Protective effect of exercise on metabolic dysfunction‑associated fatty liver disease: Potential epigenetic mechanisms (Review)
- Authors:
- Published online on: July 15, 2025 https://doi.org/10.3892/ijmm.2025.5587
- Article Number: 146
-
Copyright : © Zhang et al. This is an open access article distributed under the terms of Creative Commons Attribution License [CC BY 4.0].
Abstract
Introduction
Metabolic dysfunction-associated fatty liver disease (MAFLD) is the leading cause of chronic liver disease worldwide and affects nearly 25% of the population, with disproportionately higher prevalence in developed nations (1). The pathogenesis of MAFLD is multifactorial and driven by a complex interplay among genetic predisposition, metabolic derangements and environmental influences (2-4). Central to its development is insulin resistance, which promotes lipolysis in the adipose tissue and increases free fatty acid flux to the liver (5). Without effective treatment, MAFLD may progress to metabolic dysfunction-associated steatohepatitis (MASH), which requires secondary insults characterized by mitochondrial dysfunction, oxidative stress and lipotoxic injury (6). Furthermore, MAFLD, a hepatic manifestation of the metabolic syndrome, is closely associated with obesity, insulin resistance and type 2 diabetes (7). Critically, MAFLD independently elevates the risk and exacerbates the severity of cardiovascular diseases, particularly coronary artery disease, thereby posing a significant burden on public health systems (8).
Despite decades of research elucidating these multifaceted mechanisms, no pharmacological therapy has been approved for MAFLD treatment (9). Nonetheless, physical exercise has emerged as a remarkably potent intervention, demonstrating efficacy across various stages of MAFLD (10). Unlike pharmacological approaches that typically target single pathways, exercise exerts pleiotropic benefits across the spectrum of MAFLD pathogenesis through an orchestra of adaptive responses that remodel cellular metabolism and signaling networks (11). Importantly, the benefits of exercise persist beyond the intervention period, suggesting induction of long-lasting metabolic reprogramming (12). This sustained effect may be mediated by durable epigenetic modifications that alter the transcriptional landscape of key metabolic and inflammatory genes. Accumulating evidence has implicated epigenetic remodeling as a key mechanism underlying the lasting impact of exercise on hepatic function (13-16).
Epigenetic modifications are heritable and reversible changes in gene expression that occur without altering the underlying DNA sequences. These modifications regulate gene activity through several interrelated mechanisms, including DNA methylation, post-translational modifications of histone proteins (such as methylation and acetylation), chromatin remodeling and regulatory functions of non-coding RNAs (ncRNAs) (17). Collectively, these epigenetic processes influence chromatin architecture and transcriptional activity by modulating the accessibility of genomic regions to transcription factors and their associated cofactors, thereby governing the efficiency and specificity of gene transcription initiation and elongation (18-20). Substantial evidence has demonstrated that epigenetic modifications play a decisive role in MAFLD pathogenesis by dysregulating key pathological processes including hepatic metabolic homeostasis, inflammatory responses and fibrotic progression (21-23). Physical activity can dynamically modulate the epigenetic landscape, restore metabolic flexibility and potentially reverse disease-associated alterations (24,25). Understanding these molecular mechanisms may enable personalized exercise recommendations. The present review comprehensively summarizes current research on how epigenetic dysregulation contributes to MAFLD progression. Based on clinical and preclinical evidence, it was systematically assessed how distinct exercise modalities improve hepatic pathology through epigenetic mechanisms.
Epigenetics: An intricate yet precise regulatory network
Epigenetics, originally conceptualized by Waddington in 1942, refers to the heritable and reversible changes in gene expression that occur without altering the underlying DNA sequence (26). Epigenetic regulation of gene expression is mediated by three primary mechanisms: DNA methylation, histone modifications and ncRNAs. These mechanisms collectively establish a dynamic regulatory framework that enables the precise control of transcriptional programs (27).
DNA methylation
DNA methylation is the most extensively studied epigenetic modification in eukaryotic cells. It entails the covalent attachment of a methyl group to the fifth carbon of cytosine residues, primarily within cytosinephosphate-guanine (CpG) dinucleotides, resulting in the formation of 5-methylcytosine (5mC) (28,29). CpG sites, which are characterized by cytosine followed by guanine in the DNA sequence, are particularly prone to methylation (30). This stable and heritable epigenetic marker is strongly associated with transcriptional repression and plays a pivotal role in gene silencing. In addition to its well-established role in gene silencing, DNA methylation is critically involved in chromatin organization, maintenance of genomic stability, and key developmental processes such as X-chromosome inactivation, genomic imprinting and gametogenesis (31-33). Dynamic regulation of DNA methylation is orchestrated by two enzyme families: DNA methyltransferases (DNMTs) and ten-eleven translocation (TET) dioxygenases (34,35). Among the five known DNMTs, DNMT1, DNMT3A and DNMT3B function as the principal catalytic enzymes (36). DNMT1 maintains methylation patterns during DNA replication by recognizing hemi-methylated CpG sites, whereas DNMT3A and DNMT3B mediate de novo methylation during early development and lineage specification (37). By contrast, active DNA demethylation is orchestrated by TET enzymes, a Fe (II)- and α-ketoglutarate-dependent dioxygenases, that catalyze the iterative oxidation of 5mC to 5-hydroxymethylcytosine, 5-formylcytosine and 5-carboxylcytosine. These oxidized intermediates facilitate base excision repair via thymine DNA glycosylases, thereby enabling rapid DNA demethylation. Mammals express three TET paralogs (TET1-3) with distinct tissue distributions: TET2 and TET3 dominate in hematopoietic lineages, whereas TET1 initiates oxidative demethylation in developmental contexts (38).
Beyond the nuclear genome, accumulating evidence has revealed that mitochondrial DNA (mtDNA) is also subject to methylation modifications (39). Unlike nuclear DNA, mtDNA is circular, histone-free and organized into compact nucleoid structures (40). High-resolution mapping techniques confirmed the presence of methylation marks within mtDNA, particularly in the D-loop control region, which is a critical site governing replication and transcription initiation (41). Intriguingly, mtDNA methylation occurs not only at CpG sites but also at non-CpG sites, exhibiting a strand-specific bias predominantly affecting the light strand. This pattern diverges from the canonical nuclear methylation landscapes, suggesting specialized regulatory functions within the mitochondria (42).
Histone modifications
Post-translational modifications of histones represent a major mechanism for dynamically controlling chromatin structure and function (43,44). Highly conserved histone proteins undergo several types of covalent modifications, including acetylation, phosphorylation, methylation, ubiquitination, SUMOylation and ribosylation (45). Among these, the critical roles of histone acetylation and methylation have been particularly well characterized in modulating higher-order chromatin organization and transcriptional regulation (43,46).
Histone methylation primarily occurs at lysine residues in histones. The most studied histone methylation sites are located in histone 3, lysine corresponding to positions 4, 9, 27, 36 and 79 (H3K4, H3K9, H3K27, H3K36, and H3K79 respectively) and histone 4 K20 (H4K20) (47). Lysine residues can undergo mono-, di-, or trimethylation (48). The biological impact of these modifications varies based on the chromatin localization and the specific cell type or developmental stage (49). In general, methylation of histone residues H3K4, H3K36 and H3K79 is linked to transcriptional activation, whereas methylation of H3K9, H3K27 and H4K20 is typically associated with transcriptional repression and the formation of inactive chromatin states (48). However, enrichment of H3K9 trimethylation has been observed in the transcriptionally active euchromatic regions of genes, demonstrating that the impact of histone methylation on gene transcription is complex and nuanced (50). The epigenetic landscape of histone methylation is tightly regulated by the coordinated activities of histone methyltransferases (HMTs) and demethylases (HDMs). HMTs catalyze the transfer of methyl groups from S-adenosylmethionine to specific lysine residues on histone tails, thereby modulating chromatin structure and gene expression (51,52). The most extensively studied HMTs include the SET domain-containing lysine methyltransferase (KMT) family and SET domain-lacking Dot1 family. KMTs typically possess a conserved SET domain that is broadly preserved across species, despite differences in substrate specificity and enzymatic function. Despite their conservation, HMTs exhibit distinct substrate specificities and catalytic functions (53). Histone methylation was traditionally considered a stable modification until the discovery of lysine-specific demethylase 1 (LSD1), which revealed the existence of histone demethylation (54,55). HDMs are broadly categorized into two mechanistically distinct families. The amine oxidase family (for example, LSD1/KDM1A) removes mono- and di-methyl marks through flavin adenine dinucleotide (FAD)-dependent oxidative deamination, while JmjC domain-containing hydroxylases employ α-ketoglutarate and Fe2+ to catalyze the removal of diverse modifications including trimethylation (56).
Histone acetylation is a widely studied epigenetic modification, that plays a crucial role in regulating stem cell pluripotency, integrating metabolic signals, and forming a cross-regulatory network with DNA and histone methylation (57-59). The dysregulation of histone acetylation and deacetylation is commonly associated with various diseases, including cancer, neurodegenerative diseases and cardiovascular diseases (60-62). The most common sites of histone acetylation include lysine residues at positions 9, 14, 18 and 23 of histone H3 (H3K9, H3K14, H3K18 and H3K23, respectively), and at positions 8, 12, 16, and 20 of histone H4 (H4K8, H4K12, H4K16 and H4K20) (63). Generally, histone acetyltransferases (HATs)-mediated acetylation weakens the interaction between histones and DNA by neutralizing positive charges on histone tails, thereby promoting chromatin opening and transcriptional activation (64). Based on their catalytic mechanisms and cellular localization, HATs can be classified into two types: HAT A and HAT B. Members of the HAT A family are located in the nucleus, where they transfer acetyl groups to lysine residues after histone assembly into nucleosomes. By contrast, members of the HAT B family function in the cytoplasm by transferring acetyl groups to free histones before becoming nucleosomes (65). Histone deacetylases (HDACs) are transcriptional repressors that remove acetyl groups and restore tight binding between histones and DNA. HDACs are categorized into four classes (I-IV) based on their structural characteristics and coenzyme requirements. Classical HDACs (Class I/II/IV) are activated by Zn2+, whereas sirtuins (SIRTs)-related HDACs (Class III) are activated by NAD+ (66). Current research is focused on understanding the complex mechanisms of these enzymes, their roles in chromatin remodeling, and their potential as therapeutic targets in various diseases.
NcRNAs
In addition to DNA and histone modifications, ncRNAs have emerged as key epigenetic regulators of gene expression and chromatin organization. The human genome is pervasively transcribed, with ~75% DNA generating RNA molecules and only 2% encoding proteins. The remaining transcriptional output comprises ncRNAs that function directly as RNA entities to orchestrate complex biological processes (67). Emerging advances in epigenomics and high-throughput sequencing have identified more than 200,000 ncRNA species, establishing ncRNAs as indispensable regulators of cellular homeostasis and disease pathogenesis (68). ncRNAs are broadly classified into two major categories based on their structural or functional roles: Structural and regulatory ncRNAs. Structural ncRNAs, such as ribosomal RNA, transfer RNA, small nuclear RNAs and small nucleolar RNAs, are integral to fundamental cellular processes including ribosome assembly, protein translation and mRNA splicing. Regulatory ncRNAs, including microRNAs (miRNAs or miRs), long non-coding RNAs (lncRNAs), small interfering RNAs, PIWI-interacting RNAs and circular RNAs, primarily modulate gene expression via epigenetic, transcriptional, or post-transcriptional mechanisms (69). Certain ncRNAs such as miRNAs, small nucleolar RNAs and circular RNAs (circRNAs), demonstrate evolutionary conservation across species, whereas others, particularly lncRNAs, frequently lack sequence homology. Accumulating evidence has indicated that the expression profiles of circRNA-producing genes, circular-to-linear transcript ratios, and splice isoform patterns of circRNAs are highly cell type-specific (70-73). These observations underscore that circRNA biogenesis is not a random byproduct, but rather a conserved and tightly regulated facet of gene expression.
Among regulatory ncRNAs, miRNAs are ~22-nucleotidelong molecules that primarily function through post-transcriptional gene silencing. By binding to complementary sequences within the 3′ untranslated regions of target mRNAs, miRNAs mediate mRNA degradation or translational repression, thereby modulating diverse cellular processes such as proliferation, differentiation and apoptosis (74,75). Notably, miRNAs can be selectively incorporated into extracellular vesicles (EVs), including exosomes, and secreted into the extracellular environment to facilitate intercellular communication (76). These vesicle-containing miRNAs facilitate intercellular communication by transferring regulatory information to recipient cells, thereby influencing tissue homeostasis and contributing to disease pathogenesis, particularly in cancer, metabolic disorders and inflammatory diseases (77-79). lncRNAs, defined as transcripts longer than 200 nucleotides, display versatile mechanisms of action at the epigenetic, transcriptional and post-transcriptional levels. They serve as molecular scaffolds by recruiting chromatin-modifying complexes to specific genomic loci, thereby altering chromatin accessibility and influencing gene expression (80). lncRNAs may also act as molecular decoys, sequestering transcription factors or miRNAs to modulate downstream signaling pathways. Furthermore, some lncRNAs function as guides that direct regulatory proteins to their genomic targets or as enhancers that promote transcription by stabilizing chromosomal looping (81). Through these multifaceted mechanisms, lncRNAs critically regulate cellular identity, stress responses and disease development, highlighting their significance as potential therapeutic targets and biomarkers in various pathological conditions (82).
Molecular mechanism underlying epigenetic regulation in MAFLD
Given their dynamic and reversible nature, epigenetic modifications act as key mediators that link environmental factors to long-term changes in gene expression (83). As a central metabolic organ, the liver is particularly responsive to such epigenetic influences (17). Increasing evidence indicates that the dysregulation of epigenetic mechanisms (namely DNA methylation, histone modifications and ncRNAs) plays a pivotal role in the development and progression of MAFLD (23,84,85). These alterations affect the genes involved in lipid metabolism, insulin signaling, inflammation and fibrogenesis, thereby contributing to hepatic metabolic derangement (86,87). Current research progress was reviewed, focusing on three major epigenetic mechanisms and their specific contributions to MAFLD development (Fig. 1).
DNA methylation in MAFLD pathogenesis
In MAFLD, both global and locus-specific hypermethylation has been observed in genes that regulate lipid metabolism, mitochondrial function and inflammation (88,89). For example, hypermethylation of the peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α) promoter leads to decreased expression of this master regulator of mitochondrial biogenesis, contributing to impaired fatty acid oxidation and increased lipid accumulation (90,91). Similarly, the hypermethylation of mitochondrially encoded NADH dehydrogenase 6 (MT-ND6) correlates with decreased gene expression and disrupted oxidative phosphorylation, underscoring the impact of methylation on mitochondrial dysfunction in MAFLD (92). Genes involved in fibrogenesis, such as transforming growth factor beta 1 and platelet-derived growth factor alpha, show hypomethylation in MASH, facilitating their overexpression and promoting fibrotic remodeling (93). By contrast, tumor suppressor genes such as suppressor of cytokine signaling 1 and Ras association domain family member 1 often exhibit promoter hypermethylation, silence protective mechanisms and enabling disease progression (94). Epigenetic reprogramming also affects genes associated with insulin sensitivity and lipid homeostasis, including dipeptidyl peptidase 4, ATP citrate lyase and acyl-CoA synthetase long-chain family member 4 (95-98). These modifications not only contribute to hepatic lipid dysregulation but also influence systemic metabolic pathways.
Histone modifications and chromatin remodeling in MAFLD pathogenesis
In MAFLD, aberrant histone acetylation and methylation patterns have been linked to the altered transcription of genes involved in lipid metabolism and inflammation (99). HDAC3 is a crucial regulator of hepatic lipid homeostasis. Loss of HDAC3 activity has been associated with an increased expression of lipogenic transcription factors, exacerbating steatosis (100,101). The SIRT family, comprising NAD+-dependent deacetylases such as SIRT1, SIRT3 and SIRT6, plays a protective role against MAFLD. SIRT1 deacetylates and inhibits sterol regulatory element-binding protein-1c (SREBP-1c) and carbohydrate-responsive element-binding protein (ChREBP), thereby suppressing de novo lipogenesis (102). It also enhances fatty acid oxidation and reduces inflammation by deacetylating histones at the promoters of metabolic and inflammatory genes, such as nuclear factor-kappa B (NF-κB) and peroxisome proliferator-activated receptor alpha (PPARα) (103). SIRT3 maintains mitochondrial integrity and antioxidant defense, whereas SIRT6 modulates gluconeogenesis and lipid synthesis (104). Perturbations in these SIRTs compromise hepatic metabolic resilience (105). Histone methylation also contributes to MAFLD pathology. Elevated levels of repressive markers such as H3K9me2 and H3K36me3 are observed in MASH, correlating with the silencing of genes involved in hepatic steatosis (106). Conversely, decreased levels of activating markers such as H3K4me3 may impair the expression of genes necessary for lipid catabolism. Enzymes such as PR domain-containing protein, which regulates these histone marks, are dysregulated in susceptible populations, indicating a potential role in MAFLD susceptibility and progression (107).
NcRNAs as epigenetic regulators in MAFLD pathogenesis
NcRNAs, including miRNAs and lncRNAs, are increasingly being recognized as key epigenetic regulators of MAFLD. These molecules modulate gene expression post-transcriptionally and often function as critical nodes in metabolic and inflammatory networks (108). Among miRNAs, microRNA-122 (miR-122) is a highly conserved miRNA that is predominantly expressed in the liver and plays a critical role in the regulation of liver metabolism (109). Its downregulation in the liver tissue leads to disrupted lipid metabolism, increased inflammation and fibrosis (110,111). Paradoxically, circulating miR-122 levels were elevated in the early stages of MAFLD and MASH, likely due to enhanced secretion and hepatocellular injury-induced leakage from hepatocytes, despite the significant downregulation of hepatic miR-122 expression observed in these patients (112,113). miR-34a is expressed at low levels in hepatocytes and exerts potent regulatory control over hepatic lipid metabolism (114). It is markedly upregulated in both the liver and circulation of patients with MASH and serves as a reliable biomarker of disease severity (115). Functionally, miR-34a directly targets key metabolic regulators including SIRT1 and PPARα, thereby impairing fatty acid oxidation and promoting lipogenesis and steatotic progression (116). Moreover, miR-34a suppresses hepatocyte nuclear factor 4 alpha and attenuates AMP-activated protein kinase alpha (AMPKα) signaling, further exacerbating lipid accumulation and hepatocellular dysfunction (116,117). miR-21, a stress-responsive miRNA, is markedly elevated in the liver and circulation of patients with MASH and plays multifaceted roles in hepatic pathophysiology (118). In addition to its well-established oncogenic potential, miR-21 contributes to steatosis, inflammation and fibrosis by targeting key metabolic and regulatory pathways (118). It suppresses phosphatase and tensin homolog (PTEN), thereby enhancing de novo lipogenesis and fatty acid uptake, and downregulates PPARα, limiting lipid oxidation (119,120). Additionally, miR-21 modulates the HMG-box transcription factor 1-p53-sterol regulatory element-binding protein 1 axis, promotes hepatic stellate cell (HSC) activation, and collectively exacerbates liver inflammation and fibrogenesis (121). Additional miRNAs such as miR-192, miR-193a and miR-33 further modulate pathways involving TGF-β, AMPK and cholesterol efflux (122). Moreover, circulating miRNAs are emerging as promising non-invasive biomarkers for MAFLD diagnosis and risk stratification. Notably, miR-34a-5p has been incorporated into the NIS4 diagnostic algorithm, a blood-based composite panel, to detect MASH (123). Additionally, circulating miRNAs, such as miR-99a and miR-192, are also elevated in MAFLD and are associated with disease severity, highlighting their potential utility in monitoring disease progression (123-125). lncRNAs critically regulate glucose and lipid metabolism in MAFLD via diverse nuclear and cytoplasmic mechanisms. lncRNAs such as HOX antisense intergenic RNA and maternally expressed gene 3, promote gluconeogenesis and insulin resistance by targeting SIRT1, forkhead box O1, and microRNA-214/139 (126-128). Others, including brown-fat long ncRNA 1, metastasis-associated lung adenocarcinoma transcript 1, lnc RNA Gm15622, and lncRNA H19, enhance hepatic steatosis via the activation or stabilization of SREBP-1c and its downstream targets (129-132). Conversely, protective lncRNAs such as lncRNA suppressor of hepatic gluconeogenesis and lipogenesis, lncRNA hepatic regulator 1, and macrophage inflammation-suppressing RNA transcript 2 improve metabolic profiles by suppressing lipogenesis or restoring deacetylase activity (133-135). These findings highlight lncRNAs as central modulators of MAFLD progression and promising therapeutic targets.
Exercise and its impact on MAFLD
Epigenetic research has provided critical insights into the molecular underpinnings of MAFLD, revealing the roles of DNA methylation, histone modifications and ncRNAs in the regulation of hepatic lipid metabolism, inflammation and fibrogenesis. However, MAFLD is a multifactorial disease, and its effective prevention and treatment require a broader understanding. Lifestyle interventions, particularly physical activity, have gained increasing attention as essential components in the management of MAFLD. Physical activity is a key modulator of hepatic and systemic metabolic health (136). Structured lifestyle interventions, such as increasing daily step counts and leveraging smart-device-guided exercise programs, have been shown to enhance cardiopulmonary fitness, improve metabolic health, and promote overall physiological well-being in high-risk populations (137,138). In addition, exercise modulates gene expression profiles and elicits widespread adaptive responses, including increased endurance, enhanced efficiency of the respiratory and cardiovascular systems, and regulation of immune and neurophysiological processes (139-141). Physical inactivity is a well-established risk factor for the onset of chronic diseases, largely because of its association with adverse molecular and biochemical alterations in specific tissues (142). To elucidate the therapeutic effect of physical activity on MAFLD, the following sections review the distinct physiological and molecular effects of various exercise modalities and highlight their shared and unique mechanisms for mitigating hepatic steatosis and metabolic dysfunction.
Aerobic exercise and MAFLD improvement
Aerobic exercise, defined as sustained rhythmic physical activity that primarily depends on oxidative metabolism, is a fundamental component of a healthy lifestyle. Regular aerobic exercise elicits a wide array of systemic benefits, including enhanced cardiovascular function, improved glucose metabolism, optimized lipid profiles, and reduced systemic inflammation (143-146). In this context, aerobic exercise has gained prominence as a potent non-pharmacological intervention to prevent and ameliorate MAFLD (147). Clinically, aerobic exercise has been shown to significantly reduces liver enzyme levels (ALT and AST), decreases intrahepatic fat accumulation, and improves insulin resistance, even in the absence of significant weight loss (148). Mechanistically, aerobic exercise enhances hepatic fatty acid β-oxidation while suppressing de novo lipogenesis by downregulating lipogenic transcription factors such as SREBP-1c and upregulating PPARα (147,149). This shift in lipid metabolism reduces intrahepatic fat accumulation independent of weight loss (150). Concurrently, aerobic exercise attenuates hepatic oxidative stress by enhancing the expression of antioxidant enzymes such as superoxide dismutase, catalase and glutathione peroxidase, thereby mitigating reactive oxygen species-mediated damage (151). Inflammation, a critical driver of MAFLD progression, is also alleviated by aerobic exercise through the downregulation of pro-inflammatory cytokines such as tumor necrosis factor-alpha (TNF-α) and interleukin-1 beta (152-154). Additionally, aerobic exercise enhances hepatic autophagy, particularly lipophagy-a selective autophagic process essential for lipid droplet clearance and energy mobilization (24). Through activation of the AMPK-PPARα signaling axis, aerobic training promotes mitochondrial fatty acid oxidation, mitigates endoplasmic reticulum (ER) stress, and reduces fibrotic markers including α-smooth muscle actin and TNF-α (149,155,156). Notably, aerobic exercise significantly improves mitochondrial quality, a key pathological deficit in MAFLD, by promoting mitochondrial biogenesis, augmenting mitophagy via PTEN-induced kinase 1/Parkin RBR E3 ubiquitin protein ligase signaling, and maintaining oxidative phosphorylation (150,157). Collectively, these adaptations underscore the multifaceted therapeutic potential of aerobic exercise for halting or reversing MAFLD progression.
Resistance training and its role in MAFLD management
Resistance exercise, also known as strength training, involves repeated muscle contractions against external loads to enhance muscle strength, endurance and mass. Unlike aerobic exercise, which primarily targets cardiovascular fitness and oxidative metabolism, resistance training predominantly affects the neuromuscular system by inducing metabolic adaptations through mechanical loading, muscle hypertrophy and functional remodeling (158,159). An increasing body of evidence highlights that resistance exercise confers substantial systemic benefits, including improved insulin sensitivity, enhanced glucose uptake, favorable modulation of lipid profiles, increased resting energy expenditure, and attenuation of chronic low-grade inflammation (146,160,161). From a metabolic perspective, resistance exercise stimulates skeletal muscle glucose transporter type 4 translocation, promotes glycogen synthesis, and enhances mitochondrial oxidative capacity, thereby improving peripheral glucose disposal and energy metabolism (162). In addition, resistance training stimulates the secretion of muscle-derived cytokines (myokines), such as irisin and interleukin-6, which exert endocrine effects by regulating lipid metabolism, suppress inflammatory responses, and promoting systemic metabolic homeostasis (163). Resistance exercises have emerged as an effective and accessible intervention for MAFLD. Clinical studies have consistently demonstrated that regular resistance training significantly reduces the intrahepatic triglyceride content, improves insulin sensitivity, lowers serum liver enzyme levels (ALT and AST), and reduces visceral adiposity (164,165). Notably, simple bodyweight-based exercises such as squats and push-ups have been shown to ameliorate hepatic steatosis and improve metabolic parameters without the need for specialized equipment, thus enhancing their feasibility and applicability across diverse patient populations (166). At the molecular level, resistance exercise improves hepatic lipid metabolism by activating the AMPK signaling pathway, upregulating PPARα, and downregulating lipogenic transcription factors such as SREBPs (155). Although resistance training and aerobic exercise exhibit distinct physiological targets, emerging evidence suggests that combining both modalities yields synergistic benefits, achieving greater reductions in intrahepatic fat, systemic insulin resistance, and body fat percentage than either intervention alone. Nevertheless, even when applied independently, resistance exercise confers significant hepatoprotective effects and represents a vital component of lifestyle interventions aimed at mitigating MAFLD progression (167).
Comparative effects of high-intensity interval training (HIIT) and moderate-intensity continuous training (MICT)
HIIT, typically performed at 70-90% of the maximal heart rate, is characterized by brief bouts of vigorous effort that substantially elevate cardiovascular demand and metabolic rate (168). HIIT, a widely adopted form of high-intensity exercise, has been shown to rapidly improve cardiorespiratory fitness, enhance insulin sensitivity, promotes mitochondrial biogenesis, and elicits potent anti-inflammatory responses (169-171). These adaptations contribute to significant reductions in the risk of metabolic disorders such as type 2 diabetes, cardiovascular disease and MAFLD (172). MICT, typically conducted at 45-65% of the maximal heart rate, emphasizes sustained aerobic activity over longer durations. Although the metabolic stimulus is less intense than in HIIT, MICT effectively enhances lipid metabolism, improves glycemic control, reduces visceral adiposity, and supports cardiovascular health (168,172). It is particularly valued for its high safety profile, broad accessibility, and strong patient adherence, making it a preferred strategy for populations with limited exercise capacity or comorbidities (173). Longitudinal cohort studies have revealed that sustained engagement in vigorous physical activity from young adulthood significantly lowers the risk of developing MAFLD in midlife (174). Similarly, clinical and experimental research have shown that HIIT more effectively reduces intrahepatic triglyceride accumulation, improves glucose metabolism, and attenuates hepatic inflammation and fibrosis than MICT (175,176). Mechanistically, HIIT enhanced hepatic fatty acid oxidation, suppressed lipogenesis, and reduced the infiltration of pro-inflammatory monocyte-derived macrophages into the liver. Moreover, high-intensity exercise has been associated with favorable modulation of systemic inflammatory markers, such as C-reactive protein, whereas light-intensity physical activity appears insufficient to elicit such benefits (177). Notably, HIIT robustly activates hepatic fatty acid oxidation pathways and upregulates mitochondrial oxidative enzymes such as carnitine palmitoyl-transferase 1 alpha and β-hydroxy-acyl-CoA dehydrogenase, while promoting mitochondrial biogenesis via PGC-1α induction. These adaptations suppress hepatic lipogenesis and mitigate ER stress by downregulating the protein kinase R-like ER kinase-activating transcription factor 4-C/EBP homologous protein signaling pathway, a critical driver of hepatocellular dysfunction under lipotoxic conditions (178). MICT moderately enhances antioxidant capacity and downregulates hepatic CHOP expression, contributing to the partial relief of ER stress and hepatic lipid deposition (178). Although both MICT and HIIT reduce hepatic steatosis, HIIT often achieves more rapid and profound metabolic improvements, including improved insulin sensitivity and mitochondrial function (174). Nonetheless, moderate-intensity exercise remains a valuable strategy, particularly for individuals with lower baseline fitness or comorbidities, offering consistent and slightly lesser improvements in hepatic fat content and metabolic parameters. In conclusion, both exercise intensities confer substantial health benefits. However, the choice of intensity should be individualized based on patient characteristics, goals and tolerance.
Epigenetic mechanisms underlying exercise-mediated protection in MAFLD
Given the established roles of both epigenetic regulation and physical activity in MAFLD, increasing attention has been directed toward understanding how these two factors may interact. Although this field remains in its early stages, increasing evidence supports a mechanistic link between physical activity and epigenetic remodeling in MAFLD (179). To investigate the epigenetic mechanisms underlying exercise-mediated amelioration of MAFLD, PubMed (https://pubmed.ncbi.nlm.nih.gov/) was systematically searched using the following query: ['Non-alcoholic Fatty Liver Disease' (Mesh) OR MAFLD OR NASH OR MAFLD OR 'non-alcoholic fatty liver' OR steatosis] AND ['Exercise'(Mesh) OR 'Exercise Therapy' (Mesh) OR 'Physical Exertion' (Mesh) OR 'physical activity' OR 'aerobic exercise' OR 'resistance training'] AND ['Epigenesis, Genetic' (Mesh) OR 'DNA Methylation' (Mesh) OR 'Histone Code' (Mesh) OR 'RNA, Untranslated' (Mesh) OR epigenetic* OR 'DNA methylation' OR 'histone modification*' OR 'non-coding RNA' OR miRNA OR lncRNA]. All the retrieved records were combined, deduplicated and rigorously screened to identify studies in which exercise served as the primary intervention. Studies were further prioritized based on the explicit mechanistic exploration of exercise-induced epigenetic modifications (Table I).
Exercise-induced DNA methylation in MAFLD
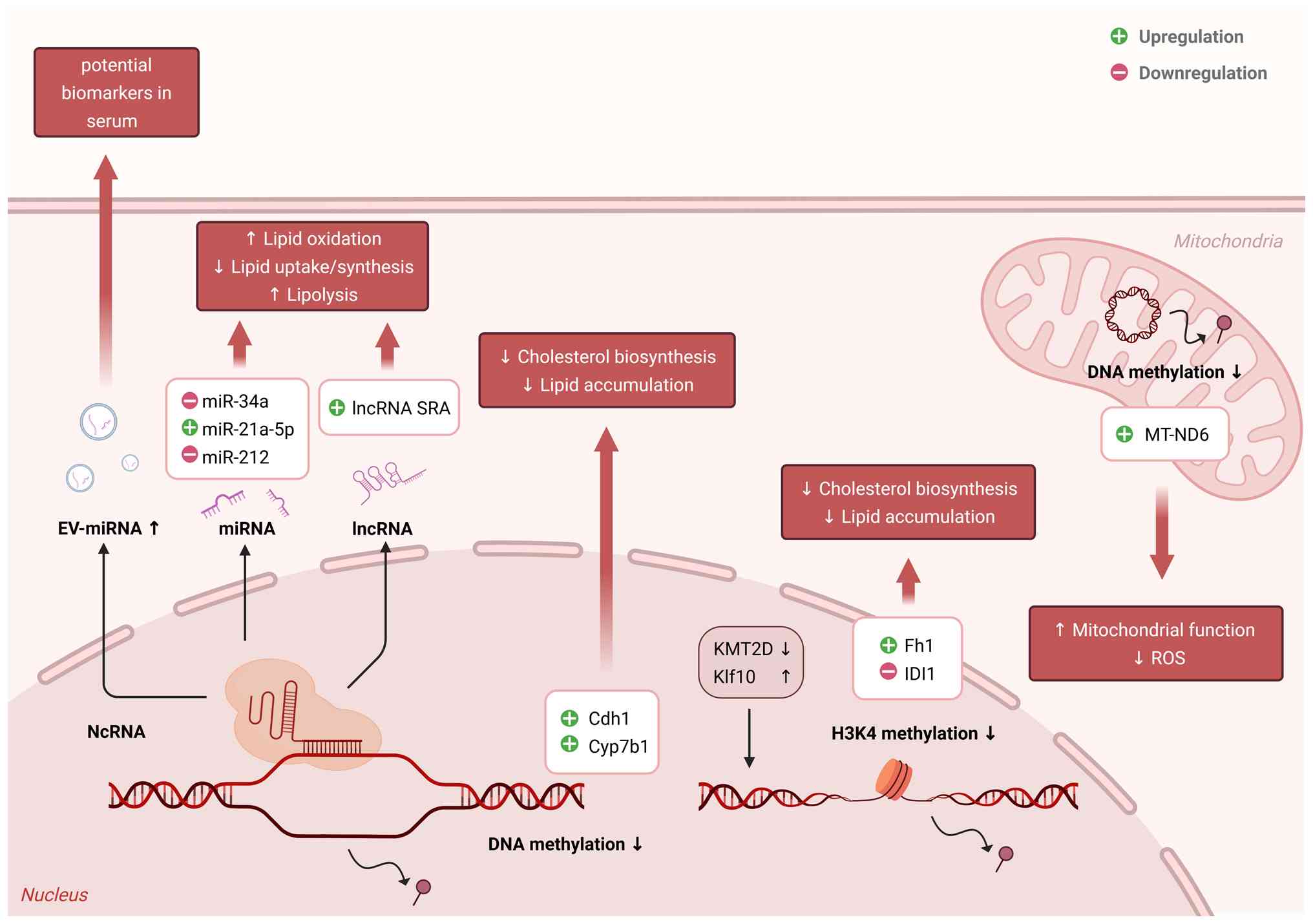
Exercise is a potent regulator of DNA methylation dynamics, influencing gene expression and cellular function (180). Notably, global DNA methylation tends to increase with age, leading to the dysregulation of gene expression and physiological deterioration (181). However, compared with sedentary individuals, those engaged in sustained physical activity exhibit a significantly slower progression of DNA methylation alterations (16). Exercise has been shown to reduce the plasma levels of folate and vitamin B12, two essential cofactors for one-carbon metabolism, thereby indirectly influencing DNA methylation stability (182,183). Notably, considerable divergence remains in the literature regarding the overall directionality of methylation changes induced by exercise. For instance, a study assessing trained young male cyclists following an acute aerobic exercise bout (4 min at 75% VO2 max followed by a 15-min time trial) reported a significant decrease in global DNA methylation levels (184). By contrast, Robson-Ansley et al (185) observed no significant changes in global DNA methylation in trained males following acute running exercise (45 min at 60% VO2 max followed by a 5-min time trial), suggesting that these changes might be negligible in certain individuals or that gains and losses in methylation could be balanced at the global level. These discrepancies may be attributed to differences in exercise modalities, sample sources and detection methodologies. Given the ongoing debate surrounding genome-wide methylation analyses, investigations into the epigenetic effects of exercise on metabolically active organs, such as the liver, may offer more physiologically relevant insights. Pirola et al (92) highlighted the critical role of mitochondrial DNA methylation in MAFLD. They demonstrated that the mitochondrial-encoded gene MT-ND6 exhibited significant hypermethylation in patients with MAFLD, leading to its transcriptional repression and correlation with disease severity. Importantly, the aforementioned study demonstrated that higher physical activity is associated with reduced hepatic MT-ND6 methylation, suggesting that exercise may enhance mitochondrial function and metabolic homeostasis via mitochondrial DNA methylation (92). Zhou et al (186) reported that a high-fat diet induced widespread pathological hypermethylation of enhancer and promoter regions in the liver, disrupting the expression of genes critical for lipid metabolism. Regular exercise reversed these aberrant DNA methylation changes, restored the expression of key metabolic genes, and restored lipid homeostasis (186). Exercise also influences several regulatory factors that play critical roles MAFLD. For instance, interval walking training was shown to epigenetically regulate the NF-κB2 gene through DNA methylation remodeling (187). In addition, high-intensity exercise has been associated with favorable DNA methylation changes in metabolic regulatory genes, contributing to improved insulin sensitivity and attenuated fatty liver disease (25,188,189). Moreover, increased DNA methylation in the promoter region of nuclear receptor co-repressor 1 has been observed in trained male mice, and this epigenetic modification appears heritable. This finding suggests that exercise may modulate the epigenetic regulation of key genes associated with MAFLD (190). However, their precise role in MAFLD pathogenesis and progression requires further investigation.
Exercise-induced histone modification in MAFLD
Despite significant advances over the past decade in defining the transcriptional responses of genes to different modes of exercise, the histone modification mechanisms underlying these changes, particularly in hepatic tissues, remain incompletely characterized. Exercise has been shown to trigger histone methylation, acetylation and phosphorylation, which are key regulators of transcription (191,192). However, these studies have largely focused on the role of histone modifications in skeletal muscle fiber type switching and improvement of cognitive function. Exercise promotes histone H3 acetylation at specific promoter regions of metabolic genes, enhance histone H3 phosphorylation to facilitate chromatin relaxation, and induces histone methylation changes associated with transcriptional activation or repression (189). Notably, emerging research has revealed that exercise-mediated regulation of histone methylation may contribute to metabolic disease prevention (193). For example, studies have demonstrated that HIIT alleviates lipid accumulation and inflammation in MAFLD models by reducing H3K4me1 mediated by KMT at the isopentenyl-diphosphate delta isomerase 1 promoter, thereby suppressing cholesterol biosynthesis and improving hepatic metabolic homeostasis (194). Exercise also induces hepatic Krüppel-like factor 10 expression via the cyclic adenosine monophosphate-protein kinase A-cAMP response element-binding protein pathway, which upregulates fumarate hydratase 1 to reduce fumarate accumulation, thereby decreasing H3K4me3 levels at lipogenic gene promoters and ameliorating lipid accumulation, inflammation and fibrosis in MASH (195). Taken together, these findings suggest that exercise modulates histone methylation dynamics at key metabolic gene loci, contributing to the attenuation of hepatic steatosis, inflammation and fibrosis. Exercise has emerged as a potent modulator of the SIRT family members, particularly SIRT1 and SIRT6, which are critical regulators of metabolic homeostasis (196). Regular aerobic, resistance and combined training upregulate hepatic SIRT1 expression in high-fat diet-induced MAFLD models, enhancing insulin sensitivity, reducing hepatic lipid accumulation, and lowering serum liver enzymes (197,198). SIRT1 activation promotes fatty acid oxidation, suppresses de novo lipogenesis via deacetylation of SREBP-1c and ChREBP, and inhibits inflammation through NF-κB signaling (199). In parallel, exercise-induced SIRT6 activation enhances mitochondrial biogenesis and shifts muscle fiber composition towards oxidative and fatigue-resistant types, thereby improving systemic metabolic resilience (200). Although the metabolic benefits of SIRT activation are well recognized, the effect of exercise-induced SIRT1 and SIRT6 modulation on hepatic histone modifications in MAFLD remains largely unexplored. SIRTs function as NAD+-dependent deacetylases targeting both histone and non-histone proteins to regulate chromatin structure and gene transcription. SIRT1 deacetylates histones H3K9 and H4K16, while SIRT6 targets H3K9 and H3K56, thereby influencing pathways linked to lipid metabolism, inflammation and genomic stability (201,202). However, the mechanism through which exercise-driven SIRT activation reprograms the hepatic epigenome to mitigate MAFLD progression remains unclear. Future studies employing high-resolution epigenomic profiling are needed to delineate the histone marks modulated by SIRT1 and SIRT6 in response to exercise and to uncover their roles in hepatic metabolic adaptation and fibrosis attenuation. Notably, lactate is a principal metabolite of glycolysis and serves as a substrate for mitochondrial respiration, thereby linking the glycolytic and oxidative metabolic pathways (203). During physical exercise and other physiological stresses, elevated lactate levels are released into the circulation, where lactate acts not only as a metabolic intermediate but also as an active signaling molecule (204). Emerging evidence suggests that lactate regulates gene expression via histone lactylation, a novel epigenetic modification that modulates chromatin structure and transcriptional activity. By influencing both transcriptional programs and cellular signaling pathways, lactate contributes to tissue adaptation, metabolic homeostasis, and inflammation control (205). Thus, histone lactylation is a potential mechanism by which exercise promotes cellular and systemic resilience. However, the specific dynamics of histone lactylation in hepatic tissues following exercise, the critical gene targets involved, and their roles in MAFLD resolution remain largely unknown. Future studies should aim to characterize the lactate-histone lactylation axis in the liver to improve understanding of its therapeutic potential in metabolic disease.
Exercise-induced ncRNAs in MAFLD
With an increasing understanding of the biological functions of ncRNAs, exercise has emerged as a potent non-pharmacological strategy to modulate ncRNA expression and drive systemic adaptation (206). Among ncRNAs, miRNAs serve as highly stable and readily detectable regulators of gene expression across multiple tissues (207). At the tissue level, skeletal muscle responds to exercise with enhanced expression of miRNA-1 and miRNA-133a, facilitating myogenic differentiation and mitochondrial biogenesis through activation of the phosphatidylinositol 3-kinase/protein kinase B and PGC-1α pathways (208,209). Exercise induces the upregulation of miR-21 and miRNA-126 in cardiac tissue, promoting physiological hypertrophy and angiogenesis and attenuating apoptosis (210,211). In adipose tissue, exercise modulates miRNAs such as miR-143, miR-145 and miR-222, favoring lipolysis and improving insulin sensitivity (212-214). Additionally, exercise-induced shifts in miR-132 enhance neurogenesis and cognitive function (215). Collectively, these systemic adaptations underscore the critical role of ncRNA networks in orchestrating the complex, multi-organ benefits of regular physical activity. Among the various organ systems affected by exercise-induced changes in miRNAs, the liver has drawn particular interest, particularly in the context of MAFLD. Exercise ameliorates MAFLD progression via miRNA-mediated mechanisms, notably by restoring the expression of key hepatic miRNAs (216). Specifically, aerobic exercise upregulates miR-33 and miR-122, two miRNAs that are suppressed in MAFLD and whose restoration leads to the inhibition of lipogenic genes such as SREBP1 and PPAR-γ, thereby attenuating hepatic lipid accumulation (217,218). Hypoxic exercise training reduces hepatic miR-378b expression in obese rats, thereby altering the FAS/CPT1A protein ratio and decreasing hepatic fatty acid oxidation, ultimately contributing to reduced fat accumulation and improved lipid profiles in diet-induced obesity (219). Furthermore, exercise downregulates miR-212, whose elevated expression promotes the suppression of fibroblast growth factor-21 (FGF-21) suppression and exacerbates hepatic steatosis (220). Collectively, these findings suggest that exercise-induced modulation of ncRNAs is a promising non-pharmacological strategy for the prevention and treatment of MAFLD, highlighting the therapeutic potential of targeting specific miRNA circuits within the context of metabolic diseases. Accumulating evidence indicates that exercise exerts potent hepatoprotective effects by modulating EV-miRNAs and lncRNAs, thereby coordinating inter-organ metabolic communication and maintaining systemic homeostasis. Aerobic exercise has been shown to remodel the circulating miRNA profile, notably downregulating EV-associated miRNAs such as miR-122, miR-192 and miR-22, leading to enhanced adipose tissue insulin sensitivity and reduced hepatic lipid accumulation (221,222). In parallel, exercise suppresses miR-33, miR-34a and miR-212, alleviating hepatic steatosis by promoting autophagy, inhibiting lipogenesis, and activating metabolic regulators such as FGF-21 (214,220,223). Emerging studies have also highlighted the role of lncRNAs, particularly exercise-induced downregulation of steroid receptor RNA activator, which activates p38 mitogen-activated protein kinase/c-Jun N-terminal kinase signaling and promotes adipose triglyceride lipase-mediated lipolysis, thereby mitigating hepatic inflammation and lipid deposition (224,225). Accumulating data has expanded the regulatory network. Long-term aerobic exercise increases adipose-derived exosomal miR-324, which targets Rho-associated coiled-coil-containing protein kinase 1 to enhance insulin sensitivity and attenuate liver fat accumulation in MAFLD (226). Similarly, exercise upregulates miR-21a-5p, suppressing key lipid metabolism genes such as fatty acid-binding protein 7, 3-Hydroxy-3-methylglutaryl-CoA reductase, acetyl-CoA acetyltransferase 1 and oxidized low-density lipoprotein receptor 1, further linking miRNA-mediated regulation to metabolic improvement (227). Beyond hepatic effects, exercise-induced changes in exosomal miRNAs, such as miR-122-5p, also contribute to vascular remodeling and skeletal muscle angiogenesis (228). However, context-dependent effects have been observed, with elevated miR-122 levels following weight-loss interventions being associated with suboptimal hepatic fat reduction, underscoring the complexity of miRNA-mediated metabolic regulation (218). In addition to altering specific miRNAs, exercise influences the miRNA-processing machinery itself, including the upregulation of Dicer1, an essential RNase involved in miRNA maturation, which may further amplify its impact on miRNA biogenesis and function (227). Moreover, the modulation of thyroid hormone-responsive miRNA networks, such as miR-383, miR-146b and miRNA-200a, provides another mechanism by which exercise restores hepatic lipid metabolism and redox balance (229). Collectively, these findings delineate a sophisticated regulatory network wherein exercise orchestrates small RNA-mediated inter-organ communication, metabolic reprogramming, and the resolution of inflammation. This emerging understanding not only deepens insights into the molecular underpinnings of exercise-induced benefits but also opens new avenues for RNA-based therapeutic strategies targeting metabolic disorders.
Challenges and perspectives in epigenetic research on exercise and MAFLD
Despite these promising advances, exploration of exercise-induced epigenetic regulation of MAFLD remains in its infancy. Studies have predominantly focused on a limited set of candidate genes or pathways, often relying on bulk tissue analyses without resolving cell-type-specific or spatially restricted epigenetic dynamics. Given the cellular heterogeneity of the liver, future investigations using single-cell epigenomic technologies and spatial transcriptomics are critical for dissecting the differential reprogramming of hepatocytes, Kupffer cells, HSCs and endothelial populations during MAFLD progression and resolution (230). Moreover, although several key epigenetic markers-such as DNA methylation, histone acetylation, and miRNA expression-have been implicated, an integrated, multi-omics understanding of how these layers interact to drive systemic metabolic adaptation is still lacking. Longitudinal studies combining epigenomics, transcriptomics, proteomics and metabolomics are essential to capture the temporal evolution of exercise-induced hepatic reprogramming and identify causal regulatory nodes amenable to therapeutic interventions. Another major limitation is the relative paucity of human studies. Much of the current knowledge has been derived from rodent models, which, while informative, may not fully recapitulate the complexity of human MAFLD pathophysiology, especially when considering genetic, dietary and lifestyle variabilities. Large-scale, well-controlled clinical trials incorporating biopsy-based liver epigenomic profiling before and after structured exercise interventions are urgently needed to validate these mechanistic findings and translate them into actionable clinical insights. Furthermore, most studies have examined the immediate or short-term epigenetic responses to exercise; however, the durability and reversibility of these modifications remain poorly understood (231). Whether exercise-induced epigenetic changes persist after cessation of training, whether they exhibit 'metabolic memory' effects, and to what extent they can counteract pre-existing pathogenic epigenetic signatures are critical questions that warrant rigorous investigation. Emerging evidence has highlighted the potential of exercise to modulate transgenerational metabolic health through epigenetic mechanisms. Maternal exercise during pregnancy protects the offspring against high-fat diet-induced MAFLD by reprogramming hepatic metabolism early in life (190). Mechanistically, this protective effect is associated with the activation of key metabolic pathways, including AMPK, PPARα and PGC1α signaling, resulting in enhanced fatty acid oxidation, suppressed lipogenesis and improved bile acid metabolism (190). Proteomic profiling revealed distinct hepatic expression patterns in the offspring of exercised dams, notably the upregulation of cytochrome P450 family 7 subfamily A member 1, a critical enzyme in cholesterol catabolism. Maternal physical activity has been proposed to stabilize the intrauterine environment through metabolic, endocrine and epigenetic pathways, thereby mitigating the susceptibility of offspring to MAFLD and other metabolic disorders (232). These findings suggest that exercise interventions during pregnancy offer a promising strategy for disrupting the intergenerational cycle of metabolic disease transmission. However, despite encouraging results from animal models, the precise epigenetic modifications involved, their persistence across generations, and their relevance in humans remain poorly understood. Understanding whether and how exercise-mediated epigenetic remodeling in the liver and germline can confer long-term protection against MAFLD across generations may provide valuable insights into refining preventive strategies. Although significant progress has been made in delineating the epigenetic mechanisms underlying the beneficial effects of exercise on MAFLD, substantial knowledge gaps remain. Future research integrating advanced epigenomic technologies, refined animal models and translational human studies is expected to play a pivotal role in uncovering the full therapeutic potential of exercise as an epigenetic intervention in metabolic liver diseases.
Conclusions
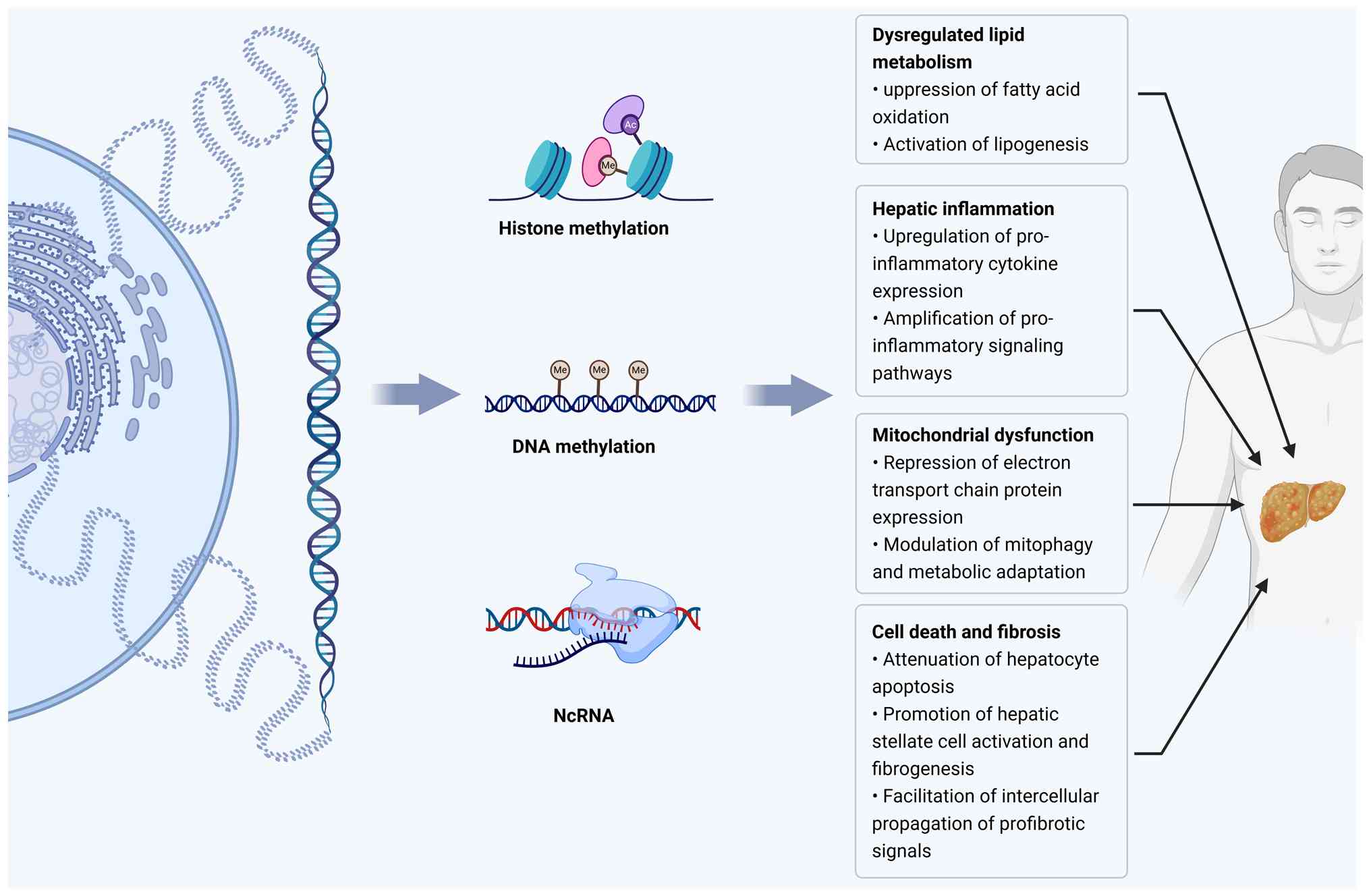
This review highlights the emerging role of exercise-induced epigenetic remodeling in MAFLD. Epigenetic dysregulation, which is characterized by altered DNA methylation landscapes, histone acetylation and methylation imbalances, and aberrant miRNA and lncRNA profiles, has been established as a central driver of hepatic lipid metabolic derangement, mitochondrial impairment, autophagic dysfunction, inflammatory activation, and fibrotic progression in MAFLD (Fig. 2). Exercise, as an effective non-pharmacological intervention, can reprogram these dysregulated epigenetic landscapes, thereby restoring mitochondrial function, reducing the hepatic lipid load, and alleviating systemic inflammation. Mechanistically, exercise modulates DNA methylation dynamics, reshapes histone modification landscapes, and reprograms the expression of ncRNAs, collectively promoting enhanced metabolic resilience. Despite these promising advances, substantial gaps remain in our understanding of exercise-induced epigenetic remodeling in MAFLD. Most available evidence is derived from animal models, which limits its translational applicability to human diseases. Further investigation with more robust clinical studies is urgently required to resolve this issue. Unraveling the epigenetic mechanisms underlying exercise-mediated hepatic protection could significantly advance the prevention and treatment of MAFLD.
Availability of data and materials
Not applicable.
Authors' contributions
YG and YZ conceptualized and designed the study and drew the figures. YW and HL collected data. All authors read and approved the final version of the manuscript. Data authentication is not applicable.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Abbreviations:
|
5mC |
5-methylcytosine |
|
AMPKα |
AMP-activated protein kinase alpha |
|
ChREBP |
carbohydrate-responsive element-binding protein |
|
circRNAs |
circular RNAs |
|
CpG |
cytosine-phosphate-guanine |
|
DNMTs |
DNA methyltransferases |
|
ER |
endoplasmic reticulum |
|
FGF-21 |
fibroblast growth factor-21 |
|
HATs |
histone acetyltransferases |
|
HDACs |
histone deacetylases |
|
HDMs |
histone demethylases |
|
HIIT |
high-intensity interval training |
|
HMTs |
histone methyltransferases |
|
HSC |
hepatic stellate cell |
|
KMT |
lysine methyltransferase |
|
lncRNAs |
long non-coding RNAs |
|
MAFLD |
metabolic dysfunction-associated fatty liver disease |
|
MALAT1 |
metastasis-associated lung adenocarcinoma transcript 1 |
|
MASH |
metabolic dysfunction-associated steatohepatitis |
|
miRNAs or miRs |
microRNAs |
|
MICT |
moderate-intensity continuous training |
|
mtDNA |
mitochondrial DNA |
|
MT-ND6 |
mitochondrially encoded NADH dehydrogenase 6 |
|
ncRNA |
non-coding RNA |
|
NF-κB |
nuclear factor-kappa B |
|
PGC-1α |
peroxisome proliferator-activated receptor gamma coactivator 1-alpha |
|
PPARα |
peroxisome proliferator-activated receptor alpha |
|
PTEN |
phosphatase and tensin homolog |
|
SIRTs |
sirtuins |
|
SREBP-1c |
sterol regulatory element-binding protein-1c |
|
TET |
ten-eleven translocation |
|
TNF-α |
tumor necrosis factor-alpha |
|
β-HAD |
β-hydroxy-acyl-CoA dehydrogenase |
Acknowledgments
Not applicable.
Funding
No funding was received.
References
|
Chen G, Ni Y, Nagata N, Xu L and Ota T: Micronutrient anti-oxidants and nonalcoholic fatty liver disease. Int J Mol Sci. 17:13792016. View Article : Google Scholar | |
|
Byrne CD and Targher G: NAFLD: A multisystem disease. J Hepatol. 62(Suppl 1): S47–S64. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Eslam M, Valenti L and Romeo S: Genetics and epigenetics of NAFLD and NASH: Clinical impact. J Hepatol. 68:268–279. 2018. View Article : Google Scholar | |
|
Schwimmer JB: Definitive diagnosis and assessment of risk for nonalcoholic fatty liver disease in children and adolescents. Semin Liver Dis. 27:312–318. 2007. View Article : Google Scholar : PubMed/NCBI | |
|
Polyzos SA, Kountouras J and Mantzoros CS: Obesity and nonalcoholic fatty liver disease: From pathophysiology to therapeutics. Metabolism. 92:82–97. 2019. View Article : Google Scholar | |
|
Targher G, Tilg H and Byrne CD: Non-alcoholic fatty liver disease: A multisystem disease requiring a multidisciplinary and holistic approach. Lancet Gastroenterol Hepatol. 6:578–588. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Younossi ZM: Non-alcoholic fatty liver disease-A global public health perspective. J Hepatol. 70:531–544. 2019. View Article : Google Scholar | |
|
Keskin M, Hayıroğlu M, Uzun AO, Güvenç TS, Şahin S and Kozan Ö: Effect of nonalcoholic fatty liver disease on in-hospital and Long-term outcomes in patients with ST-segment elevation myocardial infarction. Am J Cardiol. 120:1720–1726. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Friedman SL, Neuschwander-Tetri BA, Rinella M and Sanyal AJ: Mechanisms of NAFLD development and therapeutic strategies. Nat Med. 24:908–922. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Romero-Gómez M, Zelber-Sagi S and Trenell M: Treatment of NAFLD with diet, physical activity and exercise. J Hepatol. 67:829–846. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Kwak MS and Kim D: Non-alcoholic fatty liver disease and lifestyle modifications, focusing on physical activity. Korean J Intern Med. 33:64–74. 2018. View Article : Google Scholar : | |
|
Malkova D, Evans RD, Frayn KN, Humphreys SM, Jones PR and Hardman AE: Prior exercise and postprandial substrate extraction across the human leg. Am J Physiol Endocrinol Metab. 279:E1020–E1028. 2000. View Article : Google Scholar : PubMed/NCBI | |
|
Yaskolka Meir A, Keller M, Müller L, Bernhart SH, Tsaban G, Zelicha H, Rinott E, Kaplan A, Gepner Y, Shelef I, et al: Effects of lifestyle interventions on epigenetic signatures of liver fat: Central randomized controlled trial. Liver Int. 41:2101–2111. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Barrès R, Yan J, Egan B, Treebak JT, Rasmussen M, Fritz T, Caidahl K, Krook A, O'Gorman DJ and Zierath JR: Acute exercise remodels promoter methylation in human skeletal muscle. Cell Metab. 15:405–411. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Chen Z, Li L, Wu W, Liu Z, Huang Y, Yang L, Luo Q, Chen J, Hou Y and Song G: Exercise protects proliferative muscle satellite cells against exhaustion via the Igfbp7-Akt-mTOR axis. Theranostics. 10:6448–6466. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Fiorito G, Caini S, Palli D, Bendinelli B, Saieva C, Ermini I, Valentini V, Assedi M, Rizzolo P, Ambrogetti D, et al: DNA methylation-based biomarkers of aging were slowed down in a two-year diet and physical activity intervention trial: The DAMA study. Aging Cell. 20:e134392021. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang L, Lu Q and Chang C: Epigenetics in health and disease. Adv Exp Med Biol. 1253:3–55. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Goldberg AD, Allis CD and Bernstein E: Epigenetics: A landscape takes shape. Cell. 128:635–638. 2007. View Article : Google Scholar : PubMed/NCBI | |
|
Stefanska B, Karlic H, Varga F, Fabianowska-Majewska K and Haslberger A: Epigenetic mechanisms in anti-cancer actions of bioactive food components-the implications in cancer prevention. Br J Pharmacol. 167:279–297. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Dehan P, Kustermans G, Guenin S, Horion J, Boniver J and Delvenne P: DNA methylation and cancer diagnosis: New methods and applications. Expert Rev Mol Diagn. 9:651–657. 2009. View Article : Google Scholar : PubMed/NCBI | |
|
Zhao Q, Liu J, Deng H, Ma R, Liao JY, Liang H, Hu J, Li J, Guo Z, Cai J, et al: Targeting Mitochondria-located circRNA SCAR alleviates NASH via reducing mROS output. Cell. 183:76–93.e22. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Yang Y, Cai J, Yang X, Wang K, Sun K, Yang Z, Zhang L, Yang L, Gu C, Huang X, et al: Dysregulated m6A modification promotes lipogenesis and development of Non-alcoholic fatty liver disease and hepatocellular carcinoma. Mol Ther. 30:2342–2353. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Yang Z, Zhang S, Liu X, Shu R, Shi W, Qu W, Liu D, Cai Z, Wang Y, Cheng X, et al: Histone demethylase KDM1A promotes hepatic steatosis and inflammation by increasing chromatin accessibility in NAFLD. J Lipid Res. 65:1005132024. View Article : Google Scholar : PubMed/NCBI | |
|
Farzanegi P, Dana A, Ebrahimpoor Z, Asadi M and Azarbayjani MA: Mechanisms of beneficial effects of exercise training on non-alcoholic fatty liver disease (NAFLD): Roles of oxidative stress and inflammation. Eur J Sport Sci. 19:994–1003. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Stevanović J, Beleza J, Coxito P, Ascensão A and Magalhães J: Physical exercise and liver 'fitness': Role of mitochondrial function and epigenetics-related mechanisms in non-alcoholic fatty liver disease. Mol Metab. 32:1–14. 2020. View Article : Google Scholar | |
|
Peixoto P, Cartron PF, Serandour AA and Hervouet E: From 1957 to Nowadays: A brief history of epigenetics. Int J Mol Sci. 21:75712020. View Article : Google Scholar : PubMed/NCBI | |
|
Jablonka E: Epigenetic variations in heredity and evolution. Clin Pharmacol Ther. 92:683–688. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Coulondre C, Miller JH, Farabaugh PJ and Gilbert W: Molecular basis of base substitution hotspots in Escherichia coli. Nature. 274:775–780. 1978. View Article : Google Scholar : PubMed/NCBI | |
|
Bird AP: DNA methylation and the frequency of CpG in animal DNA. Nucleic Acids Res. 8:1499–1504. 1980. View Article : Google Scholar : PubMed/NCBI | |
|
Antequera F and Bird A: CpG islands as genomic footprints of promoters that are associated with replication origins. Curr Biol. 9:R661–R667. 1999. View Article : Google Scholar : PubMed/NCBI | |
|
Wolf SF, Dintzis S, Toniolo D, Persico G, Lunnen KD, Axelman J and Migeon BR: Complete concordance between glucose-6-phosphate dehydrogenase activity and hypomethylation of 3′CpG clusters: Implications for X chromosome dosage compensation. Nucleic Acids Res. 12:9333–9348. 1984. View Article : Google Scholar : PubMed/NCBI | |
|
Morgan RK, Wang K, Svoboda LK, Rygiel CA, Lalancette C, Cavalcante R, Bartolomei MS, Prasasya R, Neier K, Perera BPU, et al: Effects of developmental lead and phthalate exposures on DNA methylation in adult mouse blood, brain, and liver: A focus on genomic imprinting by tissue and sex. Environ Health Perspect. 132:670032024. View Article : Google Scholar : PubMed/NCBI | |
|
Liu Y, Zhang Y, Yin J, Gao Y, Li Y, Bai D, He W, Li X, Zhang P, Li R, et al: Distinct H3K9me3 and DNA methylation modifications during mouse spermatogenesis. J Biol Chem. 294:18714–18725. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Yen RW, Vertino PM, Nelkin BD, Yu JJ, el-Deiry W, Cumaraswamy A, Lennon GG, Trask BJ, Celano P and Baylin SB: Isolation and characterization of the cDNA encoding human DNA methyltransferase. Nucleic Acids Res. 20:2287–2291. 1992. View Article : Google Scholar : PubMed/NCBI | |
|
Lian H, Li WB and Jin WL: The emerging insights into catalytic or Non-catalytic roles of TET proteins in tumors and neural development. Oncotarget. 7:64512–64525. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Lyko F: The DNA methyltransferase family: A versatile toolkit for epigenetic regulation. Nat Rev Genet. 19:81–92. 2018. View Article : Google Scholar | |
|
Li E and Zhang Y: DNA methylation in mammals. Cold Spring Harb Perspect Biol. 6:a0191332014. View Article : Google Scholar : PubMed/NCBI | |
|
Melamed P, Yosefzon Y, David C, Tsukerman A and Pnueli L: Tet enzymes, variants, and differential effects on function. Front Cell Dev Biol. 6:222018. View Article : Google Scholar : PubMed/NCBI | |
|
Tábara LC, Burr SP, Frison M, Chowdhury SR, Paupe V, Nie Y, Johnson M, Villar-Azpillaga J, Viegas F, Segawa M, et al: MTFP1 controls mitochondrial fusion to regulate inner membrane quality control and maintain mtDNA levels. Cell. 187:3619–3637.e27. 2024. View Article : Google Scholar : PubMed/NCBI | |
|
Menger KE, Rodríguez-Luis A, Chapman J and Nicholls TJ: Controlling the topology of mammalian mitochondrial DNA. Open Biol. 11:2101682021. View Article : Google Scholar : PubMed/NCBI | |
|
Devall M, Soanes DM, Smith AR, Dempster EL, Smith RG, Burrage J, Iatrou A, Hannon E, Troakes C, Moore K, et al: Genome-wide characterization of mitochondrial DNA methylation in human brain. Front Endocrinol (Lausanne). 13:10591202022. View Article : Google Scholar | |
|
Patil V, Cuenin C, Chung F, Aguilera JRR, Fernandez-Jimenez N, Romero-Garmendia I, Bilbao JR, Cahais V, Rothwell J and Herceg Z: Human mitochondrial DNA is extensively methylated in a non-CpG context. Nucleic Acids Res. 47:10072–10085. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Kouzarides T: Chromatin modifications and their function. Cell. 128:693–705. 2007. View Article : Google Scholar : PubMed/NCBI | |
|
Tessarz P and Kouzarides T: Histone core modifications regulating nucleosome structure and dynamics. Nat Rev Mol Cell Biol. 15:703–708. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Zhou VW, Goren A and Bernstein BE: Charting histone modifications and the functional organization of mammalian genomes. Nat Rev Genet. 12:7–18. 2011. View Article : Google Scholar | |
|
Hansen JC: Linking genome structure and function through specific histone acetylation. ACS Chem Biol. 1:69–72. 2006. View Article : Google Scholar : PubMed/NCBI | |
|
Black JC, Van Rechem C and Whetstine JR: Histone lysine methylation dynamics: Establishment, regulation, and biological impact. Mol Cell. 48:491–507. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Hyun K, Jeon J, Park K and Kim J: Writing, erasing and reading histone lysine methylations. Exp Mol Med. 49:e3242017. View Article : Google Scholar : PubMed/NCBI | |
|
Qu P, Li L, Jin Q, Liu D, Qiao Y, Zhang Y, Sun Q, Ran S, Li Z, Liu T and Peng L: Histone methylation modification and diabetic kidney disease: Potential molecular mechanisms and therapeutic approaches (Review). Int J Mol Med. 54:1042024. View Article : Google Scholar : PubMed/NCBI | |
|
Xiong J, Nie M, Fu C, Chai X, Zhang Y, He L and Sun S: Hypoxia enhances HIF1 α transcription activity by upregulating KDM4A and mediating H3K9me3, thus inducing ferroptosis resistance in cervical cancer cells. Stem Cells Int. 2022:16088062022. View Article : Google Scholar | |
|
Song Y, Wu F and Wu J: Targeting histone methylation for cancer therapy: Enzymes, inhibitors, biological activity and perspectives. J Hematol Oncol. 9:492016. View Article : Google Scholar : PubMed/NCBI | |
|
Gong F and Miller KM: Histone methylation and the DNA damage response. Mutat Res Rev Mutat Res. 780:37–47. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Nimura K, Ura K and Kaneda Y: Histone methyltransferases: Regulation of transcription and contribution to human disease. J Mol Med (Berl). 88:1213–1220. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Allis CD, Bowen JK, Abraham GN, Glover CV and Gorovsky MA: Proteolytic processing of histone H3 in chromatin: A physiologically regulated event in Tetrahymena micronuclei. Cell. 20:55–64. 1980. View Article : Google Scholar : PubMed/NCBI | |
|
Shi Y, Lan F, Matson C, Mulligan P, Whetstine JR, Cole PA, Casero RA and Shi Y: Histone demethylation mediated by the nuclear amine oxidase homolog LSD1. Cell. 119:941–953. 2004. View Article : Google Scholar : PubMed/NCBI | |
|
Shen H, Xu W and Lan F: Histone lysine demethylases in mammalian embryonic development. Exp Mol Med. 49:e3252017. View Article : Google Scholar : PubMed/NCBI | |
|
Li L, Chen K, Wang T, Wu Y, Xing G, Chen M, Hao Z, Zhang C, Zhang J, Ma B, et al: Glis1 facilitates induction of pluripotency via an epigenome-metabolome-epigenome signalling cascade. Nat Metab. 2:882–892. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Hu Z, Lu Y, Cao J, Lin L, Chen X, Zhou Z, Pu J, Chen G, Ma X, Deng Q, et al: N-acetyltransferase NAT10 controls cell fates via connecting mRNA cytidine acetylation to chromatin signaling. Sci Adv. 10:eadh98712024. View Article : Google Scholar : PubMed/NCBI | |
|
Kronfol MM, Jahr FM, Dozmorov MG, Phansalkar PS, Xie LY, Aberg KA, McRae M, Price ET, Slattum PW, Gerk PM and McClay JL: DNA methylation and histone acetylation changes to cytochrome P450 2E1 regulation in normal aging and impact on rates of drug metabolism in the liver. Geroscience. 42:819–832. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Lin Y, Lin A, Cai L, Huang W, Yan S, Wei Y, Ruan X, Fang W, Dai X, Cheng J, et al: ACSS2-dependent histone acetylation improves cognition in mouse model of Alzheimer's disease. Mol Neurodegener. 18:472023. View Article : Google Scholar : PubMed/NCBI | |
|
Wu Y, Tang L, Huang H, Yu Q, Hu B, Wang G, Ge F, Yin T, Li S and Yu X: Phosphoglycerate dehydrogenase activates PKM2 to phosphorylate histone H3T11 and attenuate cellular senescence. Nat Commun. 14:13232023. View Article : Google Scholar : PubMed/NCBI | |
|
Chen G, Zhu X, Li J, Zhang Y, Wang X, Zhang R, Qin X, Chen X, Wang J, Liao W, et al: Celastrol inhibits lung cancer growth by triggering histone acetylation and acting synergically with HDAC inhibitors. Pharmacol Res. 185:1064872022. View Article : Google Scholar : PubMed/NCBI | |
|
Turner BM: Histone acetylation and an epigenetic code. Bioessays. 22:836–845. 2000. View Article : Google Scholar : PubMed/NCBI | |
|
Shvedunova M and Akhtar A: Modulation of cellular processes by histone and non-histone protein acetylation. Nat Rev Mol Cell Biol. 23:329–349. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Shen Y, Wei W and Zhou DX: Histone acetylation enzymes coordinate metabolism and gene expression. Trends Plant Sci. 20:614–621. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Wang P, Wang Z and Liu J: Role of HDACs in normal and malignant hematopoiesis. Mol Cancer. 19:52020. View Article : Google Scholar : PubMed/NCBI | |
|
Esteller M: Non-coding RNAs in human disease. Nat Rev Genet. 12:861–874. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
Wessels HH, Stirn A, Méndez-Mancilla A, Kim EJ, Hart SK, Knowles DA and Sanjana NE: Prediction of on-target and off-target activity of CRISPR-Cas13d guide RNAs using deep learning. Nat Biotechnol. 42:628–637. 2024. View Article : Google Scholar | |
|
Lodde V, Murgia G, Simula ER, Steri M, Floris M and Idda ML: Long noncoding RNAs and circular RNAs in autoimmune diseases. Biomolecules. 10:10442020. View Article : Google Scholar : PubMed/NCBI | |
|
Statello L, Guo CJ, Chen LL and Huarte M: Gene regulation by long non-coding RNAs and its biological functions. Nat Rev Mol Cell Biol. 22:96–118. 2021. View Article : Google Scholar | |
|
Greene J, Baird AM, Brady L, Lim M, Gray SG, McDermott R and Finn SP: Circular RNAs: Biogenesis, function and role in human diseases. Front Mol Biosci. 4:382017. View Article : Google Scholar : PubMed/NCBI | |
|
Gebert LFR and MacRae IJ: Regulation of microRNA function in animals. Nat Rev Mol Cell Biol. 20:21–37. 2019. View Article : Google Scholar : | |
|
Bratkovič T, Božič J and Rogelj B: Functional diversity of small nucleolar RNAs. Nucleic Acids Res. 48:1627–1651. 2020. View Article : Google Scholar | |
|
Yao Q, He T, Liao JY, Liao R, Wu X, Lin L and Xiao G: Noncoding RNAs in skeletal development and disorders. Biol Res. 57:162024. View Article : Google Scholar : PubMed/NCBI | |
|
Horvitz HR and Sulston JE: Isolation and genetic characterization of cell-lineage mutants of the nematode Caenorhabditis elegans. Genetics. 96:435–454. 1980. View Article : Google Scholar : PubMed/NCBI | |
|
Röszer T: MicroRNA profile of mouse Adipocyte-derived extracellular vesicles. Cells. 13:12982024. View Article : Google Scholar : PubMed/NCBI | |
|
Kingreen T, Kewitz-Hempel S, Rohde C, Hause G, Sunderkötter C and Gerloff D: Extracellular vesicles from highly invasive melanoma subpopulations increase the invasive capacity of less invasive melanoma cells through mir-1246-mediated inhibition of CCNG2. Cell Commun Signal. 22:4422024. View Article : Google Scholar : PubMed/NCBI | |
|
Kmiotek-Wasylewska K, Bobis-Wozowicz S, Karnas E, Orpel M, Woźnicka O, Madeja Z, Dawn B and Zuba-Surma EK: Anti-inflammatory, Anti-fibrotic and Pro-cardiomyogenic effects of genetically engineered extracellular vesicles enriched in miR-1 and miR-199a on human cardiac fibroblasts. Stem Cell Rev Rep. 19:2756–2773. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Li Y, Meng Y, Zhu X, Van Wijnen A, Eirin A and Lerman LO: Metabolic syndrome is associated with altered mRNA and miRNA content in human circulating extracellular vesicles. Front Endocrinol (Lausanne). 12:6875862021. View Article : Google Scholar : PubMed/NCBI | |
|
Venkatesh J, Wasson MD, Brown JM, Fernando W and Marcato P: LncRNA-miRNA axes in breast cancer: Novel points of interaction for strategic attack. Cancer Lett. 509:81–88. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Han JJ: LncRNAs: The missing link to senescence nuclear architecture. Trends Biochem Sci. 48:618–628. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Herman AB, Tsitsipatis D and Gorospe M: Integrated lncRNA function upon genomic and epigenomic regulation. Mol Cell. 82:2252–2266. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang X, Asllanaj E, Amiri M, Portilla-Fernandez E, Bramer WM, Nano J, Voortman T, Pan Q and Ghanbari M: Deciphering the role of epigenetic modifications in fatty liver disease: A systematic review. Eur J Clin Invest. 51:e134792021. View Article : Google Scholar : | |
|
Wei L, Yang X, Wang J, Wang Z, Wang Q, Ding Y and Yu A: H3K18 lactylation of senescent microglia potentiates brain aging and Alzheimer's disease through the NFκB signaling pathway. J Neuroinflammation. 20:2082023. View Article : Google Scholar | |
|
Huang MY, Xuan F, Liu W and Cui HJ: MINA controls proliferation and tumorigenesis of glioblastoma by epigenetically regulating cyclins and CDKs via H3K9me3 demethylation. Oncogene. 36:387–396. 2017. View Article : Google Scholar | |
|
Miao F, Gonzalo IG, Lanting L and Natarajan R: In vivo chromatin remodeling events leading to inflammatory gene transcription under diabetic conditions. J Biol Chem. 279:18091–18097. 2004. View Article : Google Scholar : PubMed/NCBI | |
|
Bricambert J, Miranda J, Benhamed F, Girard J, Postic C and Dentin R: Salt-inducible kinase 2 links transcriptional coactivator p300 phosphorylation to the prevention of ChREBP-dependent hepatic steatosis in mice. J Clin Invest. 120:4316–4331. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Brunt EM, Wong VW, Nobili V, Day CP, Sookoian S, Maher JJ, Bugianesi E, Sirlin CB, Neuschwander-Tetri BA and Rinella ME: Nonalcoholic fatty liver disease. Nat Rev Dis Primers. 1:150802015. View Article : Google Scholar : PubMed/NCBI | |
|
Tryndyak VP, Han T, Muskhelishvili L, Fuscoe JC, Ross SA, Beland FA and Pogribny IP: Coupling global methylation and gene expression profiles reveal key pathophysiological events in liver injury induced by a methyl-deficient diet. Mol Nutr Food Res. 55:411–418. 2011. View Article : Google Scholar | |
|
Lee JH, Friso S and Choi SW: Epigenetic mechanisms underlying the link between non-alcoholic fatty liver diseases and nutrition. Nutrients. 6:3303–3325. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Sookoian S, Rosselli MS, Gemma C, Burgueño AL, Fernández Gianotti T, Castaño GO and Pirola CJ: Epigenetic regulation of insulin resistance in nonalcoholic fatty liver disease: Impact of liver methylation of the peroxisome proliferator-activated receptor γ coactivator 1α promoter. Hepatology. 52:1992–2000. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Pirola CJ, Gianotti TF, Burgueño AL, Rey-Funes M, Loidl CF, Mallardi P, Martino JS, Castaño GO and Sookoian S: Epigenetic modification of liver mitochondrial DNA is associated with histological severity of nonalcoholic fatty liver disease. Gut. 62:1356–1363. 2013. View Article : Google Scholar | |
|
Zeybel M, Hardy T, Robinson SM, Fox C, Anstee QM, Ness T, Masson S, Mathers JC, French J, White S and Mann J: Differential DNA methylation of genes involved in fibrosis progression in Non-alcoholic fatty liver disease and alcoholic liver disease. Clin Epigenetics. 7:252015. View Article : Google Scholar : PubMed/NCBI | |
|
Nishida N, Yada N, Hagiwara S, Sakurai T, Kitano M and Kudo M: Unique features associated with hepatic oxidative DNA damage and DNA methylation in non-alcoholic fatty liver disease. J Gastroenterol Hepatol. 31:1646–1653. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Baumeier C, Schlüter L, Saussenthaler S, Laeger T, Rödiger M, Alaze SA, Fritsche L, Häring HU, Stefan N, Fritsche A, et al: Elevated hepatic DPP4 activity promotes insulin resistance and non-alcoholic fatty liver disease. Mol Metab. 6:1254–1263. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Baumeier C, Saussenthaler S, Kammel A, Jähnert M, Schlüter L, Hesse D, Canouil M, Lobbens S, Caiazzo R, Raverdy V, et al: Hepatic DPP4 DNA methylation associates with fatty liver. Diabetes. 66:25–35. 2017. View Article : Google Scholar | |
|
Zhang RN, Pan Q, Zheng RD, Mi YQ, Shen F, Zhou D, Chen GY, Zhu CY and Fan JG: Genome-wide analysis of DNA methylation in human peripheral leukocytes identifies potential biomarkers of nonalcoholic fatty liver disease. Int J Mol Med. 42:443–452. 2018.PubMed/NCBI | |
|
Ahrens M, Ammerpohl O, von Schönfels W, Kolarova J, Bens S, Itzel T, Teufel A, Herrmann A, Brosch M, Hinrichsen H, et al: DNA methylation analysis in nonalcoholic fatty liver disease suggests Distinct disease-specific and remodeling signatures after bariatric surgery. Cell Metab. 18:296–302. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Sun C, Fan JG and Qiao L: Potential epigenetic mechanism in non-alcoholic Fatty liver disease. Int J Mol Sci. 16:5161–5179. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Emmett MJ and Lazar MA: Integrative regulation of physiology by histone deacetylase 3. Nat Rev Mol Cell Biol. 20:102–115. 2019. View Article : Google Scholar : | |
|
Sun Z, Miller RA, Patel RT, Chen J, Dhir R, Wang H, Zhang D, Graham MJ, Unterman TG, Shulman GI, et al: Hepatic Hdac3 promotes gluconeogenesis by repressing lipid synthesis and sequestration. Nat Med. 18:934–942. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Wang RH, Li C and Deng CX: Liver steatosis and increased ChREBP expression in mice carrying a liver specific SIRT1 null mutation under a normal feeding condition. Int J Biol Sci. 6:682–690. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Jin M, Shen Y, Pan T, Zhu T, Li X, Xu F, Betancor MB, Jiao L, Tocher DR and Zhou Q: Dietary betaine mitigates hepatic steatosis and inflammation induced by a High-Fat-Diet by modulating the Sirt1/Srebp-1/Pparα pathway in juvenile black seabream (Acanthopagrus schlegelii). Front Immunol. 12:6947202021. View Article : Google Scholar | |
|
Zeng C and Chen M: Progress in nonalcoholic fatty liver disease: SIRT family regulates mitochondrial biogenesis. Biomolecules. 12:10792022. View Article : Google Scholar : PubMed/NCBI | |
|
Nassir F and Ibdah JA: Sirtuins and nonalcoholic fatty liver disease. World J Gastroenterol. 22:10084–10092. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Kim JH, Jung DY, Kim HR and Jung MH: Histone H3K9 demethylase JMJD2B plays a role in LXRα-dependent lipogenesis. Int J Mol Sci. 21:83132020. View Article : Google Scholar | |
|
Pogribny IP, Tryndyak VP, Bagnyukova TV, Melnyk S, Montgomery B, Ross SA, Latendresse JR, Rusyn I and Beland FA: Hepatic epigenetic phenotype predetermines individual susceptibility to hepatic steatosis in mice fed a lipogenic methyl-deficient diet. J Hepatol. 51:176–186. 2009. View Article : Google Scholar : PubMed/NCBI | |
|
Gjorgjieva M, Sobolewski C, Dolicka D, Correia de Sousa M and Foti M: miRNAs and NAFLD: From pathophysiology to therapy. Gut. 68:2065–2079. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Bandiera S, Pfeffer S, Baumert TF and Zeisel MB: miR-122-a key factor and therapeutic target in liver disease. J Hepatol. 62:448–457. 2015. View Article : Google Scholar | |
|
Cheung O, Puri P, Eicken C, Contos MJ, Mirshahi F, Maher JW, Kellum JM, Min H, Luketic VA and Sanyal AJ: Nonalcoholic steatohepatitis is associated with altered hepatic MicroRNA expression. Hepatology. 48:1810–1820. 2008. View Article : Google Scholar : PubMed/NCBI | |
|
Pirola CJ, Fernández Gianotti T, Castaño GO, Mallardi P, San Martino J, Mora Gonzalez Lopez Ledesma M, Flichman D, Mirshahi F, Sanyal AJ and Sookoian S: Circulating microRNA signature in non-alcoholic fatty liver disease: From serum Non-coding RNAs to liver histology and disease pathogenesis. Gut. 64:800–812. 2015. View Article : Google Scholar | |
|
Hsu SH, Wang B, Kota J, Yu J, Costinean S, Kutay H, Yu L, Bai S, La Perle K, Chivukula RR, et al: Essential metabolic, anti-inflammatory, and anti-tumorigenic functions of miR-122 in liver. J Clin Invest. 122:2871–2883. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Tsai WC, Hsu SD, Hsu CS, Lai TC, Chen SJ, Shen R, Huang Y, Chen HC, Lee CH, Tsai TF, et al: MicroRNA-122 plays a critical role in liver homeostasis and hepatocarcinogenesis. J Clin Invest. 122:2884–2897. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Liu CH, Ampuero J, Gil-Gómez A, Montero-Vallejo R, Rojas Á, Muñoz-Hernández R, Gallego-Durán R and Romero-Gómez M: miRNAs in patients with non-alcoholic fatty liver disease: A systematic review and meta-analysis. J Hepatol. 69:1335–1348. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Liu XL, Pan Q, Zhang RN, Shen F, Yan SY, Sun C, Xu ZJ, Chen YW and Fan JG: Disease-specific miR-34a as diagnostic marker of non-alcoholic steatohepatitis in a Chinese population. World J Gastroenterol. 22:9844–9852. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Ding J, Li M, Wan X, Jin X, Chen S, Yu C and Li Y: Effect of miR-34a in regulating steatosis by targeting PPARα expression in nonalcoholic fatty liver disease. Sci Rep. 5:137292015. View Article : Google Scholar | |
|
Xu Y, Zalzala M, Xu J, Li Y, Yin L and Zhang Y: A metabolic stress-inducible miR-34a-HNF4α pathway regulates lipid and lipoprotein metabolism. Nat Commun. 6:74662015. View Article : Google Scholar | |
|
Calo N, Ramadori P, Sobolewski C, Romero Y, Maeder C, Fournier M, Rantakari P, Zhang FP, Poutanen M, Dufour JF, et al: Stress-activated miR-21/miR-21* in hepatocytes promotes lipid and glucose metabolic disorders associated with High-fat diet consumption. Gut. 65:1871–1881. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Meng F, Henson R, Wehbe-Janek H, Ghoshal K, Jacob ST and Patel T: MicroRNA-21 regulates expression of the PTEN tumor suppressor gene in human hepatocellular cancer. Gastroenterology. 133:647–658. 2007. View Article : Google Scholar : PubMed/NCBI | |
|
Loyer X, Paradis V, Hénique C, Vion AC, Colnot N, Guerin CL, Devue C, On S, Scetbun J, Romain M, et al: Liver microRNA-21 is overexpressed in non-alcoholic steatohepatitis and contributes to the disease in experimental models by inhibiting PPARα expression. Gut. 65:1882–1894. 2016. View Article : Google Scholar | |
|
Wu H, Ng R, Chen X, Steer CJ and Song G: MicroRNA-21 is a potential link between non-alcoholic fatty liver disease and hepatocellular carcinoma via modulation of the HBP1-p53-Srebp1c pathway. Gut. 65:1850–1860. 2016. View Article : Google Scholar | |
|
Atic AI, Thiele M, Munk A and Dalgaard LT: Circulating miRNAs associated with nonalcoholic fatty liver disease. Am J Physiol Cell Physiol. 324:C588–C602. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Hendy OM, Rabie H, El Fouly A, Abdel-Samiee M, Abdelmotelb N, Elshormilisy AA, Allam M, Ali ST, Bahaa El-Deen NM, Abdelsattar S and Mohamed SM: The circulating Micro-RNAs (-122, -34a and -99a) as predictive biomarkers for Non-alcoholic fatty liver diseases. Diabetes Metab Syndr Obes. 12:2715–2723. 2019. View Article : Google Scholar | |
|
Liu XL, Pan Q, Cao HX, Xin FZ, Zhao ZH, Yang RX, Zeng J, Zhou H and Fan JG: Lipotoxic Hepatocyte-derived exosomal MicroRNA 192-5p activates macrophages through Rictor/Akt/Forkhead box transcription factor O1 signaling in nonalcoholic fatty liver disease. Hepatology. 72:454–469. 2020. View Article : Google Scholar | |
|
Becker PP, Rau M, Schmitt J, Malsch C, Hammer C, Bantel H, Müllhaupt B and Geier A: Performance of Serum microRNAs-122, -192 and -21 as biomarkers in patients with Non-alcoholic steatohepatitis. PLoS One. 10:e01426612015. View Article : Google Scholar | |
|
Li M, Guo Y, Wang XJ, Duan BH and Li L: HOTAIR participates in hepatic insulin resistance via regulating SIRT1. Eur Rev Med Pharmacol Sci. 22:7883–7890. 2018.PubMed/NCBI | |
|
Zhu X, Wu YB, Zhou J and Kang DM: Upregulation of lncRNA MEG3 promotes hepatic insulin resistance via increasing FoxO1 expression. Biochem Biophys Res Commun. 469:319–325. 2016. View Article : Google Scholar | |
|
Zhu X, Li H, Wu Y, Zhou J, Yang G and Wang W: lncRNA MEG3 promotes hepatic insulin resistance by serving as a competing endogenous RNA of miR-214 to regulate ATF4 expression. Int J Mol Med. 43:345–357. 2019. | |
|
Zhao XY, Xiong X, Liu T, Mi L, Peng X, Rui C, Guo L, Li S, Li X and Lin JD: Long noncoding RNA licensing of obesity-linked hepatic lipogenesis and NAFLD pathogenesis. Nat Commun. 9:29862018. View Article : Google Scholar : PubMed/NCBI | |
|
Yan C, Chen J and Chen N: Long noncoding RNA MALAT1 promotes hepatic steatosis and insulin resistance by increasing nuclear SREBP-1c protein stability. Sci Rep. 6:226402016. View Article : Google Scholar : PubMed/NCBI | |
|
Ma M, Duan R, Shen L, Liu M, Ji Y, Zhou H, Li C, Liang T, Li X and Guo L: The lncRNA Gm15622 stimulates SREBP-1c expression and hepatic lipid accumulation by sponging the miR-742-3p in mice. J Lipid Res. 61:1052–1064. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang N, Geng T, Wang Z, Zhang R, Cao T, Camporez JP, Cai SY, Liu Y, Dandolo L, Shulman GI, et al: Elevated hepatic expression of H19 long noncoding RNA contributes to diabetic hyperglycemia. JCI Insight. 3:e1203042018. View Article : Google Scholar : PubMed/NCBI | |
|
Wang J, Yang W, Chen Z, Chen J, Meng Y, Feng B, Sun L, Dou L, Li J, Cui Q and Yang J: Long noncoding RNA lncSHGL recruits hnRNPA1 to suppress hepatic gluconeogenesis and lipogenesis. Diabetes. 67:581–593. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Li D, Cheng M, Niu Y, Chi X, Liu X, Fan J, Fan H, Chang Y and Yang W: Identification of a novel human long non-coding RNA that regulates hepatic lipid metabolism by inhibiting SREBP-1c. Int J Biol Sci. 13:349–357. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang B, Li H, Li D, Sun H, Li M and Hu H: Long noncoding RNA Mirt2 upregulates USP10 expression to suppress hepatic steatosis by sponging miR-34a-5p. Gene. 700:139–148. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Berzigotti A, Saran U and Dufour JF: Physical activity and liver diseases. Hepatology. 63:1026–1040. 2016. View Article : Google Scholar | |
|
Hayıroğlu M, Çınar T, Cilli Hayıroğlu S, Şaylık F, Uzun M and Tekkeşin A: The role of smart devices and mobile application on the change in peak VO2 in patients with high cardiovascular risk: A sub-study of the LIGHT randomised clinical trial. Acta Cardiol. 78:1000–1005. 2023. View Article : Google Scholar | |
|
Hayıroğlu M, Çınar T, Çinier G, Karakaya A, Yıldırım M, Güney BÇ, Öz A, Gündoğmuş PD, Ösken A, Özkan A, et al: The effect of 1-year mean step count on the change in the atherosclerotic cardiovascular disease risk calculation in patients with high cardiovascular risk: A sub-study of the LIGHT randomized clinical trial. Kardiol Pol. 79:1140–1142. 2021. View Article : Google Scholar | |
|
Su P, Chen JG and Tang DH: Exercise against nonalcoholic fatty liver disease: Possible role and mechanism of lipophagy. Life Sci. 327:1218372023. View Article : Google Scholar : PubMed/NCBI | |
|
Garber CE, Blissmer B, Deschenes MR, Franklin BA, Lamonte MJ, Lee IM, Nieman DC and Swain DP; American College of Sports Medicine: American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: Guidance for prescribing exercise. Med Sci Sports Exerc. 43:1334–1359. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
Simpson RJ, Campbell JP, Gleeson M, Krüger K, Nieman DC, Pyne DB, Turner JE and Walsh NP: Can exercise affect immune function to increase susceptibility to infection? Exerc Immunol Rev. 26:8–22. 2020.PubMed/NCBI | |
|
Carbone S, Del Buono MG, Ozemek C and Lavie CJ: Obesity, risk of diabetes and role of physical activity, exercise training and cardiorespiratory fitness. Prog Cardiovasc Dis. 62:327–333. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Liu S, Liu Y, Liu Z, Hu Y and Jiang M: A review of the signaling pathways of aerobic and anaerobic exercise on atherosclerosis. J Cell Physiol. 238:866–879. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
McKie GL and Wright DC: Biochemical adaptations in white adipose tissue following aerobic exercise: From mitochondrial biogenesis to browning. Biochem J. 477:1061–1081. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Richter EA and Hargreaves M: Exercise, GLUT4, and skeletal muscle glucose uptake. Physiol Rev. 93:993–1017. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
El Assar M, Álvarez-Bustos A, Sosa P, Angulo J and Rodríguez-Mañas L: Effect of physical Activity/exercise on oxidative stress and inflammation in muscle and vascular aging. Int J Mol Sci. 23:87132022. View Article : Google Scholar : PubMed/NCBI | |
|
Guo R, Liong EC, So KF, Fung ML and Tipoe GL: Beneficial mechanisms of aerobic exercise on hepatic lipid metabolism in Non-alcoholic fatty liver disease. Hepatobiliary Pancreat Dis Int. 14:139–144. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Mohammad Rahimi GR and Attarzadeh Hosseini SR: Effect of aerobic exercise alone or in conjunction with diet on liver function, insulin resistance and lipids in Non-alcoholic fatty liver disease. Biol Res Nurs. 24:259–276. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Diniz TA, de Lima Junior EA, Teixeira AA, Biondo LA, da Rocha LAF, Valadão IC, Silveira LS, Cabral-Santos C, de Souza CO and Rosa Neto JC: Aerobic training improves NAFLD markers and insulin resistance through AMPK-PPAR-α signaling in obese mice. Life Sci. 266:1188682021. View Article : Google Scholar | |
|
Keating SE, Hackett DA, George J and Johnson NA: Exercise and non-alcoholic fatty liver disease: A systematic review and meta-analysis. J Hepatol. 57:157–166. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Hajighasem A, Farzanegi P and Mazaheri Z: Effects of combined therapy with resveratrol, continuous and interval exercises on apoptosis, oxidative stress, and inflammatory biomarkers in the liver of old rats with Non-alcoholic fatty liver disease. Arch Physiol Biochem. 125:142–149. 2019. View Article : Google Scholar | |
|
Sohet FM, Neyrinck AM, Pachikian BD, de Backer FC, Bindels LB, Niklowitz P, Menke T, Cani PD and Delzenne NM: Coenzyme Q10 supplementation lowers hepatic oxidative stress and inflammation associated with diet-induced obesity in mice. Biochem Pharmacol. 78:1391–1400. 2009. View Article : Google Scholar : PubMed/NCBI | |
|
Xiao J, Ching YP, Liong EC, Nanji AA, Fung ML and Tipoe GL: Garlic-derived S-allylmercaptocysteine is a hepato-protective agent in non-alcoholic fatty liver disease in vivo animal model. Eur J Nutr. 52:179–191. 2013. View Article : Google Scholar : | |
|
Xiao J, Ho CT, Liong EC, Nanji AA, Leung TM, Lau TY, Fung ML and Tipoe GL: Epigallocatechin gallate attenuates fibrosis, oxidative stress, and inflammation in non-alcoholic fatty liver disease rat model through TGF/SMAD, PI3 K/Akt/FoxO1, and NF-kappa B pathways. Eur J Nutr. 53:187–199. 2014. View Article : Google Scholar | |
|
Qi F, Li T, Deng Q and Fan A: The impact of aerobic and anaerobic exercise interventions on the management and outcomes of non-alcoholic fatty liver disease. Physiol Res. 73:671–686. 2024. View Article : Google Scholar : PubMed/NCBI | |
|
Li H, Dun Y, Zhang W, You B, Liu Y, Fu S, Qiu L, Cheng J, Ripley-Gonzalez JW and Liu S: Exercise improves lipid droplet metabolism disorder through activation of AMPK-mediated lipophagy in NAFLD. Life Sci. 273:1193142021. View Article : Google Scholar : PubMed/NCBI | |
|
Zou YY, Tang XB, Chen ZL, Liu B, Zheng L, Song MY, Xiao Q, Zhou ZQ, Peng XY and Tang CF: Exercise intervention improves mitochondrial quality in non-alcoholic fatty liver disease zebrafish. Front Endocrinol (Lausanne). 14:11624852023. View Article : Google Scholar : PubMed/NCBI | |
|
McLeod JC, Currier BS, Lowisz CV and Phillips SM: The influence of resistance exercise training prescription variables on skeletal muscle mass, strength, and physical function in healthy adults: An umbrella review. J Sport Health Sci. 13:47–60. 2024. View Article : Google Scholar : | |
|
Schoenfeld BJ, Ogborn D and Krieger JW: Effects of resistance training frequency on measures of muscle hypertrophy: A systematic review and Meta-analysis. Sports Med. 46:1689–1697. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Consitt LA, Dudley C and Saxena G: Impact of endurance and resistance training on skeletal muscle glucose metabolism in older adults. Nutrients. 11:26362019. View Article : Google Scholar : PubMed/NCBI | |
|
Fedewa MV, Gist NH, Evans EM and Dishman RK: Exercise and insulin resistance in youth: A meta-analysis. Pediatrics. 133:e163–e174. 2014. View Article : Google Scholar | |
|
Ivy JL: Muscle insulin resistance amended with exercise training: Role of GLUT4 expression. Med Sci Sports Exerc. 36:1207–1211. 2004.PubMed/NCBI | |
|
Mazur-Bialy AI, Pocheć E and Zarawski M: Anti-inflammatory properties of irisin, mediator of physical activity, are connected with TLR4/MyD88 signaling pathway activation. Int J Mol Sci. 18:7012017. View Article : Google Scholar : PubMed/NCBI | |
|
Hashida R, Kawaguchi T, Bekki M, Omoto M, Matsuse H, Nago T, Takano Y, Ueno T, Koga H, George J, et al: Aerobic vs. resistance exercise in non-alcoholic fatty liver disease: A systematic review. J Hepatol. 66:142–152. 2017. View Article : Google Scholar | |
|
Xue Y, Peng Y, Zhang L, Ba Y, Jin G and Liu G: Effect of different exercise modalities on nonalcoholic fatty liver disease: A systematic review and network meta-analysis. Sci Rep. 14:62122024. View Article : Google Scholar : PubMed/NCBI | |
|
Takahashi A, Abe K, Usami K, Imaizumi H, Hayashi M, Okai K, Kanno Y, Tanji N, Watanabe H and Ohira H: Simple resistance exercise helps patients with Non-alcoholic fatty liver disease. Int J Sports Med. 36:848–852. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Yang HJ, Hong YP, Yoon TY, Ryoo JH, Choi JM and Oh CM: Independent and synergistic associations of aerobic physical activity and resistance exercise with nonalcoholic fatty liver disease. Gut Liver. 17:600–609. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Gripp F, Nava RC, Cassilhas RC, Esteves EA, Magalhães COD, Dias-Peixoto MF, de Castro Magalhães F and Amorim FT: HIIT is superior than MICT on cardiometabolic health during training and detraining. Eur J Appl Physiol. 121:159–172. 2021. View Article : Google Scholar | |
|
Ryan BJ, Schleh MW, Ahn C, Ludzki AC, Gillen JB, Varshney P, Van Pelt DW, Pitchford LM, Chenevert TL, Gioscia-Ryan RA, et al: Moderate-intensity exercise and High-intensity interval training affect insulin sensitivity similarly in obese adults. J Clin Endocrinol Metab. 105:e2941–e2959. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Han C, Lu P and Yan SZ: Effects of high-intensity interval training on mitochondrial supercomplex assembly and biogenesis, mitophagy, and the AMP-activated protein kinase pathway in the soleus muscle of aged female rats. Exp Gerontol. 158:1116482022. View Article : Google Scholar | |
|
Wadley AJ, Chen YW, Lip GY, Fisher JP and Aldred S: Low volume-high intensity interval exercise elicits antioxidant and anti-inflammatory effects in humans. J Sports Sci. 34:1–9. 2016. View Article : Google Scholar | |
|
Su L, Fu J, Sun S, Zhao G, Cheng W, Dou C and Quan M: Effects of HIIT and MICT on cardiovascular risk factors in adults with overweight and/or obesity: A meta-analysis. PLoS One. 14:e02106442019. View Article : Google Scholar : PubMed/NCBI | |
|
Gu S, Du X, Wang D, Yu Y and Guo S: Effects of high intensity interval training versus moderate intensity continuous training on exercise capacity and quality of life in patients with heart failure: A systematic review and Meta-analysis. PLoS One. 18:e02903622023. View Article : Google Scholar : PubMed/NCBI | |
|
de Brito JN, McDonough DJ, Mathew M, VanWagner LB, Schreiner PJ, Gabriel KP, Jacobs DR Jr, Terry JG, Carr JJ and Pereira MA: Young adult physical activity trajectories and midlife nonalcoholic fatty liver disease. JAMA Netw Open. 6:e23389522023. View Article : Google Scholar : PubMed/NCBI | |
|
Fredrickson G, Barrow F, Dietsche K, Parthiban P, Khan S, Robert S, Demirchian M, Rhoades H, Wang H, Adeyi O and Revelo XS: Exercise of high intensity ameliorates hepatic inflammation and the progression of NASH. Mol Metab. 53:1012702021. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang HJ, He J, Pan LL, Ma ZM, Han CK, Chen CS, Chen Z, Han HW, Chen S, Sun Q, et al: Effects of moderate and vigorous exercise on nonalcoholic fatty liver disease: A Randomized clinical trial. JAMA Intern Med. 176:1074–1082. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Harden JE, Tabacu L and Reynolds LJ: Physical activity intensity and markers of inflammation in those with non-alcoholic fatty liver disease. Diabetes Res Clin Pract. 207:1110472024. View Article : Google Scholar | |
|
Yuan Z, Xiao-Wei L, Juan W, Xiu-Juan L, Nian-Yun Z and Lei S: HIIT and MICT attenuate high-fat diet-induced hepatic lipid accumulation and ER stress via the PERK-ATF4-CHOP signaling pathway. J Physiol Biochem. 78:641–652. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Hyun J and Jung Y: DNA Methylation in nonalcoholic fatty liver disease. Int J Mol Sci. 21:81382020. View Article : Google Scholar : PubMed/NCBI | |
|
Moore LD, Le T and Fan G: DNA methylation and its basic function. Neuropsychopharmacology. 38:23–38. 2013. View Article : Google Scholar | |
|
Jones MJ, Goodman SJ and Kobor MS: DNA methylation and healthy human aging. Aging Cell. 14:924–932. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Kim YN, Hwang JH and Cho YO: The effects of exercise training and acute exercise duration on plasma folate and vitamin B12. Nutr Res Pract. 10:161–166. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Brunaud L, Alberto JM, Ayav A, Gérard P, Namour F, Antunes L, Braun M, Bronowicki JP, Bresler L and Guéant JL: Effects of vitamin B12 and folate deficiencies on DNA methylation and carcinogenesis in rat liver. Clin Chem Lab Med. 41:1012–1019. 2003. View Article : Google Scholar : PubMed/NCBI | |
|
Hunter DJ, James L, Hussey B, Wadley AJ, Lindley MR and Mastana SS: Impact of aerobic exercise and fatty acid supplementation on global and gene-specific DNA methylation. Epigenetics. 14:294–309. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Robson-Ansley PJ, Saini A, Toms C, Ansley L, Walshe IH, Nimmo MA and Curtin JA: Dynamic changes in dna methylation status in peripheral blood Mononuclear cells following an acute bout of exercise: Potential impact of exercise-induced elevations in interleukin-6 concentration. J Biol Regul Homeost Agents. 28:407–417. 2014.PubMed/NCBI | |
|
Zhou D, Hlady RA, Schafer MJ, White TA, Liu C, Choi JH, Miller JD, Roberts LR, LeBrasseur NK and Robertson KD: High fat diet and exercise lead to a disrupted and pathogenic DNA methylome in mouse liver. Epigenetics. 12:55–69. 2017. View Article : Google Scholar : | |
|
Zhang Y, Hashimoto S, Fujii C, Hida S, Ito K, Matsumura T, Sakaizawa T, Morikawa M, Masuki S, Nose H, et al: NFκB2 gene as a novel candidate that epigenetically responds to interval walking training. Int J Sports Med. 36:769–775. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Światowy WJ, Drzewiecka H, Kliber M, Sąsiadek M, Karpiński P, Pławski A and Jagodziński PP: Physical Activity and DNA Methylation in Humans. Int J Mol Sci. 22:129892021. View Article : Google Scholar : PubMed/NCBI | |
|
King-Himmelreich TS, Schramm S, Wolters MC, Schmetzer J, Möser CV, Knothe C, Resch E, Peil J, Geisslinger G and Niederberger E: The impact of endurance exercise on global and AMPK gene-specific DNA methylation. Biochem Biophys Res Commun. 474:284–290. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Freitas-Dias R, Lima TI, Costa-Junior JM, Gonçalves LM, Araujo HN, Paula FMM, Santos GJ, Branco RCS, Ou K, Kaestner KH, et al: Offspring from trained male mice inherit improved muscle mitochondrial function through PPAR co-repressor modulation. Life Sci. 291:1202392022. View Article : Google Scholar | |
|
McGee SL and Hargreaves M: Epigenetics and Exercise. Trends Endocrinol Metab. 30:636–645. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Mallett G: The effect of exercise and physical activity on skeletal muscle epigenetics and metabolic adaptations. Eur J Appl Physiol. 125:611–627. 2025. View Article : Google Scholar : PubMed/NCBI | |
|
Stols-Gonçalves D, Tristão LS, Henneman P and Nieuwdorp M: Epigenetic markers and Microbiota/Metabolite-Induced epigenetic modifications in the pathogenesis of obesity, metabolic syndrome, type 2 diabetes, and Non-alcoholic fatty liver disease. Curr Diab Rep. 19:312019. View Article : Google Scholar : PubMed/NCBI | |
|
Fan X, Wang H, Wang W, Shen J and Wang Z: Exercise training alleviates cholesterol and lipid accumulation in mice with non-alcoholic steatohepatitis: Reduction of KMT2D-mediated histone methylation of IDI1. Exp Cell Res. 442:1142652024. View Article : Google Scholar : PubMed/NCBI | |
|
Luo HY, Mu WJ, Chen M, Zhu JY, Li Y, Li S, Yan LJ, Li RY, Yin MT, Li X, et al: Hepatic Klf10-Fh1 axis promotes exercise-mediated amelioration of NASH in mice. Metabolism. 155:1559162024. View Article : Google Scholar : PubMed/NCBI | |
|
Villanova L, Vernucci E, Pucci B, Pellegrini L, Nebbioso M, Mauri C, Marfe G, Spataro A, Fini M, Banfi G, et al: Influence of age and physical exercise on sirtuin activity in humans. J Biol Regul Homeost Agents. 27:497–507. 2013.PubMed/NCBI | |
|
Yang J, Félix-Soriano E, Martínez-Gayo A, Ibañez-Santos J, Sáinz N, Martínez JA and Moreno-Aliaga MJ: SIRT1 and FOXO1 role on MASLD risk: Effects of DHA-rich n-3 PUFA supplementation and exercise in aged obese female mice and in Post-menopausal Overweight/obese women. J Physiol Biochem. 80:697–712. 2024. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang Y, Shan M, Ding X, Sun H, Qiu F and Shi L: Maternal exercise represses Nox4 via SIRT1 to prevent vascular oxidative stress and endothelial dysfunction in SHR offspring. Front Endocrinol (Lausanne). 14:12191942023. View Article : Google Scholar : PubMed/NCBI | |
|
Nikroo H, Hosseini SRA, Fathi M, Sardar MA and Khazaei M: The effect of aerobic, resistance, and combined training on PPAR-α, SIRT1 gene expression, and insulin resistance in high-fat diet-induced NAFLD male rats. Physiol Behav. 227:1131492020. View Article : Google Scholar | |
|
Song MY, Han CY, Moon YJ, Lee JH, Bae EJ and Park BH: Sirt6 reprograms myofibers to oxidative type through CREB-dependent Sox6 suppression. Nat Commun. 13:18082022. View Article : Google Scholar : PubMed/NCBI | |
|
Tian C, Huang R and Xiang M: SIRT1: Harnessing multiple pathways to hinder NAFLD. Pharmacol Res. 203:1071552024. View Article : Google Scholar : PubMed/NCBI | |
|
Hou T, Tian Y, Cao Z, Zhang J, Feng T, Tao W, Sun H, Wen H, Lu X, Zhu Q, et al: Cytoplasmic SIRT6-mediated ACSL5 deacetylation impedes nonalcoholic fatty liver disease by facilitating hepatic fatty acid oxidation. Mol Cell. 82:4099–4115.e4099. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Hall MM, Rajasekaran S, Thomsen TW and Peterson AR: Lactate: Friend or Foe. PM R. 8(Suppl 3): S8–S15. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Harris RT and Dudley GA: Exercise alters the distribution of ammonia and lactate in blood. J Appl Physiol (1985). 66:313–317. 1989. View Article : Google Scholar : PubMed/NCBI | |
|
Han H, Zhao Y, Du J, Wang S, Yang X, Li W, Song J, Zhang S, Zhang Z, Tan Y, et al: Exercise improves cognitive dysfunction and neuroinflammation in mice through Histone H3 lactylation in microglia. Immun Ageing. 20:632023. View Article : Google Scholar : PubMed/NCBI | |
|
Widmann M, Nieß AM and Munz B: Physical exercise and epigenetic modifications in skeletal muscle. Sports Med. 49:509–523. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Dos Santos JAC, Veras ASC, Batista VRG, Tavares MEA, Correia RR, Suggett CB and Teixeira GR: Physical exercise and the functions of microRNAs. Life Sci. 304:1207232022. View Article : Google Scholar : PubMed/NCBI | |
|
Antunes-Correa LM, Trevizan PF, Bacurau AVN, Ferreira-Santos L, Gomes JLP, Urias U, Oliveira PA, Alves MJNN, de Almeida DR, Brum PC, et al: Effects of aerobic and inspiratory training on skeletal muscle microRNA-1 and downstream-associated pathways in patients with heart failure. J Cachexia Sarcopenia Muscle. 11:89–102. 2020. View Article : Google Scholar | |
|
Chen JF, Mandel EM, Thomson JM, Wu Q, Callis TE, Hammond SM, Conlon FL and Wang DZ: The role of microRNA-1 and microRNA-133 in skeletal muscle proliferation and differentiation. Nat Genet. 38:228–233. 2006. View Article : Google Scholar | |
|
Scisciola L, Benedetti R, Chianese U, Fontanella RA, Del Gaudio N, Marfella R, Surina, Altucci L, Barbieri M, Paolisso G, et al: The pivotal role of miRNA-21 in myocardial metabolic flexibility in response to short- and long-term high glucose treatment: Evidence in human cardiomyocyte cell line. Diabetes Res Clin Pract. 191:1100662022. View Article : Google Scholar : PubMed/NCBI | |
|
Da Silva ND Jr, Fernandes T, Soci UP, Monteiro AW, Phillips MI and De Oliveira EM: Swimming training in rats increases cardiac MicroRNA-126 expression and angiogenesis. Med Sci Sports Exerc. 44:1453–1462. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Wilson RA, Stathis CG, Hayes A and Cooke MB: Intermittent fasting and High-intensity exercise elicit Sexual-dimorphic and Tissue-specific adaptations in Diet-induced obese mice. Nutrients. 12:17642020. View Article : Google Scholar : PubMed/NCBI | |
|
Kristensen MM, Davidsen PK, Vigelsø A, Hansen CN, Jensen LJ, Jessen N, Bruun JM, Dela F and Helge JW: miRNAs in human subcutaneous adipose tissue: Effects of weight loss induced by hypocaloric diet and exercise. Obesity (Silver Spring). 25:572–580. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Lionett S, Kiel IA, Camera DM, Vanky E, Parr EB, Lydersen S, Hawley JA and Moholdt T: Circulating and adipose Tissue miRNAs in women with polycystic ovary syndrome and responses to High-intensity interval training. Front Physiol. 11:9042020. View Article : Google Scholar : PubMed/NCBI | |
|
Xu B, Zhang Y, Du XF, Li J, Zi HX, Bu JW, Yan Y, Han H and Du JL: Neurons secrete miR-132-containing exosomes to regulate brain vascular integrity. Cell Res. 27:882–897. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Panera N, Gnani D, Crudele A, Ceccarelli S, Nobili V and Alisi A: MicroRNAs as controlled systems and controllers in non-alcoholic fatty liver disease. World J Gastroenterol. 20:15079–15086. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Ghareghani P, Shanaki M, Ahmadi S, Khoshdel AR, Rezvan N, Meshkani R, Delfan M and Gorgani-Firuzjaee S: Aerobic endurance training improves nonalcoholic fatty liver disease (NAFLD) features via miR-33 dependent autophagy induction in high fat diet fed mice. Obes Res Clin Pract. 12:80–89. 2018. View Article : Google Scholar | |
|
Kalaki-Jouybari F, Shanaki M, Delfan M, Gorgani-Firouzjae S and Khakdan S: High-intensity interval training (HIIT) alleviated NAFLD feature via miR-122 induction in liver of high-fat high-fructose diet induced diabetic rats. Arch Physiol Biochem. 126:242–249. 2020. View Article : Google Scholar | |
|
Lu YL, Jing W, Feng LS, Zhang L, Xu JF, You TJ and Zhao J: Effects of hypoxic exercise training on microRNA expression and lipid metabolism in obese rat livers. J Zhejiang Univ Sci B. 15:820–829. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Xiao J, Bei Y, Liu J, Dimitrova-Shumkovska J, Kuang D, Zhou Q, Li J, Yang Y, Xiang Y, Wang F, et al: miR-212 downregulation contributes to the protective effect of exercise against non-alcoholic fatty liver via targeting FGF-21. J Cell Mol Med. 20:204–216. 2016. View Article : Google Scholar | |
|
Wang M, Xue Q, Li X, Krohn K, Ziesche S, Ceglarek U, Blüher M, Keller M, Yaskolka Meir A, Heianza Y, et al: Circulating levels of microRNA-122 and hepatic fat change in response to Weight-Loss interventions: CENTRAL Trial. J Clin Endocrinol Metab. 107:e1899–e1906. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
de Mendonça M, Rocha KC, de Sousa É, Pereira BMV, Oyama LM and Rodrigues AC: Aerobic exercise training regulates serum extracellular vesicle miRNAs linked to obesity to promote their beneficial effects in mice. Am J Physiol Endocrinol Metab. 319:E579–E591. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Wang Z, Zhu Y, Xia L, Li J, Song M and Yang C: Exercise-Induced ADAR2 protects against nonalcoholic fatty liver disease through miR-34a. Nutrients. 15:1212022. View Article : Google Scholar | |
|
Wu B, Ding J, Chen A, Song Y, Xu C, Tian F and Zhao J: Aerobic exercise improves adipogenesis in diet-induced obese mice via the lncSRA/p38/JNK/PPARγ pathway. Nutr Res. 105:20–32. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Wu B, Xu C, Tian Y, Zeng Y, Yan F, Chen A, Zhao J and Chen L: Aerobic exercise promotes the expression of ATGL and attenuates inflammation to improve hepatic steatosis via lncRNA SRA. Sci Rep. 12:53702022. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang Y, Wei Q, Geng X and Fang G: Long-term aerobic exercise enhances hepatoprotection in MAFLD by modulating exosomal miR-324 via ROCK1. Metabolites. 14:6922024. View Article : Google Scholar : PubMed/NCBI | |
|
Zhao J, Song Y, Zeng Y, Chen L, Yan F, Chen A, Wu B and Wang Y: Improvement of hyperlipidemia by aerobic exercise in mice through a regulatory effect of miR-21a-5p on its target genes. Sci Rep. 11:119662021. View Article : Google Scholar : PubMed/NCBI | |
|
Lou J, Wu J, Feng M, Dang X, Wu G, Yang H, Wang Y, Li J, Zhao Y, Shi C, et al: Exercise promotes angiogenesis by enhancing endothelial cell fatty acid utilization via liver-derived extracellular vesicle miR-122-5p. Journal of sport and health science. 11:495–508. 2022. View Article : Google Scholar : | |
|
Xia SF, Jiang YY, Qiu YY, Huang W and Wang J: Role of diets and exercise in ameliorating obesity-related hepatic steatosis: Insights at the microRNA-dependent thyroid hormone synthesis and action. Life Sci. 242:1171822020. View Article : Google Scholar | |
|
Abdelmalek MF: Nonalcoholic fatty liver disease: Another leap forward. Nat Rev Gastroenterol Hepatol. 18:85–86. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Gruet M, Saynor ZL, Urquhart DS and Radtke T: Rethinking physical exercise training in the modern era of cystic fibrosis: A step towards optimising Short-term efficacy and long-term engagement. J Cyst Fibros. 21:e83–e98. 2022. View Article : Google Scholar | |
|
Stevanović-Silva J, Beleza J, Coxito P, Costa RC, Ascensão A and Magalhães J: Fit mothers for a healthy future: Breaking the intergenerational cycle of non-alcoholic fatty liver disease with maternal exercise. Eur J Clin Invest. 52:e135962022. View Article : Google Scholar |