Pulmonary angiosarcoma in an HIV‑positive patient presenting with hemoptysis and multisystem involvement: Report of a rare case
- Authors:
- Published online on: August 19, 2025 https://doi.org/10.3892/mi.2025.261
- Article Number: 62
-
Copyright : © Maule et al. This is an open access article distributed under the terms of Creative Commons Attribution License [CC BY 4.0].
Abstract
Introduction
Angiosarcoma is a rare and highly aggressive cancer originating from the endothelial cells of blood and lymphatic vessels, comprising <1% of sarcoma cases (1-3). This malignancy can affect any part of the body, but pulmonary involvement is particularly rare (4). Although the majority of cases occur spontaneously, risk factors such as radiation, chronic lymphedema, exposure to vinyl chloride and genetic syndromes have been documented in the literature (3). Primary pulmonary angiosarcoma is an aggressive but rare cancer of the pulmonary arteries that is malignant in nature. Secondary pulmonary angiosarcoma where lung metastasis from some other origin, such as the heart or breast occurs more often (4,5). Metastatic pulmonary angiosarcoma often presents with non-specific symptoms, complicating and delaying the diagnosis (4,6). Given the clinical and pathological similarities between primary and metastatic pulmonary angiosarcoma, excluding other tumor sites is often necessary for diagnosing primary pulmonary angiosarcoma (5). Angiosarcoma is the most common malignancy affecting the heart, and the lungs are the most common site of malignancy (7). Early detection plays a critical role in the workup and management of pulmonary angiosarcoma and having a high clinical suspicion augments early detection (4).
In pulmonary angiosarcoma, hemoptysis is a critical and alarming symptom indicative of tumor invasion into the pulmonary vasculature (8-11). Hemoptysis as an initial presentation is rare and is often accompanied by other symptoms related to other metastatic sites or systemic involvement (8-10). The non-specific presentation of primary angiosarcoma is even more complex in patients with other underlying comorbidities. In immunocompromised patients, hemoptysis can occur due to opportunistic infections (~80%), or other malignancies related to immunodeficiency, such as Kaposi's sarcoma (12,13). This overlapping symptomatology between pulmonary angiosarcoma, infections, and other malignancies can further delay diagnosis and appropriate treatment, further complicating the clinical picture. In terms of radiographic evidence, the review compiled by Yogi et al (14) indicated that the halo sign was observed in 58% of patients on a computed tomography (CT) scan of the chest. Among the patients of 28 cases of primary pulmonary angiosarcoma summarized in the study by Yogi et al (14), 50% of patients (14/28) had multiple nodules (14). Solitary lesions appeared in 39.3% of patients (11/28). They also noted that patients with multiple nodules had more aggressive courses and poorer prognosis compared to their single-lesioned counterparts (14).
The present study describes the case a case of metastatic pulmonary angiosarcoma in an human immunodeficiency virus (HIV)-positive patient presenting with hemoptysis and multisystem involvement. This serves to highlight the diagnostic challenges associated with this condition and the importance of considering rare malignancies in differential diagnoses, particularly in patients with complex medical histories.
Case report
A 36-year-old African American male patient with HIV on highly active antiretroviral therapy who initially presented to the HCA Florida North Florida Hospital Emergency Department with 3 weeks of progressive hemoptysis (day 1). He reported recent exposure to mold following flooding and renovations in his apartment. His symptoms began as a mild, intermittent cough with clear sputum, which progressed to hemoptysis, shortness of breath, fatigue, post-tussive chest and back pain, and palpitations. Upon his arrival, he was alert but frail, with conjunctival pallor, borderline tachycardia and coarse breath sounds.
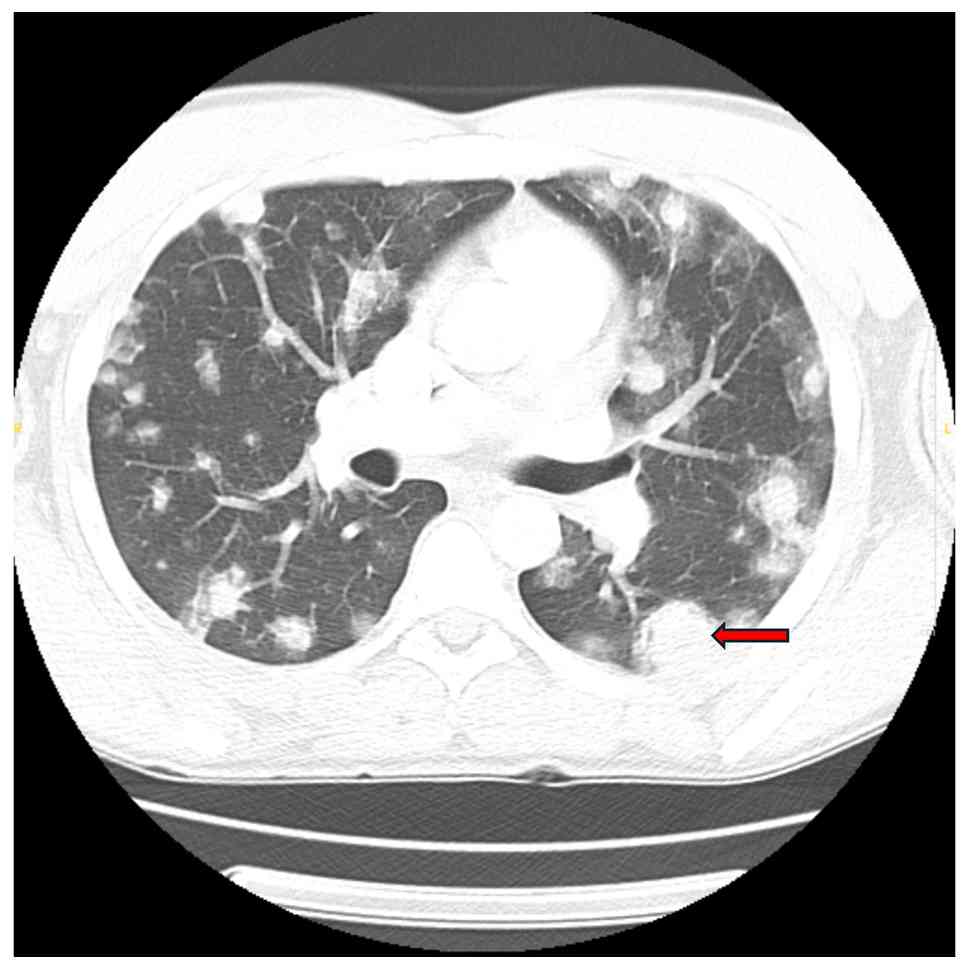
Initial laboratory tests revealed microcytic anemia (Hgb, 8.4 g/dl), thrombocytopenia (platelets, 52 K/µl) and markedly elevated D-dimer levels (19,119 ng/ml). The CD4 count was 447, and the HIV viral load was undetectable. Imaging upon admission revealed innumerable bilateral pulmonary nodules with peripheral ground-glass halos, the largest measuring 29x24 mm in the left lower lobe, along with enlarged hilar lymph nodes (up to 14x15 mm on the left and 14x21 mm on the right), hepatic lesions and lytic bone lesions, all suggestive of metastatic disease (Fig. 1). Given his immunocompromised state, mold exposure history and imaging findings, initial concerns included opportunistic fungal infections, Kaposi's sarcoma and metastatic malignancy.
A robotic-assisted bronchoscopy with transbronchial biopsy and bronchoalveolar lavage (BAL) was performed on day 3. Blood was noted throughout the tracheobronchial tree, although no focal bleeding source was identified. The BAL fluid culture grew Haemophilus influenzae, and he was commenced on ceftriaxone 2 g daily for 5 days (day 6). Fungal, Acid-fast bacillus and tuberculosis workups were negative. Transthoracic echocardiography on day 8 revealed a large (7.4x3.3 cm) mobile mass on the atrial side of the tricuspid valve, initially raising concern for culture-negative endocarditis. In the setting of HIV, this prompted empiric treatment with continuation of ceftriaxone 2 g daily and vancomycin 1 g every 8 h, and serological testing for Bartonella, Q fever and Brucella was ordered. A dental evaluation was also pursued to assess for potential infectious sources. Blood cultures remained negative throughout his hospitalization periods.
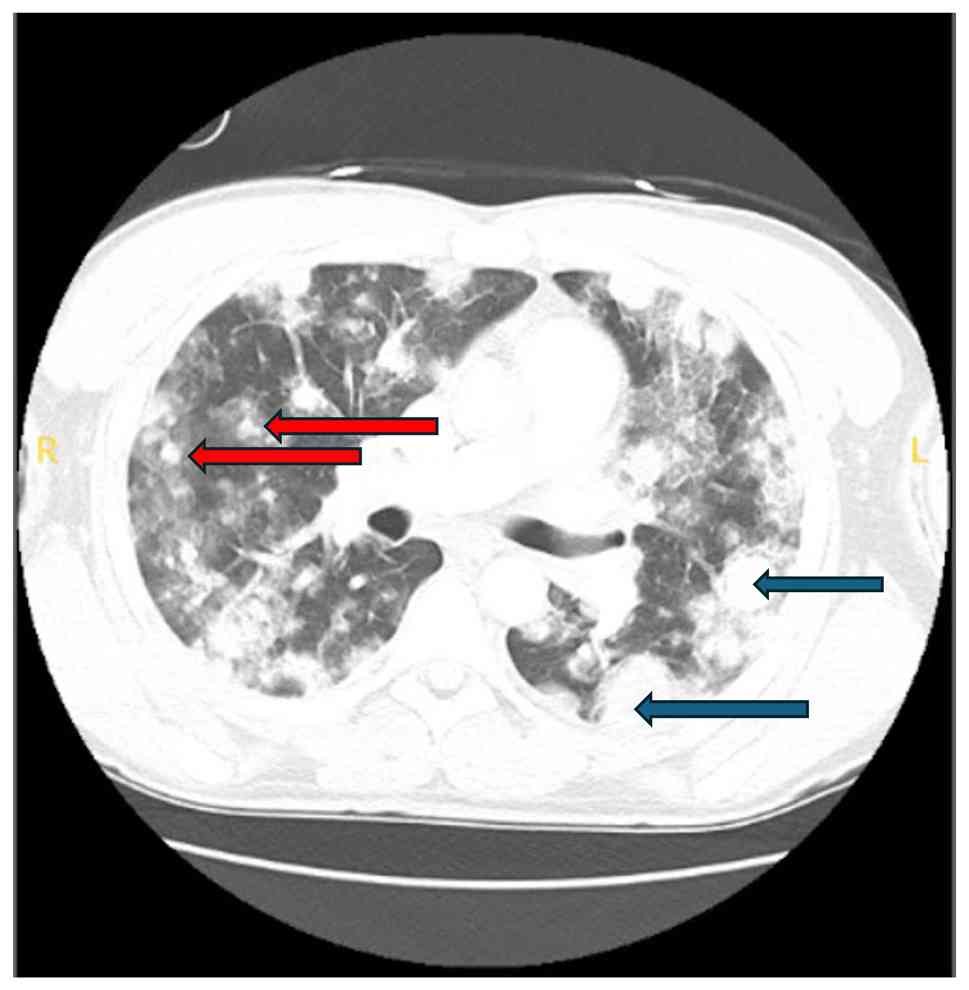
A CT scan on day 14 revealed an interval increase in the size and number of nodules, with the largest left-sided mass now measuring 4.2x3.1 cm, and a right-sided mass along the major fissure measuring 2.9x1.8 cm, along with new right hilar adenopathy measuring up to 2.3x1.6 cm (Fig. 2).
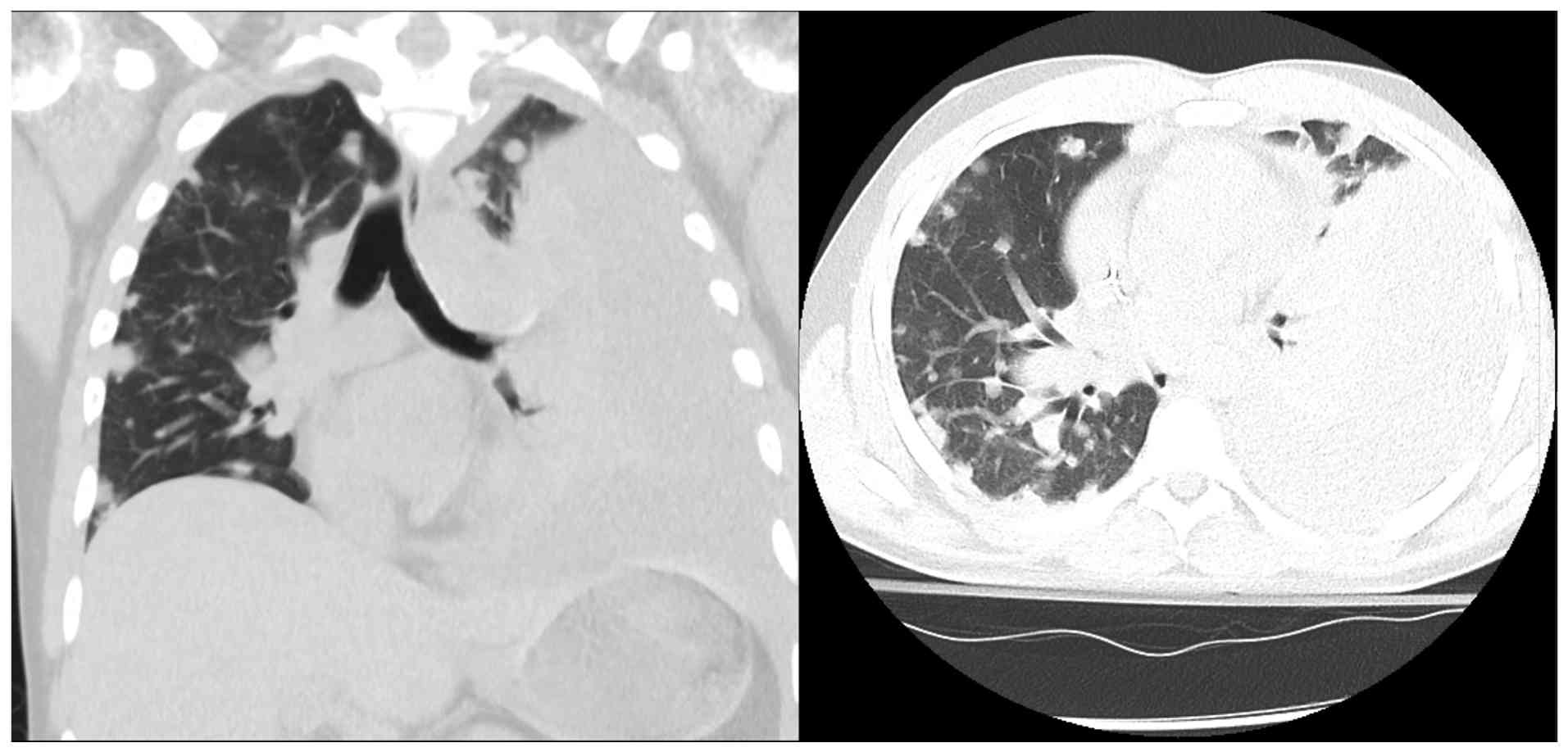
The patient departed from the hospital day 16, against medical advice (AMA), prior to the biopsy results, citing fatigue with the prolonged workup. He returned 5 days later (day 21) with worsening hemoptysis and dyspnea. Laboratory tests revealed a hemoglobin level of 6.6 g/dl and a platelet count of 37 K/µl. He was transfused and stabilized. The pathological analysis of the initial biopsy sample, reviewed at a tertiary center, confirmed stage IV pulmonary angiosarcoma with immunohistochemistry positivity for ERG, CD31, CD34 and CD117, and negativity for STAT6, HHV8 and ALK (Fig. 3). These results excluded more common HIV-associated malignancies, such as Kaposi's sarcoma and ruled out inflammatory myofibroblastic tumor and solitary fibrous tumor.
The Oncology Department initiated weekly paclitaxel therapy, 160 mg once weekly (3 weeks on, 1 week off) on day 24, a first-line agent with demonstrated efficacy in angiosarcoma. He initially tolerated the first dose, but again departed from the hospital, AMA, on day 29 before receiving subsequent treatment. He returned several days later (day 39) with severe dyspnea and was found to have a large left pleural effusion. Chest tube placement on day 45 drained 1.35 liters of bloody fluid, consistent with hemothorax, a common complication of angiosarcoma due to tumor-induced vascular fragility. Cytology from both the pleural and later pericardial effusions observed on the CT scan was negative for malignancy, with no malignant cells identified (Fig. 4).
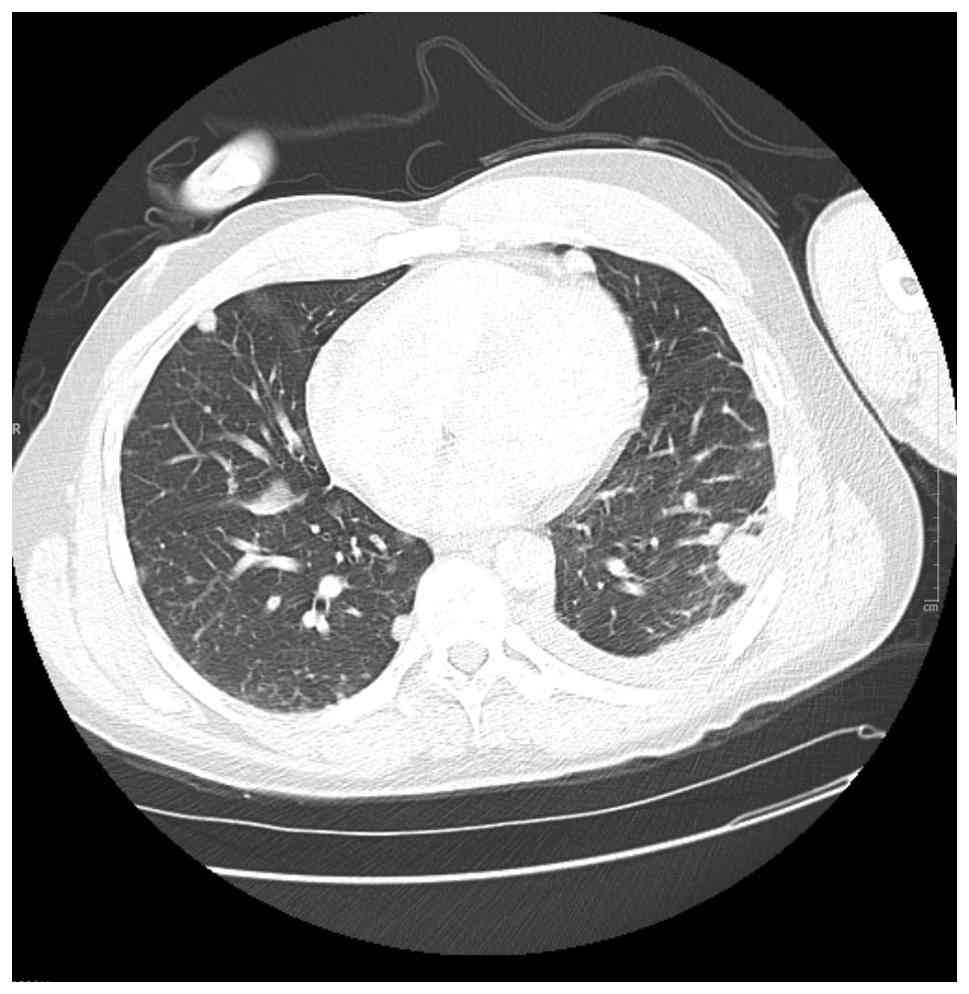
During his second attempt at paclitaxel treatment on day 41, he experienced a grade 2 infusion reaction characterized by dyspnea and hypoxia, necessitating the discontinuation of treatment. Given the reaction severity and limited access to close outpatient monitoring, he was transitioned to doxorubicin 60 mg/m2. He received his first dose of doxorubicin on day 50 and tolerated it well. He continued with a second cycle on day 64 without major complications. Repeat CT imaging following two cycles of doxorubicin (day 70) demonstrated a marked improvement in pulmonary and hepatic metastases (Fig. 5), supporting a degree of chemotherapeutic responsiveness.
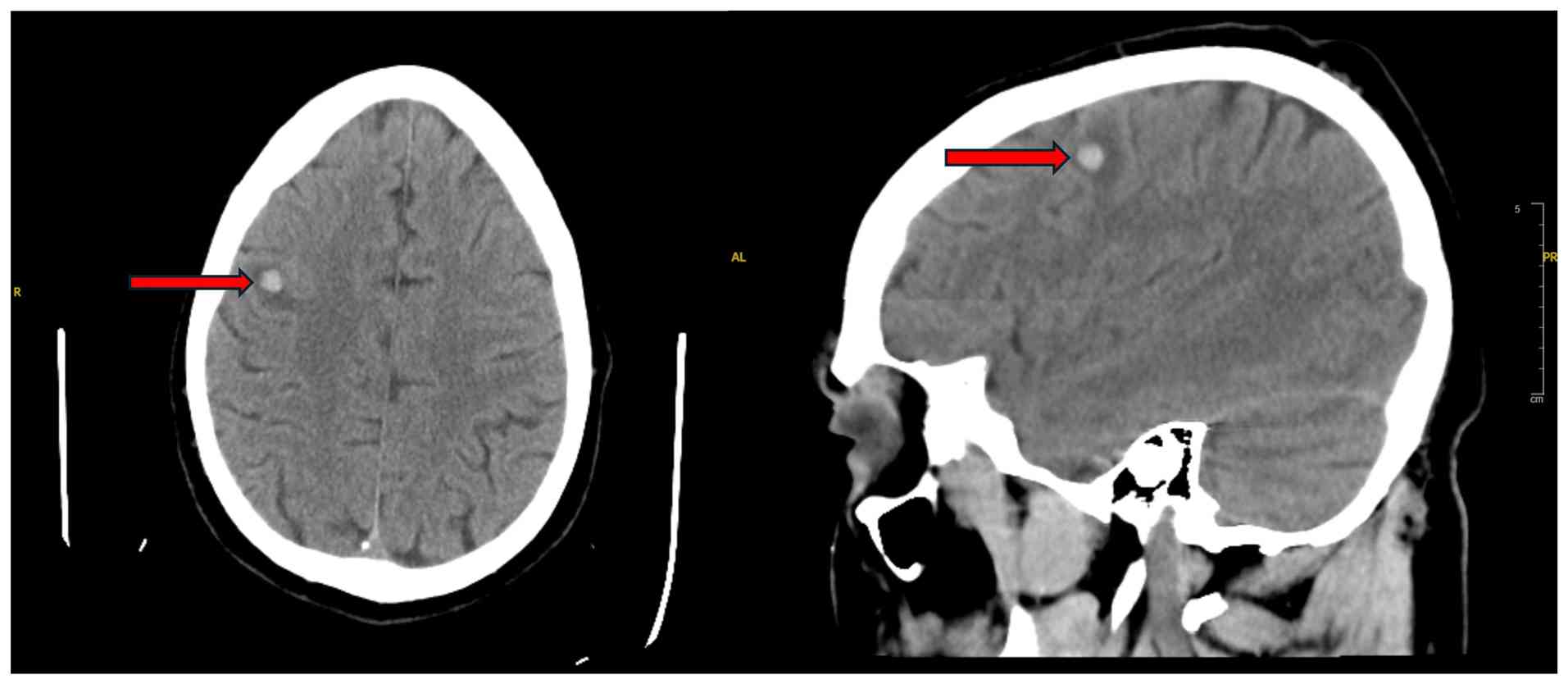
Despite early signs of treatment benefits, his course was complicated by a progressive pericardial effusion. On day 106 of hospitalization, shortly after using the bathroom, he experienced acute abdominal pain, diaphoresis, hypotension and oxygen desaturation. A rapid response was called, and a bedside echocardiogram revealed cardiac tamponade with right ventricular collapse. He was urgently transferred to the cardiovascular intensive care unit, where emergent pericardiocentesis was performed, draining 800 ml hemorrhagic fluid. He was stabilized with supportive care and was commenced on colchicine 0.6 mg twice daily, and ibuprofen 800 mg every 8 h for pericardial inflammation. Both medications were continued for 14 days while admitted, and he was discharged with the same regimen. A CT head scan performed during this admission revealed a right frontal hemorrhagic metastasis with surrounding edema (Fig. 6).
Although discharged home, he returned repeatedly over the following weeks with hemoptysis, shortness of breath and refractory metastatic pain. He was readmitted for fluid reaccumulation (day 85), requiring a second pericardiocentesis (day 90). Over time, his performance status declined. He initially enrolled in hospice care, but later revoked his do-not-resuscitate order (DNR) status and pursued further treatment following perceived clinical improvement. Despite this, the disease continued to progress, and he again decompensated. He and his family engaged in comprehensive goals-of-care discussions with the palliative care team. Expressing concern about suffering and being a burden to his loved ones, he opted for comfort-centered care and formally signed a DNR (day 109). He was discharged to hospice care on hospital day 135, where he later passed away peacefully. The clinical timeline is summarized in Table I.
Discussion
Pulmonary angiosarcoma is a malignant, yet rare disease that has non-specific lung symptoms that lead to delay in diagnosis and attributability to more usual conditions (15-17). The case in the present study depicts the diagnostic and therapeutic challenges of the disease. The non-specificity of symptoms of hemoptysis, cough and dyspnea renders pulmonary angiosarcoma a clinical imitator that frequently imitates infectious pneumonia, tuberculosis and other malignancies (18,19). In immunocompromised patients, such as in patients with HIV, the initial suspicion tends to lean toward infectious disease rather than uncommon primary pulmonary malignancy.
Radiologically, pulmonary angiosarcoma tends to exhibit numerous pulmonary nodules in 50% of cases that possess characteristics of nodules, ground-glass opacities, or halo signs, a presentation that is typically associated with cases of fungal or hemorrhagic metastases (20,21). Tricuspid valve mass in this context was yet another clue for the differential diagnoses of cardiac angiosarcoma or metastatic disease. Primary pulmonary angiosarcoma could also be a possibility, as it has been shown to manifest as either single or multiple pulmonary nodules (22).
Histopathologic confirmation remained the gold standard, with strong positivity for CD31, ERG and CD34 supporting the diagnosis of angiosarcoma. These vascular markers are commonly expressed in endothelial malignancies and help distinguish angiosarcoma from other sarcomas and primary lung tumors. Negative staining for STAT6, ALK and HHV8 effectively excluded solitary fibrous tumors, inflammatory myofibroblastic tumors and Kaposi's sarcoma, respectively (23-27). CD117 (c-KIT), while also positive in this case, is less specific and can be expressed in a range of neoplasms, including gastrointestinal stromal tumors, seminomas and some melanomas. In angiosarcoma, CD117 expression has been reported but is considered supportive rather than definitive. Therefore, its diagnostic utility lies in being interpreted alongside more endothelial-specific markers like CD31 and ERG (28,29).
Pulmonary angiosarcoma is associated with a poor prognosis, particularly in patients with more than one pulmonary mass, who have much poorer outcomes than patients with a solitary mass (15). Accelerated disease course and poor responsiveness to chemotherapy account for poor survival in these patients (22). Paclitaxel remains a first-line chemotherapeutic agent with demonstrated efficacy in angiosarcoma; however, the patient described herein was unable to tolerate it, necessitating a switch to doxorubicin (22). While no standardized treatment regimen has been established, therapy selection is often guided more by patient tolerability than by effectiveness (22).
A unique characteristic of this case was the presence of a tricuspid valve mass that manifested either by direct invasion of the heart or by seeding from the primary tumor. Although primary cardiac angiosarcomas have their characteristic localization in the right atrium, secondary cardiac disease is generally more appreciated in the context of disseminated disease (30). This presentation provides an element of complexity to treatment as cardiac metastases have greater thrombotic potential and hemodynamic impairment (31). Hemorrhagic features of the effusions in angiosarcoma are in accordance with tumor-caused vascular fragility that leads to intrapulmonary and pericardial hemorrhage (32).
In conclusion, pulmonary angiosarcoma is an exceedingly rare and aggressive malignancy that presents significant diagnostic and therapeutic challenges, often mimicking infectious or other malignant processes. The present case report illustrates the diagnostic complexity of pulmonary angiosarcoma in the setting of HIV, where extensive pulmonary and cardiac involvement contributes to a poor prognosis, and highlights the importance of maintaining a high index of suspicion for angiosarcoma in immunocompromised patients presenting with unexplained pulmonary nodules, hemoptysis and cardiac abnormalities.
Acknowledgements
The authors would like to express their gratitude sincere appreciation to the HCA North Florida Hospital for their support and assistance towards the completion of this project with mentorship and access patient charts. The resources and support provided by the hospital was invaluable in facilitating this study.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be requested from the corresponding author.
Authors' contributions
All authors (GM, SP, HH, MK, AR and LJ) were responsible for the clinical workup, literature review and drafting of the manuscript. All authors (GM, SP, HH, MK, AR and LJ) contributed to the case interpretation and manuscript revision. All authors have read and approved the final version of the manuscript to be published. All authors (GM, SP, HH, MK, AR and LJ) confirm the authenticity of all the raw data.
Ethics approval and consent to participate
The present case report was conducted in accordance with institutional guidelines. Consent was obtained from the patient for described herein for the presentation of his case.
Patient consent for publication
Written informed consent was obtained from the patient for the publication of this case report and any accompanying images.
Competing interests
The authors declare that they have no competing interests.
Use of artificial intelligence tools
During the preparation of this work, AI tools were used to improve the readability and language of the manuscript or to generate images, and subsequently, the authors revised and edited the content produced by the AI tools as necessary, taking full responsibility for the ultimate content of the present manuscript.
References
|
Young RJ, Brown NJ, Reed MW, Hughes D and Woll PJ: Angiosarcoma. Lancet Oncol. 11:983–991. 2010.PubMed/NCBI View Article : Google Scholar | |
|
Zhang J, Gong H, Wang Y, Zhang G and Hou P: Angiosarcoma of the visceral organs: A morphological, immunohistochemical, and C-MYC status analysis. Pathol Res Pract. 238(154118)2022.PubMed/NCBI View Article : Google Scholar | |
|
Florou V and Wilky BA: Current management of angiosarcoma: Recent advances and lessons from the past. Curr Treat Options Oncol. 22(61)2021.PubMed/NCBI View Article : Google Scholar | |
|
Khalid K, Khan A, Lomiguen CM and Chin J: Clinical detection of primary pulmonary angiosarcoma. Cureus. 13(e17059)2021.PubMed/NCBI View Article : Google Scholar | |
|
Obeso Carillo GA, García Fontán EM, Cañizares Carretero MÁ and Pérez Pedrosa A: Primary pulmonary angiosarcoma, an exceptional neoplasm with a poor prognosis: Reports of two cases and review of the literature. Gen Thorac Cardiovasc Surg. 61:643–647. 2013.PubMed/NCBI View Article : Google Scholar | |
|
Darawsha F, Kramer R, Raanani E and Saute M: Angiosarcoma of the main pulmonary artery-hand-made conduit reconstruction. Interact Cardiovasc Thorac Surg. 35(35)2022.PubMed/NCBI View Article : Google Scholar | |
|
Jain G, Mukhopadhyay S, Kurien S, Yusuf J, Tyagi S and Jain R: Ruptured cardiac angiosarcoma with pulmonary metastases: A rare disease with a common (mis)diagnosis! Indian Heart. J. 64:603–606. 2012.PubMed/NCBI View Article : Google Scholar | |
|
Shimabukuro I, Yatera K, Noguchi S, Kawanami Y, Iwanami T, Nishida C, Yamasaki K, Kawanami T, Ishimoto H, So T, et al: Primary pulmonary angiosarcoma presenting with hemoptysis and Ground-glass opacity: A case report and literature review. Tohoku J Exp Med. 237:273–278. 2015.PubMed/NCBI View Article : Google Scholar | |
|
Piechuta A, Przybyłowski T, Szołkowska M and Krenke R: Hemoptysis in a patient with multifocal primary pulmonary angiosarcoma. Pneumonol Alergol Pol. 84:283–289. 2016.PubMed/NCBI View Article : Google Scholar | |
|
Pan Z, An Z, Li Y and Zhou J: Diffuse alveolar hemorrhage due to metastatic angiosarcoma of the lung: A case report. Oncol Lett. 10:3853–3855. 2015.PubMed/NCBI View Article : Google Scholar | |
|
Chen J, Li C, Tian Y and He Y: 18F-FDG PET/CT findings in a rare case of primary pulmonary angiosarcoma. Japanese J Clin Oncol. 52:286–287. 2022.PubMed/NCBI View Article : Google Scholar | |
|
Nelson JE and Forman M: Hemoptysis in HIV-infected patients. Chest. 110:737–743. 1996.PubMed/NCBI View Article : Google Scholar | |
|
Aboulafia DM: The Epidemiologic, pathologic, and clinical features of AIDS-associated pulmonary Kaposi's sarcoma. Chest. 117:1128–1145. 2000.PubMed/NCBI View Article : Google Scholar | |
|
Yogi A, Miyara T, Ogawa K, Iraha S, Matori S, Haranaga S and Murayama S: Pulmonary metastases from angiosarcoma: A spectrum of CT findings. Acta Radiol. 57:41–46. 2016.PubMed/NCBI View Article : Google Scholar | |
|
Sofia J, Moreira N, Ferreira MJ and Antunes M: Angiosarcoma arising from the main pulmonary artery mimicking pulmonary thromboembolism. Arq Bras Cardiol. 109:90–91. 2017.PubMed/NCBI View Article : Google Scholar : (In English, Portuguese). | |
|
Kim JB, Kim SH, Lim SY, Roh SY, Cho G, Song HJ, Park S, Lee J and Kim SJ: Primary angiosarcoma of the pulmonary trunk mimicking pulmonary thromboembolism. Echocardiography. 27:E23–E26. 2010.PubMed/NCBI View Article : Google Scholar | |
|
Hsing JM, Thakkar SG, Borden EC and Budd GT: Intimal pulmonary artery sarcoma presenting as dyspnea. Case report. Int Semin Surg Oncol. 4(14)2007.PubMed/NCBI View Article : Google Scholar | |
|
Scheffel H, Stolzmann P, Plass A, Weber A, Prêtre R, Marincek B and Alkadhi H: Primary intimal pulmonary artery sarcoma: A diagnostic challenge. J Thorac Cardiovasc Surg. 135:949–950. 2008.PubMed/NCBI View Article : Google Scholar | |
|
Lakmal Samarasinghe, Manoharan A, Dinusha Dharmaratna, Nathani Z and Cedeno H: Angiosarcoma of lung: A diagnostic challenge. CHEST J. 164:A4525–6. 2023. | |
|
Saito M, Saraya T, Oda M, Minamishima T, Kongoji K, Isomura A, Fujiwara M, Soejima K and Ishii H: Rapidly progressive respiratory failure with multiple halo signs on computed tomography in a patient with primary cardiac angiosarcoma derived from the right atrium: A case report. BMC Pulm Med. 20(321)2020.PubMed/NCBI View Article : Google Scholar | |
|
Luan T, Hao J, Gu Y, He P, Li Y, Wang L, Deng H, Guan W, Lin X, Xie X, et al: A clinical analysis and literature review of eleven cases with primary pulmonary angiosarcoma. BMC Cancer. 24(1597)2024.PubMed/NCBI View Article : Google Scholar | |
|
Miettinen M, Wang Z, Sarlomo-Rikala M, Abdullaev Z, Pack SD and Fetsch JF: ERG expression in epithelioid sarcoma. Am J Surg Pathol. 37:1580–1585. 2013.PubMed/NCBI View Article : Google Scholar | |
|
Sullivan HC, Edgar MA, Cohen C, Kovach CK, HooKim K and Reid MD: The utility of ERG, CD31 and CD34 in the cytological diagnosis of angiosarcoma: An analysis of 25 cases. J Clin Pathol. 68:44–50. 2014.PubMed/NCBI View Article : Google Scholar | |
|
Yoshida A, Tsuta K, Ohno M, Yoshida M, Narita Y, Kawai A, Asamura H and Kushima R: STAT6 Immunohistochemistry is helpful in the diagnosis of solitary fibrous tumors. Am J Surg Pathol. 38:552–559. 2014.PubMed/NCBI View Article : Google Scholar | |
|
Lobato F: Mesenchymal tumors of the digestive system. https://atlasgeneticsoncology.org/solid-tumor/209310. Accessed March 22, 2025. | |
|
Miettinen M: Immunohistochemistry of soft tissue tumours-review with emphasis on 10 markers. Histopathology. 64:101–118. 2014.PubMed/NCBI View Article : Google Scholar | |
|
Ren Y, Zhu M, Liu Y, Diao X and Zhang Y: Primary pulmonary angiosarcoma: Three case reports and literature review. Thoracic Cancer. 7:607–613. 2016.PubMed/NCBI View Article : Google Scholar | |
|
Miettinen M and Lasota J: KIT (CD117): A review on expression in normal and neoplastic tissues, and mutations and their clinicopathologic correlation. Appl Immunohistochem Mol Morphol. 13:205–220. 2005.PubMed/NCBI View Article : Google Scholar | |
|
Darre T, Djiwa T, N'Timon B, Simgban P, Tchaou M and Napo-Koura G: Breast primary angiosarcoma: A clinicopathologic and imaging study of a series cases. Breast Cancer (Auckl). 16(11782234221086726)2022.PubMed/NCBI View Article : Google Scholar | |
|
Yamashita H, Higashida T, Huchioka A, Asakawa Y, Nambu A, Ohyatsu S, Kohyama T, Takahashi M, Hayashi T and Tago M: Cardiac angiosarcoma with metastatic to lung, brain, and bone. Radiol Case Rep. 19:473–478. 2023.PubMed/NCBI View Article : Google Scholar | |
|
Le AN, Nguyen AV, Nguyen TN, Kirkpatrick JN, Nguyen HT and Thu T: Cardiac metastasis mimicking STEMI-impact of point-of-care ultrasound on clinical decision-making: A case report. Front Cardiovasc Med. 10(1098154)2013.PubMed/NCBI View Article : Google Scholar | |
|
Chen FF, Jiang SF, Dong C, Che Y, Du LY, Li ZY, Yang ZQ, Zhao YC and Liu Y: Case Report: Thromboembolism and hemorrhagic pericardial Effusion-The Janus face of primary pericardial angiosarcoma. Front Cardiovasc Med. 7(618146)2021.PubMed/NCBI View Article : Google Scholar |