glucose transporter 1 |
|
KA |
kainic acid |
|
Kir4.1 |
inwardly rectifying potassium channel 4.1 |
|
LEV |
levetiracetam |
|
MB |
microbubble |
|
MCT |
monocarboxylate transporter |
|
MLCK |
myosin light chain kinase |
|
MMP |
matrix metalloproteinase |
|
MRI |
magnetic resonance imaging |
|
MRP |
multidrug resistance protein |
|
NIR |
near-infrared |
|
PDGFRβ |
platelet-derived growth factor receptor β |
|
P-gp |
P-glycoprotein |
|
PKC |
protein kinase C |
|
rhEPO |
recombinant human erythropoietin |
|
SE |
status epilepticus |
|
SHH |
sonic hedgehog |
|
Tf |
transferrin |
|
TGF-βR |
transforming growth factor β |
|
receptor; TJ |
tight junction |
|
VEGFR |
vascular endothelial growth factor |
Acknowledgments
Not applicable.
Funding
This review was supported in part by a grant from the National Natural Science Foundation of China (no. 82060253).
References
|
Thijs RD, Surges R, O'Brien TJ and Sander JW: Epilepsy in adults. Lancet. 393:689–701. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
World Health Organization: Epilepsy: A public health imperative: Summary. World Health Organization; 2019 | |
|
Shao LR, Habela CW and Stafstrom CE: Pediatric epilepsy mechanisms: Expanding the paradigm of excitation/inhibition imbalance. Children (Basel). 6:232019.PubMed/NCBI | |
|
Fisher RS, Cross JH, French JA, Higurashi N, Hirsch E, Jansen FE, Lagae L, Moshé SL, Peltola J, Roulet Perez E, et al: Operational classification of seizure types by the international league against epilepsy: Position paper of the ILAE commission for classification and terminology. Epilepsia. 58:522–530. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Chen Z, Brodie MJ, Liew D and Kwan P: Treatment outcomes in patients with newly diagnosed epilepsy treated with established and new antiepileptic drugs: A 30-year longitudinal cohort study. JAMA Neurol. 75:279–286. 2018. View Article : Google Scholar : | |
|
Cendes F, Sakamoto AC, Spreafico R, Bingaman W and Becker AJ: Epilepsies associated with hippocampal sclerosis. Acta Neuropathol. 128:21–37. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Liu X, Zhang Y, Zhao Y, Zhang Q and Han F: The neurovascular unit dysfunction in the molecular mechanisms of epileptogenesis and targeted therapy. Neurosci Bull. 40:621–634. 2024. View Article : Google Scholar : PubMed/NCBI | |
|
Devinsky O, Vezzani A, O'Brien TJ, Jette N, Scheffer IE, de Curtis M and Perucca P: Epilepsy Nat Rev Dis Primers. 4:180242018. View Article : Google Scholar | |
|
Sultana B, Panzini MA, Veilleux Carpentier A, Comtois J, Rioux B, Gore G, Bauer PR, Kwon CS, Jetté N, Josephson CB and Keezer MR: Incidence and prevalence of drug-resistant epilepsy: A systematic review and meta-analysis. Neurology. 96:805–817. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Kantanen AM, Reinikainen M, Parviainen I and Kälviäinen R: Long-term outcome of refractory status epilepticus in adults: A retrospective population-based study. Epilepsy Res. 133:13–21. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Strzelczyk A, Griebel C, Lux W, Rosenow F and Reese JP: The Burden of severely drug-refractory epilepsy: A comparative longitudinal evaluation of mortality, morbidity, resource use, and cost using german health insurance data. Front Neurol. 8:7122017. View Article : Google Scholar | |
|
Wang T, Wang J, Dou Y, Yan W, Ding D, Lu G, Ma J, Zhou Y, Li T, Zhou S, et al: Clinical characteristics and prognosis in a large paediatric cohort with status epilepticus. Seizure. 80:5–11. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Tian L, Li Y, Xue X, Wu M, Liu F, Hao X and Zhou D: Super-refractory status epilepticus in West China. Acta Neurol Scand. 132:1–6. 2015. View Article : Google Scholar | |
|
Al-Otaibi FA, Hamani C and Lozano AM: Neuromodulation in epilepsy. Neurosurgery. 69:957–979. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
Dadas A and Janigro D: Breakdown of blood brain barrier as a mechanism of post-traumatic epilepsy. Neurobiol Dis. 123:20–26. 2019. View Article : Google Scholar : | |
|
Baghirov H: Receptor-mediated transcytosis of macromolecules across the blood-brain barrier. Expert Opin Drug Deliv. 20:1699–1711. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Chaves JCS, Dando SJ, White AR and Oikari LE: Blood-brain barrier transporters: An overview of function, dysfunction in Alzheimer's disease and strategies for treatment. Biochim Biophys Acta Mol Basis Dis. 1870:1669672024. View Article : Google Scholar | |
|
Patabendige A and Janigro D: The role of the blood-brain barrier during neurological disease and infection. Biochem Soc Trans. 51:613–626. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Vazana U, Veksler R, Pell GS, Prager O, Fassler M, Chassidim Y, Roth Y, Shahar H, Zangen A, Raccah R, et al: Glutamate-mediated blood-brain barrier opening: Implications for neuroprotection and drug delivery. J Neurosci. 36:7727–7739. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Leandro K, Bicker J, Alves G, Falcão A and Fortuna A: ABC transporters in drug-resistant epilepsy: Mechanisms of upregulation and therapeutic approaches. Pharmacol Res. 144:357–376. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Celentano C, Carotenuto L, Miceli F, Carleo G, Corrado B, Baroli G, Iervolino S, Vecchione R, Taglialatela M and Barrese V: Kv7 channel activation reduces brain endothelial cell permeability and prevents kainic acid-induced blood-brain barrier damage. Am J Physiol Cell Physiol. 326:C893–C904. 2024. View Article : Google Scholar : PubMed/NCBI | |
|
Reimann F: Liver diseases and pernicious anemia; clinical and physiopathological study of the behavior of the anti-pernicious factor in the body. Blut. 4:261–279. 1958.In German. View Article : Google Scholar : PubMed/NCBI | |
|
Bentivoglio M and Kristensson K: Tryps and trips: Cell trafficking across the 100-year-old blood-brain barrier. Trends Neurosci. 37:325–333. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Reese TS and Karnovsky MJ: Fine structural localization of a blood-brain barrier to exogenous peroxidase. J Cell Biol. 34:207–217. 1967. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang S, Gan L, Cao F, Wang H, Gong P, Ma C, Ren L, Lin Y and Lin X: The barrier and interface mechanisms of the brain barrier, and brain drug delivery. Brain Res Bull. 190:69–83. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Yan L, Moriarty RA and Stroka KM: Recent progress and new challenges in modeling of human pluripotent stem cell-derived blood-brain barrier. Theranostics. 11:10148–10170. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Ushiyama A, Kataoka H and Iijima T: Glycocalyx and its involvement in clinical pathophysiologies. J Intensive Care. 4:592016. View Article : Google Scholar : PubMed/NCBI | |
|
Iba T and Levy JH: Derangement of the endothelial glycocalyx in sepsis. J Thromb Haemost. 17:283–294. 2019. View Article : Google Scholar | |
|
Liu R, Collier JM, Abdul-Rahman NH, Capuk O, Zhang Z and Begum G: Dysregulation of Ion channels and transporters and blood-brain barrier dysfunction in Alzheimer's disease and vascular dementia. Aging Dis. 15:1748–1770. 2024.PubMed/NCBI | |
|
Nguyen B, Bix G and Yao Y: Basal lamina changes in neurodegenerative disorders. Mol Neurodegener. 16:812021. View Article : Google Scholar : PubMed/NCBI | |
|
Armulik A, Genové G, Mäe M, Nisancioglu MH, Wallgard E, Niaudet C, He L, Norlin J, Lindblom P, Strittmatter K, et al: Pericytes regulate the blood-brain barrier. Nature. 468:557–561. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Gaceb A, Özen I, Padel T, Barbariga M and Paul G: Pericytes secrete pro-regenerative molecules in response to platelet-derived growth factor-BB. J Cereb Blood Flow Metab. 38:45–57. 2018. View Article : Google Scholar : | |
|
Dabravolski SA, Andreeva ER, Eremin II, Markin AM, Nadelyaeva II, Orekhov AN and Melnichenko AA: The role of pericytes in regulation of innate and adaptive immunity. Biomedicines. 11:6002023. View Article : Google Scholar : PubMed/NCBI | |
|
Giovannoni F and Quintana FJ: The role of astrocytes in CNS inflammation. Trends Immunol. 41:805–819. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Abbott NJ, Rönnbäck L and Hansson E: Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 7:41–53. 2006. View Article : Google Scholar | |
|
Díaz-Castro B, Robel S and Mishra A: Astrocyte endfeet in brain function and pathology: Open questions. Annu Rev Neurosci. 46:101–121. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Alvarez JI, Dodelet-Devillers A, Kebir H, Ifergan I, Fabre PJ, Terouz S, Sabbagh M, Wosik K, Bourbonnière L, Bernard M, et al: The hedgehog pathway promotes blood-brain barrier integrity and CNS immune quiescence. Science. 334:1727–1731. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
Xing G, Zhao T, Zhang X, Li H, Li X, Cui P, Li M, Li D, Zhang N and Jiang W: Astrocytic Sonic hedgehog alleviates intracerebral hemorrhagic brain injury via modulation of blood-brain barrier integrity. Front Cell Neurosci. 14:5756902020. View Article : Google Scholar : PubMed/NCBI | |
|
Takahashi S: Metabolic contribution and cerebral blood flow regulation by astrocytes in the neurovascular unit. Cells. 11:8132022. View Article : Google Scholar : PubMed/NCBI | |
|
Cornford EM and Oldendorf WH: Epilepsy and the blood-brain barrier. Adv Neurol. 44:787–812. 1986.PubMed/NCBI | |
|
Leroy C, Roch C, Koning E, Namer IJ and Nehlig A: In the lithium-pilocarpine model of epilepsy, brain lesions are not linked to changes in blood-brain barrier permeability: An autoradiographic study in adult and developing rats. Exp Neurol. 182:361–372. 2003. View Article : Google Scholar : PubMed/NCBI | |
|
Saija A, Princi P, Pisani A, Santoro G, De Pasquale R, Massi M and Costa G: Blood-brain barrier dysfunctions following systemic injection of kainic acid in the rat. Life Sci. 51:467–477. 1992. View Article : Google Scholar : PubMed/NCBI | |
|
Librizzi L, Noè F, Vezzani A, de Curtis M and Ravizza T: Seizure-induced brain-borne inflammation sustains seizure recurrence and blood-brain barrier damage. Ann Neurol. 72:82–90. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Michalak Z, Sano T, Engel T, Miller-Delaney SFC, Lerner-Natoli M and Henshall DC: Spatio-temporally restricted blood-brain barrier disruption after intra-amygdala kainic acid-induced status epilepticus in mice. Epilepsy Res. 103:167–179. 2013. View Article : Google Scholar | |
|
Löscher W: Epilepsy and alterations of the blood-brain barrier: Cause or consequence of epileptic seizures or both? Handb Exp Pharmacol. 273:331–350. 2022. View Article : Google Scholar | |
|
Castañeda-Cabral JL, Colunga-Durán A, Ureña-Guerrero ME, Beas-Zárate C, Nuñez-Lumbreras MLA, Orozco-Suárez S, Alonso-Vanegas M, Guevara-Guzmán R, Deli MA, Valle-Dorado MG, et al: Expression of VEGF- and tight junction-related proteins in the neocortical microvasculature of patients with drug-resistant temporal lobe epilepsy. Microvasc Res. 132:1040592020. View Article : Google Scholar : PubMed/NCBI | |
|
Morin-Brureau M, Lebrun A, Rousset MC, Fagni L, Bockaert J, de Bock F and Lerner-Natoli M: Epileptiform activity induces vascular remodeling and zonula occludens 1 downregulation in organotypic hippocampal cultures: Role of VEGF signaling pathways. J Neurosci. 31:10677–10688. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
Rempe RG, Hartz AMS, Soldner ELB, Sokola BS, Alluri SR, Abner EL, Kryscio RJ, Pekcec A, Schlichtiger J and Bauer B: Matrix metalloproteinase-mediated blood-brain barrier dysfunction in epilepsy. J Neurosci. 38:4301–4315. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Bronisz E, Cudna A, Wierzbicka A and Kurkowska-Jastrzębska I: Blood-brain barrier-associated proteins are elevated in serum of epilepsy patients. Cells. 12:3682023. View Article : Google Scholar : PubMed/NCBI | |
|
Lai M, Zou W, Han Z, Zhou L, Qiu Z, Chen J, Zhang S, Lai P, Li K, Zhang Y, et al: Tsc1 regulates tight junction independent of mTORC1. Proc Natl Acad Sci USA. 118:e20208911182021. View Article : Google Scholar : PubMed/NCBI | |
|
Guo D, Zhang B, Han L, Rensing NR and Wong M: Cerebral vascular and blood brain-barrier abnormalities in a mouse model of epilepsy and tuberous sclerosis complex. Epilepsia. 65:483–496. 2024. View Article : Google Scholar : | |
|
Ogaki A, Ikegaya Y and Koyama R: Vascular abnormalities and the role of vascular endothelial growth factor in the epileptic brain. Front Pharmacol. 11:202020. View Article : Google Scholar : PubMed/NCBI | |
|
Sweeney MD, Zhao Z, Montagne A, Nelson AR and Zlokovic BV: Blood-brain barrier: From physiology to disease and back. Physiol Rev. 99:21–78. 2019. View Article : Google Scholar : | |
|
Klement W, Blaquiere M, Zub E, deBock F, Boux F, Barbier E, Audinat E, Lerner-Natoli M and Marchi N: A pericyte-glia scarring develops at the leaky capillaries in the hippocampus during seizure activity. Epilepsia. 60:1399–1411. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Prager O, Kamintsky L, Hasam-Henderson LA, Schoknecht K, Wuntke V, Papageorgiou I, Swolinsky J, Muoio V, Bar-Klein G, Vazana U, et al: Seizure-induced microvascular injury is associated with impaired neurovascular coupling and blood-brain barrier dysfunction. Epilepsia. 60:322–336. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Li Q, Li QQ, Jia JN, Liu ZQ, Zhou HH and Mao XY: Targeting gap junction in epilepsy: Perspectives and challenges. Biomed Pharmacother. 109:57–65. 2019. View Article : Google Scholar | |
|
Haruwaka K, Ikegami A, Tachibana Y, Ohno N, Konishi H, Hashimoto A, Matsumoto M, Kato D, Ono R, Kiyama H, et al: Dual microglia effects on blood brain barrier permeability induced by systemic inflammation. Nat Commun. 10:58162019. View Article : Google Scholar : PubMed/NCBI | |
|
Park H, Choi SH, Kong MJ and Kang TC: Dysfunction of 67-kDa laminin receptor disrupts bbb integrity via impaired dystrophin/AQP4 complex and p38 MAPK/VEGF activation following status epilepticus. Front Cell Neurosci. 13:2362019. View Article : Google Scholar : PubMed/NCBI | |
|
Zhu H, Dai R, Zhou Y, Fu H and Meng Q: TLR2 ligand Pam3CSK4 regulates MMP-2/9 expression by MAPK/NF-κB signaling pathways in primary brain microvascular endothelial cells. Neurochem Res. 43:1897–1904. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Yazarlou F, Lipovich L and Loeb JA: Emerging roles of long non-coding RNAs in human epilepsy. Epilepsia. 65:1491–1511. 2024. View Article : Google Scholar : PubMed/NCBI | |
|
Du Y, Chi X and An W: Downregulation of microRNA-200c-3p reduces damage of hippocampal neurons in epileptic rats by upregulating expression of RECK and inactivating the AKT signaling pathway. Chem Biol Interact. 307:223–233. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Meijer WC and Gorter JA: Role of blood-brain barrier dysfunction in the development of poststroke epilepsy. Epilepsia. 65:2519–2536. 2024. View Article : Google Scholar : PubMed/NCBI | |
|
Ngo A, Royer J, Rodriguez-Cruces R, Xie K, DeKraker J, Auer H, Tavakol S, Lam J, Schrader DV, Dudley RWR, et al: Associations of cerebral blood flow patterns with gray and white matter structure in patients with temporal lobe epilepsy. Neurology. 103:e2095282024. View Article : Google Scholar : PubMed/NCBI | |
|
Musaeus CS, Kjaer TW, Lindberg U, Vestergaard MB, Bo H, Larsson W, Press DZ, Andersen BB, Høgh P, Kidmose P, et al: Subclinical epileptiform discharges in Alzheimer's disease are associated with increased hippocampal blood flow. Alzheimers Res Ther. 16:802024. View Article : Google Scholar : PubMed/NCBI | |
|
Chai AB, Callaghan R and Gelissen IC: Regulation of P-glycoprotein in the brain. Int J Mol Sci. 23:146672022. View Article : Google Scholar : PubMed/NCBI | |
|
Tishler DM, Weinberg KI, Hinton DR, Barbaro N, Annett GM and Raffel C: MDR1 gene expression in brain of patients with medically intractable epilepsy. Epilepsia. 36:1–6. 1995. View Article : Google Scholar : PubMed/NCBI | |
|
Lazarowski A, Ramos AJ, García-Rivello H, Brusco A and Girardi E: Neuronal and glial expression of the multidrug resistance gene product in an experimental epilepsy model. Cell Mol Neurobiol. 24:77–85. 2004. View Article : Google Scholar : PubMed/NCBI | |
|
Hartz AMS, Pekcec A, Soldner ELB, Zhong Y, Schlichtiger J and Bauer B: P-gp protein expression and transport activity in rodent seizure models and human epilepsy. Mol Pharm. 14:999–1011. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Liu JYW, Thom M, Catarino CB, Mar tinian L, Figarella-Branger D, Bartolomei F, Koepp M and Sisodiya SM: Neuropathology of the blood-brain barrier and pharmaco-resistance in human epilepsy. Brain. 135:3115–3133. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
van Vliet EA, Redeker S, Aronica E, Edelbroek PM and Gorter JA: Expression of multidrug transporters MRP1, MRP2, and BCRP shortly after status epilepticus, during the latent period, and in chronic epileptic rats. Epilepsia. 46:1569–1580. 2005. View Article : Google Scholar : PubMed/NCBI | |
|
Aronica E, Gorter JA, Redeker S, van Vliet EA, Ramkema M, Scheffer GL, Scheper RJ, van der Valk P, Leenstra S, Baayen JC, et al: Localization of breast cancer resistance protein (BCRP) in microvessel endothelium of human control and epileptic brain. Epilepsia. 46:849–857. 2005. View Article : Google Scholar : PubMed/NCBI | |
|
Aronica E, Gorter JA, Jansen GH, van Veelen CW, van Rijen PC, Leenstra S, Ramkema M, Scheffer GL, Scheper RJ and Troost D: Expression and cellular distribution of multidrug transporter proteins in two major causes of medically intractable epilepsy: Focal cortical dysplasia and glioneuronal tumors. Neuroscience. 118:417–429. 2003. View Article : Google Scholar : PubMed/NCBI | |
|
Ferreira A, Rodrigues M, Fortuna A, Falcão A and Alves G: Flavonoid compounds as reversing agents of the P-glycoprotein-mediated multidrug resistance: An in vitro evaluation with focus on antiepileptic drugs. Food Res Int. 103:110–120. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Gil-Martins E, Barbosa DJ, Silva V, Remião F and Silva R: Dysfunction of ABC transporters at the blood-brain barrier: Role in neurological disorders. Pharmacol Ther. 213:1075542020. View Article : Google Scholar : PubMed/NCBI | |
|
Vijay N and Morris ME: Role of monocarboxylate transporters in drug delivery to the brain. Curr Pharm Des. 20:1487–1498. 2014. View Article : Google Scholar : | |
|
Lauritzen F, Perez EL, Melillo ER, Roh JM, Zaveri HP, Lee TS, Wang Y, Bergersen LH and Eid T: Altered expression of brain monocarboxylate transporter 1 in models of temporal lobe epilepsy. Neurobiol Dis. 45:165–176. 2012. View Article : Google Scholar : | |
|
van Vliet EA, Otte WM, Gorter JA, Dijkhuizen RM and Wadman WJ: Longitudinal assessment of blood-brain barrier leakage during epileptogenesis in rats. A quantitative MRI study Neurobiol Dis. 63:74–84. 2014. View Article : Google Scholar | |
|
Bar-Klein G, Lublinsky S, Kamintsky L, Noyman I, Veksler R, Dalipaj H, Senatorov VV Jr, Swissa E, Rosenbach D, Elazary N, et al: Imaging blood-brain barrier dysfunction as a biomarker for epileptogenesis. Brain. 140:1692–1705. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Scott G, Mahmud M, Owen DR and Johnson MR: Microglial positron emission tomography (PET) imaging in epilepsy: Applications, opportunities and pitfalls. Seizure. 44:42–47. 2017. View Article : Google Scholar | |
|
Gruenbaum BF, Zlotnik A, Fleidervish I, Frenkel A and Boyko M: Glutamate neurotoxicity and destruction of the blood-brain barrier: Key pathways for the development of neuropsychiatric consequences of TBI and their potential treatment strategies. Int J Mol Sci. 23:96282022. View Article : Google Scholar : PubMed/NCBI | |
|
Barker-Haliski M and White HS: Glutamatergic mechanisms associated with seizures and epilepsy. Cold Spring Harb Perspect Med. 5:a0228632015. View Article : Google Scholar : PubMed/NCBI | |
|
Hogan-Cann AD and Anderson CM: Physiological roles of non-neuronal NMDA receptors. Trends Pharmacol Sci. 37:750–767. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Kim KS, Jeon MT, Kim ES, Lee CH and Kim DG: Activation of NMDA receptors in brain endothelial cells increases transcellular permeability. Fluids Barriers CNS. 19:702022. View Article : Google Scholar : PubMed/NCBI | |
|
Sharp CD, Hines I, Houghton J, Warren A, Jackson TH IV, Jawahar A, Nanda A, Elrod JW, Long A, Chi A, et al: Glutamate causes a loss in human cerebral endothelial barrier integrity through activation of NMDA receptor. Am J Physiol Heart Circ Physiol. 285:H2592–H2598. 2003. View Article : Google Scholar : PubMed/NCBI | |
|
Chen JT, Chen TG, Chang YC, Chen CY and Chen RM: Roles of NMDARs in maintenance of the mouse cerebrovascular endothelial cell-constructed tight junction barrier. Toxicology. 339:40–50. 2016. View Article : Google Scholar | |
|
Yang T, Liu YW, Zhao L, Wang H, Yang N, Dai SS and He F: Metabotropic glutamate receptor 5 deficiency inhibits neutrophil infiltration after traumatic brain injury in mice. Sci Rep. 7:99982017. View Article : Google Scholar : PubMed/NCBI | |
|
Ren J, Yang T, Liu H, Ma P, Zhou M, Li J, Li T, Sun J, He W, Xu L, et al: Metabotropic glutamate receptor 5 promotes blood-brain barrier recovery after traumatic brain injury. Exp Neurol. 374:1146912024. View Article : Google Scholar : PubMed/NCBI | |
|
Liu J, Feng X, Wang Y, Xia X and Zheng JC: Astrocytes: GABAceptive and GABAergic cells in the brain. Front Cell Neurosci. 16:8924972022. View Article : Google Scholar : PubMed/NCBI | |
|
Kopeikina E, Dukhinova M, Yung AWY, Veremeyko T, Kuznetsova IS, Lau TYB, Levchuk K and Ponomarev ED: Platelets promote epileptic seizures by modulating brain serotonin level, enhancing neuronal electric activity, and contributing to neuroinflammation and oxidative stress. Prog Neurobiol. 188:1017832020. View Article : Google Scholar : PubMed/NCBI | |
|
Wang Y, Zhu Y, Wang J, Dong L, Liu S, Li S and Wu Q: Purinergic signaling: A gatekeeper of blood-brain barrier permeation. Front Pharmacol. 14:11127582023. View Article : Google Scholar : PubMed/NCBI | |
|
Zhao H, Zhang X, Dai Z, Feng Y, Li Q, Zhang JH, Liu X, Chen Y and Feng H: P2X7 receptor suppression preserves blood-brain barrier through inhibiting RhoA activation after experimental intracerebral hemorrhage in rats. Sci Rep. 6:232862016. View Article : Google Scholar : PubMed/NCBI | |
|
Wang K, Sun M, Juan Z, Zhang J, Sun Y, Wang G, Wang C, Li Y, Kong W, Fan L, et al: The improvement of sepsis-associated encephalopathy by P2X7R inhibitor through inhibiting the Omi/HtrA2 apoptotic signaling pathway. Behav Neurol. 2022:37773512022. View Article : Google Scholar : PubMed/NCBI | |
|
van Vliet EA, Otte WM, Wadman WJ, Aronica E, Kooij G, de Vries HE, Dijkhuizen RM and Gorter JA: Blood-brain barrier leakage after status epilepticus in rapamycin-treated rats II: Potential mechanisms. Epilepsia. 57:70–78. 2016. View Article : Google Scholar | |
|
Feng L, Shu Y, Wu Q, Liu T, Long H, Yang H, Li Y and Xiao B: EphA4 may contribute to microvessel remodeling in the hippocampal CA1 and CA3 areas in a mouse model of temporal lobe epilepsy. Mol Med Rep. 15:37–46. 2017. View Article : Google Scholar : | |
|
Rigau V, Morin M, Rousset MC, de Bock F, Lebrun A, Coubes P, Picot MC, Baldy-Moulinier M, Bockaert J, Crespel A and Lerner-Natoli M: Angiogenesis is associated with blood-brain barrier permeability in temporal lobe epilepsy. Brain. 130:1942–1956. 2007. View Article : Google Scholar : PubMed/NCBI | |
|
Hoeben A, Landuyt B, Highley MS, Wildiers H, Van Oosterom AT and De Bruijn EA: Vascular endothelial growth factor and angiogenesis. Pharmacol Rev. 56:549–580. 2004. View Article : Google Scholar : PubMed/NCBI | |
|
Waldbaum S and Patel M: Mitochondrial dysfunction and oxidative stress: A contributing link to acquired epilepsy? J Bioenerg Biomembr. 42:449–455. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Pugh CW and Ratcliffe PJ: Regulation of angiogenesis by hypoxia: Role of the HIF system. Nat Med. 9:677–684. 2003. View Article : Google Scholar : PubMed/NCBI | |
|
Marchi N, Granata T, Ghosh C and Janigro D: Blood-brain barrier dysfunction and epilepsy: Pathophysiologic role and therapeutic approaches. Epilepsia. 53:1877–1886. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Wengert ER and Patel MK: The role of the persistent sodium current in epilepsy. Epilepsy Curr. 21:40–47. 2021. View Article : Google Scholar : | |
|
Di Cristo G, Awad PN, Hamidi S and Avoli M: KCC2, epileptiform synchronization, and epileptic disorders. Prog Neurobiol. 162:1–16. 2018. View Article : Google Scholar | |
|
Xu JH and Tang FR: Voltage-dependent calcium channels, calcium binding proteins, and their interaction in the pathological process of epilepsy. Int J Mol Sci. 19:27352018. View Article : Google Scholar : PubMed/NCBI | |
|
Dalal PJ, Muller WA and Sullivan DP: Endothelial cell calcium signaling during barrier function and inflammation. Am J Pathol. 190:535–542. 2020. View Article : Google Scholar : | |
|
Wu J, Yang J, Yu M, Sun W, Han Y, Lu X, Jin C, Wu S and Cai Y: Lanthanum chloride causes blood-brain barrier disruption through intracellular calcium-mediated RhoA/Rho kinase signaling and myosin light chain kinase. Metallomics. 12:2075–2083. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Luh C, Feiler S, Frauenknecht K, Meyer S, Lubomirov LT, Neulen A and Thal SC: The contractile apparatus is essential for the integrity of the blood-brain barrier after experimental subarachnoid hemorrhage. Transl Stroke Res. 10:534–545. 2019. View Article : Google Scholar : | |
|
Hubbard JA, Szu JI, Yonan JM and Binder DK: Regulation of astrocyte glutamate transporter-1 (GLT1) and aquaporin-4 (AQP4) expression in a model of epilepsy. Exp Neurol. 283:85–96. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Alvestad S, Hammer J, Hoddevik EH, Skare Ø, Sonnewald U, Amiry-Moghaddam M and Ottersen OP: Mislocalization of AQP4 precedes chronic seizures in the kainate model of temporal lobe epilepsy. Epilepsy Res. 105:30–41. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Salman MM, Sheilabi MA, Bhattacharyya D, Kitchen P, Conner AC, Bill RM, Woodroofe MN, Conner MT and Princivalle AP: Transcriptome analysis suggests a role for the differential expression of cerebral aquaporins and the MAPK signalling pathway in human temporal lobe epilepsy. Eur J Neurosci. 46:2121–2132. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Vandebroek A and Yasui M: Regulation of AQP4 in the central nervous system. Int J Mol Sci. 21:16032020. View Article : Google Scholar : PubMed/NCBI | |
|
Bonosi L, Benigno UE, Musso S, Giardina K, Gerardi RM, Brunasso L, Costanzo R, Paolini F, Buscemi F, Avallone C, et al: The role of aquaporins in epileptogenesis-a systematic review. Int J Mol Sci. 24:119232023. View Article : Google Scholar : PubMed/NCBI | |
|
Zhu DD, Yang G, Huang YL, Zhang T, Sui AR, Li N, Su WH, Sun HL, Gao JJ, Ntim M, et al: AQP4-A25Q point mutation in mice depolymerizes orthogonal arrays of particles and decreases polarized expression of AQP4 protein in astrocytic endfeet at the blood-brain barrier. J Neurosci. 42:8169–8183. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Lin X, Peng Y, Guo Z, He W, Guo W, Feng J, Lu L, Liu Q and Xu P: Short-chain fatty acids suppresses astrocyte activation by amplifying Trp-AhR-AQP4 signaling in experimental autoimmune encephalomyelitis mice. Cell Mol Life Sci. 81:2932024. View Article : Google Scholar : PubMed/NCBI | |
|
Lee DJ, Hsu MS, Seldin MM, Arellano JL and Binder DK: Decreased expression of the glial water channel aquaporin-4 in the intrahippocampal kainic acid model of epileptogenesis. Exp Neurol. 235:246–255. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Murphy TR, Davila D, Cuvelier N, Young LR, Lauderdale K, Binder DK and Fiacco TA: Hippocampal and cortical pyramidal neurons swell in parallel with astrocytes during acute hypoosmolar stress. Front Cell Neurosci. 11:2752017. View Article : Google Scholar : PubMed/NCBI | |
|
Seiffert E, Dreier JP, Ivens S, Bechmann I, Tomkins O, Heinemann U and Friedman A: Lasting blood-brain barrier disruption induces epileptic focus in the rat somatosensory cortex. J Neurosci. 24:7829–7836. 2004. View Article : Google Scholar : PubMed/NCBI | |
|
van Vliet EA, da Costa Araújo S, Redeker S, van Schaik R, Aronica E and Gorter JA: Blood-brain barrier leakage may lead to progression of temporal lobe epilepsy. Brain. 130:521–534. 2007. View Article : Google Scholar | |
|
Savotchenko A, Klymenko M, Shypshyna M and Isaev D: The role of thrombin in early-onset seizures. Front Cell Neurosci. 17:11010062023. View Article : Google Scholar : PubMed/NCBI | |
|
Greene C, Hanley N, Reschke CR, Reddy A, Mäe MA, Connolly R, Behan C, O'Keeffe E, Bolger I, Hudson N, et al: Microvascular stabilization via blood-brain barrier regulation prevents seizure activity. Nat Commun. 13:20032022. View Article : Google Scholar | |
|
Liu XX, Yang L, Shao LX, He Y, Wu G, Bao YH, Lu NN, Gong DM, Lu YP, Cui TT, et al: Endothelial Cdk5 deficit leads to the development of spontaneous epilepsy through CXCL1/CXCR2-mediated reactive astrogliosis. J Exp Med. 217:e201809922020. View Article : Google Scholar : | |
|
Salar S, Maslarova A, Lippmann K, Nichtweiss J, Weissberg I, Sheintuch L, Kunz WS, Shorer Z, Friedman A and Heinemann U: Blood-brain barrier dysfunction can contribute to pharmacoresistance of seizures. Epilepsia. 55:1255–1263. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Lapilover EG, Lippmann K, Salar S, Maslarova A, Dreier JP, Heinemann U and Friedman A: Peri-infarct blood-brain barrier dysfunction facilitates induction of spreading depolarization associated with epileptiform discharges. Neurobiol Dis. 48:495–506. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Frigerio F, Frasca A, Weissberg I, Parrella S, Friedman A, Vezzani A and Noé FM: Long-lasting pro-ictogenic effects induced in vivo by rat brain exposure to serum albumin in the absence of concomitant pathology. Epilepsia. 53:1887–1897. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Salar S, Lapilover E, Müller J, Hollnagel JO, Lippmann K, Friedman A and Heinemann U: Synaptic plasticity in area CA1 of rat hippocampal slices following intraventricular application of albumin. Neurobiol Dis. 91:155–165. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Noé FM, Bellistri E, Colciaghi F, Cipelletti B, Battaglia G, de Curtis M and Librizzi L: Kainic acid-induced albumin leak across the blood-brain barrier facilitates epileptiform hyperexcitability in limbic regions. Epilepsia. 57:967–976. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Kinboshi M, Ikeda A and Ohno Y: Role of astrocytic inwardly rectifying potassium (Kir) 4.1 channels in epileptogenesis. Front Neurol. 11:6266582020. View Article : Google Scholar | |
|
Szu JI and Binder DK: Mechanisms underlying aquaporin-4 subcellular mislocalization in epilepsy. Front Cell Neurosci. 16:9005882022. View Article : Google Scholar : PubMed/NCBI | |
|
David Y, Cacheaux LP, Ivens S, Lapilover E, Heinemann U, Kaufer D and Friedman A: Astrocytic dysfunction in epileptogenesis: Consequence of altered potassium and glutamate homeostasis? J Neurosci. 29:10588–10599. 2009. View Article : Google Scholar : PubMed/NCBI | |
|
Wallraff A, Köhling R, Heinemann U, Theis M, Willecke K and Steinhäuser C: The impact of astrocytic gap junctional coupling on potassium buffering in the hippocampus. J Neurosci. 26:5438–5447. 2006. View Article : Google Scholar : PubMed/NCBI | |
|
Vezzani A, Ravizza T, Bedner P, Aronica E, Steinhäuser C and Boison D: Astrocytes in the initiation and progression of epilepsy. Nat Rev Neurol. 18:707–722. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Qiu YM, Zhang CL, Chen AQ, Wang HL, Zhou YF, Li YN and Hu B: Immune cells in the BBB disruption after acute ischemic stroke: Targets for immune therapy? Front Immunol. 12:6787442021. View Article : Google Scholar : PubMed/NCBI | |
|
Yang J, Ran M, Li H, Lin Y, Ma K, Yang Y, Fu X and Yang S: New insight into neurological degeneration: Inflammatory cytokines and blood-brain barrier. Front Mol Neurosci. 15:10139332022. View Article : Google Scholar : PubMed/NCBI | |
|
Broekaart DWM, Anink JJ, Baayen JC, Idema S, de Vries HE, Aronica E, Gorter JA and van Vliet EA: Activation of the innate immune system is evident throughout epileptogenesis and is associated with blood-brain barrier dysfunction and seizure progression. Epilepsia. 59:1931–1944. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Di Nunzio M, Di Sapia R, Sorrentino D, Kebede V, Cerovic M, Gullotta GS, Bacigaluppi M, Audinat E, Marchi N, Ravizza T and Vezzani A: Microglia proliferation plays distinct roles in acquired epilepsy depending on disease stages. Epilepsia. 62:1931–1945. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Kamaşak T, Dilber B, Yaman SÖ, Durgut BD, Kurt T, Çoban E, Arslan EA, Şahin S, Karahan SC and Cansu A: HMGB-1, TLR4, IL-1R1, TNF-α, and IL-1β: Novel epilepsy markers? Epileptic Disord. 22:183–193. 2020. View Article : Google Scholar | |
|
Greene C, Hanley N and Campbell M: Claudin-5: Gatekeeper of neurological function. Fluids Barriers CNS. 16:32019. View Article : Google Scholar : PubMed/NCBI | |
|
Clark PR, Kim RK, Pober JS and Kluger MS: Tumor necrosis factor disrupts claudin-5 endothelial tight junction barriers in two distinct NF-κB-dependent phases. PLoS One. 10:e01200752015. View Article : Google Scholar | |
|
Khan A, Ni W, Lopez-Giraldez F, Kluger MS, Pober JS and Pierce RW: Tumor necrosis factor-induced ArhGEF10 selectively activates RhoB contributing to human microvascular endothelial cell tight junction disruption. FASEB J. 35:e216272021. View Article : Google Scholar : PubMed/NCBI | |
|
Tsai MM, Chen JL, Lee TH, Liu H, Shanmugam V and Hsieh HL: Brain protective effect of resveratrol via ameliorating interleukin-1β-induced MMP-9-mediated disruption of ZO-1 arranged integrity. Biomedicines. 10:12702022. View Article : Google Scholar | |
|
Manu DR, Slevin M, Barcutean L, Forro T, Boghitoiu T and Balasa R: Astrocyte involvement in blood-brain barrier function: A critical update highlighting novel, complex, neurovascular interactions. Int J Mol Sci. 24:171462023. View Article : Google Scholar : PubMed/NCBI | |
|
Viviani B, Bartesaghi S, Gardoni F, Vezzani A, Behrens MM, Bartfai T, Binaglia M, Corsini E, Di Luca M, Galli CL and Marinovich M: Interleukin-1beta enhances NMDA receptor-mediated intracellular calcium increase through activation of the Src family of kinases. J Neurosci. 23:8692–8700. 2003. View Article : Google Scholar : PubMed/NCBI | |
|
Mukhtar I: Inflammatory and immune mechanisms underlying epileptogenesis and epilepsy: From pathogenesis to treatment target. Seizure. 82:65–79. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Yang F, Zhao K, Zhang X, Zhang J and Xu B: ATP induces disruption of tight junction proteins via IL-1 beta-dependent MMP-9 activation of human blood-brain barrier in vitro. Neural Plast. 2016:89285302016. View Article : Google Scholar : PubMed/NCBI | |
|
Qiu J, Xu J, Zheng Y, Wei Y, Zhu X, Lo EH, Moskowitz MA and Sims JR: High-mobility group box 1 promotes metalloproteinase-9 upregulation through Toll-like receptor 4 after cerebral ischemia. Stroke. 41:2077–2082. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Kim EJ, Park SY, Baek SE, Jang MA, Lee WS, Bae SS, Kim K and Kim CD: HMGB1 Increases IL-1β production in vascular smooth muscle cells via NLRP3 inflammasome. Front Physiol. 9:3132018. View Article : Google Scholar | |
|
de Jong JM, Broekaart DWM, Bongaarts A, Mühlebner A, Mills JD, van Vliet EA and Aronica E: Altered extracellular matrix as an alternative risk factor for epileptogenicity in brain tumors. Biomedicines. 10:24752022. View Article : Google Scholar : PubMed/NCBI | |
|
Su J, Yin J, Qin W, Sha S, Xu J and Jiang C: Role for pro-inflammatory cytokines in regulating expression of GABA transporter type 1 and 3 in specific brain regions of kainic acid-induced status epilepticus. Neurochem Res. 40:621–627. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Fu CY, He XY, Li XF, Zhang X, Huang ZW, Li J, Chen M and Duan CZ: Nefiracetam attenuates pro-inflammatory cytokines and GABA transporter in specific brain regions of rats with post-ischemic seizures. Cell Physiol Biochem. 37:2023–2031. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Olmos G and Lladó J: Tumor necrosis factor alpha: A link between neuroinflammation and excitotoxicity. Mediators Inflamm. 2014:8612312014. View Article : Google Scholar : PubMed/NCBI | |
|
Roseti C, van Vliet EA, Cifelli P, Ruffolo G, Baayen JC, Di Castro MA, Bertollini C, Limatola C, Aronica E, Vezzani A and Palma E: GABAA currents are decreased by IL-1β in epileptogenic tissue of patients with temporal lobe epilepsy: Implications for ictogenesis. Neurobiol Dis. 82:311–320. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Librizzi L, Vila Verde D, Colciaghi F, Deleo F, Regondi MC, Costanza M, Cipelletti B and de Curtis M: Peripheral blood mononuclear cell activation sustains seizure activity. Epilepsia. 62:1715–1728. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Cresto N, Janvier A and Marchi N: From neurons to the neuro-glio-vascular unit: Seizures and brain homeostasis in networks. Rev Neurol (Paris). 179:308–315. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Thirupathi A and Chang YZ: Brain iron metabolism and CNS diseases. Adv Exp Med Biol. 1173:1–19. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Tang S, Gao P, Chen H, Zhou X, Ou Y and He Y: The role of iron, its metabolism and ferroptosis in traumatic brain injury. Front Cell Neurosci. 14:5907892020. View Article : Google Scholar : PubMed/NCBI | |
|
Chiueh CC: Iron overload, oxidative stress, and axonal dystrophy in brain disorders. Pediatr Neurol. 25:138–147. 2001. View Article : Google Scholar : PubMed/NCBI | |
|
Willmore LJ, Sypert GW and Munson JB: Recurrent seizures induced by cortical iron injection: a model of posttraumatic epilepsy. Ann Neurol. 4:329–336. 1978. View Article : Google Scholar : PubMed/NCBI | |
|
Willmore LJ, Sypert GW, Munson JV and Hurd RW: Chronic focal epileptiform discharges induced by injection of iron into rat and cat cortex. Science. 200:1501–1503. 1978. View Article : Google Scholar : PubMed/NCBI | |
|
Zimmer TS, David B, Broekaart DWM, Schidlowski M, Ruffolo G, Korotkov A, van der Wel NN, van Rijen PC, Mühlebner A, van Hecke W, et al: Seizure-mediated iron accumulation and dysregulated iron metabolism after status epilepticus and in temporal lobe epilepsy. Acta Neuropathol. 142:729–759. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Ikeda M: Iron overload without the C282Y mutation in patients with epilepsy. J Neurol Neurosurg Psychiatry. 70:551–553. 2001. View Article : Google Scholar : PubMed/NCBI | |
|
Dusek P, Hofer T, Alexander J, Roos PM and Aaseth JO: Cerebral iron deposition in neurodegeneration. Biomolecules. 12:7142022. View Article : Google Scholar : PubMed/NCBI | |
|
Bagwe-Parab S and Kaur G: Molecular targets and therapeutic interventions for iron induced neurodegeneration. Brain Res Bull. 156:1–9. 2020. View Article : Google Scholar | |
|
Wang F, Guo L, Wu Z, Zhang T, Dong D and Wu B: The Clock gene regulates kainic acid-induced seizures through inhibiting ferroptosis in mice. J Pharm Pharmacol. 74:1640–1650. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Mao XY, Zhou HH and Jin WL: Ferroptosis induction in pentylenetetrazole kindling and pilocarpine-induced epileptic seizures in mice. Front Neurosci. 13:7212019. View Article : Google Scholar : PubMed/NCBI | |
|
Huang L, Liu H and Liu S: Insight into the role of ferroptosis in epilepsy. J Integr Neurosci. 23:1132024. View Article : Google Scholar : PubMed/NCBI | |
|
Vezzani A, French J, Bartfai T and Baram TZ: The role of inflammation in epilepsy. Nat Rev Neurol. 7:31–40. 2011. View Article : Google Scholar | |
|
Montagne A, Zhao Z and Zlokovic BV: Alzheimer's disease: A matter of blood-brain barrier dysfunction? J Exp Med. 214:3151–3169. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Profaci CP, Munji RN, Pulido RS and Daneman R: The blood-brain barrier in health and disease: Important unanswered questions. J Exp Med. 217:e201900622020. View Article : Google Scholar : PubMed/NCBI | |
|
Lendahl U, Nilsson P and Betsholtz C: Emerging links between cerebrovascular and neurodegenerative diseases-a special role for pericytes. EMBO Rep. 20:e480702019. View Article : Google Scholar | |
|
Bell RD, Winkler EA, Sagare AP, Singh I, LaRue B, Deane R and Zlokovic BV: Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging. Neuron. 68:409–427. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Davalos D, Ryu JK, Merlini M, Baeten KM, Le Moan N, Petersen MA, Deerinck TJ, Smirnoff DS, Bedard C, Hakozaki H, et al: Fibrinogen-induced perivascular microglial clustering is required for the development of axonal damage in neuroinflammation. Nat Commun. 3:12272012. View Article : Google Scholar : PubMed/NCBI | |
|
Marchi N, Angelov L, Masaryk T, Fazio V, Granata T, Hernandez N, Hallene K, Diglaw T, Franic L, Najm I and Janigro D: Seizure-promoting effect of blood-brain barrier disruption. Epilepsia. 48:732–742. 2007. View Article : Google Scholar : PubMed/NCBI | |
|
Oby E and Janigro D: The blood-brain barrier and epilepsy. Epilepsia. 47:1761–1774. 2006. View Article : Google Scholar : PubMed/NCBI | |
|
van Vliet EA, Ndode-Ekane XE, Lehto LJ, Gorter JA, Andrade P, Aronica E, Gröhn O and Pitkänen A: Long-lasting blood-brain barrier dysfunction and neuroinflammation after traumatic brain injury. Neurobiol Dis. 145:1050802020. View Article : Google Scholar : PubMed/NCBI | |
|
Zhao Y, Liu Y, Xu Y, Li K, Zhou L, Qiao H, Xu Q and Zhao J: The role of ferroptosis in blood-brain barrier injury. Cell Mol Neurobiol. 43:223–236. 2023. View Article : Google Scholar | |
|
Yang LT, Anthony G and Kaufer D: Inflammatory astrocytic TGFβ signaling induced by blood-brain barrier dysfunction drives epileptogenesis. Noebels JL, Avoli M, Rogawski MA, Vezzani A and Delgado-Escueta AV: Jasper's Basic Mechanisms of the Epilepsies. 5th edition. New York: Oxford University Press; 2024 | |
|
van Vliet EA, Aronica E and Gorter JA: Blood-brain barrier dysfunction, seizures and epilepsy. Semin Cell Dev Biol. 38:26–34. 2015. View Article : Google Scholar | |
|
Belinskaia DA, Voronina PA, Shmurak VI, Jenkins RO and Goncharov NV: Serum albumin in health and disease: Esterase, antioxidant, transporting and signaling properties. Int J Mol Sci. 22:103182021. View Article : Google Scholar : PubMed/NCBI | |
|
Wang Y: Leaky blood-brain barrier: A double whammy for the brain. Epilepsy Currents. 20:165–167. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Luo WD, Min JW, Huang WX, Wang X, Peng YY, Han S, Yin J, Liu WH, He XH and Peng BW: Vitexin reduces epilepsy after hypoxic ischemia in the neonatal brain via inhibition of NKCC1. J Neuroinflammation. 15:1862018. View Article : Google Scholar : PubMed/NCBI | |
|
Ahishali B, Kaya M, Orhan N, Arican N, Ekizoglu O, Elmas I, Kucuk M, Kemikler G, Kalayci R and Gurses C: Effects of levetiracetam on blood-brain barrier disturbances following hyperthermia-induced seizures in rats with cortical dysplasia. Life Sci. 87:609–619. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Shaikh TG, Hasan SFS, Ahmed H, Kazi AI and Mansoor R: The role of angiotensin receptor blockers in treating epilepsy: A review. Neurol Sci. 45:1437–1445. 2024. View Article : Google Scholar | |
|
Rodriguez-Ortiz CJ, Thorwald MA, Rodriguez R, Mejias-Ortega M, Kieu Z, Maitra N, Hawkins C, Valenzuela J, Peng M, Nishiyama A, et al: Angiotensin receptor blockade with olmesartan alleviates brain pathology in obese OLETF rats. Clin Exp Pharmacol Physiol. 50:228–237. 2023. View Article : Google Scholar : | |
|
Pereira MGAG, Becari C, Oliveira JAC, Salgado MCO, Garcia-Cairasco N and Costa-Neto CM: Inhibition of the renin-angiotensin system prevents seizures in a rat model of epilepsy. Clin Sci (Lond). 119:477–482. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Winkler EA, Sengillo JD, Sagare AP, Zhao Z, Ma Q, Zuniga E, Wang Y, Zhong Z, Sullivan JS, Griffin JH, et al: Blood-spinal cord barrier disruption contributes to early motor-neuron degeneration in ALS-model mice. Proc Natl Acad Sci USA. 111:E1035–E1042. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Cui J, Liu X and Chow LMC: Flavonoids as P-gp inhibitors: A systematic review of SARs. Curr Med Chem. 26:4799–4831. 2019. View Article : Google Scholar | |
|
Deng X, Shao Y, Xie Y, Feng Y, Wu M, Wang M and Chen Y: MicroRNA-146a-5p downregulates the expression of P-glycoprotein in rats with lithium-pilocarpine-induced status epilepticus. Biol Pharm Bull. 42:744–750. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Merelli A, Ramos AJ, Lazarowski A and Auzmendi J: Convulsive stress mimics brain hypoxia and promotes the P-glycoprotein (P-gp) and erythropoietin receptor overexpression. Recombinant human erythropoietin effect on P-gp activity. Front Neurosci. 13:7502019. View Article : Google Scholar : PubMed/NCBI | |
|
Montanari F and Ecker GF: Prediction of drug-ABC-transporter interaction-recent advances and future challenges. Adv Drug Deliv Rev. 86:17–26. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Yu J and Ragueneau-Majlessi I: In Vitro-to-in vivo extrapolation of transporter inhibition data for drugs approved by the US food and drug administration in 2018. Clin Transl Sci. 13:693–699. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Faal T, Phan DTT, Davtyan H, Scarfone VM, Varady E, Blurton-Jones M, Hughes CCW and Inlay MA: Induction of mesoderm and neural crest-derived pericytes from human pluripotent stem cells to study blood-brain barrier interactions. Stem Cell Reports. 12:451–460. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Chen B: Cell therapy rejuvenates the neuroglial-vascular unit. Neural Regen Res. 21: View Article : Google Scholar : 2025. | |
|
Dahalia M, Gupta S, Majid H, Vohora D and Nidhi: Pirfenidone regulates seizures through the HMGB1/TLR4 axis to improve cognitive functions and modulate oxidative stress and neurotransmitters in PTZ-induced kindling in mice. Front Pharmacol. 15:15280322025. View Article : Google Scholar : PubMed/NCBI | |
|
Wang Q, Qin B, Yu H, Yu H, Zhang X, Li M, Zhou Y, Diao L and Liu H: Dingxian pill alleviates hippocampal neuronal apoptosis in epileptic mice through TNF-α/TNFR1 signaling pathway inhibition. J Ethnopharmacol. 334:1185792024. View Article : Google Scholar | |
|
Chen S, Chen R, Luo M, Luo Y, Ma X, Zhao H and Xu Z: Increased seizure susceptibility in the collagen-induced arthritis mouse model depends on neuronal IL-1R1. Life Sci. 369:1235372025. View Article : Google Scholar : PubMed/NCBI | |
|
Wu L, Zhu Y, Qin Y, Yuan H, Zhang L, Lu T, Chen Q and Hu A: Conditional knockout of IL-1R1 in endothelial cells attenuates seizures and neurodegeneration via inhibiting neuroinflammation mediated by Nrf2/HO-1/NLRP3 signaling in status epilepticus model. Mol Neurobiol. 61:4289–4303. 2024. View Article : Google Scholar : | |
|
Walker LE, Sills GJ, Jorgensen A, Alapirtti T, Peltola J, Brodie MJ, Marson AG, Vezzani A and Pirmohamed M: High-mobility group box 1 as a predictive biomarker for drug-resistant epilepsy: A proof-of-concept study. Epilepsia. 63:e1–e6. 2022. View Article : Google Scholar | |
|
Zhao J, Zheng Y, Liu K, Chen J, Lai N, Fei F, Shi J, Xu C, Wang S, Nishibori M, et al: HMGB1 is a therapeutic target and biomarker in diazepam-refractory status epilepticus with wide time window. Neurotherapeutics. 17:710–721. 2020. View Article : Google Scholar : | |
|
Rana A and Musto AE: The role of inflammation in the development of epilepsy. J Neuroinflammation. 15:1442018. View Article : Google Scholar : PubMed/NCBI | |
|
Mariette X, Matucci-Cerinic M, Pavelka K, Taylor P, van Vollenhoven R, Heatley R, Walsh C, Lawson R, Reynolds A and Emery P: Malignancies associated with tumour necrosis factor inhibitors in registries and prospective observational studies: A systematic review and meta-analysis. Ann Rheum Dis. 70:1895–1904. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
Vezzani A, Balosso S and Ravizza T: Neuroinflammatory pathways as treatment targets and biomarkers in epilepsy. Nat Rev Neurol. 15:459–472. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Ravizza T and Vezzani A: Pharmacological targeting of brain inflammation in epilepsy: Therapeutic perspectives from experimental and clinical studies. Epilepsia Open. 3(Suppl 2): S133–S142. 2018. View Article : Google Scholar | |
|
Soltani Khaboushan A, Yazdanpanah N and Rezaei N: Neuroinflammation and proinflammatory cytokines in epileptogenesis. Mol Neurobiol. 59:1724–1743. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Dilena R, Mauri E, Aronica E, Bernasconi P, Bana C, Cappelletti C, Carrabba G, Ferrero S, Giorda R, Guez S, et al: Therapeutic effect of Anakinra in the relapsing chronic phase of febrile infection-related epilepsy syndrome. Epilepsia Open. 4:344–350. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Costagliola G, Depietri G, Michev A, Riva A, Foiadelli T, Savasta S, Bonuccelli A, Peroni D, Consolini R, Marseglia GL, et al: Targeting inflammatory mediators in epilepsy: A systematic review of its molecular basis and clinical applications. Front Neurol. 13:7412442022. View Article : Google Scholar : PubMed/NCBI | |
|
Zhong X, Na Y, Yin S, Yan C, Gu J, Zhang N and Geng F: Cell membrane biomimetic nanoparticles with potential in treatment of Alzheimer's disease. Molecules. 28:23362023. View Article : Google Scholar : PubMed/NCBI | |
|
Qin Y, Fan W, Chen H, Yao N, Tang W, Tang J, Yuan W, Kuai R, Zhang Z, Wu Y and He Q: In vitro and in vivo investigation of glucose-mediated brain-targeting liposomes. J Drug Target. 18:536–549. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Xie F, Yao N, Qin Y, Zhang Q, Chen H, Yuan M, Tang J, Li X, Fan W, Zhang Q, et al: Investigation of glucose-modified liposomes using polyethylene glycols with different chain lengths as the linkers for brain targeting. Int J Nanomedicine. 7:163–175. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Zhou Y, Zhu F, Liu Y, Zheng M, Wang Y, Zhang D, Anraku Y, Zou Y, Li J, Wu H, et al: Blood-brain barrier-penetrating siRNA nanomedicine for Alzheimer's disease therapy. Sci Adv. 6:eabc70312020. View Article : Google Scholar : PubMed/NCBI | |
|
Anraku Y, Kuwahara H, Fukusato Y, Mizoguchi A, Ishii T, Nitta K, Matsumoto Y, Toh K, Miyata K, Uchida S, et al: Glycaemic control boosts glucosylated nanocarrier crossing the BBB into the brain. Nat Commun. 8:10012017. View Article : Google Scholar : PubMed/NCBI | |
|
Fu X, Li J, Wu Y, Mao C and Jiang Y: PAR2 deficiency tunes inflammatory microenvironment to magnify STING signalling for mitigating cancer metastasis via anionic CRISPR/Cas9 nanoparticles. J Control Release. 363:733–746. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Meng R, Hao S, Sun C, Hou Z, Hou Y, Wang L, Deng P, Deng J, Yang Y, Xia H, et al: Reverse-QTY code design of active human serum albumin self-assembled amphiphilic nanoparticles for effective anti-tumor drug doxorubicin release in mice. Proc Natl Acad Sci USA. 120:e22201731202023. View Article : Google Scholar : PubMed/NCBI | |
|
Gu GJ, Chung H, Park JY, Yoo R, Im HJ, Choi H, Lee YS and Seok SH: Mannosylated-serum albumin nanoparticle imaging to monitor tumor-associated macrophages under anti-PD1 treatment. J Nanobiotechnology. 21:312023. View Article : Google Scholar : PubMed/NCBI | |
|
Deng Z, Wang J, Xiao Y, Li F, Niu L, Liu X, Meng L and Zheng H: Ultrasound-mediated augmented exosome release from astrocytes alleviates amyloid-β-induced neurotoxicity. Theranostics. 11:4351–4362. 2021. View Article : Google Scholar : | |
|
Qu F, Wang P, Zhang K, Shi Y, Li Y, Li C, Lu J, Liu Q and Wang X: Manipulation of mitophagy by 'all-in-one' nanosensitizer augments sonodynamic glioma therapy. Autophagy. 16:1413–1435. 2020. View Article : Google Scholar | |
|
Gasca-Salas C, Fernández-Rodríguez B, Pineda-Pardo JA, Rodríguez-Rojas R, Obeso I, Hernández-Fernández F, Del Álamo M, Mata D, Guida P, Ordás-Bandera C, et al: Blood-brain barrier opening with focused ultrasound in Parkinson's disease dementia. Nat Commun. 12:7792021. View Article : Google Scholar : PubMed/NCBI | |
|
Ozdas MS, Shah AS, Johnson PM, Patel N, Marks M, Yasar TB, Stalder U, Bigler L, von der Behrens W, Sirsi SR and Yanik MF: Non-invasive molecularly-specific millimeter-resolution manipulation of brain circuits by ultrasound-mediated aggregation and uncaging of drug carriers. Nat Commun. 11:49292020. View Article : Google Scholar : PubMed/NCBI | |
|
Chen J, Yuan M, Madison CA, Eitan S and Wang Y: Blood-brain barrier crossing using magnetic stimulated nanoparticles. J Control Release. 345:557–571. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Gupta R, Chauhan A, Kaur T, Kuanr BK and Sharma D: Transmigration of magnetite nanoparticles across the blood-brain barrier in a rodent model: Influence of external and alternating magnetic fields. Nanoscale. 14:17589–17606. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang Q, Yang L, Zheng Y, Wu X, Chen X, Fei F, Gong Y, Tan B, Chen Q, Wang Y, et al: Electro-responsive micelle-based universal drug delivery system for on-demand therapy in epilepsy. J Control Release. 360:759–771. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang Y, Wu X, Ding J, Su B, Chen Z, Xiao Z, Wu C, Wei D, Sun J, Luo F, et al: Wireless-powering deep brain stimulation platform based on 1D-structured magnetoelectric nanochains applied in antiepilepsy treatment. ACS Nano. 17:15796–15809. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Gao S, Zheng M, Ren X, Tang Y and Liang X: Local hyperthermia in head and neck cancer: Mechanism, application and advance. Oncotarget. 7:57367–57378. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Wu D, Fei F, Zhang Q, Wang X, Gong Y, Chen X, Zheng Y, Tan B, Xu C, Xie H, et al: Nanoengineered on-demand drug delivery system improves efficacy of pharmacotherapy for epilepsy. Sci Adv. 8:eabm33812022. View Article : Google Scholar : PubMed/NCBI | |
|
Yang D, Ren Q, Nie J, Zhang Y, Wu H, Chang Z, Wang B, Dai J and Fang Y: Black phosphorus flake-enabled wireless neuromodulation for epilepsy treatment. Nano Lett. 24:1052–1061. 2024. View Article : Google Scholar | |
|
Hou Q, Wang L, Xiao F, Wang L, Liu X, Zhu L, Lu Y, Zheng W and Jiang X: Dual targeting nanoparticles for epilepsy therapy. Chem Sci. 13:12913–12920. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Chen SG, Tsai CH, Lin CJ, Lee CC, Yu HY, Hsieh TH and Liu HL: Transcranial focused ultrasound pulsation suppresses pentylenetetrazol induced epilepsy in vivo. Brain Stimul. 13:35–46. 2020. View Article : Google Scholar | |
|
Lee CC, Chou CC, Hsiao FJ, Chen YH, Lin CF, Chen CJ, Peng SJ, Liu HL and Yu HY: Pilot study of focused ultrasound for drug-resistant epilepsy. Epilepsia. 63:162–175. 2022. View Article : Google Scholar | |
|
Gustafson HH, Holt-Casper D, Grainger DW and Ghandehari H: Nanoparticle uptake: The phagocyte problem. Nano Today. 10:487–510. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Dobrovolskaia MA and McNeil SE: Immunological properties of engineered nanomaterials. Nat Nanotechnol. 2:469–478. 2007. View Article : Google Scholar | |
|
Singh N, Manshian B, Jenkins GJS, Griffiths SM, Williams PM, Maffeis TGG, Wright CJ and Doak SH: NanoGenotoxicology: The DNA damaging potential of engineered nanomaterials. Biomaterials. 30:3891–3914. 2009. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang J, Yan F, Zhang W, He L, Li Y, Zheng S, Wang Y, Yu T, Du L, Shen Y and He W: Biosynthetic gas vesicles combined with focused ultrasound for blood-brain barrier opening. Int J Nanomedicine. 17:6759–6772. 2022. View Article : Google Scholar | |
|
Mainprize T, Lipsman N, Huang Y, Meng Y, Bethune A, Ironside S, Heyn C, Alkins R, Trudeau M, Sahgal A, et al: Blood-brain barrier opening in primary brain tumors with non-invasive MR-guided focused ultrasound: A clinical safety and feasibility study. Sci Rep. 9:3212019. View Article : Google Scholar : PubMed/NCBI | |
|
Lipsman N, Meng Y, Bethune AJ, Huang Y, Lam B, Masellis M, Herrmann N, Heyn C, Aubert I, Boutet A, et al: Blood-brain barrier opening in Alzheimer's disease using MR-guided focused ultrasound. Nat Commun. 9:23362018. View Article : Google Scholar : PubMed/NCBI | |
|
Pineda-Pardo JA, Gasca-Salas C, Fernández-Rodríguez B, Rodríguez-Rojas R, Del Álamo M, Obeso I, Hernández-Fernández F, Trompeta C, Martínez-Fernández R, Matarazzo M, et al: Striatal blood-brain barrier opening in Parkinson's disease dementia: A pilot exploratory study. Mov Disord. 37:2057–2065. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Gkountas AA, Polychronopoulos ND, Sofiadis GN, Karvelas EG, Spyrou LA and Sarris IE: Simulation of magnetic nanoparticles crossing through a simplified blood-brain barrier model for Glioblastoma multiforme treatment. Comput Methods Programs Biomed. 212:1064772021. View Article : Google Scholar : PubMed/NCBI | |
|
Bencsik A, Lestaevel P and Guseva Canu I: Nano- and neuro-toxicology: An emerging discipline. Prog Neurobiol. 160:45–63. 2018. View Article : Google Scholar | |
|
Xiong X, Sun Y, Sattiraju A, Jung Y, Mintz A, Hayasaka S and Li KC: Remote spatiotemporally controlled and biologically selective permeabilization of blood-brain barrier. J Control Release. 217:113–120. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Chen W, Yu H, Hao Y, Liu W, Wang R, Huang Y, Wu J, Feng L, Guan Y, Huang L and Qian K: Comprehensive metabolic fingerprints characterize neuromyelitis optica spectrum disorder by nanoparticle-enhanced laser desorption/ionization mass spectrometry. ACS Nano. 17:19779–19792. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Lyu X, Liu J, Gou Y, Sun S, Hao J and Cui Y: Development and validation of a machine learning-based model of ischemic stroke risk in the Chinese elderly hypertensive population. VIEW. 5:202400592024. View Article : Google Scholar | |
|
Yuan Y, Zhang X, Wang Y, Li H, Qi Z, Du Z, Chu YH, Feng D, Xie Q, Song J, et al: Multimodal data integration using deep learning predicts overall survival of patients with glioma. VIEW. 5:202400012024. View Article : Google Scholar | |
|
Ying X, Wang Y, Liang J, Yue J, Xu C, Lu L, Xu Z, Gao J, Du Y and Chen Z: Angiopep-conjugated electro-responsive hydrogel nanoparticles: Therapeutic potential for epilepsy. Angew Chem Int Ed Engl. 53:12436–12440. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Geng C, Ren X, Cao P, Chu X, Wei P, Liu Q, Lu Y, Fu B, Li W, Li Y and Zhao G: Macrophage membrane-biomimetic nanoparticles target inflammatory microenvironment for epilepsy treatment. Theranostics. 14:6652–6670. 2024. View Article : Google Scholar : | |
|
Fang Z, Chen S, Qin J, Chen B, Ni G, Chen Z, Zhou J, Li Z, Ning Y, Wu C and Zhou L: Pluronic P85-coated poly(butylcyanoacrylate) nanoparticles overcome phenytoin resistance in P-glycoprotein overexpressing rats with lithium-pilocarpine-induced chronic temporal lobe epilepsy. Biomaterials. 97:110–121. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Zybina A, Anshakova A, Malinovskaya J, Melnikov P, Baklaushev V, Chekhonin V, Maksimenko O, Titov S, Balabanyan V, Kreuter J, et al: Nanoparticle-based delivery of carbamazepine: A promising approach for the treatment of refractory epilepsy. Int J Pharm. 547:10–23. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Zhao J, Ye Z, Yang J, Zhang Q, Shan W, Wang X, Wang Z, Ye S, Zhou X, Shao Z and Ren L: Nanocage encapsulation improves antiepileptic efficiency of phenytoin. Biomaterials. 240:1198492020. View Article : Google Scholar : PubMed/NCBI | |
|
Chu PC, Yu HY, Lee CC, Fisher R and Liu HL: Pulsed-focused ultrasound provides long-term suppression of epileptiform bursts in the kainic acid-induced epilepsy rat model. Neurotherapeutics. 19:1368–1380. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Krishna V, Mindel J, Sammartino F, Block C, Dwivedi AK, Van Gompel JJ, Fountain N and Fisher R: A phase 1 open-label trial evaluating focused ultrasound unilateral anterior thalamotomy for focal onset epilepsy. Epilepsia. 64:831–842. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Choi T, Koo M, Joo J, Kim T, Shon YM and Park J: Bidirectional neuronal control of epileptiform activity by repetitive transcranial focused ultrasound stimulations. Adv Sci (Weinh). 11:e23024042024. View Article : Google Scholar | |
|
Chu PC, Huang CS, Ing SZ, Yu HY, Fisher RS and Liu HL: Pulsed focused ultrasound reduces hippocampal volume loss and improves behavioral performance in the kainic acid rat model of epilepsy. Neurotherapeutics. 20:502–517. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang Y, Buckmaster PS, Qiu L, Wang J, Keunen O, Ghobadi SN, Huang A, Hou Q, Li N, Narang S, et al: Non-invasive, neurotoxic surgery reduces seizures in a rat model of temporal lobe epilepsy. Exp Neurol. 343:1137612021. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang M, Li B, Liu Y, Tang R, Lang Y, Huang Q and He J: Different modes of low-frequency focused ultrasound-mediated attenuation of epilepsy based on the topological theory. Micromachines (Basel). 12. pp. 10012021, View Article : Google Scholar |