Peripheral biomarkers predict survival in patients with glioblastoma treated with temozolomide
- Authors:
- Published online on: April 17, 2025 https://doi.org/10.3892/mco.2025.2851
- Article Number: 56
-
Copyright: © Zhang et al. This is an open access article distributed under the terms of Creative Commons Attribution License.
Metrics:
Total
Views: 0 (Spandidos Publications: | PMC Statistics:
)
Total PDF Downloads: 0 (Spandidos Publications: | PMC Statistics:
)
Abstract
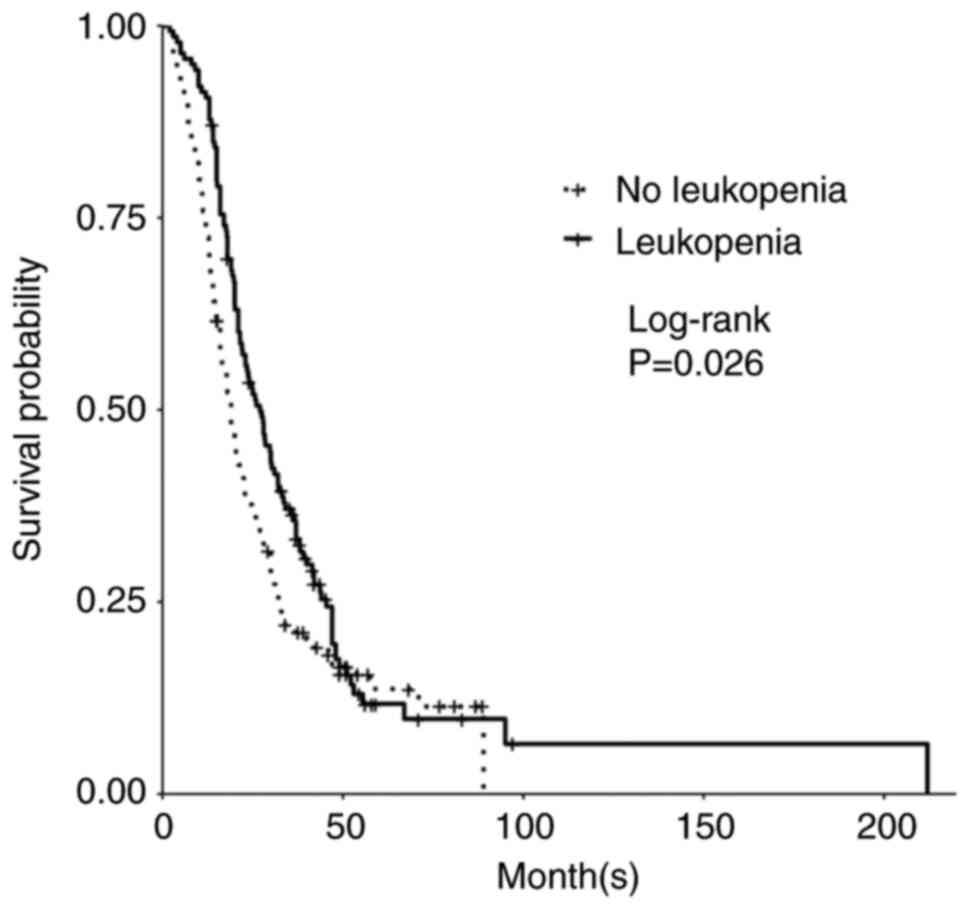
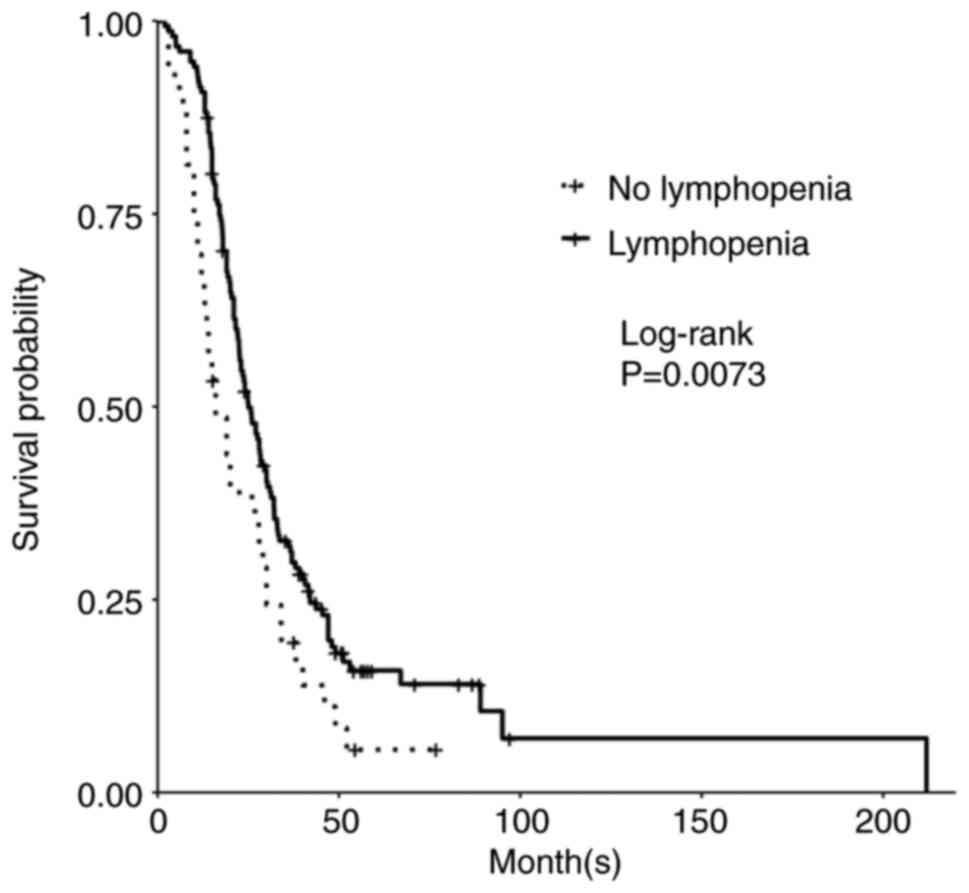
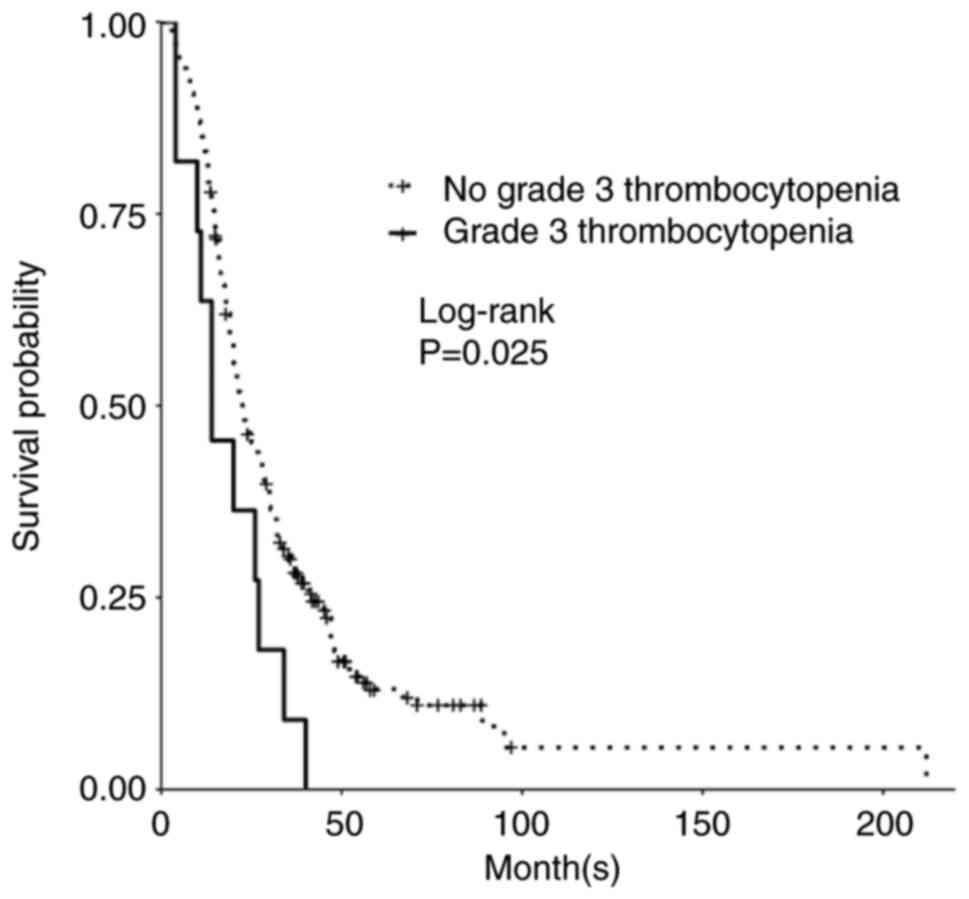
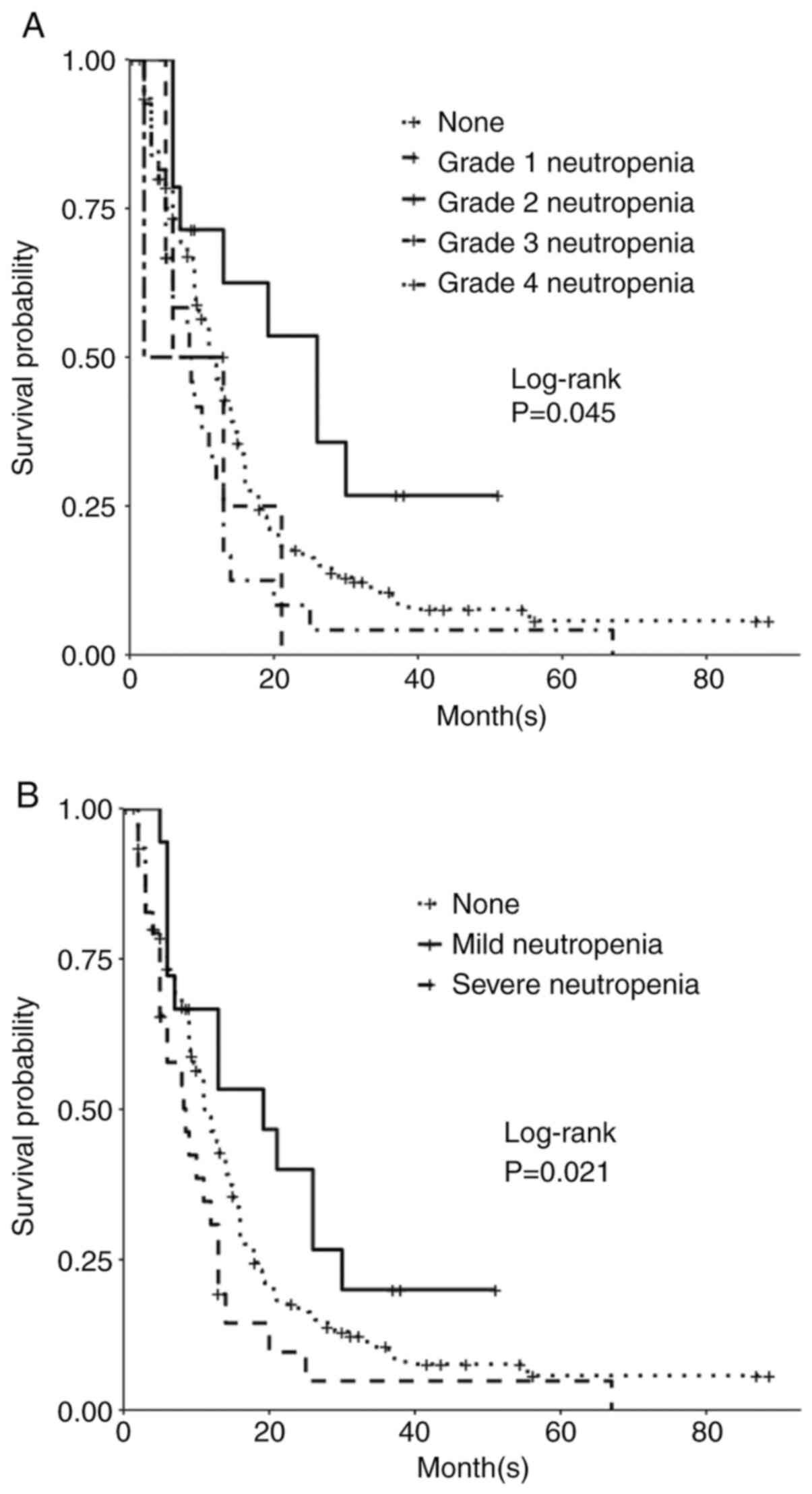
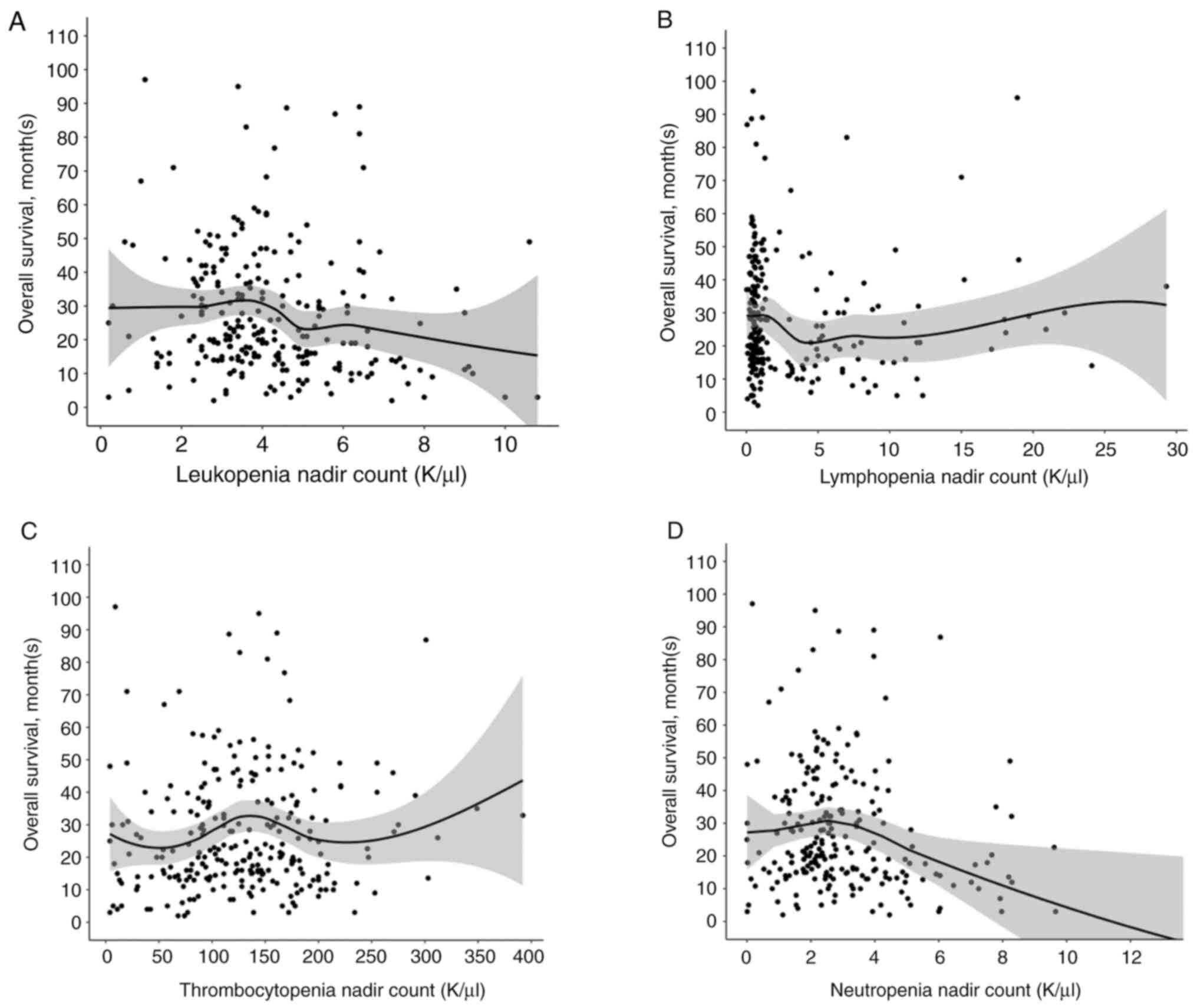
Grade 4 gliomas, including glioblastoma, isocitrate dehydrogenase (IDH)‑wild‑type [GBM (IDH wt)], and astrocytoma, IDH‑mutant, grade 4, are among the most aggressive primary brain tumors. The standard of care for GBM (IDH wt) and astrocytoma, IDH‑mutant, grade 4 is maximum safe resection and radiation plus oral temozolomide (TMZ) followed by six cycles of TMZ. The study objective was to identify peripheral biomarkers that predict favorable outcomes and stratify patients likely to respond to treatment. Adults with biopsy‑confirmed glioblastoma (based upon the 2016 World Health Organization classification) treated at Beth Israel Deaconess Medical Center (Boston, USA) between January 2018 and November 2021 were identified. Data on laboratory values (white blood cells, absolute neutrophil count, absolute lymphocyte count, red blood cells, hemoglobin and platelet count), molecular markers, including IDH1 R132H and methylguanine‑DNA methyltransferase promoter methylation, and progression‑free survival (PFS) and overall survival (OS) were collected retrospectively. Data were combined with those from two prior studies, resulting in a total of 263 patients. Leukopenia development during TMZ treatment was associated with increased PFS (P=0.008) and OS (P=0.03). Lymphopenia development during TMZ treatment was associated with increased PFS (P=0.05) and OS (P=0.007). Grade 3 thrombocytopenia during TMZ treatment was associated with decreased PFS (P=0.01) and OS (P=0.02). Patients who developed leukopenia alone during treatment had an increased OS compared with those with only lymphopenia development and those with both lymphopenia and leukopenia development (P=0.007). Lower baseline lymphocyte counts (<0.7 K/µl) prior to treatment was associated with improved OS (P=0.007), while increased baseline neutrophil counts (≥10.0 K/µl) prior to initiation of treatment were associated with worse OS (P=0.002). In conclusion, TMZ exposure may result in a leukocyte predominant bone marrow effect vs. a platelet predominant bone marrow effect. Clinically, leukopenia could indicate adequate TMZ dosing, with thrombocytopenia serving as a limiting factor in the ability to continue TMZ. Baseline counts may offer insights into which patients will benefit the most from treatment.