S-glutathionylation modification of proteins and the association with cellular death (Review)
- Authors:
- Xiongxing Sun
- Le Xie
- Shiliang Wang
- Shanshan Zeng
- Lingying Wu
- Xukun Tang
- Jiajian Zhu
- Shigao Lin
- Tenghui Hu
- Lin Jia
- Xia Li
- Songqing Zhang
- Jun Deng
- Dahua Wu
-
Affiliations: Graduate School of Hunan University of Chinese Medicine, Changsha, Hunan 410208, P.R. China, Hunan Provincial Hospital of Integrated Traditional Chinese and Western Medicine (The Affiliated Hospital of Hunan Academy of Traditional Chinese Medicine), Changsha, Hunan 410060, P.R. China, Shimen County Traditional Chinese Medicine Hospital, Changde, Hunan 415300, P.R. China, Xiangxi Tujia and Miao Autonomous Prefecture Ethnic Traditional Chinese Medicine Hospital, Jishou, Hunan 416000, P.R. China - Published online on: August 22, 2025 https://doi.org/10.3892/mi.2025.263
- Article Number: 64
-
Copyright : © Sun et al. This is an open access article distributed under the terms of Creative Commons Attribution License [CC BY 4.0].
This article is mentioned in:
Abstract
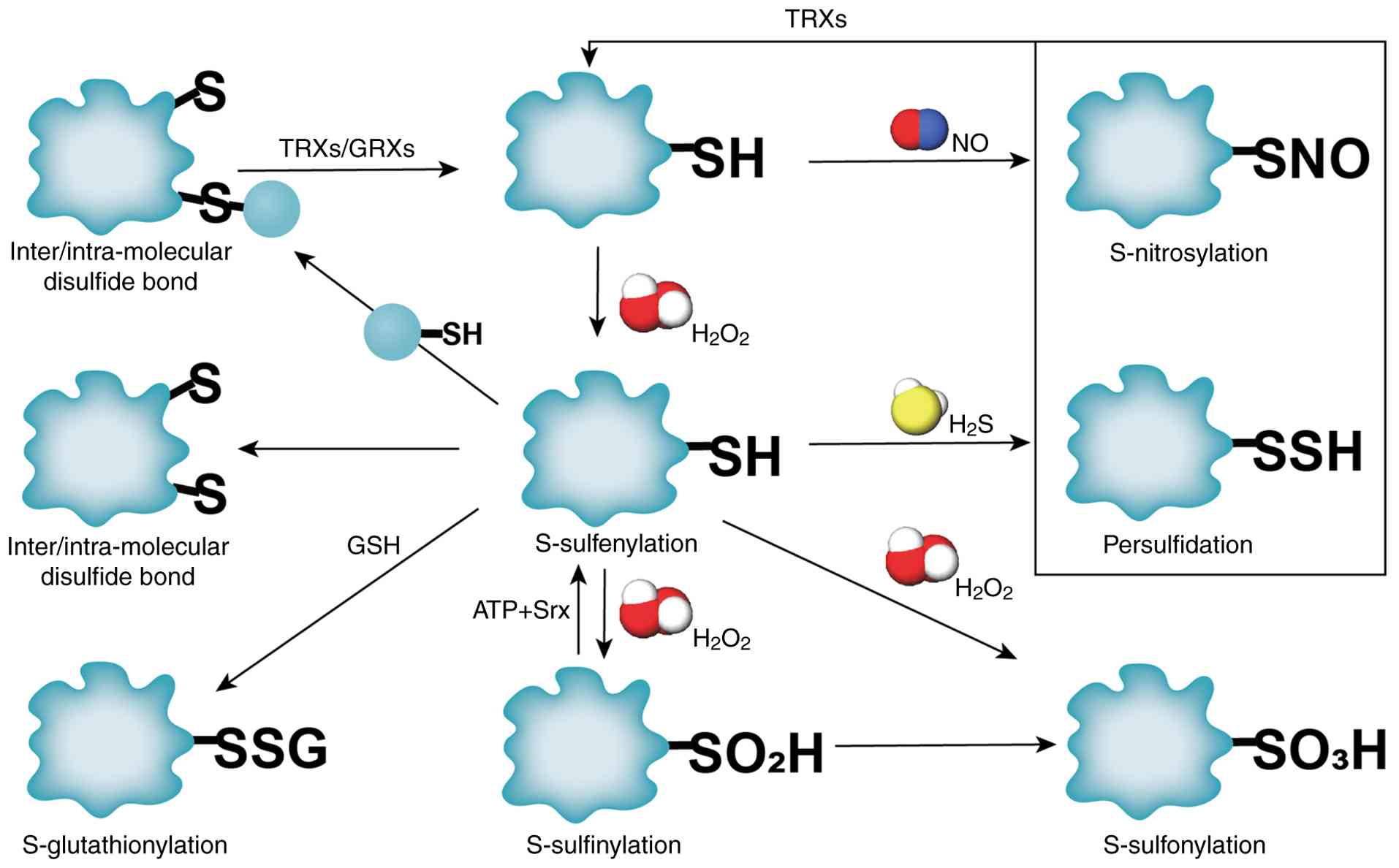 |
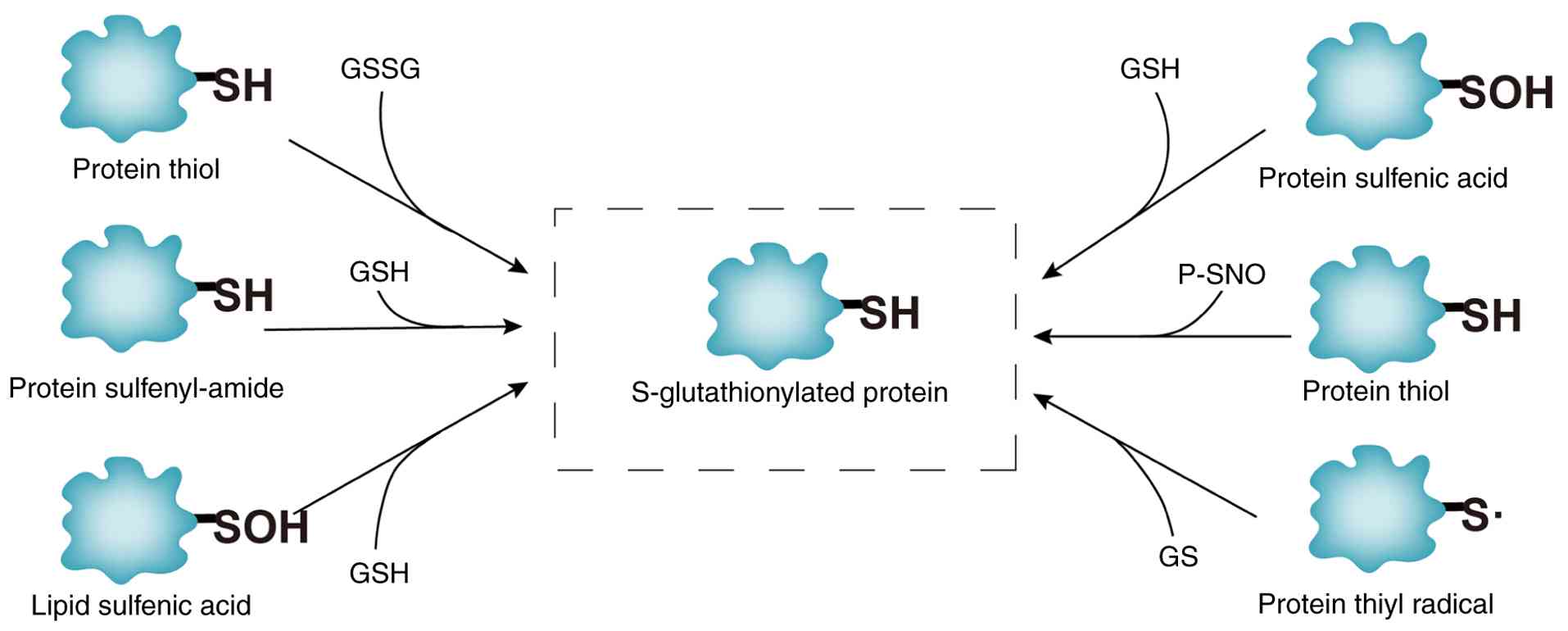 |
|
Mohammadi SA, Najafi H, Zolgharnian S, Sharifian S and Asasian-Kolur N: Biological oxidation methods for the removal of organic and inorganic contaminants from wastewater: A comprehensive review. Sci Total Environ. 843(157026)2022.PubMed/NCBI View Article : Google Scholar | |
|
Jakubczyk K, Dec K, Kałduńska J, Kawczuga D, Kochman J and Janda K: Reactive oxygen species-sources, functions, oxidative damage. Pol Merkur Lekarski. 48:124–127. 2020.PubMed/NCBI | |
|
Jomova K, Raptova R, Alomar SY, Alwasel SH, Nepovimova E, Kuca K and Valko M: Reactive oxygen species, toxicity, oxidative stress, and antioxidants: Chronic diseases and aging. Arch Toxicol. 97:2499–2574. 2023.PubMed/NCBI View Article : Google Scholar | |
|
Noguchi N, Saito Y and Niki E: Actions of thiols, persulfides, and polysulfides as free radical scavenging antioxidants. Antioxid Redox Signal. 39:728–743. 2023.PubMed/NCBI View Article : Google Scholar | |
|
Tan M, Yin Y, Ma X, Zhang J, Pan W, Tan M, Zhao Y, Yang T, Jiang T and Li H: Glutathione system enhancement for cardiac protection: Pharmacological options against oxidative stress and ferroptosis. Cell Death Dis. 14(131)2023.PubMed/NCBI View Article : Google Scholar | |
|
Xiao W and Loscalzo J: Metabolic responses to reductive stress. Antioxid Redox Signal. 32:1330–1347. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Kalinina EV and Gavriliuk LA: Glutathione synthesis in cancer cells. Biochemistry (Mosc). 85:895–907. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Wang F, Yuan Q, Chen F, Pang J, Pan C, Xu F and Chen Y: Fundamental mechanisms of the cell death caused by nitrosative stress. Front Cell Dev Biol. 9(742483)2021.PubMed/NCBI View Article : Google Scholar | |
|
Diaz-Vivancos P, de Simone A, Kiddle G and Foyer CH: Glutathione-linking cell proliferation to oxidative stress. Free Radic Biol Med. 89:1154–1164. 2015.PubMed/NCBI View Article : Google Scholar | |
|
Giustarini D, Milzani A, Dalle-Donne I and Rossi R: How to increase cellular glutathione. Antioxidants (Basel). 12(1094)2023.PubMed/NCBI View Article : Google Scholar | |
|
Corpas FJ, González-Gordo S, Rodríguez-Ruiz M, Muñoz-Vargas MA and Palma JM: Thiol-based oxidative posttranslational modifications (OxiPTMs) of plant proteins. Plant Cell Physiol. 63:889–900. 2022.PubMed/NCBI View Article : Google Scholar | |
|
Hofmann F: The cGMP system: Components and function. Biol Chem. 401:447–469. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Hasan MM, Khatun MS and Kurata H: A comprehensive review of in silico analysis for protein S-sulfenylation sites. Protein Pept Lett. 25:815–821. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Corti A, Franzini M, Scataglini I and Pompella A: Mechanisms and targets of the modulatory action of S-nitrosoglutathione (GSNO) on inflammatory cytokines expression. Arch Biochem Biophys. 562:80–91. 2014.PubMed/NCBI View Article : Google Scholar | |
|
Fu L, Liu K, Ferreira RB, Carroll KS and Yang J: Proteome-wide analysis of cysteine S-sulfenylation using a benzothiazine-based probe. Curr Protoc Protein Sci. 95(e76)2019.PubMed/NCBI View Article : Google Scholar | |
|
Li C, Chen X, Zhang S, Liang C, Ma X, Zhang R and Yan H: Glutaredoxin 1 protects lens epithelial cells from epithelial-mesenchymal transition by preventing casein kinase 1α S-glutathionylation during posterior capsular opacification. Redox Biol. 62(102676)2023.PubMed/NCBI View Article : Google Scholar | |
|
Zhang J, Ye ZW, Singh S, Townsend DM and Tew KD: An evolving understanding of the S-glutathionylation cycle in pathways of redox regulation. Free Radic Biol Med. 120:204–216. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Holmgren A: Hydrogen donor system for Escherichia coli ribonucleoside-diphosphate reductase dependent upon glutathione. Proc Natl Acad Sci USA. 73:2275–2279. 1976.PubMed/NCBI View Article : Google Scholar | |
|
Fernandes AP and Holmgren A: Glutaredoxins: Glutathione-dependent redox enzymes with functions far beyond a simple thioredoxin backup system. Antioxid Redox Signal. 6:63–74. 2004.PubMed/NCBI View Article : Google Scholar | |
|
Ogata FT, Branco V, Vale FF and Coppo L: Glutaredoxin: Discovery, redox defense and much more. Redox Biol. 43(101975)2021.PubMed/NCBI View Article : Google Scholar | |
|
Lillig CH, Berndt C and Holmgren A: Glutaredoxin systems. Biochim Biophys Acta. 1780:1304–1317. 2008.PubMed/NCBI View Article : Google Scholar | |
|
Gladyshev VN, Liu A, Novoselov SV, Krysan K, Sun QA, Kryukov VM, Kryukov GV and Lou MF: Identification and characterization of a new mammalian glutaredoxin (thioltransferase), Grx2. J Biol Chem. 276:30374–30380. 2001.PubMed/NCBI View Article : Google Scholar | |
|
Abdalla M, Eltayb WA and Yousif A: Comparison of structures among Saccharomyces cerevisiae Grxs proteins. Genes Environ. 40(17)2018.PubMed/NCBI View Article : Google Scholar | |
|
Matsui R, Ferran B, Oh A, Croteau D, Shao D, Han J, Pimentel DR and Bachschmid MM: Redox regulation via glutaredoxin-1 and protein S-glutathionylation. Antioxid Redox Signal. 32:677–700. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Sevilla F, Martí MC, De Brasi-Velasco S and Jiménez A: Redox regulation, thioredoxins, and glutaredoxins in retrograde signalling and gene transcription. J Exp Bot. 74:5955–5969. 2023.PubMed/NCBI View Article : Google Scholar | |
|
Yang Y, Liao Z and Xiao Q: Metformin ameliorates skeletal muscle atrophy in Grx1 KO mice by regulating intramuscular lipid accumulation and glucose utilization. Biochem Biophys Res Commun. 533:1226–1232. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Li Y, Liang K, Yuan L, Gao J, Wei L and Zhao L: The role of thioredoxin and glutathione systems in arsenic-induced liver injury in rats under glutathione depletion. Int J Environ Health Res. 34:547–563. 2024.PubMed/NCBI View Article : Google Scholar | |
|
Chen X, Chen Y, Li C, Li J, Zhang S, Liang C, Deng Q, Guo Z, Guo C and Yan H: Glutaredoxin 2 protects lens epithelial cells from epithelial-mesenchymal transition by suppressing mitochondrial oxidative stress-related upregulation of integrin-linked kinase. Exp Eye Res. 234(109609)2023.PubMed/NCBI View Article : Google Scholar | |
|
Ai Y, Meng Y, Yan B, Zhou Q and Wang X: The biochemical pathways of apoptotic, necroptotic, pyroptotic, and ferroptotic cell death. Mol Cell. 84:170–179. 2024.PubMed/NCBI View Article : Google Scholar | |
|
Vignane T and Filipovic MR: Emerging chemical biology of protein persulfidation. Antioxid Redox Signal. 39:19–39. 2023.PubMed/NCBI View Article : Google Scholar | |
|
Benhar M: Oxidants, antioxidants and thiol redox switches in the control of regulated cell death pathways. Antioxidants (Basel). 9(309)2020.PubMed/NCBI View Article : Google Scholar | |
|
Liu T, Sun L, Zhang Y, Wang Y and Zheng J: Imbalanced GSH/ROS and sequential cell death. J Biochem Mol Toxicol. 36(e22942)2022.PubMed/NCBI View Article : Google Scholar | |
|
Liaghati A, Pileggi CA, Parmar G, Patten DA, Hadzimustafic N, Cuillerier A, Menzies KJ, Burelle Y and Harper ME: Grx2 regulates skeletal muscle mitochondrial structure and autophagy. Front Physiol. 12(604210)2021.PubMed/NCBI View Article : Google Scholar | |
|
D'Arcy MS: Cell death: A review of the major forms of apoptosis, necrosis and autophagy. Cell Biol Int. 43:582–592. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Nie C, Tian C, Zhao L, Petit PX, Mehrpour M and Chen Q: Cysteine 62 of Bax is critical for its conformational activation and its proapoptotic activity in response to H2O2-induced apoptosis. J Biol Chem. 283:15359–15369. 2008.PubMed/NCBI View Article : Google Scholar | |
|
Adluri RS, Thirunavukkarasu M, Zhan L, Dunna NR, Akita Y, Selvaraju V, Otani H, Sanchez JA, Ho YS and Maulik N: Glutaredoxin-1 overexpression enhances neovascularization and diminishes ventricular remodeling in chronic myocardial infarction. PLoS One. 7(e34790)2012.PubMed/NCBI View Article : Google Scholar | |
|
Corteselli E, Aboushousha R and Janssen-Heininger Y: S-glutathionylation-controlled apoptosis of lung epithelial cells; potential implications for lung fibrosis. Antioxidants (Basel). 11(1789)2022.PubMed/NCBI View Article : Google Scholar | |
|
Pan S and Berk BC: Glutathiolation regulates tumor necrosis factor-alpha-induced caspase-3 cleavage and apoptosis: Key role for glutaredoxin in the death pathway. Circ Res. 100:213–219. 2007.PubMed/NCBI View Article : Google Scholar | |
|
Sun X, Ye C, Deng Q, Chen J and Guo C: Contribution of glutaredoxin-1 to Fas s-glutathionylation and inflammation in ethanol-induced liver injury. Life Sci. 264(118678)2021.PubMed/NCBI View Article : Google Scholar | |
|
Zhang J, Guo J, Yang N, Huang Y, Hu T and Rao C: Endoplasmic reticulum stress-mediated cell death in liver injury. Cell Death Dis. 13(1051)2022.PubMed/NCBI View Article : Google Scholar | |
|
Ye ZW, Zhang J, Aslam M, Blumental-Perry A, Tew KD and Townsend DM: Protein disulfide isomerase family mediated redox regulation in cancer. Adv Cancer Res. 160:83–106. 2023.PubMed/NCBI View Article : Google Scholar | |
|
He M, Hu J, Fang T, Tang W, Lv B, Yang B and Xia J: Protein convertase subtilisin/Kexin type 9 inhibits hepatocellular carcinoma growth by interacting with GSTP1 and suppressing the JNK signaling pathway. Cancer Biol Med. 19:90–103. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Feng Y, Chen Y, Wu X, Chen J, Zhou Q, Liu B, Zhang L and Yi C: Interplay of energy metabolism and autophagy. Autophagy. 20:4–14. 2024.PubMed/NCBI View Article : Google Scholar | |
|
Jeong SJ and Oh GT: Unbalanced redox with autophagy in cardiovascular disease. J Lipid Atheroscler. 12:132–151. 2023.PubMed/NCBI View Article : Google Scholar | |
|
Li N, Wang J, Zang X, Wang Z, Zhang T, Zhao B, Miao J and Lin Z: H2S probe CPC inhibits autophagy and promotes apoptosis by inhibiting glutathionylation of Keap1 at Cys434. Apoptosis. 26:111–131. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Mancilla H, Maldonado R, Cereceda K, Villarroel-Espíndola F, Montes de Oca M, Angulo C, Castro MA, Slebe JC, Vera JC, Lavandero S and Concha II: Glutathione depletion induces spermatogonial cell autophagy. J Cell Biochem. 116:2283–2292. 2015.PubMed/NCBI View Article : Google Scholar | |
|
Cianfruglia L, Perrelli A, Fornelli C, Magini A, Gorbi S, Salzano AM, Antognelli C, Retta F, Benedetti V, Cassoni P, et al: KRIT1 loss-of-function associated with cerebral cavernous malformation disease leads to enhanced S-glutathionylation of distinct structural and regulatory proteins. Antioxidants (Basel). 8(27)2019.PubMed/NCBI View Article : Google Scholar | |
|
Armeni T, Ercolani L, Urbanelli L, Magini A, Magherini F, Pugnaloni A, Piva F, Modesti A, Emiliani C and Principato G: Cellular redox imbalance and changes of protein S-glutathionylation patterns are associated with senescence induced by oncogenic H-ras. PLoS One. 7(e52151)2012.PubMed/NCBI View Article : Google Scholar | |
|
Mallén-Ponce MJ and Pérez-Pérez ME: Redox-mediated activation of ATG3 promotes ATG8 lipidation and autophagy progression in Chlamydomonas reinhardtii. Plant Physiol. 194:359–375. 2023.PubMed/NCBI View Article : Google Scholar | |
|
Foerster EG, Mukherjee T, Cabral-Fernandes L, Rocha JDB, Girardin SE and Philpott DJ: How autophagy controls the intestinal epithelial barrier. Autophagy. 18:86–103. 2022.PubMed/NCBI View Article : Google Scholar | |
|
Lee SR, Yang KS, Kwon J, Lee C, Jeong W and Rhee SG: Reversible inactivation of the tumor suppressor PTEN by H2O2. J Biol Chem. 277:20336–20342. 2002.PubMed/NCBI View Article : Google Scholar | |
|
Zhang J, Tripathi DN, Jing J, Alexander A, Kim J, Powell RT, Dere R, Tait-Mulder J, Lee JH, Paull TT, et al: ATM functions at the peroxisome to induce pexophagy in response to ROS. Nat Cell Biol. 17:1259–1269. 2015.PubMed/NCBI View Article : Google Scholar | |
|
Wang H, Wang N, Xu D, Ma Q, Chen Y, Xu S, Xia Q, Zhang Y, Prehn JHM, Wang G and Ying Z: Oxidation of multiple MiT/TFE transcription factors links oxidative stress to transcriptional control of autophagy and lysosome biogenesis. Autophagy. 16:1683–1696. 2020.PubMed/NCBI View Article : Google Scholar | |
|
López-Grueso MJ, Lagal DJ, García-Jiménez ÁF, Tarradas RM, Carmona-Hidalgo B, Peinado J, Requejo-Aguilar R, Bárcena JA and Padilla CA: Knockout of PRDX6 induces mitochondrial dysfunction and cell cycle arrest at G2/M in HepG2 hepatocarcinoma cells. Redox Biol. 37(101737)2020.PubMed/NCBI View Article : Google Scholar | |
|
Murata H, Ihara Y, Nakamura H, Yodoi J, Sumikawa K and Kondo T: Glutaredoxin exerts an antiapoptotic effect by regulating the redox state of Akt. J Biol Chem. 278:50226–50233. 2003.PubMed/NCBI View Article : Google Scholar | |
|
López-Grueso MJ, González-Ojeda R, Requejo-Aguilar R, McDonagh B, Fuentes-Almagro CA, Muntané J, Bárcena JA and Padilla CA: Thioredoxin and glutaredoxin regulate metabolism through different multiplex thiol switches. Redox Biol. 21(101049)2019.PubMed/NCBI View Article : Google Scholar | |
|
Ye K, Chen Z and Xu Y: The double-edged functions of necroptosis. Cell Death Dis. 14(163)2023.PubMed/NCBI View Article : Google Scholar | |
|
Che L, Yang CL, Chen Y, Wu ZL, Du ZB, Wu JS, Gan CL, Yan SP, Huang J, Guo NJ, et al: Mitochondrial redox-driven mitofusin 2 S-glutathionylation promotes neuronal necroptosis via disrupting ER-mitochondria crosstalk in cadmium-induced neurotoxicity. Chemosphere. 262(127878)2021.PubMed/NCBI View Article : Google Scholar | |
|
Bozonet SM, Magon NJ, Schwartfeger AJ, Konigstorfer A, Heath SG, Vissers MCM, Morris VK, Göbl C, Murphy JM, Salvesen GS and Hampton MB: Oxidation of caspase-8 by hypothiocyanous acid enables TNF-mediated necroptosis. J Biol Chem. 299(104792)2023.PubMed/NCBI View Article : Google Scholar | |
|
Gorelenkova Miller O, Behring JB, Siedlak SL, Jiang S, Matsui R, Bachschmid MM, Zhu X and Mieyal JJ: Upregulation of glutaredoxin-1 activates microglia and promotes neurodegeneration: Implications for Parkinson's disease. Antioxid Redox Signal. 25:967–982. 2016.PubMed/NCBI View Article : Google Scholar | |
|
Thawkar BS and Kaur G: Inhibitors of NF-κB and P2X7/NLRP3/Caspase 1 pathway in microglia: Novel therapeutic opportunities in neuroinflammation induced early-stage Alzheimer's disease. J Neuroimmunol. 326:62–74. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Shao R, Lou X, Xue J, Yang Y, Ning D, Chen G and Jiang L: Thioredoxin-1 regulates IRE1α to ameliorate sepsis-induced NLRP3 inflammasome activation and oxidative stress in Raw 264.7 cell. Immunopharmacol Immunotoxicol. 45:277–286. 2023.PubMed/NCBI View Article : Google Scholar | |
|
Wang Y, Shi P, Chen Q, Huang Z, Zou D, Zhang J, Gao X and Lin Z: Mitochondrial ROS promote macrophage pyroptosis by inducing GSDMD oxidation. J Mol Cell Biol. 11:1069–1082. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Newton K, Strasser A, Kayagaki N and Dixit VM: Cell death. Cell. 187:235–256. 2024.PubMed/NCBI View Article : Google Scholar | |
|
Chen T, Liang L, Wang Y, Li X and Yang C: Ferroptosis and cuproptposis in kidney diseases: Dysfunction of cell metabolism. Apoptosis. 29:289–302. 2024.PubMed/NCBI View Article : Google Scholar | |
|
Lee J, You JH, Shin D and Roh JL: Inhibition of glutaredoxin 5 predisposes cisplatin-resistant head and neck cancer cells to Ferroptosis. Theranostics. 10:7775–7786. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Seco-Cervera M, González-Cabo P, Pallardó FV, Romá-Mateo C and García-Giménez JL: Thioredoxin and glutaredoxin systems as potential targets for the development of new treatments in Friedreich's ataxia. Antioxidants (Basel). 9(1257)2020.PubMed/NCBI View Article : Google Scholar | |
|
Liu X, Zhuang L and Gan B: Disulfidptosis: Disulfide stress-induced cell death. Trends Cell Biol. 34:327–337. 2024.PubMed/NCBI View Article : Google Scholar | |
|
Elftmaoui Z and Bignon E: Robust AMBER force field parameters for glutathionylated cysteines. Int J Mol Sci. 24(15022)2023.PubMed/NCBI View Article : Google Scholar | |
|
Guan L, Mao Z, Yang S, Wu G, Chen Y, Yin L, Qi Y, Han L and Xu L: Dioscin alleviates Alzheimer's disease through regulating RAGE/NOX4 mediated oxidative stress and inflammation. Biomed Pharmacother. 152(113248)2022.PubMed/NCBI View Article : Google Scholar | |
|
Rani P, Krishnan S and Rani Cathrine C: Study on analysis of peripheral biomarkers for Alzheimer's disease diagnosis. Front Neurol. 8(328)2017.PubMed/NCBI View Article : Google Scholar | |
|
Akterin S, Cowburn RF, Miranda-Vizuete A, Jiménez A, Bogdanovic N, Winblad B and Cedazo-Minguez A: Involvement of glutaredoxin-1 and thioredoxin-1 in beta-amyloid toxicity and Alzheimer's disease. Cell Death Differ. 13:1454–1465. 2006.PubMed/NCBI View Article : Google Scholar | |
|
Kommaddi RP, Tomar DS, Karunakaran S, Bapat D, Nanguneri S, Ray A, Schneider BL, Nair D and Ravindranath V: Glutaredoxin1 diminishes amyloid beta-mediated oxidation of F-actin and reverses cognitive deficits in an Alzheimer's disease mouse model. Antioxid Redox Signal. 31:1321–1338. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Dionísio PA, Amaral JD and Rodrigues CMP: Oxidative stress and regulated cell death in Parkinson's disease. Ageing Res Rev. 67(101263)2021.PubMed/NCBI View Article : Google Scholar | |
|
Johnson WM, Yao C, Siedlak SL, Wang W, Zhu X, Caldwell GA, Wilson-Delfosse AL, Mieyal JJ and Chen SG: Glutaredoxin deficiency exacerbates neurodegeneration in C. elegans models of Parkinson's disease. Hum Mol Genet. 24:1322–1335. 2015.PubMed/NCBI View Article : Google Scholar | |
|
Xu J, Kao SY, Lee FJ, Song W, Jin LW and Yankner BA: Dopamine-dependent neurotoxicity of alpha-synuclein: A mechanism for selective neurodegeneration in Parkinson disease. Nat Med. 8:600–606. 2002.PubMed/NCBI View Article : Google Scholar | |
|
Johnson WM, Golczak M, Choe K, Curran PL, Miller OG, Yao C, Wang W, Lin J, Milkovic NM, Ray A, et al: Regulation of DJ-1 by glutaredoxin 1 in vivo: Implications for Parkinson's disease. Biochemistry. 55:4519–4532. 2016.PubMed/NCBI View Article : Google Scholar | |
|
Tokarew JM, El-Kodsi DN, Lengacher NA, Fehr TK, Nguyen AP, Shutinoski B, O'Nuallain B, Jin M, Khan JM, Ng ACH, et al: Age-associated insolubility of parkin in human midbrain is linked to redox balance and sequestration of reactive dopamine metabolites. Acta Neuropathol. 141:725–754. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Mailloux RJ, Xuan JY, McBride S, Maharsy W, Thorn S, Holterman CE, Kennedy CR, Rippstein P, deKemp R, da Silva J, et al: Glutaredoxin-2 is required to control oxidative phosphorylation in cardiac muscle by mediating deglutathionylation reactions. J Biol Chem. 289:14812–14828. 2014.PubMed/NCBI View Article : Google Scholar | |
|
Zima AV and Mazurek SR: Functional impact of ryanodine receptor oxidation on intracellular calcium regulation in the heart. Rev Physiol Biochem Pharmacol. 171:39–62. 2016.PubMed/NCBI View Article : Google Scholar | |
|
Wegener JW, Wagdi A, Wagner E, Katschinski DM, Hasenfuss G, Bruegmann T and Lehnart SE: The RyR2-R2474S mutation sensitizes cardiomyocytes and hearts to catecholaminergic stress-induced oxidation of the mitochondrial glutathione pool. Front Physiol. 12(777770)2021.PubMed/NCBI View Article : Google Scholar | |
|
Rashdan NA, Shrestha B and Pattillo CB: S-glutathionylation, friend or foe in cardiovascular health and disease. Redox Biol. 37(101693)2020.PubMed/NCBI View Article : Google Scholar | |
|
Mizuno M, Matsuzaki T, Ozeki N, Katano H, Koga H, Takebe T, Yoshikawa HY and Sekiya I: Cell membrane fluidity and ROS resistance define DMSO tolerance of cryopreserved synovial MSCs and HUVECs. Stem Cell Res Ther. 13(177)2022.PubMed/NCBI View Article : Google Scholar | |
|
Duan R, Pan H, Li D, Liao S and Han B: Ergothioneine improves myocardial remodeling and heart function after acute myocardial infarction via S-glutathionylation through the NF-ĸB dependent Wnt5a-sFlt-1 pathway. Eur J Pharmacol. 950(175759)2023.PubMed/NCBI View Article : Google Scholar | |
|
Fan X, Dong T, Yan K, Ci X and Peng L: PM2.5 increases susceptibility to acute exacerbation of COPD via NOX4/Nrf2 redox imbalance-mediated mitophagy. Redox Biol. 59(102587)2023.PubMed/NCBI View Article : Google Scholar | |
|
Kuipers I, Guala AS, Aesif SW, Konings G, Bouwman FG, Mariman EC, Wouters EF, Janssen-Heininger YM and Reynaert NL: Cigarette smoke targets glutaredoxin 1, increasing s-glutathionylation and epithelial cell death. Am J Respir Cell Mol Biol. 45:931–937. 2011.PubMed/NCBI View Article : Google Scholar | |
|
Chia SB, Nolin JD, Aboushousha R, Erikson C, Irvin CG, Poynter ME, van der Velden J, Taatjes DJ, van der Vliet A, Anathy V, et al: Glutaredoxin deficiency promotes activation of the transforming growth factor beta pathway in airway epithelial cells, in association with fibrotic airway remodeling. Redox Biol. 37(101720)2020.PubMed/NCBI View Article : Google Scholar | |
|
Guo Y, Liu Y, Zhao S, Xu W, Li Y, Zhao P, Wang D, Cheng H, Ke Y and Zhang X: Oxidative stress-induced FABP5 S-glutathionylation protects against acute lung injury by suppressing inflammation in macrophages. Nat Commun. 12(7094)2021.PubMed/NCBI View Article : Google Scholar | |
|
Zhang D, Wang X, Chen S, Chen S, Yu W, Liu X, Yang G, Tao Y, Tang X, Bu D, et al: Endogenous hydrogen sulfide sulfhydrates IKKβ at cysteine 179 to control pulmonary artery endothelial cell inflammation. Clin Sci (Lond). 133:2045–2059. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Reynaert NL, van der Vliet A, Guala AS, McGovern T, Hristova M, Pantano C, Heintz NH, Heim J, Ho YS, Matthews DE, et al: Dynamic redox control of NF-kappaB through glutaredoxin-regulated S-glutathionylation of inhibitory kappaB kinase beta. Proc Natl Acad Sci USA. 103:13086–13091. 2006.PubMed/NCBI View Article : Google Scholar | |
|
Zhang X, Liu P, Zhang C, Chiewchengchol D, Zhao F, Yu H, Li J, Kambara H, Luo KY, Venkataraman A, et al: Positive regulation of interleukin-1β bioactivity by physiological ROS-mediated cysteine S-glutathionylation. Cell Rep. 20:224–235. 2017.PubMed/NCBI View Article : Google Scholar | |
|
Anathy V, Lahue KG, Chapman DG, Chia SB, Casey DT, Aboushousha R, van der Velden JLJ, Elko E, Hoffman SM, McMillan DH, et al: Reducing protein oxidation reverses lung fibrosis. Nat Med. 24:1128–1135. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Shahmarvand N, Nagy A, Shahryari J and Ohgami RS: Mutations in the signal transducer and activator of transcription family of genes in cancer. Cancer Sci. 109:926–933. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Anastasiou D, Poulogiannis G, Asara JM, Boxer MB, Jiang JK, Shen M, Bellinger G, Sasaki AT, Locasale JW, Auld DS, et al: Inhibition of pyruvate kinase M2 by reactive oxygen species contributes to cellular antioxidant responses. Science. 334:1278–1283. 2011.PubMed/NCBI View Article : Google Scholar | |
|
Li X, Ma Y, Wu J, Ni M, Chen A, Zhou Y, Dai W, Chen Z, Jiang R, Ling Y, et al: Thiol oxidative stress-dependent degradation of transglutaminase2 via protein S-glutathionylation sensitizes 5-fluorouracil therapy in 5-fluorouracil-resistant colorectal cancer cells. Drug Resist Updat. 67(100930)2023.PubMed/NCBI View Article : Google Scholar | |
|
Zhang L, Zhang J, Ye Z, Manevich Y, Townsend DM, Marshall DT and Tew KD: S-glutathionylated serine proteinase inhibitors as biomarkers for radiation exposure in prostate cancer patients. Sci Rep. 9(13792)2019.PubMed/NCBI View Article : Google Scholar | |
|
Zhang J, Ye ZW, Chen W, Culpepper J, Jiang H, Ball LE, Mehrotra S, Blumental-Perry A, Tew KD and Townsend DM: Altered redox regulation and S-glutathionylation of BiP contribute to bortezomib resistance in multiple myeloma. Free Radic Biol Med. 160:755–767. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Abo M and Weerapana E: Chemical probes for redox signaling and oxidative stress. Antioxid Redox Signal. 30:1369–1386. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Xiao H, Jedrychowski MP, Schweppe DK, Huttlin EL, Yu Q, Heppner DE, Li J, Long J, Mills EL, Szpyt J, et al: A quantitative tissue-specific landscape of protein redox regulation during aging. Cell. 180:968–983.e24. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Subramani J, Kundumani-Sridharan V and Das KC: Chaperone-mediated autophagy of eNOS in myocardial ischemia-reperfusion injury. Circ Res. 129:930–945. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Wu X, Liu L, Zheng Q, Ye H, Yang H, Hao H and Li P: Dihydrotanshinone I preconditions myocardium against ischemic injury via PKM2 glutathionylation sensitive to ROS. Acta Pharm Sin B. 13:113–127. 2023.PubMed/NCBI View Article : Google Scholar |









