Protective effect of exercise on metabolic dysfunction‑associated fatty liver disease: Potential epigenetic mechanisms (Review)
- Authors:
- Yanhua Zhang
- Yuqin Wei
- Huan Liu
- Yanju Guo
-
Affiliations: School of Journalism and Communication, Wuhan Sports University, Wuhan, Hubei 430000, P.R. China, School of Physical Education and Health, Hubei Business College, Wuhan, Hubei 430000, P.R. China, College of Sports Medicine, Wuhan Sports University, Wuhan, Hubei 430000, P.R. China - Published online on: July 15, 2025 https://doi.org/10.3892/ijmm.2025.5587
- Article Number: 146
-
Copyright : © Zhang et al. This is an open access article distributed under the terms of Creative Commons Attribution License [CC BY 4.0].
This article is mentioned in:
Abstract
 |
 |
|
Chen G, Ni Y, Nagata N, Xu L and Ota T: Micronutrient anti-oxidants and nonalcoholic fatty liver disease. Int J Mol Sci. 17:13792016. View Article : Google Scholar | |
|
Byrne CD and Targher G: NAFLD: A multisystem disease. J Hepatol. 62(Suppl 1): S47–S64. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Eslam M, Valenti L and Romeo S: Genetics and epigenetics of NAFLD and NASH: Clinical impact. J Hepatol. 68:268–279. 2018. View Article : Google Scholar | |
|
Schwimmer JB: Definitive diagnosis and assessment of risk for nonalcoholic fatty liver disease in children and adolescents. Semin Liver Dis. 27:312–318. 2007. View Article : Google Scholar : PubMed/NCBI | |
|
Polyzos SA, Kountouras J and Mantzoros CS: Obesity and nonalcoholic fatty liver disease: From pathophysiology to therapeutics. Metabolism. 92:82–97. 2019. View Article : Google Scholar | |
|
Targher G, Tilg H and Byrne CD: Non-alcoholic fatty liver disease: A multisystem disease requiring a multidisciplinary and holistic approach. Lancet Gastroenterol Hepatol. 6:578–588. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Younossi ZM: Non-alcoholic fatty liver disease-A global public health perspective. J Hepatol. 70:531–544. 2019. View Article : Google Scholar | |
|
Keskin M, Hayıroğlu M, Uzun AO, Güvenç TS, Şahin S and Kozan Ö: Effect of nonalcoholic fatty liver disease on in-hospital and Long-term outcomes in patients with ST-segment elevation myocardial infarction. Am J Cardiol. 120:1720–1726. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Friedman SL, Neuschwander-Tetri BA, Rinella M and Sanyal AJ: Mechanisms of NAFLD development and therapeutic strategies. Nat Med. 24:908–922. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Romero-Gómez M, Zelber-Sagi S and Trenell M: Treatment of NAFLD with diet, physical activity and exercise. J Hepatol. 67:829–846. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Kwak MS and Kim D: Non-alcoholic fatty liver disease and lifestyle modifications, focusing on physical activity. Korean J Intern Med. 33:64–74. 2018. View Article : Google Scholar : | |
|
Malkova D, Evans RD, Frayn KN, Humphreys SM, Jones PR and Hardman AE: Prior exercise and postprandial substrate extraction across the human leg. Am J Physiol Endocrinol Metab. 279:E1020–E1028. 2000. View Article : Google Scholar : PubMed/NCBI | |
|
Yaskolka Meir A, Keller M, Müller L, Bernhart SH, Tsaban G, Zelicha H, Rinott E, Kaplan A, Gepner Y, Shelef I, et al: Effects of lifestyle interventions on epigenetic signatures of liver fat: Central randomized controlled trial. Liver Int. 41:2101–2111. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Barrès R, Yan J, Egan B, Treebak JT, Rasmussen M, Fritz T, Caidahl K, Krook A, O'Gorman DJ and Zierath JR: Acute exercise remodels promoter methylation in human skeletal muscle. Cell Metab. 15:405–411. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Chen Z, Li L, Wu W, Liu Z, Huang Y, Yang L, Luo Q, Chen J, Hou Y and Song G: Exercise protects proliferative muscle satellite cells against exhaustion via the Igfbp7-Akt-mTOR axis. Theranostics. 10:6448–6466. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Fiorito G, Caini S, Palli D, Bendinelli B, Saieva C, Ermini I, Valentini V, Assedi M, Rizzolo P, Ambrogetti D, et al: DNA methylation-based biomarkers of aging were slowed down in a two-year diet and physical activity intervention trial: The DAMA study. Aging Cell. 20:e134392021. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang L, Lu Q and Chang C: Epigenetics in health and disease. Adv Exp Med Biol. 1253:3–55. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Goldberg AD, Allis CD and Bernstein E: Epigenetics: A landscape takes shape. Cell. 128:635–638. 2007. View Article : Google Scholar : PubMed/NCBI | |
|
Stefanska B, Karlic H, Varga F, Fabianowska-Majewska K and Haslberger A: Epigenetic mechanisms in anti-cancer actions of bioactive food components-the implications in cancer prevention. Br J Pharmacol. 167:279–297. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Dehan P, Kustermans G, Guenin S, Horion J, Boniver J and Delvenne P: DNA methylation and cancer diagnosis: New methods and applications. Expert Rev Mol Diagn. 9:651–657. 2009. View Article : Google Scholar : PubMed/NCBI | |
|
Zhao Q, Liu J, Deng H, Ma R, Liao JY, Liang H, Hu J, Li J, Guo Z, Cai J, et al: Targeting Mitochondria-located circRNA SCAR alleviates NASH via reducing mROS output. Cell. 183:76–93.e22. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Yang Y, Cai J, Yang X, Wang K, Sun K, Yang Z, Zhang L, Yang L, Gu C, Huang X, et al: Dysregulated m6A modification promotes lipogenesis and development of Non-alcoholic fatty liver disease and hepatocellular carcinoma. Mol Ther. 30:2342–2353. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Yang Z, Zhang S, Liu X, Shu R, Shi W, Qu W, Liu D, Cai Z, Wang Y, Cheng X, et al: Histone demethylase KDM1A promotes hepatic steatosis and inflammation by increasing chromatin accessibility in NAFLD. J Lipid Res. 65:1005132024. View Article : Google Scholar : PubMed/NCBI | |
|
Farzanegi P, Dana A, Ebrahimpoor Z, Asadi M and Azarbayjani MA: Mechanisms of beneficial effects of exercise training on non-alcoholic fatty liver disease (NAFLD): Roles of oxidative stress and inflammation. Eur J Sport Sci. 19:994–1003. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Stevanović J, Beleza J, Coxito P, Ascensão A and Magalhães J: Physical exercise and liver 'fitness': Role of mitochondrial function and epigenetics-related mechanisms in non-alcoholic fatty liver disease. Mol Metab. 32:1–14. 2020. View Article : Google Scholar | |
|
Peixoto P, Cartron PF, Serandour AA and Hervouet E: From 1957 to Nowadays: A brief history of epigenetics. Int J Mol Sci. 21:75712020. View Article : Google Scholar : PubMed/NCBI | |
|
Jablonka E: Epigenetic variations in heredity and evolution. Clin Pharmacol Ther. 92:683–688. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Coulondre C, Miller JH, Farabaugh PJ and Gilbert W: Molecular basis of base substitution hotspots in Escherichia coli. Nature. 274:775–780. 1978. View Article : Google Scholar : PubMed/NCBI | |
|
Bird AP: DNA methylation and the frequency of CpG in animal DNA. Nucleic Acids Res. 8:1499–1504. 1980. View Article : Google Scholar : PubMed/NCBI | |
|
Antequera F and Bird A: CpG islands as genomic footprints of promoters that are associated with replication origins. Curr Biol. 9:R661–R667. 1999. View Article : Google Scholar : PubMed/NCBI | |
|
Wolf SF, Dintzis S, Toniolo D, Persico G, Lunnen KD, Axelman J and Migeon BR: Complete concordance between glucose-6-phosphate dehydrogenase activity and hypomethylation of 3′CpG clusters: Implications for X chromosome dosage compensation. Nucleic Acids Res. 12:9333–9348. 1984. View Article : Google Scholar : PubMed/NCBI | |
|
Morgan RK, Wang K, Svoboda LK, Rygiel CA, Lalancette C, Cavalcante R, Bartolomei MS, Prasasya R, Neier K, Perera BPU, et al: Effects of developmental lead and phthalate exposures on DNA methylation in adult mouse blood, brain, and liver: A focus on genomic imprinting by tissue and sex. Environ Health Perspect. 132:670032024. View Article : Google Scholar : PubMed/NCBI | |
|
Liu Y, Zhang Y, Yin J, Gao Y, Li Y, Bai D, He W, Li X, Zhang P, Li R, et al: Distinct H3K9me3 and DNA methylation modifications during mouse spermatogenesis. J Biol Chem. 294:18714–18725. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Yen RW, Vertino PM, Nelkin BD, Yu JJ, el-Deiry W, Cumaraswamy A, Lennon GG, Trask BJ, Celano P and Baylin SB: Isolation and characterization of the cDNA encoding human DNA methyltransferase. Nucleic Acids Res. 20:2287–2291. 1992. View Article : Google Scholar : PubMed/NCBI | |
|
Lian H, Li WB and Jin WL: The emerging insights into catalytic or Non-catalytic roles of TET proteins in tumors and neural development. Oncotarget. 7:64512–64525. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Lyko F: The DNA methyltransferase family: A versatile toolkit for epigenetic regulation. Nat Rev Genet. 19:81–92. 2018. View Article : Google Scholar | |
|
Li E and Zhang Y: DNA methylation in mammals. Cold Spring Harb Perspect Biol. 6:a0191332014. View Article : Google Scholar : PubMed/NCBI | |
|
Melamed P, Yosefzon Y, David C, Tsukerman A and Pnueli L: Tet enzymes, variants, and differential effects on function. Front Cell Dev Biol. 6:222018. View Article : Google Scholar : PubMed/NCBI | |
|
Tábara LC, Burr SP, Frison M, Chowdhury SR, Paupe V, Nie Y, Johnson M, Villar-Azpillaga J, Viegas F, Segawa M, et al: MTFP1 controls mitochondrial fusion to regulate inner membrane quality control and maintain mtDNA levels. Cell. 187:3619–3637.e27. 2024. View Article : Google Scholar : PubMed/NCBI | |
|
Menger KE, Rodríguez-Luis A, Chapman J and Nicholls TJ: Controlling the topology of mammalian mitochondrial DNA. Open Biol. 11:2101682021. View Article : Google Scholar : PubMed/NCBI | |
|
Devall M, Soanes DM, Smith AR, Dempster EL, Smith RG, Burrage J, Iatrou A, Hannon E, Troakes C, Moore K, et al: Genome-wide characterization of mitochondrial DNA methylation in human brain. Front Endocrinol (Lausanne). 13:10591202022. View Article : Google Scholar | |
|
Patil V, Cuenin C, Chung F, Aguilera JRR, Fernandez-Jimenez N, Romero-Garmendia I, Bilbao JR, Cahais V, Rothwell J and Herceg Z: Human mitochondrial DNA is extensively methylated in a non-CpG context. Nucleic Acids Res. 47:10072–10085. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Kouzarides T: Chromatin modifications and their function. Cell. 128:693–705. 2007. View Article : Google Scholar : PubMed/NCBI | |
|
Tessarz P and Kouzarides T: Histone core modifications regulating nucleosome structure and dynamics. Nat Rev Mol Cell Biol. 15:703–708. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Zhou VW, Goren A and Bernstein BE: Charting histone modifications and the functional organization of mammalian genomes. Nat Rev Genet. 12:7–18. 2011. View Article : Google Scholar | |
|
Hansen JC: Linking genome structure and function through specific histone acetylation. ACS Chem Biol. 1:69–72. 2006. View Article : Google Scholar : PubMed/NCBI | |
|
Black JC, Van Rechem C and Whetstine JR: Histone lysine methylation dynamics: Establishment, regulation, and biological impact. Mol Cell. 48:491–507. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Hyun K, Jeon J, Park K and Kim J: Writing, erasing and reading histone lysine methylations. Exp Mol Med. 49:e3242017. View Article : Google Scholar : PubMed/NCBI | |
|
Qu P, Li L, Jin Q, Liu D, Qiao Y, Zhang Y, Sun Q, Ran S, Li Z, Liu T and Peng L: Histone methylation modification and diabetic kidney disease: Potential molecular mechanisms and therapeutic approaches (Review). Int J Mol Med. 54:1042024. View Article : Google Scholar : PubMed/NCBI | |
|
Xiong J, Nie M, Fu C, Chai X, Zhang Y, He L and Sun S: Hypoxia enhances HIF1 α transcription activity by upregulating KDM4A and mediating H3K9me3, thus inducing ferroptosis resistance in cervical cancer cells. Stem Cells Int. 2022:16088062022. View Article : Google Scholar | |
|
Song Y, Wu F and Wu J: Targeting histone methylation for cancer therapy: Enzymes, inhibitors, biological activity and perspectives. J Hematol Oncol. 9:492016. View Article : Google Scholar : PubMed/NCBI | |
|
Gong F and Miller KM: Histone methylation and the DNA damage response. Mutat Res Rev Mutat Res. 780:37–47. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Nimura K, Ura K and Kaneda Y: Histone methyltransferases: Regulation of transcription and contribution to human disease. J Mol Med (Berl). 88:1213–1220. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Allis CD, Bowen JK, Abraham GN, Glover CV and Gorovsky MA: Proteolytic processing of histone H3 in chromatin: A physiologically regulated event in Tetrahymena micronuclei. Cell. 20:55–64. 1980. View Article : Google Scholar : PubMed/NCBI | |
|
Shi Y, Lan F, Matson C, Mulligan P, Whetstine JR, Cole PA, Casero RA and Shi Y: Histone demethylation mediated by the nuclear amine oxidase homolog LSD1. Cell. 119:941–953. 2004. View Article : Google Scholar : PubMed/NCBI | |
|
Shen H, Xu W and Lan F: Histone lysine demethylases in mammalian embryonic development. Exp Mol Med. 49:e3252017. View Article : Google Scholar : PubMed/NCBI | |
|
Li L, Chen K, Wang T, Wu Y, Xing G, Chen M, Hao Z, Zhang C, Zhang J, Ma B, et al: Glis1 facilitates induction of pluripotency via an epigenome-metabolome-epigenome signalling cascade. Nat Metab. 2:882–892. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Hu Z, Lu Y, Cao J, Lin L, Chen X, Zhou Z, Pu J, Chen G, Ma X, Deng Q, et al: N-acetyltransferase NAT10 controls cell fates via connecting mRNA cytidine acetylation to chromatin signaling. Sci Adv. 10:eadh98712024. View Article : Google Scholar : PubMed/NCBI | |
|
Kronfol MM, Jahr FM, Dozmorov MG, Phansalkar PS, Xie LY, Aberg KA, McRae M, Price ET, Slattum PW, Gerk PM and McClay JL: DNA methylation and histone acetylation changes to cytochrome P450 2E1 regulation in normal aging and impact on rates of drug metabolism in the liver. Geroscience. 42:819–832. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Lin Y, Lin A, Cai L, Huang W, Yan S, Wei Y, Ruan X, Fang W, Dai X, Cheng J, et al: ACSS2-dependent histone acetylation improves cognition in mouse model of Alzheimer's disease. Mol Neurodegener. 18:472023. View Article : Google Scholar : PubMed/NCBI | |
|
Wu Y, Tang L, Huang H, Yu Q, Hu B, Wang G, Ge F, Yin T, Li S and Yu X: Phosphoglycerate dehydrogenase activates PKM2 to phosphorylate histone H3T11 and attenuate cellular senescence. Nat Commun. 14:13232023. View Article : Google Scholar : PubMed/NCBI | |
|
Chen G, Zhu X, Li J, Zhang Y, Wang X, Zhang R, Qin X, Chen X, Wang J, Liao W, et al: Celastrol inhibits lung cancer growth by triggering histone acetylation and acting synergically with HDAC inhibitors. Pharmacol Res. 185:1064872022. View Article : Google Scholar : PubMed/NCBI | |
|
Turner BM: Histone acetylation and an epigenetic code. Bioessays. 22:836–845. 2000. View Article : Google Scholar : PubMed/NCBI | |
|
Shvedunova M and Akhtar A: Modulation of cellular processes by histone and non-histone protein acetylation. Nat Rev Mol Cell Biol. 23:329–349. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Shen Y, Wei W and Zhou DX: Histone acetylation enzymes coordinate metabolism and gene expression. Trends Plant Sci. 20:614–621. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Wang P, Wang Z and Liu J: Role of HDACs in normal and malignant hematopoiesis. Mol Cancer. 19:52020. View Article : Google Scholar : PubMed/NCBI | |
|
Esteller M: Non-coding RNAs in human disease. Nat Rev Genet. 12:861–874. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
Wessels HH, Stirn A, Méndez-Mancilla A, Kim EJ, Hart SK, Knowles DA and Sanjana NE: Prediction of on-target and off-target activity of CRISPR-Cas13d guide RNAs using deep learning. Nat Biotechnol. 42:628–637. 2024. View Article : Google Scholar | |
|
Lodde V, Murgia G, Simula ER, Steri M, Floris M and Idda ML: Long noncoding RNAs and circular RNAs in autoimmune diseases. Biomolecules. 10:10442020. View Article : Google Scholar : PubMed/NCBI | |
|
Statello L, Guo CJ, Chen LL and Huarte M: Gene regulation by long non-coding RNAs and its biological functions. Nat Rev Mol Cell Biol. 22:96–118. 2021. View Article : Google Scholar | |
|
Greene J, Baird AM, Brady L, Lim M, Gray SG, McDermott R and Finn SP: Circular RNAs: Biogenesis, function and role in human diseases. Front Mol Biosci. 4:382017. View Article : Google Scholar : PubMed/NCBI | |
|
Gebert LFR and MacRae IJ: Regulation of microRNA function in animals. Nat Rev Mol Cell Biol. 20:21–37. 2019. View Article : Google Scholar : | |
|
Bratkovič T, Božič J and Rogelj B: Functional diversity of small nucleolar RNAs. Nucleic Acids Res. 48:1627–1651. 2020. View Article : Google Scholar | |
|
Yao Q, He T, Liao JY, Liao R, Wu X, Lin L and Xiao G: Noncoding RNAs in skeletal development and disorders. Biol Res. 57:162024. View Article : Google Scholar : PubMed/NCBI | |
|
Horvitz HR and Sulston JE: Isolation and genetic characterization of cell-lineage mutants of the nematode Caenorhabditis elegans. Genetics. 96:435–454. 1980. View Article : Google Scholar : PubMed/NCBI | |
|
Röszer T: MicroRNA profile of mouse Adipocyte-derived extracellular vesicles. Cells. 13:12982024. View Article : Google Scholar : PubMed/NCBI | |
|
Kingreen T, Kewitz-Hempel S, Rohde C, Hause G, Sunderkötter C and Gerloff D: Extracellular vesicles from highly invasive melanoma subpopulations increase the invasive capacity of less invasive melanoma cells through mir-1246-mediated inhibition of CCNG2. Cell Commun Signal. 22:4422024. View Article : Google Scholar : PubMed/NCBI | |
|
Kmiotek-Wasylewska K, Bobis-Wozowicz S, Karnas E, Orpel M, Woźnicka O, Madeja Z, Dawn B and Zuba-Surma EK: Anti-inflammatory, Anti-fibrotic and Pro-cardiomyogenic effects of genetically engineered extracellular vesicles enriched in miR-1 and miR-199a on human cardiac fibroblasts. Stem Cell Rev Rep. 19:2756–2773. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Li Y, Meng Y, Zhu X, Van Wijnen A, Eirin A and Lerman LO: Metabolic syndrome is associated with altered mRNA and miRNA content in human circulating extracellular vesicles. Front Endocrinol (Lausanne). 12:6875862021. View Article : Google Scholar : PubMed/NCBI | |
|
Venkatesh J, Wasson MD, Brown JM, Fernando W and Marcato P: LncRNA-miRNA axes in breast cancer: Novel points of interaction for strategic attack. Cancer Lett. 509:81–88. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Han JJ: LncRNAs: The missing link to senescence nuclear architecture. Trends Biochem Sci. 48:618–628. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Herman AB, Tsitsipatis D and Gorospe M: Integrated lncRNA function upon genomic and epigenomic regulation. Mol Cell. 82:2252–2266. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
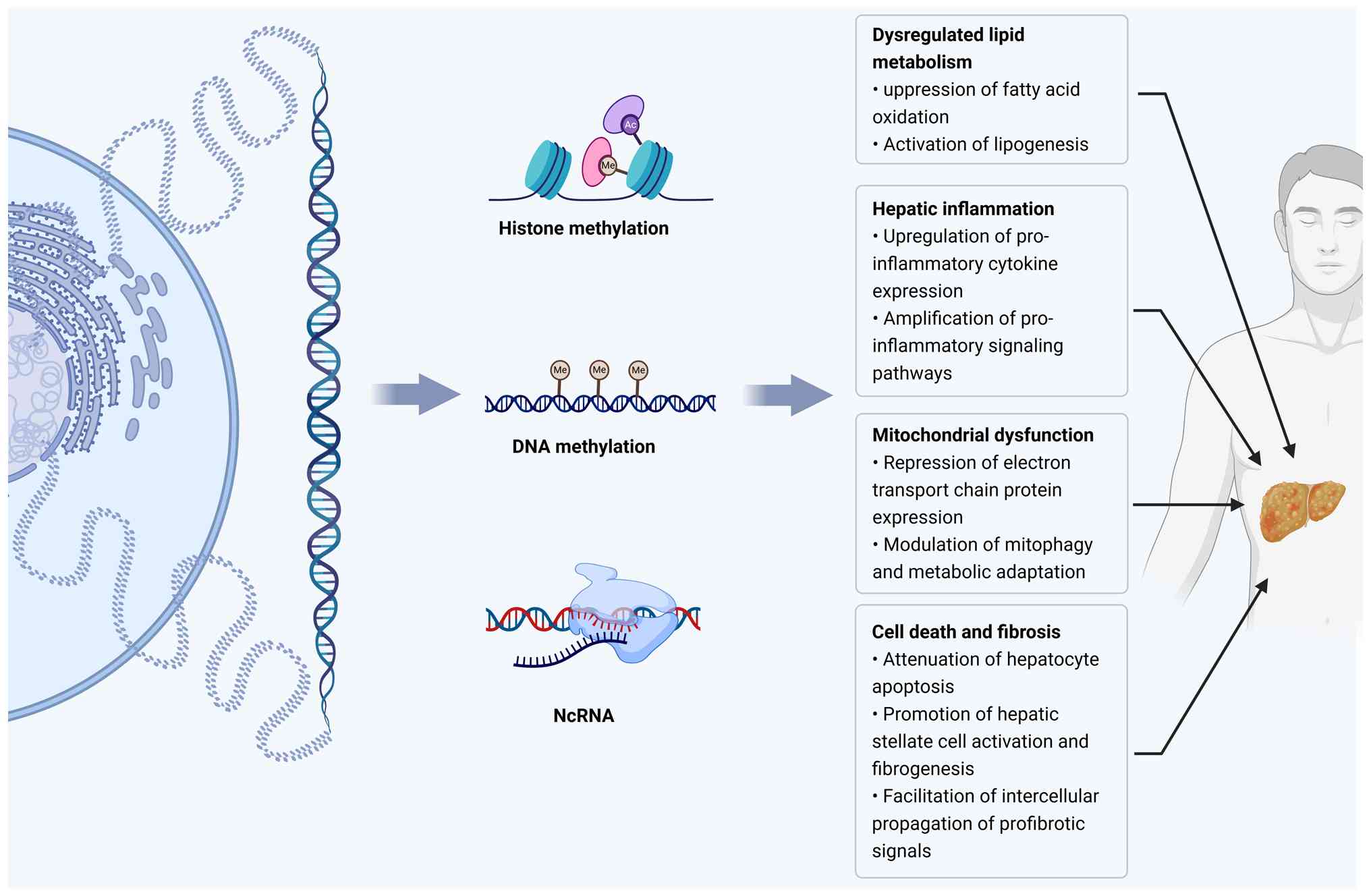
Zhang X, Asllanaj E, Amiri M, Portilla-Fernandez E, Bramer WM, Nano J, Voortman T, Pan Q and Ghanbari M: Deciphering the role of epigenetic modifications in fatty liver disease: A systematic review. Eur J Clin Invest. 51:e134792021. View Article : Google Scholar : | |
|
Wei L, Yang X, Wang J, Wang Z, Wang Q, Ding Y and Yu A: H3K18 lactylation of senescent microglia potentiates brain aging and Alzheimer's disease through the NFκB signaling pathway. J Neuroinflammation. 20:2082023. View Article : Google Scholar | |
|
Huang MY, Xuan F, Liu W and Cui HJ: MINA controls proliferation and tumorigenesis of glioblastoma by epigenetically regulating cyclins and CDKs via H3K9me3 demethylation. Oncogene. 36:387–396. 2017. View Article : Google Scholar | |
|
Miao F, Gonzalo IG, Lanting L and Natarajan R: In vivo chromatin remodeling events leading to inflammatory gene transcription under diabetic conditions. J Biol Chem. 279:18091–18097. 2004. View Article : Google Scholar : PubMed/NCBI | |
|
Bricambert J, Miranda J, Benhamed F, Girard J, Postic C and Dentin R: Salt-inducible kinase 2 links transcriptional coactivator p300 phosphorylation to the prevention of ChREBP-dependent hepatic steatosis in mice. J Clin Invest. 120:4316–4331. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Brunt EM, Wong VW, Nobili V, Day CP, Sookoian S, Maher JJ, Bugianesi E, Sirlin CB, Neuschwander-Tetri BA and Rinella ME: Nonalcoholic fatty liver disease. Nat Rev Dis Primers. 1:150802015. View Article : Google Scholar : PubMed/NCBI | |
|
Tryndyak VP, Han T, Muskhelishvili L, Fuscoe JC, Ross SA, Beland FA and Pogribny IP: Coupling global methylation and gene expression profiles reveal key pathophysiological events in liver injury induced by a methyl-deficient diet. Mol Nutr Food Res. 55:411–418. 2011. View Article : Google Scholar | |
|
Lee JH, Friso S and Choi SW: Epigenetic mechanisms underlying the link between non-alcoholic fatty liver diseases and nutrition. Nutrients. 6:3303–3325. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Sookoian S, Rosselli MS, Gemma C, Burgueño AL, Fernández Gianotti T, Castaño GO and Pirola CJ: Epigenetic regulation of insulin resistance in nonalcoholic fatty liver disease: Impact of liver methylation of the peroxisome proliferator-activated receptor γ coactivator 1α promoter. Hepatology. 52:1992–2000. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Pirola CJ, Gianotti TF, Burgueño AL, Rey-Funes M, Loidl CF, Mallardi P, Martino JS, Castaño GO and Sookoian S: Epigenetic modification of liver mitochondrial DNA is associated with histological severity of nonalcoholic fatty liver disease. Gut. 62:1356–1363. 2013. View Article : Google Scholar | |
|
Zeybel M, Hardy T, Robinson SM, Fox C, Anstee QM, Ness T, Masson S, Mathers JC, French J, White S and Mann J: Differential DNA methylation of genes involved in fibrosis progression in Non-alcoholic fatty liver disease and alcoholic liver disease. Clin Epigenetics. 7:252015. View Article : Google Scholar : PubMed/NCBI | |
|
Nishida N, Yada N, Hagiwara S, Sakurai T, Kitano M and Kudo M: Unique features associated with hepatic oxidative DNA damage and DNA methylation in non-alcoholic fatty liver disease. J Gastroenterol Hepatol. 31:1646–1653. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Baumeier C, Schlüter L, Saussenthaler S, Laeger T, Rödiger M, Alaze SA, Fritsche L, Häring HU, Stefan N, Fritsche A, et al: Elevated hepatic DPP4 activity promotes insulin resistance and non-alcoholic fatty liver disease. Mol Metab. 6:1254–1263. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Baumeier C, Saussenthaler S, Kammel A, Jähnert M, Schlüter L, Hesse D, Canouil M, Lobbens S, Caiazzo R, Raverdy V, et al: Hepatic DPP4 DNA methylation associates with fatty liver. Diabetes. 66:25–35. 2017. View Article : Google Scholar | |
|
Zhang RN, Pan Q, Zheng RD, Mi YQ, Shen F, Zhou D, Chen GY, Zhu CY and Fan JG: Genome-wide analysis of DNA methylation in human peripheral leukocytes identifies potential biomarkers of nonalcoholic fatty liver disease. Int J Mol Med. 42:443–452. 2018.PubMed/NCBI | |
|
Ahrens M, Ammerpohl O, von Schönfels W, Kolarova J, Bens S, Itzel T, Teufel A, Herrmann A, Brosch M, Hinrichsen H, et al: DNA methylation analysis in nonalcoholic fatty liver disease suggests Distinct disease-specific and remodeling signatures after bariatric surgery. Cell Metab. 18:296–302. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Sun C, Fan JG and Qiao L: Potential epigenetic mechanism in non-alcoholic Fatty liver disease. Int J Mol Sci. 16:5161–5179. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Emmett MJ and Lazar MA: Integrative regulation of physiology by histone deacetylase 3. Nat Rev Mol Cell Biol. 20:102–115. 2019. View Article : Google Scholar : | |
|
Sun Z, Miller RA, Patel RT, Chen J, Dhir R, Wang H, Zhang D, Graham MJ, Unterman TG, Shulman GI, et al: Hepatic Hdac3 promotes gluconeogenesis by repressing lipid synthesis and sequestration. Nat Med. 18:934–942. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Wang RH, Li C and Deng CX: Liver steatosis and increased ChREBP expression in mice carrying a liver specific SIRT1 null mutation under a normal feeding condition. Int J Biol Sci. 6:682–690. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Jin M, Shen Y, Pan T, Zhu T, Li X, Xu F, Betancor MB, Jiao L, Tocher DR and Zhou Q: Dietary betaine mitigates hepatic steatosis and inflammation induced by a High-Fat-Diet by modulating the Sirt1/Srebp-1/Pparα pathway in juvenile black seabream (Acanthopagrus schlegelii). Front Immunol. 12:6947202021. View Article : Google Scholar | |
|
Zeng C and Chen M: Progress in nonalcoholic fatty liver disease: SIRT family regulates mitochondrial biogenesis. Biomolecules. 12:10792022. View Article : Google Scholar : PubMed/NCBI | |
|
Nassir F and Ibdah JA: Sirtuins and nonalcoholic fatty liver disease. World J Gastroenterol. 22:10084–10092. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Kim JH, Jung DY, Kim HR and Jung MH: Histone H3K9 demethylase JMJD2B plays a role in LXRα-dependent lipogenesis. Int J Mol Sci. 21:83132020. View Article : Google Scholar | |
|
Pogribny IP, Tryndyak VP, Bagnyukova TV, Melnyk S, Montgomery B, Ross SA, Latendresse JR, Rusyn I and Beland FA: Hepatic epigenetic phenotype predetermines individual susceptibility to hepatic steatosis in mice fed a lipogenic methyl-deficient diet. J Hepatol. 51:176–186. 2009. View Article : Google Scholar : PubMed/NCBI | |
|
Gjorgjieva M, Sobolewski C, Dolicka D, Correia de Sousa M and Foti M: miRNAs and NAFLD: From pathophysiology to therapy. Gut. 68:2065–2079. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Bandiera S, Pfeffer S, Baumert TF and Zeisel MB: miR-122-a key factor and therapeutic target in liver disease. J Hepatol. 62:448–457. 2015. View Article : Google Scholar | |
|
Cheung O, Puri P, Eicken C, Contos MJ, Mirshahi F, Maher JW, Kellum JM, Min H, Luketic VA and Sanyal AJ: Nonalcoholic steatohepatitis is associated with altered hepatic MicroRNA expression. Hepatology. 48:1810–1820. 2008. View Article : Google Scholar : PubMed/NCBI | |
|
Pirola CJ, Fernández Gianotti T, Castaño GO, Mallardi P, San Martino J, Mora Gonzalez Lopez Ledesma M, Flichman D, Mirshahi F, Sanyal AJ and Sookoian S: Circulating microRNA signature in non-alcoholic fatty liver disease: From serum Non-coding RNAs to liver histology and disease pathogenesis. Gut. 64:800–812. 2015. View Article : Google Scholar | |
|
Hsu SH, Wang B, Kota J, Yu J, Costinean S, Kutay H, Yu L, Bai S, La Perle K, Chivukula RR, et al: Essential metabolic, anti-inflammatory, and anti-tumorigenic functions of miR-122 in liver. J Clin Invest. 122:2871–2883. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Tsai WC, Hsu SD, Hsu CS, Lai TC, Chen SJ, Shen R, Huang Y, Chen HC, Lee CH, Tsai TF, et al: MicroRNA-122 plays a critical role in liver homeostasis and hepatocarcinogenesis. J Clin Invest. 122:2884–2897. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Liu CH, Ampuero J, Gil-Gómez A, Montero-Vallejo R, Rojas Á, Muñoz-Hernández R, Gallego-Durán R and Romero-Gómez M: miRNAs in patients with non-alcoholic fatty liver disease: A systematic review and meta-analysis. J Hepatol. 69:1335–1348. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Liu XL, Pan Q, Zhang RN, Shen F, Yan SY, Sun C, Xu ZJ, Chen YW and Fan JG: Disease-specific miR-34a as diagnostic marker of non-alcoholic steatohepatitis in a Chinese population. World J Gastroenterol. 22:9844–9852. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Ding J, Li M, Wan X, Jin X, Chen S, Yu C and Li Y: Effect of miR-34a in regulating steatosis by targeting PPARα expression in nonalcoholic fatty liver disease. Sci Rep. 5:137292015. View Article : Google Scholar | |
|
Xu Y, Zalzala M, Xu J, Li Y, Yin L and Zhang Y: A metabolic stress-inducible miR-34a-HNF4α pathway regulates lipid and lipoprotein metabolism. Nat Commun. 6:74662015. View Article : Google Scholar | |
|
Calo N, Ramadori P, Sobolewski C, Romero Y, Maeder C, Fournier M, Rantakari P, Zhang FP, Poutanen M, Dufour JF, et al: Stress-activated miR-21/miR-21* in hepatocytes promotes lipid and glucose metabolic disorders associated with High-fat diet consumption. Gut. 65:1871–1881. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Meng F, Henson R, Wehbe-Janek H, Ghoshal K, Jacob ST and Patel T: MicroRNA-21 regulates expression of the PTEN tumor suppressor gene in human hepatocellular cancer. Gastroenterology. 133:647–658. 2007. View Article : Google Scholar : PubMed/NCBI | |
|
Loyer X, Paradis V, Hénique C, Vion AC, Colnot N, Guerin CL, Devue C, On S, Scetbun J, Romain M, et al: Liver microRNA-21 is overexpressed in non-alcoholic steatohepatitis and contributes to the disease in experimental models by inhibiting PPARα expression. Gut. 65:1882–1894. 2016. View Article : Google Scholar | |
|
Wu H, Ng R, Chen X, Steer CJ and Song G: MicroRNA-21 is a potential link between non-alcoholic fatty liver disease and hepatocellular carcinoma via modulation of the HBP1-p53-Srebp1c pathway. Gut. 65:1850–1860. 2016. View Article : Google Scholar | |
|
Atic AI, Thiele M, Munk A and Dalgaard LT: Circulating miRNAs associated with nonalcoholic fatty liver disease. Am J Physiol Cell Physiol. 324:C588–C602. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Hendy OM, Rabie H, El Fouly A, Abdel-Samiee M, Abdelmotelb N, Elshormilisy AA, Allam M, Ali ST, Bahaa El-Deen NM, Abdelsattar S and Mohamed SM: The circulating Micro-RNAs (-122, -34a and -99a) as predictive biomarkers for Non-alcoholic fatty liver diseases. Diabetes Metab Syndr Obes. 12:2715–2723. 2019. View Article : Google Scholar | |
|
Liu XL, Pan Q, Cao HX, Xin FZ, Zhao ZH, Yang RX, Zeng J, Zhou H and Fan JG: Lipotoxic Hepatocyte-derived exosomal MicroRNA 192-5p activates macrophages through Rictor/Akt/Forkhead box transcription factor O1 signaling in nonalcoholic fatty liver disease. Hepatology. 72:454–469. 2020. View Article : Google Scholar | |
|
Becker PP, Rau M, Schmitt J, Malsch C, Hammer C, Bantel H, Müllhaupt B and Geier A: Performance of Serum microRNAs-122, -192 and -21 as biomarkers in patients with Non-alcoholic steatohepatitis. PLoS One. 10:e01426612015. View Article : Google Scholar | |
|
Li M, Guo Y, Wang XJ, Duan BH and Li L: HOTAIR participates in hepatic insulin resistance via regulating SIRT1. Eur Rev Med Pharmacol Sci. 22:7883–7890. 2018.PubMed/NCBI | |
|
Zhu X, Wu YB, Zhou J and Kang DM: Upregulation of lncRNA MEG3 promotes hepatic insulin resistance via increasing FoxO1 expression. Biochem Biophys Res Commun. 469:319–325. 2016. View Article : Google Scholar | |
|
Zhu X, Li H, Wu Y, Zhou J, Yang G and Wang W: lncRNA MEG3 promotes hepatic insulin resistance by serving as a competing endogenous RNA of miR-214 to regulate ATF4 expression. Int J Mol Med. 43:345–357. 2019. | |
|
Zhao XY, Xiong X, Liu T, Mi L, Peng X, Rui C, Guo L, Li S, Li X and Lin JD: Long noncoding RNA licensing of obesity-linked hepatic lipogenesis and NAFLD pathogenesis. Nat Commun. 9:29862018. View Article : Google Scholar : PubMed/NCBI | |
|
Yan C, Chen J and Chen N: Long noncoding RNA MALAT1 promotes hepatic steatosis and insulin resistance by increasing nuclear SREBP-1c protein stability. Sci Rep. 6:226402016. View Article : Google Scholar : PubMed/NCBI | |
|
Ma M, Duan R, Shen L, Liu M, Ji Y, Zhou H, Li C, Liang T, Li X and Guo L: The lncRNA Gm15622 stimulates SREBP-1c expression and hepatic lipid accumulation by sponging the miR-742-3p in mice. J Lipid Res. 61:1052–1064. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang N, Geng T, Wang Z, Zhang R, Cao T, Camporez JP, Cai SY, Liu Y, Dandolo L, Shulman GI, et al: Elevated hepatic expression of H19 long noncoding RNA contributes to diabetic hyperglycemia. JCI Insight. 3:e1203042018. View Article : Google Scholar : PubMed/NCBI | |
|
Wang J, Yang W, Chen Z, Chen J, Meng Y, Feng B, Sun L, Dou L, Li J, Cui Q and Yang J: Long noncoding RNA lncSHGL recruits hnRNPA1 to suppress hepatic gluconeogenesis and lipogenesis. Diabetes. 67:581–593. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Li D, Cheng M, Niu Y, Chi X, Liu X, Fan J, Fan H, Chang Y and Yang W: Identification of a novel human long non-coding RNA that regulates hepatic lipid metabolism by inhibiting SREBP-1c. Int J Biol Sci. 13:349–357. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang B, Li H, Li D, Sun H, Li M and Hu H: Long noncoding RNA Mirt2 upregulates USP10 expression to suppress hepatic steatosis by sponging miR-34a-5p. Gene. 700:139–148. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Berzigotti A, Saran U and Dufour JF: Physical activity and liver diseases. Hepatology. 63:1026–1040. 2016. View Article : Google Scholar | |
|
Hayıroğlu M, Çınar T, Cilli Hayıroğlu S, Şaylık F, Uzun M and Tekkeşin A: The role of smart devices and mobile application on the change in peak VO2 in patients with high cardiovascular risk: A sub-study of the LIGHT randomised clinical trial. Acta Cardiol. 78:1000–1005. 2023. View Article : Google Scholar | |
|
Hayıroğlu M, Çınar T, Çinier G, Karakaya A, Yıldırım M, Güney BÇ, Öz A, Gündoğmuş PD, Ösken A, Özkan A, et al: The effect of 1-year mean step count on the change in the atherosclerotic cardiovascular disease risk calculation in patients with high cardiovascular risk: A sub-study of the LIGHT randomized clinical trial. Kardiol Pol. 79:1140–1142. 2021. View Article : Google Scholar | |
|
Su P, Chen JG and Tang DH: Exercise against nonalcoholic fatty liver disease: Possible role and mechanism of lipophagy. Life Sci. 327:1218372023. View Article : Google Scholar : PubMed/NCBI | |
|
Garber CE, Blissmer B, Deschenes MR, Franklin BA, Lamonte MJ, Lee IM, Nieman DC and Swain DP; American College of Sports Medicine: American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: Guidance for prescribing exercise. Med Sci Sports Exerc. 43:1334–1359. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
Simpson RJ, Campbell JP, Gleeson M, Krüger K, Nieman DC, Pyne DB, Turner JE and Walsh NP: Can exercise affect immune function to increase susceptibility to infection? Exerc Immunol Rev. 26:8–22. 2020.PubMed/NCBI | |
|
Carbone S, Del Buono MG, Ozemek C and Lavie CJ: Obesity, risk of diabetes and role of physical activity, exercise training and cardiorespiratory fitness. Prog Cardiovasc Dis. 62:327–333. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Liu S, Liu Y, Liu Z, Hu Y and Jiang M: A review of the signaling pathways of aerobic and anaerobic exercise on atherosclerosis. J Cell Physiol. 238:866–879. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
McKie GL and Wright DC: Biochemical adaptations in white adipose tissue following aerobic exercise: From mitochondrial biogenesis to browning. Biochem J. 477:1061–1081. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Richter EA and Hargreaves M: Exercise, GLUT4, and skeletal muscle glucose uptake. Physiol Rev. 93:993–1017. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
El Assar M, Álvarez-Bustos A, Sosa P, Angulo J and Rodríguez-Mañas L: Effect of physical Activity/exercise on oxidative stress and inflammation in muscle and vascular aging. Int J Mol Sci. 23:87132022. View Article : Google Scholar : PubMed/NCBI | |
|
Guo R, Liong EC, So KF, Fung ML and Tipoe GL: Beneficial mechanisms of aerobic exercise on hepatic lipid metabolism in Non-alcoholic fatty liver disease. Hepatobiliary Pancreat Dis Int. 14:139–144. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Mohammad Rahimi GR and Attarzadeh Hosseini SR: Effect of aerobic exercise alone or in conjunction with diet on liver function, insulin resistance and lipids in Non-alcoholic fatty liver disease. Biol Res Nurs. 24:259–276. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Diniz TA, de Lima Junior EA, Teixeira AA, Biondo LA, da Rocha LAF, Valadão IC, Silveira LS, Cabral-Santos C, de Souza CO and Rosa Neto JC: Aerobic training improves NAFLD markers and insulin resistance through AMPK-PPAR-α signaling in obese mice. Life Sci. 266:1188682021. View Article : Google Scholar | |
|
Keating SE, Hackett DA, George J and Johnson NA: Exercise and non-alcoholic fatty liver disease: A systematic review and meta-analysis. J Hepatol. 57:157–166. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Hajighasem A, Farzanegi P and Mazaheri Z: Effects of combined therapy with resveratrol, continuous and interval exercises on apoptosis, oxidative stress, and inflammatory biomarkers in the liver of old rats with Non-alcoholic fatty liver disease. Arch Physiol Biochem. 125:142–149. 2019. View Article : Google Scholar | |
|
Sohet FM, Neyrinck AM, Pachikian BD, de Backer FC, Bindels LB, Niklowitz P, Menke T, Cani PD and Delzenne NM: Coenzyme Q10 supplementation lowers hepatic oxidative stress and inflammation associated with diet-induced obesity in mice. Biochem Pharmacol. 78:1391–1400. 2009. View Article : Google Scholar : PubMed/NCBI | |
|
Xiao J, Ching YP, Liong EC, Nanji AA, Fung ML and Tipoe GL: Garlic-derived S-allylmercaptocysteine is a hepato-protective agent in non-alcoholic fatty liver disease in vivo animal model. Eur J Nutr. 52:179–191. 2013. View Article : Google Scholar : | |
|
Xiao J, Ho CT, Liong EC, Nanji AA, Leung TM, Lau TY, Fung ML and Tipoe GL: Epigallocatechin gallate attenuates fibrosis, oxidative stress, and inflammation in non-alcoholic fatty liver disease rat model through TGF/SMAD, PI3 K/Akt/FoxO1, and NF-kappa B pathways. Eur J Nutr. 53:187–199. 2014. View Article : Google Scholar | |
|
Qi F, Li T, Deng Q and Fan A: The impact of aerobic and anaerobic exercise interventions on the management and outcomes of non-alcoholic fatty liver disease. Physiol Res. 73:671–686. 2024. View Article : Google Scholar : PubMed/NCBI | |
|
Li H, Dun Y, Zhang W, You B, Liu Y, Fu S, Qiu L, Cheng J, Ripley-Gonzalez JW and Liu S: Exercise improves lipid droplet metabolism disorder through activation of AMPK-mediated lipophagy in NAFLD. Life Sci. 273:1193142021. View Article : Google Scholar : PubMed/NCBI | |
|
Zou YY, Tang XB, Chen ZL, Liu B, Zheng L, Song MY, Xiao Q, Zhou ZQ, Peng XY and Tang CF: Exercise intervention improves mitochondrial quality in non-alcoholic fatty liver disease zebrafish. Front Endocrinol (Lausanne). 14:11624852023. View Article : Google Scholar : PubMed/NCBI | |
|
McLeod JC, Currier BS, Lowisz CV and Phillips SM: The influence of resistance exercise training prescription variables on skeletal muscle mass, strength, and physical function in healthy adults: An umbrella review. J Sport Health Sci. 13:47–60. 2024. View Article : Google Scholar : | |
|
Schoenfeld BJ, Ogborn D and Krieger JW: Effects of resistance training frequency on measures of muscle hypertrophy: A systematic review and Meta-analysis. Sports Med. 46:1689–1697. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Consitt LA, Dudley C and Saxena G: Impact of endurance and resistance training on skeletal muscle glucose metabolism in older adults. Nutrients. 11:26362019. View Article : Google Scholar : PubMed/NCBI | |
|
Fedewa MV, Gist NH, Evans EM and Dishman RK: Exercise and insulin resistance in youth: A meta-analysis. Pediatrics. 133:e163–e174. 2014. View Article : Google Scholar | |
|
Ivy JL: Muscle insulin resistance amended with exercise training: Role of GLUT4 expression. Med Sci Sports Exerc. 36:1207–1211. 2004.PubMed/NCBI | |
|
Mazur-Bialy AI, Pocheć E and Zarawski M: Anti-inflammatory properties of irisin, mediator of physical activity, are connected with TLR4/MyD88 signaling pathway activation. Int J Mol Sci. 18:7012017. View Article : Google Scholar : PubMed/NCBI | |
|
Hashida R, Kawaguchi T, Bekki M, Omoto M, Matsuse H, Nago T, Takano Y, Ueno T, Koga H, George J, et al: Aerobic vs. resistance exercise in non-alcoholic fatty liver disease: A systematic review. J Hepatol. 66:142–152. 2017. View Article : Google Scholar | |
|
Xue Y, Peng Y, Zhang L, Ba Y, Jin G and Liu G: Effect of different exercise modalities on nonalcoholic fatty liver disease: A systematic review and network meta-analysis. Sci Rep. 14:62122024. View Article : Google Scholar : PubMed/NCBI | |
|
Takahashi A, Abe K, Usami K, Imaizumi H, Hayashi M, Okai K, Kanno Y, Tanji N, Watanabe H and Ohira H: Simple resistance exercise helps patients with Non-alcoholic fatty liver disease. Int J Sports Med. 36:848–852. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Yang HJ, Hong YP, Yoon TY, Ryoo JH, Choi JM and Oh CM: Independent and synergistic associations of aerobic physical activity and resistance exercise with nonalcoholic fatty liver disease. Gut Liver. 17:600–609. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Gripp F, Nava RC, Cassilhas RC, Esteves EA, Magalhães COD, Dias-Peixoto MF, de Castro Magalhães F and Amorim FT: HIIT is superior than MICT on cardiometabolic health during training and detraining. Eur J Appl Physiol. 121:159–172. 2021. View Article : Google Scholar | |
|
Ryan BJ, Schleh MW, Ahn C, Ludzki AC, Gillen JB, Varshney P, Van Pelt DW, Pitchford LM, Chenevert TL, Gioscia-Ryan RA, et al: Moderate-intensity exercise and High-intensity interval training affect insulin sensitivity similarly in obese adults. J Clin Endocrinol Metab. 105:e2941–e2959. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Han C, Lu P and Yan SZ: Effects of high-intensity interval training on mitochondrial supercomplex assembly and biogenesis, mitophagy, and the AMP-activated protein kinase pathway in the soleus muscle of aged female rats. Exp Gerontol. 158:1116482022. View Article : Google Scholar | |
|
Wadley AJ, Chen YW, Lip GY, Fisher JP and Aldred S: Low volume-high intensity interval exercise elicits antioxidant and anti-inflammatory effects in humans. J Sports Sci. 34:1–9. 2016. View Article : Google Scholar | |
|
Su L, Fu J, Sun S, Zhao G, Cheng W, Dou C and Quan M: Effects of HIIT and MICT on cardiovascular risk factors in adults with overweight and/or obesity: A meta-analysis. PLoS One. 14:e02106442019. View Article : Google Scholar : PubMed/NCBI | |
|
Gu S, Du X, Wang D, Yu Y and Guo S: Effects of high intensity interval training versus moderate intensity continuous training on exercise capacity and quality of life in patients with heart failure: A systematic review and Meta-analysis. PLoS One. 18:e02903622023. View Article : Google Scholar : PubMed/NCBI | |
|
de Brito JN, McDonough DJ, Mathew M, VanWagner LB, Schreiner PJ, Gabriel KP, Jacobs DR Jr, Terry JG, Carr JJ and Pereira MA: Young adult physical activity trajectories and midlife nonalcoholic fatty liver disease. JAMA Netw Open. 6:e23389522023. View Article : Google Scholar : PubMed/NCBI | |
|
Fredrickson G, Barrow F, Dietsche K, Parthiban P, Khan S, Robert S, Demirchian M, Rhoades H, Wang H, Adeyi O and Revelo XS: Exercise of high intensity ameliorates hepatic inflammation and the progression of NASH. Mol Metab. 53:1012702021. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang HJ, He J, Pan LL, Ma ZM, Han CK, Chen CS, Chen Z, Han HW, Chen S, Sun Q, et al: Effects of moderate and vigorous exercise on nonalcoholic fatty liver disease: A Randomized clinical trial. JAMA Intern Med. 176:1074–1082. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Harden JE, Tabacu L and Reynolds LJ: Physical activity intensity and markers of inflammation in those with non-alcoholic fatty liver disease. Diabetes Res Clin Pract. 207:1110472024. View Article : Google Scholar | |
|
Yuan Z, Xiao-Wei L, Juan W, Xiu-Juan L, Nian-Yun Z and Lei S: HIIT and MICT attenuate high-fat diet-induced hepatic lipid accumulation and ER stress via the PERK-ATF4-CHOP signaling pathway. J Physiol Biochem. 78:641–652. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Hyun J and Jung Y: DNA Methylation in nonalcoholic fatty liver disease. Int J Mol Sci. 21:81382020. View Article : Google Scholar : PubMed/NCBI | |
|
Moore LD, Le T and Fan G: DNA methylation and its basic function. Neuropsychopharmacology. 38:23–38. 2013. View Article : Google Scholar | |
|
Jones MJ, Goodman SJ and Kobor MS: DNA methylation and healthy human aging. Aging Cell. 14:924–932. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Kim YN, Hwang JH and Cho YO: The effects of exercise training and acute exercise duration on plasma folate and vitamin B12. Nutr Res Pract. 10:161–166. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Brunaud L, Alberto JM, Ayav A, Gérard P, Namour F, Antunes L, Braun M, Bronowicki JP, Bresler L and Guéant JL: Effects of vitamin B12 and folate deficiencies on DNA methylation and carcinogenesis in rat liver. Clin Chem Lab Med. 41:1012–1019. 2003. View Article : Google Scholar : PubMed/NCBI | |
|
Hunter DJ, James L, Hussey B, Wadley AJ, Lindley MR and Mastana SS: Impact of aerobic exercise and fatty acid supplementation on global and gene-specific DNA methylation. Epigenetics. 14:294–309. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Robson-Ansley PJ, Saini A, Toms C, Ansley L, Walshe IH, Nimmo MA and Curtin JA: Dynamic changes in dna methylation status in peripheral blood Mononuclear cells following an acute bout of exercise: Potential impact of exercise-induced elevations in interleukin-6 concentration. J Biol Regul Homeost Agents. 28:407–417. 2014.PubMed/NCBI | |
|
Zhou D, Hlady RA, Schafer MJ, White TA, Liu C, Choi JH, Miller JD, Roberts LR, LeBrasseur NK and Robertson KD: High fat diet and exercise lead to a disrupted and pathogenic DNA methylome in mouse liver. Epigenetics. 12:55–69. 2017. View Article : Google Scholar : | |
|
Zhang Y, Hashimoto S, Fujii C, Hida S, Ito K, Matsumura T, Sakaizawa T, Morikawa M, Masuki S, Nose H, et al: NFκB2 gene as a novel candidate that epigenetically responds to interval walking training. Int J Sports Med. 36:769–775. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Światowy WJ, Drzewiecka H, Kliber M, Sąsiadek M, Karpiński P, Pławski A and Jagodziński PP: Physical Activity and DNA Methylation in Humans. Int J Mol Sci. 22:129892021. View Article : Google Scholar : PubMed/NCBI | |
|
King-Himmelreich TS, Schramm S, Wolters MC, Schmetzer J, Möser CV, Knothe C, Resch E, Peil J, Geisslinger G and Niederberger E: The impact of endurance exercise on global and AMPK gene-specific DNA methylation. Biochem Biophys Res Commun. 474:284–290. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Freitas-Dias R, Lima TI, Costa-Junior JM, Gonçalves LM, Araujo HN, Paula FMM, Santos GJ, Branco RCS, Ou K, Kaestner KH, et al: Offspring from trained male mice inherit improved muscle mitochondrial function through PPAR co-repressor modulation. Life Sci. 291:1202392022. View Article : Google Scholar | |
|
McGee SL and Hargreaves M: Epigenetics and Exercise. Trends Endocrinol Metab. 30:636–645. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Mallett G: The effect of exercise and physical activity on skeletal muscle epigenetics and metabolic adaptations. Eur J Appl Physiol. 125:611–627. 2025. View Article : Google Scholar : PubMed/NCBI | |
|
Stols-Gonçalves D, Tristão LS, Henneman P and Nieuwdorp M: Epigenetic markers and Microbiota/Metabolite-Induced epigenetic modifications in the pathogenesis of obesity, metabolic syndrome, type 2 diabetes, and Non-alcoholic fatty liver disease. Curr Diab Rep. 19:312019. View Article : Google Scholar : PubMed/NCBI | |
|
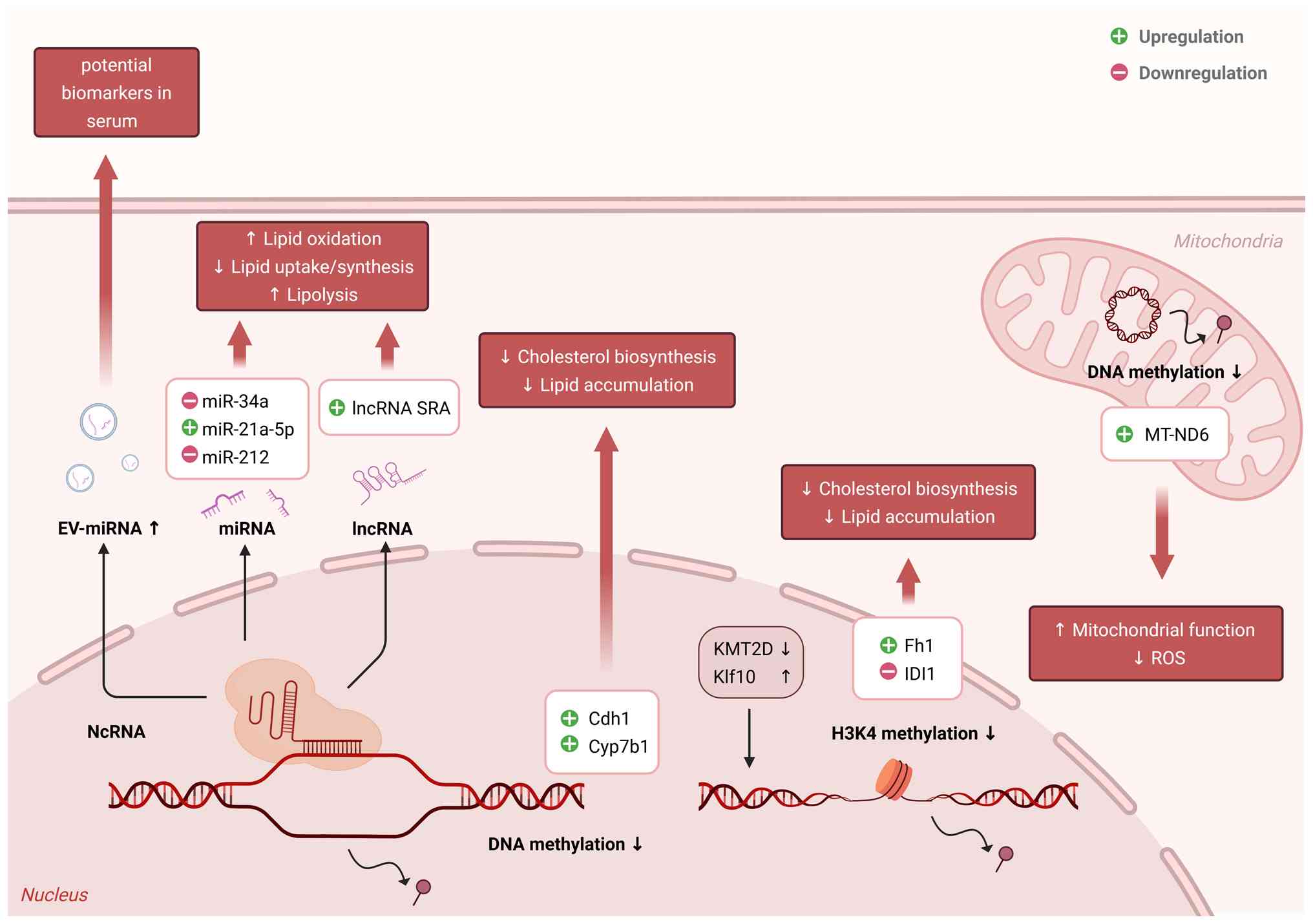
Fan X, Wang H, Wang W, Shen J and Wang Z: Exercise training alleviates cholesterol and lipid accumulation in mice with non-alcoholic steatohepatitis: Reduction of KMT2D-mediated histone methylation of IDI1. Exp Cell Res. 442:1142652024. View Article : Google Scholar : PubMed/NCBI | |
|
Luo HY, Mu WJ, Chen M, Zhu JY, Li Y, Li S, Yan LJ, Li RY, Yin MT, Li X, et al: Hepatic Klf10-Fh1 axis promotes exercise-mediated amelioration of NASH in mice. Metabolism. 155:1559162024. View Article : Google Scholar : PubMed/NCBI | |
|
Villanova L, Vernucci E, Pucci B, Pellegrini L, Nebbioso M, Mauri C, Marfe G, Spataro A, Fini M, Banfi G, et al: Influence of age and physical exercise on sirtuin activity in humans. J Biol Regul Homeost Agents. 27:497–507. 2013.PubMed/NCBI | |
|
Yang J, Félix-Soriano E, Martínez-Gayo A, Ibañez-Santos J, Sáinz N, Martínez JA and Moreno-Aliaga MJ: SIRT1 and FOXO1 role on MASLD risk: Effects of DHA-rich n-3 PUFA supplementation and exercise in aged obese female mice and in Post-menopausal Overweight/obese women. J Physiol Biochem. 80:697–712. 2024. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang Y, Shan M, Ding X, Sun H, Qiu F and Shi L: Maternal exercise represses Nox4 via SIRT1 to prevent vascular oxidative stress and endothelial dysfunction in SHR offspring. Front Endocrinol (Lausanne). 14:12191942023. View Article : Google Scholar : PubMed/NCBI | |
|
Nikroo H, Hosseini SRA, Fathi M, Sardar MA and Khazaei M: The effect of aerobic, resistance, and combined training on PPAR-α, SIRT1 gene expression, and insulin resistance in high-fat diet-induced NAFLD male rats. Physiol Behav. 227:1131492020. View Article : Google Scholar | |
|
Song MY, Han CY, Moon YJ, Lee JH, Bae EJ and Park BH: Sirt6 reprograms myofibers to oxidative type through CREB-dependent Sox6 suppression. Nat Commun. 13:18082022. View Article : Google Scholar : PubMed/NCBI | |
|
Tian C, Huang R and Xiang M: SIRT1: Harnessing multiple pathways to hinder NAFLD. Pharmacol Res. 203:1071552024. View Article : Google Scholar : PubMed/NCBI | |
|
Hou T, Tian Y, Cao Z, Zhang J, Feng T, Tao W, Sun H, Wen H, Lu X, Zhu Q, et al: Cytoplasmic SIRT6-mediated ACSL5 deacetylation impedes nonalcoholic fatty liver disease by facilitating hepatic fatty acid oxidation. Mol Cell. 82:4099–4115.e4099. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Hall MM, Rajasekaran S, Thomsen TW and Peterson AR: Lactate: Friend or Foe. PM R. 8(Suppl 3): S8–S15. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Harris RT and Dudley GA: Exercise alters the distribution of ammonia and lactate in blood. J Appl Physiol (1985). 66:313–317. 1989. View Article : Google Scholar : PubMed/NCBI | |
|
Han H, Zhao Y, Du J, Wang S, Yang X, Li W, Song J, Zhang S, Zhang Z, Tan Y, et al: Exercise improves cognitive dysfunction and neuroinflammation in mice through Histone H3 lactylation in microglia. Immun Ageing. 20:632023. View Article : Google Scholar : PubMed/NCBI | |
|
Widmann M, Nieß AM and Munz B: Physical exercise and epigenetic modifications in skeletal muscle. Sports Med. 49:509–523. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Dos Santos JAC, Veras ASC, Batista VRG, Tavares MEA, Correia RR, Suggett CB and Teixeira GR: Physical exercise and the functions of microRNAs. Life Sci. 304:1207232022. View Article : Google Scholar : PubMed/NCBI | |
|
Antunes-Correa LM, Trevizan PF, Bacurau AVN, Ferreira-Santos L, Gomes JLP, Urias U, Oliveira PA, Alves MJNN, de Almeida DR, Brum PC, et al: Effects of aerobic and inspiratory training on skeletal muscle microRNA-1 and downstream-associated pathways in patients with heart failure. J Cachexia Sarcopenia Muscle. 11:89–102. 2020. View Article : Google Scholar | |
|
Chen JF, Mandel EM, Thomson JM, Wu Q, Callis TE, Hammond SM, Conlon FL and Wang DZ: The role of microRNA-1 and microRNA-133 in skeletal muscle proliferation and differentiation. Nat Genet. 38:228–233. 2006. View Article : Google Scholar | |
|
Scisciola L, Benedetti R, Chianese U, Fontanella RA, Del Gaudio N, Marfella R, Surina, Altucci L, Barbieri M, Paolisso G, et al: The pivotal role of miRNA-21 in myocardial metabolic flexibility in response to short- and long-term high glucose treatment: Evidence in human cardiomyocyte cell line. Diabetes Res Clin Pract. 191:1100662022. View Article : Google Scholar : PubMed/NCBI | |
|
Da Silva ND Jr, Fernandes T, Soci UP, Monteiro AW, Phillips MI and De Oliveira EM: Swimming training in rats increases cardiac MicroRNA-126 expression and angiogenesis. Med Sci Sports Exerc. 44:1453–1462. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Wilson RA, Stathis CG, Hayes A and Cooke MB: Intermittent fasting and High-intensity exercise elicit Sexual-dimorphic and Tissue-specific adaptations in Diet-induced obese mice. Nutrients. 12:17642020. View Article : Google Scholar : PubMed/NCBI | |
|
Kristensen MM, Davidsen PK, Vigelsø A, Hansen CN, Jensen LJ, Jessen N, Bruun JM, Dela F and Helge JW: miRNAs in human subcutaneous adipose tissue: Effects of weight loss induced by hypocaloric diet and exercise. Obesity (Silver Spring). 25:572–580. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Lionett S, Kiel IA, Camera DM, Vanky E, Parr EB, Lydersen S, Hawley JA and Moholdt T: Circulating and adipose Tissue miRNAs in women with polycystic ovary syndrome and responses to High-intensity interval training. Front Physiol. 11:9042020. View Article : Google Scholar : PubMed/NCBI | |
|
Xu B, Zhang Y, Du XF, Li J, Zi HX, Bu JW, Yan Y, Han H and Du JL: Neurons secrete miR-132-containing exosomes to regulate brain vascular integrity. Cell Res. 27:882–897. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Panera N, Gnani D, Crudele A, Ceccarelli S, Nobili V and Alisi A: MicroRNAs as controlled systems and controllers in non-alcoholic fatty liver disease. World J Gastroenterol. 20:15079–15086. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Ghareghani P, Shanaki M, Ahmadi S, Khoshdel AR, Rezvan N, Meshkani R, Delfan M and Gorgani-Firuzjaee S: Aerobic endurance training improves nonalcoholic fatty liver disease (NAFLD) features via miR-33 dependent autophagy induction in high fat diet fed mice. Obes Res Clin Pract. 12:80–89. 2018. View Article : Google Scholar | |
|
Kalaki-Jouybari F, Shanaki M, Delfan M, Gorgani-Firouzjae S and Khakdan S: High-intensity interval training (HIIT) alleviated NAFLD feature via miR-122 induction in liver of high-fat high-fructose diet induced diabetic rats. Arch Physiol Biochem. 126:242–249. 2020. View Article : Google Scholar | |
|
Lu YL, Jing W, Feng LS, Zhang L, Xu JF, You TJ and Zhao J: Effects of hypoxic exercise training on microRNA expression and lipid metabolism in obese rat livers. J Zhejiang Univ Sci B. 15:820–829. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Xiao J, Bei Y, Liu J, Dimitrova-Shumkovska J, Kuang D, Zhou Q, Li J, Yang Y, Xiang Y, Wang F, et al: miR-212 downregulation contributes to the protective effect of exercise against non-alcoholic fatty liver via targeting FGF-21. J Cell Mol Med. 20:204–216. 2016. View Article : Google Scholar | |
|
Wang M, Xue Q, Li X, Krohn K, Ziesche S, Ceglarek U, Blüher M, Keller M, Yaskolka Meir A, Heianza Y, et al: Circulating levels of microRNA-122 and hepatic fat change in response to Weight-Loss interventions: CENTRAL Trial. J Clin Endocrinol Metab. 107:e1899–e1906. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
de Mendonça M, Rocha KC, de Sousa É, Pereira BMV, Oyama LM and Rodrigues AC: Aerobic exercise training regulates serum extracellular vesicle miRNAs linked to obesity to promote their beneficial effects in mice. Am J Physiol Endocrinol Metab. 319:E579–E591. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Wang Z, Zhu Y, Xia L, Li J, Song M and Yang C: Exercise-Induced ADAR2 protects against nonalcoholic fatty liver disease through miR-34a. Nutrients. 15:1212022. View Article : Google Scholar | |
|
Wu B, Ding J, Chen A, Song Y, Xu C, Tian F and Zhao J: Aerobic exercise improves adipogenesis in diet-induced obese mice via the lncSRA/p38/JNK/PPARγ pathway. Nutr Res. 105:20–32. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Wu B, Xu C, Tian Y, Zeng Y, Yan F, Chen A, Zhao J and Chen L: Aerobic exercise promotes the expression of ATGL and attenuates inflammation to improve hepatic steatosis via lncRNA SRA. Sci Rep. 12:53702022. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang Y, Wei Q, Geng X and Fang G: Long-term aerobic exercise enhances hepatoprotection in MAFLD by modulating exosomal miR-324 via ROCK1. Metabolites. 14:6922024. View Article : Google Scholar : PubMed/NCBI | |
|
Zhao J, Song Y, Zeng Y, Chen L, Yan F, Chen A, Wu B and Wang Y: Improvement of hyperlipidemia by aerobic exercise in mice through a regulatory effect of miR-21a-5p on its target genes. Sci Rep. 11:119662021. View Article : Google Scholar : PubMed/NCBI | |
|
Lou J, Wu J, Feng M, Dang X, Wu G, Yang H, Wang Y, Li J, Zhao Y, Shi C, et al: Exercise promotes angiogenesis by enhancing endothelial cell fatty acid utilization via liver-derived extracellular vesicle miR-122-5p. Journal of sport and health science. 11:495–508. 2022. View Article : Google Scholar : | |
|
Xia SF, Jiang YY, Qiu YY, Huang W and Wang J: Role of diets and exercise in ameliorating obesity-related hepatic steatosis: Insights at the microRNA-dependent thyroid hormone synthesis and action. Life Sci. 242:1171822020. View Article : Google Scholar | |
|
Abdelmalek MF: Nonalcoholic fatty liver disease: Another leap forward. Nat Rev Gastroenterol Hepatol. 18:85–86. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Gruet M, Saynor ZL, Urquhart DS and Radtke T: Rethinking physical exercise training in the modern era of cystic fibrosis: A step towards optimising Short-term efficacy and long-term engagement. J Cyst Fibros. 21:e83–e98. 2022. View Article : Google Scholar | |
|
Stevanović-Silva J, Beleza J, Coxito P, Costa RC, Ascensão A and Magalhães J: Fit mothers for a healthy future: Breaking the intergenerational cycle of non-alcoholic fatty liver disease with maternal exercise. Eur J Clin Invest. 52:e135962022. View Article : Google Scholar |










