S-glutathionylation modification of proteins and the association with cellular death (Review)
- Authors:
- Published online on: August 22, 2025 https://doi.org/10.3892/mi.2025.263
- Article Number: 64
-
Copyright : © Sun et al. This is an open access article distributed under the terms of Creative Commons Attribution License [CC BY 4.0].
Abstract
1. Analysis of protein S-glutathionylation
Biological oxidation, the process of energy substrate breakdown, generates ATP and reactive oxygen species (ROS), including superoxide anion (O2-), hydroxyl radical (·OH) and hydrogen peroxide (H2O2). While low ROS levels sustain redox equilibrium, excessive ROS induce oxidative stress, disrupting lipid membranes, proteins, DNA and cytoskeletal integrity (1). Mitochondria-derived ROS are pivotal in redox signaling and pathology (2). Cellular redox balance relies on enzymatic [e.g., superoxide dismutase and glutathione peroxidase (GPX)] and non-enzymatic [e.g., glutathione (GSH)] antioxidants to neutralize ROS (3). The dysregulation of oxidant-antioxidant systems underpins diverse diseases, from neurodegeneration to cancer.
Structure and biological roles of GSH
GSH, a γ-glutamyl-cysteinyl-glycine tripeptide, is the primary endogenous antioxidant. Synthesized via glutamate-cysteine ligase (the rate-limiting enzyme) and GSH synthetase, GSH functions as a key antioxidant. It scavenges ROS through the nucleophilic attack by the thiol group (-SH) of its cysteine residue (4). ROS attack protein thiols, forming sulfenic acid (-SOH), which may progress to irreversible sulfinic (-SO2H) or sulfonic (-SO3H) acids, impairing protein function (5). GSH combats oxidative stress by directly scavenging ROS (e.g., H2O2, ONOO-) and by reducing oxidized thiol groups on other molecules (6,7).
The GSH/GSH disulfide (GSSG) ratio reflects cellular redox status: a high ratio (>100:1) signifies a reduced state, while under severe oxidative stress, this ratio is markedly reduced to a range of 1:1 to 10:1(8). Tissue-specific GSH levels vary, peaking in the liver (highest detoxification demand) and declining in adipose tissue (9). Compartmental redox potentials further regulate function; for example, the endoplasmic reticulum maintains an oxidizing redox environment (approximately -180 mV), characterized by a lower GSH/GSSG ratio (typically 1:1-3:1) compared to the cytosol (approximately -220 to -260 mV; GSH/GSSG >100:1), facilitating disulfide bond formation during protein folding (10).
Protein oxidative thiol modifications. Types of thiol oxidative modifications
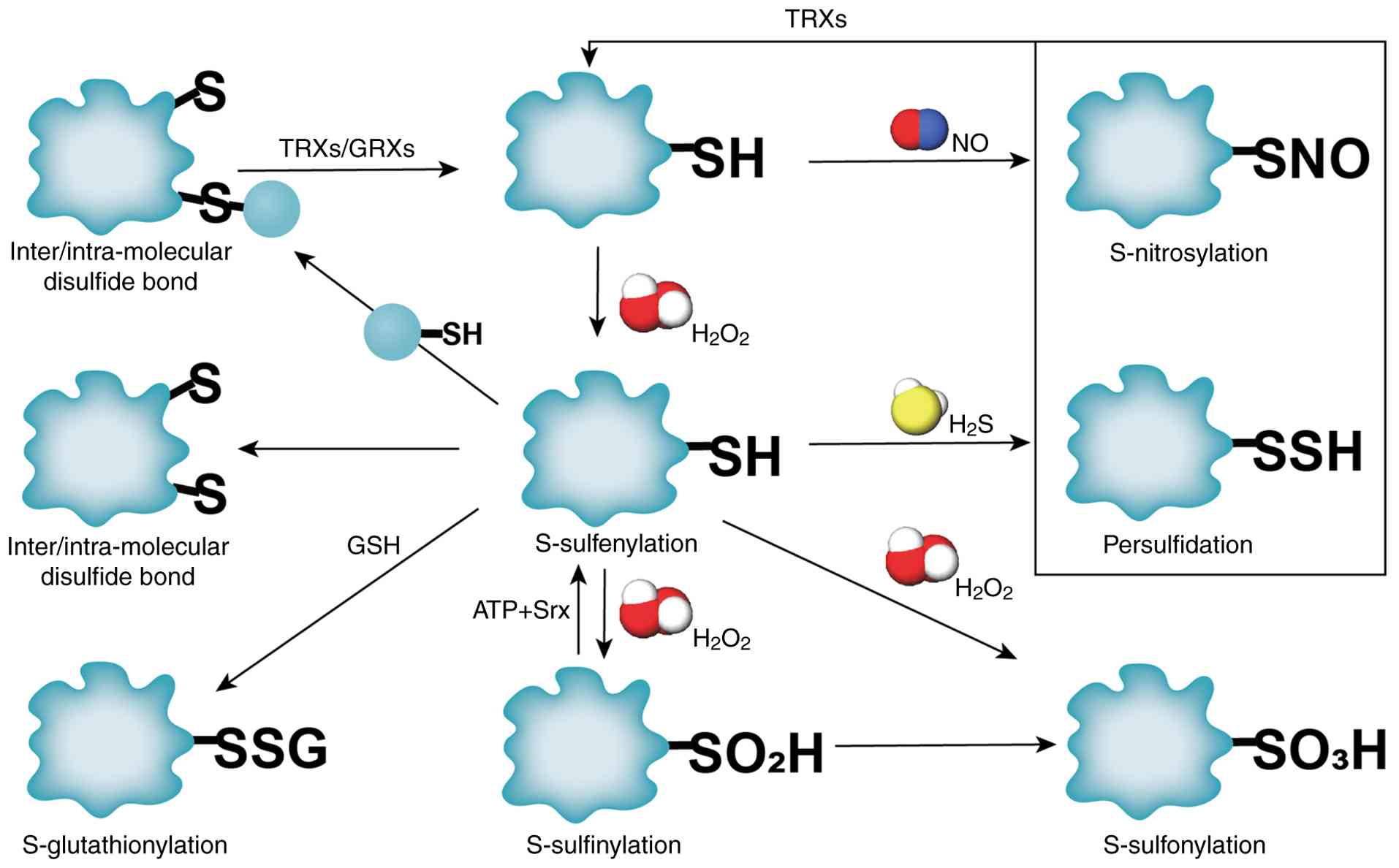
ROS induce dynamic oxidative post-translational modifications on cysteine residues, serving as redox switches to regulate protein conformation, signaling, and cellular homeostasis (11). Key reversible modifications include the following: i) S-glutathionylation (SSG), where GSH adduct formation occurs via thiol-disulfide exchange. ii) S-nitrosylation (SNO), where nitric oxide (NO)-mediated covalent binding to thiols occurs, modulating synaptic transmission and mitochondrial function (12). iii) S-sulfenylation (SOH), where the initial oxidation product of cysteine by H2O2, functions as a precursor for SSG or disulfide bonds (13). These modifications are tightly regulated by reductases [e.g., S-nitrosoglutathione (GSNO) reductase for SNO removal] and cellular redox state (14). Persistent oxidative stress drives irreversible oxidation to -SO2H or sulfonic-SO3H acids, disrupting protein function and promoting pathological outcomes (e.g., neurodegeneration and metabolic dysfunction) (15). Gasotransmitters, such as NO and H2S, further fine-tune redox signaling: H2S preferentially reacts with SOH to form persulfides (-SSH), protecting thiols from overoxidation (13). Key metabolic enzymes regulate these processes, as depicted in Fig. 1.
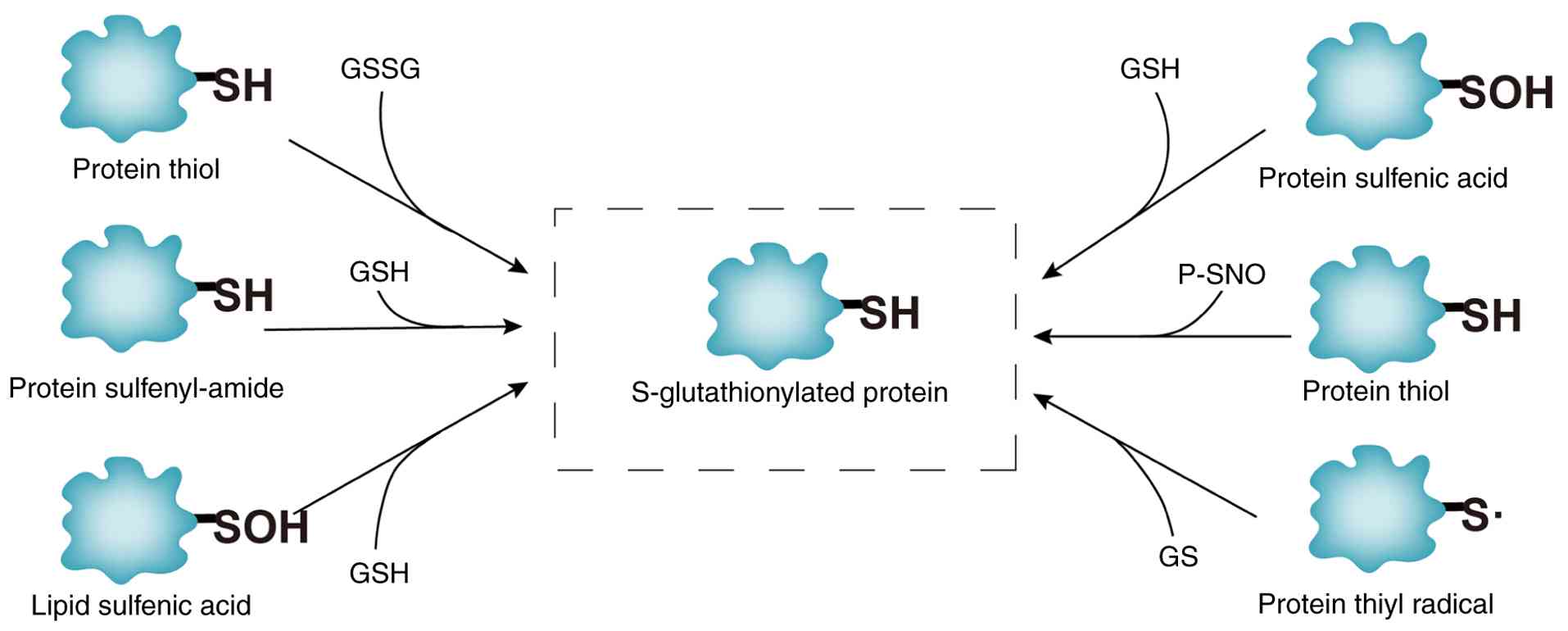
Molecular mechanisms of protein SSG modification. SSG modification, mediated by GSH, involves the covalent attachment of GSH to cysteine thiols, forming mixed disulfide bonds. This redox-sensitive process regulates protein activity, protects cysteine residues from irreversible oxidation and maintains thiol/disulfide balance (16). As illustrated in Fig. 2, SSG formation occurs through six pathways: i) Thiol-disulfide exchange: Direct reaction between protein thiols and oxidized glutathione (GSSG). ii) SOH intermediates: Protein sulfenic acid (P-SOH) reacts with GSH or GSNO (GSH-SOH). iii) Sulfinamide intermediates: Thiol metabolites (e.g., P-SNOR) interact with GSH. iv) Sulfur radical pathways: ROS/RNS generate sulfur radicals (S·), leading to disulfide fomation. v) SOH lipid mediation: Lipid-SOH intermediates react with GSH. vi) SNO intermediates: GSNO facilitates the conversion of thiol-nitrosylated proteins (P-SNO) to SSG (17). Although intermediates vary across pathways, all converge on redox exchange between GSH/GSSG and protein thiols, as depicted in Fig. 2. SSG dynamics are critical for redox signaling and stress adaptation, with glutaredoxins (GRXs) playing a central role in reversing these modifications (as described below).
GRXs in the regulation of SSG modifications
GRXs, first identified in Escherichia coli by Holmgren in 1976(18), are GSH-dependent oxidoreductases critical for reversing SSG (19). GRXs catalyze deglutathionylation via two mechanisms: The monothiol pathway (single active-site cysteine reacts with GSH to release GSSG) and the dithiol pathway (two cysteines form intermolecular disulfides) (20). Their conserved Cys-X-X-Cys motif enables binding to GSH; however, it limits the reduction of sulfonic acids (-SO3H) or intermolecular disulfides (21).
Mammals express two isoforms: GRX1 (cytoplasmic) and GRX2 (mitochondrial/nuclear), sharing 34% homology (22). GRX1 predominantly utilizes the monothiol pathway, maintaining iron homeostasis and 2Fe-2S cluster assembly, essential for electron transport and anti-apoptotic functions (23). GRX2, although less abundant, exhibits enhanced Fe-S cluster synthesis and mitochondrial SSG regulation, sustaining antioxidant capacity under oxidative overload (24). Both isoforms dynamically regulate SSG levels based on cellular GSH/GSSG ratios. Oxidative stress reduces this ratio, overwhelming GRX activity and driving irreversible protein oxidation linked to cell death (25).
Despite the non-essential role of GRX1 in viability (GRX1-/- mice exhibit a normal lifespan under standard conditions) (26), as it modulates disease-specific pathways. For example, GRX1 deficiency exacerbates hepatic lipid dysregulation via sirtuin1 glutathionylation (27), while GRX2 loss impairs mitochondrial redox balance, accelerating lens epithelial-mesenchymal transition and cataract formation through ILK/AKT/GSK-3β dysregulation (28). Therapeutic restoration of GRX1 in pulmonary fibrosis models reduces pathological SSG accumulation, highlighting its potential as a redox-targeted therapy (24).
2. Thiol redox modulations in programmed cell death
Programmed cell death (PCD), including apoptosis, necroptosis, pyroptosis, autophagy and ferroptosis, is a genetically regulated process essential for tissue homeostasis and pathogen defense (29). In contrast to accidental necrosis, PCD eliminates superfluous or damaged cells through precise molecular cascades (e.g., caspase activation, inflammasome signaling). Cellular redox imbalance, driven by excessive ROS, disrupts thiol homeostasis by oxidizing critical cysteine residues in proteins (e.g., caspases, BAX and GPX4) and small molecules such as GSH (30). Reversible thiol modifications, particularly SSG, act as redox switches to regulate PCD execution. For instance, SSG modulates death receptor signaling (e.g., FAS activation), caspase activity, and antioxidant defense systems (e.g., GPX4 in ferroptosis), as summarized in Table I (31).
SSG dynamically balances pro-survival and pro-death signals. Under oxidative stress, diminished GRX activity impairs deglutathionylation, leading to persistent SSG accumulation. This disrupts redox-sensitive pathways (e.g., RAS/ERK and NLRP3 inflammasome) and shifts cellular fate toward PCD (32). GRX dysfunction further exacerbates mitochondrial SSG overload, impairing electron transport chain complexes and amplifying ROS-driven damage (33). Thus, SSG serves as both a protective mechanism (shielding thiols from irreversible oxidation) and a pathogenic trigger (sustaining oxidative stress), depending on cellular context and modification dynamics.
SSG modification and cell apoptosis
Apoptosis, characterized by cell shrinkage, chromatin condensation and caspase activation, is regulated through intrinsic (mitochondrial), extrinsic (death receptor) and endoplasmic reticulum (ER) stress pathways (34). The intrinsic pathway involves mitochondrial permeability transition pore (MPTP) opening, controlled by BCL-2 family proteins (e.g., BAX/BAK). Oxidative stress induces SSG of BAX at Cys62, promoting mitochondrial translocation and caspase-9/3 activation, although the conformational effects remain unclear (35). GRX1 overexpression mitigates apoptosis in myocardial infarction models by restoring BCL-2/BAX balance (36).
The extrinsic pathway is initiated by death receptors (e.g., FAS and TNFR) with redox-sensitive cysteine-rich extracellular domains. SSG of FAS at Cys294 enhances FASL binding, accelerating caspase-8/3 activation (37). Similarly, SSG of pro-caspase-3 (Cys184/220) inhibits its activation, while TNF-α-induced GRX1 downregulation shifts this balance toward apoptosis (38). In ethanol-exposed GRX1-deficient mice, FAS-SSG accumulation drives hepatocyte apoptosis via NF-κB and AKT dysregulation (39).
ER stress-mediated apoptosis arises from misfolded protein aggregation and Ca²+ imbalance, activating CHOP and JNK pathways (40). SSG of ER chaperones (e.g., BiP) at Cys420/441 modulates ATPase activity and protein folding, paradoxically suppressing myeloma cell apoptosis (41). Conversely, glutathione S-transferase Pi (GSTP)1 promotes ER stress-induced apoptosis in liver cancer via the glutathionylation of calreticulin and sarco/endoplasmic reticulum Ca2+-ATPase (SERCA), inhibiting JNK survival signals (42). While SSG generally enhances apoptosis, its role in ER protein quality control may contextually oppose cell death.
SSG modification and autophagy
Autophagy, a lysosome-dependent degradation process, includes macroautophagy, microautophagy and chaperone-mediated autophagy, playing dual roles in cell survival and death (43). Oxidative stress (e.g., hypoxia and ischemia/reperfusion) induces autophagy to degrade damaged organelles and proteins, while autophagy itself regulates redox balance by clearing oxidized products (44). SSG functions as a redox switch in autophagy modulation. For instance, H2S-induced SSG of KEAP1 at Cys434 disrupts KEAP1-NRF2 binding, promoting autophagy gene expression (45). Conversely, GSH depletion in cancer cells reduces SSG, leading to oxidative stress and autophagy activation (46). The loss of KRIT1 increases the SSG of chaperones and cytoskeletal proteins, impairing autophagic flux and quality control (47). Oncogenic H-RAS12 enhances GAPDH SSG, depleting GSH and triggering autophagy-independent of ROS accumulation (48).
SSG also negatively regulates autophagy. The SSG of ATG3 and ATG7 inhibits LC3 lipidation, a critical step in autophagosome maturation (49). Similarly, the SSG of AMPKα (Cys299/304) and SENP3 (Cys243/274) disrupts Beclin1 and PtdIns3K complex formation, impairing autophagosome assembly (50). PTEN SSG at Cys124/71 inhibits its phosphorylation, activating AKT/mTOR and suppressing autophagy, potentially driving pathological cell proliferation (51). Conversely, the SSG of ATM at Cys2991 promotes peroxisomal autophagy via PEX5 ubiquitination (52). The MiT/TFE transcription factors (e.g., TFEB) are regulated by SSG, enhancing lysosomal biogenesis and autophagy initiation (53).
GRXs are central to autophagy regulation by reversing SSG. GRX1 deficiency in liver cancer cells increases oxidative modifications and autophagic flux, while PRDX6-mediated GRX1 upregulation suppresses autophagy (54). GRX1 also protects against oxidative stress by maintaining AKT phosphorylation and mTORC1 activity, inhibiting autophagy in ischemic myocardial cells (55). GRX2, crucial for mitochondrial autophagy, stabilizes mitochondrial dynamics and ultrastructure. GRX2-/- mice exhibit reduced GSH/GSSG ratios and increased mitophagy, highlighting its role in redox homeostasis (33). These findings underscore the ‘thiol switch-autophagy cascade’ as a therapeutic target in diseases like cancer and neurodegeneration (56).
SSG modification and other forms of cell death
Necroptosis, a caspase-independent PCD, is triggered by RIPK3-mediated phosphorylation of MLKL, leading to membrane rupture and inflammatory damage-associated molecular pattern release (57). The SSG of mitochondrial fusion protein MFN2 disrupts mitochondria-ER crosstalk, promoting necroptosis in cadmium-induced neurotoxicity. This modification is reversible by cytoplasmic GRX1, but not mitochondrial GRX2, highlighting compartment-specific redox regulation (58). Caspase-8, a necroptosis inhibitor, undergoes intermolecular SSG at Cys360/409 under low thiocyanate conditions, impairing its ability to suppress RIPK3 phosphorylation (59). In models of Parkinson's disease (PD), GRXs paradoxically enhance microglial necroptosis via TNF-α/NF-κB upregulation, suggesting context-dependent roles (60).
Pyroptosis, driven by gasdermin D (GSDMD) cleavage and pore formation, amplifies inflammation through the release of IL-1β (61). Redox modifications regulate pyroptosis: The SSG of NLRP3 at Cys483 inhibits inflammasome activation, while thioredoxin (TRX)-1 reduces NLRP3 cysteine reactivity, attenuating sepsis-induced pyroptosis (61). The active thiols of GSDMD (Cys38/56/268/467) are susceptible to oxidative modifications that enhance caspase-1-mediated cleavage, linking mitochondrial ROS to inflammatory death (63). However, the mechanistic interplay between glutathionylation and pyroptosis remains underexplored.
Ferroptosis, an iron-dependent lipid peroxidation process, is tightly linked to GSH metabolism. System Xc- (SLC7A11-dependent cystine uptake) sustains GSH synthesis, while GPX4 utilizes GSH to neutralize lipid hydroperoxides (64). GRX2, via Fe-S cluster assembly, mitigates ferroptosis by maintaining mitochondrial redox balance (65). GSH depletion (e.g., erastin treatment) or GRX5 silencing induces iron overload and ferroptosis, sensitizing cancer cells to chemotherapy (66). In hereditary ataxia, FXN deficiency impairs Fe-S biogenesis, reducing GRX/TRX activity; NRF2 activators (e.g., sulforaphane) may counteract this defect (67).
A newly identified death modality, disulfidptosis, arises from NADPH depletion-induced disulfide stress. SLC7A11 overexpression under glucose starvation promotes aberrant actin cytoskeleton SSG, triggering cytoskeletal collapse and membrane detachment (68). Unlike ferroptosis or apoptosis, disulfidptosis is uniquely potentiated by thiol oxidants (e.g., diamide) and unresolved by GRX-mediated redox repair, suggesting distinct therapeutic vulnerabilities.
3. Protein SSG modifications in disease pathogenesis
SSG regulates cell growth, differentiation and apoptosis by modulating enzymatic activity, protein conformation and stability through redox-sensitive mechanisms (69). Advances in redox proteomics have linked SSG dysregulation to neurodegenerative, cardiovascular, respiratory and malignant diseases, as summarized in Table II.
Neurodegenerative diseases
Mitochondrial oxidative stress in Alzheimer's disease (AD) drives Aβ accumulation and tau hyperphosphorylation, exacerbating neuronal damage (70). Reduced GSH/GSSG ratios in patients with AD are associated with disease severity, while SSG levels of cortical proteins (e.g., GAPDH and α-enolase) are elevated, impairing synaptic function (71). The expression of GRX1 and GRX2 is markedly reduced in the brains of patients with AD, particularly in the CA1 region, contributing to F-actin destabilization and memory deficits (72). APP/PS1 transgenic mice overexpressing GRX1 exhibit a restored synaptic plasticity and cognitive function, highlighting therapeutic potential (73).
PD is characterized by dopaminergic neuron loss and α-synuclein aggregation, exacerbated by GSH depletion in the substantia nigra (74). There is evidence to indicate that oxidative stress induces the SSG of α-synuclein, which alters its conformational stability and promotes pathological oligomerization (75). GRX1 deficiency exacerbates this process, leading to enhanced α-synuclein toxicity and dopaminergic neurodegeneration in C. elegans models of PD (76). The SSG of DJ-1 at Cys106 enhances its mitochondrial localization and ROS scavenging, whereas GRX1 upregulation in MPTP models paradoxically accelerates respiratory chain dysfunction and neuron death (77). Conversely, GRX2 promotes Fe-S cluster assembly, mitigating oxidative damage, while Parkin deglutathionylation rescues proteasomal dysfunction, underscoring context-dependent GRX roles (78).
Cardiovascular system diseases
In cardiomyocytes, mitochondrial and sarcoplasmic reticulum redox dynamics are critical for energy metabolism and contractility. GRX2 mitigates oxidative stress by reducing SSG of NADPH oxidase subunits (NDUFS1/NDUFV1), suppressing mitochondrial ROS overproduction linked to left ventricular hypertrophy and hypertension (79). The sarcoplasmic reticulum Ca²+ channel RyR2 undergoes SSG under oxidative stress, which initially protects against calcium overload in ischemia-reperfusion injury, whereas it becomes maladaptive in sustained oxidative environments (e.g., catecholamine-induced arrhythmias) (80). The R2474S RyR2 mutation exacerbates SSG-mediated mitochondrial oxidation, increasing ventricular arrhythmia susceptibility (81).
Atherosclerosis progression is associated with low-density lipoprotein ApoB100 SSG levels, promoting endothelial dysfunction and plaque instability (82). GRX1 inhibition (e.g., 2-AAPA) attenuates endothelial-mesenchymal transition by reducing SSG, suggesting therapeutic potential in vascular remodeling (83). In myocardial infarction, the SSG of SERCA and Na+/K+ ATPase α-subunit impairs calcium handling and action potential generation, exacerbating contractile dysfunction and oxidative damage (46). Pharmacological agents such as ergothioneine acid reduce the SSG of NF-κB-dependent Wnt5a-sFlt1, improving post-MI outcomes by preserving GRX1 activity and myocardial integrity (84).
Respiratory system diseases
Chronic obstructive pulmonary disease (COPD), driven by exogenous oxidants (e.g., cigarette smoke) and endogenous ROS/nitric oxide synthase (NOS), is characterized by macrophage/neutrophil infiltration, epithelial cell death and fibrosis (85). Cigarette smoke-induced SSG accumulation in lung proteins promotes alveolar epithelial apoptosis, while GRX1 overexpression rescues cell survival and reduces airway inflammation (86). GRX1 deficiency exacerbates COPD progression by elevating TGF-β levels, collagen deposition and basal cell plasticity, accelerating lung remodeling (87).
In acute lung injury (ALI), oxidative stress reduces GRX1 expression, impairing redox homeostasis. The SSG of FABP5 at Cys127 activates PPARβ/δ, suppressing macrophage inflammation and alleviating H2O2-induced ALI (88). Asthma pathogenesis involves NF-κB-mediated airway inflammation, where the SSG of IKKβ at Cys179 inhibits pro-inflammatory chemokine production (89). GRX1-/- mice exhibit attenuated LPS-induced cytokine release (IL-1β and TNF-α) and macrophage dysfunction, suggesting the dual role of GRX1 in the regulation of inflammation (90). Notably, the SSG of IL-1β at Cys188 directly reduces its inflammatory activity, highlighting a self-limiting redox checkpoint (91). The therapeutic administration of recombinant GRX1 reverses pathological SSG, providing promise for chronic lung diseases (92).
Malignant tumors
Cancer cells exhibit metabolic reprogramming (Warburg effect) and redox adaptation to sustain proliferation under oxidative stress. SSG modulates tumor progression by regulating enzymes critical for glycolysis, drug resistance and protein stability (93). For example, SSG of pyruvate kinase M2 (PKM2) at Cys358 suppresses its activity, attenuating glycolytic flux and oxidative stress in small-cell lung cancer (94). Conversely, the SSG of transglutaminase 2 at Cys193 promotes its degradation, sensitizing colon cancer cells to 5-fluorouracil by restoring apoptosis (95).
Clinically, SSG levels of plasma proteins (e.g., serpin A1/A3) are associated with radiotherapy efficacy in prostate cancer, serving as potential biomarkers (96). In breast cancer, oxidized Hsp90 (with reduced SSG) is associated with a poor treatment response, while the SSG of GSTP at Cys411/420 counteracts bortezomib resistance in multiple myeloma by impairing ATPase binding and protein folding (97). These findings position SSG as a dual regulator of tumor survival and therapeutic vulnerability, with targeted cysteine modification offering novel avenues for anticancer drug development.
The therapeutic targeting of SSG modifications confronts significant pharmacological constraints. Target specificity is hindered by functional divergence among GRX isoforms, particularly GRX1 which exhibits opposing effects in pulmonary protection vs. neurodegenerative exacerbation. The reversible instability of SSG modifications, demonstrated in the context-dependent roles of RyR2 glutathionylation during cardiac injury, compromises sustained efficacy. Direct clinical biomarkers for pathological SSG sites remain undeveloped, restricting clinical monitoring beyond indirect redox indicators such as GSH/GSSG ratios. Additionally, detection sensitivity suffers from technical limitations in capturing low-abundance labile SSG modifications in situ. Addressing these barriers will enhance the translational rigor of SSG-targeted therapies and strategically guide future mechanistic and therapeutic development.
4. Summary and future perspectives
ROS-mediated cysteine modifications, particularly SSG, serve as dynamic redox switches regulating cellular signaling, death pathways and stress adaptation. SSG dynamics are governed by the GSH/GSSG ratio, GRX activity and oxidative stress intensity. While moderate SSG protects against irreversible cysteine oxidation (e.g., -SOH/-SO2H), severe oxidative stress drives pathological overoxidation, triggering cell senescence or death (98). The complexity of thiol redox regulation necessitates advanced redox proteomics to map tissue- and site-specific SSG modifications, given their low abundance and instability (99).
SSG critically modulates diverse cell death modalities (apoptosis, autophagy, pyroptosis, etc.) by altering protein conformation, membrane integrity and mitochondrial function, often amplifying inflammatory cascades (31). Therapeutic strategies targeting SSG show promise: Recombinant TRX or N-acetylcysteine reduces ischemic reperfusion injury by restoring endothelial NOS activity (100), while tanshinone IIA, a traditional Chinese medicine component, protects against myocardial ischemia via PKM2 glutathionylation at Cys423/424(101). Glutaredoxin mimetics, such as para-aminobenzoic acid-conjugated glutaredoxin peptide enhance deglutathionylation activity, showing efficacy in reducing pathological SSG accumulation in pulmonary fibrosis models by emulating the catalytic function of GRX1(24). Complementary thioredoxin mimetics exemplified by TXM-CB3 indirectly support GRX systems through the replenishment of reducing equivalents, attenuating mitochondrial SSG overload in neurodegenerative contexts (78). Concurrently, thiol-targeted pharmacologic agents enable precise cysteine redox modulation: Controlled thiol oxidants such as diamide induce protective SSG, but risk off-target overoxidation; reversible covalent inhibitors, such as IBD-0063 selectively engage hyperreactive cysteines including PKM2 Cys358 for anticancer effects (94); natural electrophiles typified by sulforaphane activate NRF2 to upregulate endogenous GRX and glutathione systems, countering ferroptosis in redox-deficient pathologies (67). Notwithstanding promising preclinical outcomes, clinical translation confronts limitations in tissue selectivity, transient efficacy, and biomarker availability. Future research is required to prioritize mechanistic studies to elucidate the crosstalk between SSG and programmed cell death pathways, particularly its organelle-specific redox regulation in mitochondria, endoplasmic reticulum, and lysosomes. Translational efforts must focus on developing GRX mimetics and thiol-targeted pharmacological agents to restore SSG homeostasis in neurodegenerative disorders, cancer, and cardiovascular diseases. Additionally, integrative strategies combining traditional medicine-derived thiol modulators (e.g., tanshinone IIA) with advanced redox proteomics could unveil novel therapeutic targets and optimize precision medicine approaches for redox-related pathologies.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the National Natural Science Foundation of China General Program (grant no. 8237153821); the National Natural Science Foundation of China Youth Fund Project (grant no. 82104831); the Hunan Province Science and Technology Innovation Plan Project (grant no. 2023RC3215); the Hunan Province Health Commission Research Program Project (grant no. B202303077689); and the Hunan Innovative Province Construction Special Project (grant no. 2023JJ40397).
Availability of data and materials
Not applicable.
Authors' contributions
All authors (XS, LX, SW, SZ, LW, XT, JZ, SL, TH, LJ, XL, SZ, JD and DW) were involved in the conceptualization of the study. XS and DW was also involved in the writing and preparation of the original draft. LX, SW, SZ, LW, XT, JZ, SL, TH, LJ, XL and SZ were involved in the writing, reviewing and editing of the manuscript. JD supervised the study and was also involved in project administration. TH, SZ and SL also involved in funding acquisition. All authors have read and agreed to the published version of the manuscript. Data authentication is not applicable.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
|
Mohammadi SA, Najafi H, Zolgharnian S, Sharifian S and Asasian-Kolur N: Biological oxidation methods for the removal of organic and inorganic contaminants from wastewater: A comprehensive review. Sci Total Environ. 843(157026)2022.PubMed/NCBI View Article : Google Scholar | |
|
Jakubczyk K, Dec K, Kałduńska J, Kawczuga D, Kochman J and Janda K: Reactive oxygen species-sources, functions, oxidative damage. Pol Merkur Lekarski. 48:124–127. 2020.PubMed/NCBI | |
|
Jomova K, Raptova R, Alomar SY, Alwasel SH, Nepovimova E, Kuca K and Valko M: Reactive oxygen species, toxicity, oxidative stress, and antioxidants: Chronic diseases and aging. Arch Toxicol. 97:2499–2574. 2023.PubMed/NCBI View Article : Google Scholar | |
|
Noguchi N, Saito Y and Niki E: Actions of thiols, persulfides, and polysulfides as free radical scavenging antioxidants. Antioxid Redox Signal. 39:728–743. 2023.PubMed/NCBI View Article : Google Scholar | |
|
Tan M, Yin Y, Ma X, Zhang J, Pan W, Tan M, Zhao Y, Yang T, Jiang T and Li H: Glutathione system enhancement for cardiac protection: Pharmacological options against oxidative stress and ferroptosis. Cell Death Dis. 14(131)2023.PubMed/NCBI View Article : Google Scholar | |
|
Xiao W and Loscalzo J: Metabolic responses to reductive stress. Antioxid Redox Signal. 32:1330–1347. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Kalinina EV and Gavriliuk LA: Glutathione synthesis in cancer cells. Biochemistry (Mosc). 85:895–907. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Wang F, Yuan Q, Chen F, Pang J, Pan C, Xu F and Chen Y: Fundamental mechanisms of the cell death caused by nitrosative stress. Front Cell Dev Biol. 9(742483)2021.PubMed/NCBI View Article : Google Scholar | |
|
Diaz-Vivancos P, de Simone A, Kiddle G and Foyer CH: Glutathione-linking cell proliferation to oxidative stress. Free Radic Biol Med. 89:1154–1164. 2015.PubMed/NCBI View Article : Google Scholar | |
|
Giustarini D, Milzani A, Dalle-Donne I and Rossi R: How to increase cellular glutathione. Antioxidants (Basel). 12(1094)2023.PubMed/NCBI View Article : Google Scholar | |
|
Corpas FJ, González-Gordo S, Rodríguez-Ruiz M, Muñoz-Vargas MA and Palma JM: Thiol-based oxidative posttranslational modifications (OxiPTMs) of plant proteins. Plant Cell Physiol. 63:889–900. 2022.PubMed/NCBI View Article : Google Scholar | |
|
Hofmann F: The cGMP system: Components and function. Biol Chem. 401:447–469. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Hasan MM, Khatun MS and Kurata H: A comprehensive review of in silico analysis for protein S-sulfenylation sites. Protein Pept Lett. 25:815–821. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Corti A, Franzini M, Scataglini I and Pompella A: Mechanisms and targets of the modulatory action of S-nitrosoglutathione (GSNO) on inflammatory cytokines expression. Arch Biochem Biophys. 562:80–91. 2014.PubMed/NCBI View Article : Google Scholar | |
|
Fu L, Liu K, Ferreira RB, Carroll KS and Yang J: Proteome-wide analysis of cysteine S-sulfenylation using a benzothiazine-based probe. Curr Protoc Protein Sci. 95(e76)2019.PubMed/NCBI View Article : Google Scholar | |
|
Li C, Chen X, Zhang S, Liang C, Ma X, Zhang R and Yan H: Glutaredoxin 1 protects lens epithelial cells from epithelial-mesenchymal transition by preventing casein kinase 1α S-glutathionylation during posterior capsular opacification. Redox Biol. 62(102676)2023.PubMed/NCBI View Article : Google Scholar | |
|
Zhang J, Ye ZW, Singh S, Townsend DM and Tew KD: An evolving understanding of the S-glutathionylation cycle in pathways of redox regulation. Free Radic Biol Med. 120:204–216. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Holmgren A: Hydrogen donor system for Escherichia coli ribonucleoside-diphosphate reductase dependent upon glutathione. Proc Natl Acad Sci USA. 73:2275–2279. 1976.PubMed/NCBI View Article : Google Scholar | |
|
Fernandes AP and Holmgren A: Glutaredoxins: Glutathione-dependent redox enzymes with functions far beyond a simple thioredoxin backup system. Antioxid Redox Signal. 6:63–74. 2004.PubMed/NCBI View Article : Google Scholar | |
|
Ogata FT, Branco V, Vale FF and Coppo L: Glutaredoxin: Discovery, redox defense and much more. Redox Biol. 43(101975)2021.PubMed/NCBI View Article : Google Scholar | |
|
Lillig CH, Berndt C and Holmgren A: Glutaredoxin systems. Biochim Biophys Acta. 1780:1304–1317. 2008.PubMed/NCBI View Article : Google Scholar | |
|
Gladyshev VN, Liu A, Novoselov SV, Krysan K, Sun QA, Kryukov VM, Kryukov GV and Lou MF: Identification and characterization of a new mammalian glutaredoxin (thioltransferase), Grx2. J Biol Chem. 276:30374–30380. 2001.PubMed/NCBI View Article : Google Scholar | |
|
Abdalla M, Eltayb WA and Yousif A: Comparison of structures among Saccharomyces cerevisiae Grxs proteins. Genes Environ. 40(17)2018.PubMed/NCBI View Article : Google Scholar | |
|
Matsui R, Ferran B, Oh A, Croteau D, Shao D, Han J, Pimentel DR and Bachschmid MM: Redox regulation via glutaredoxin-1 and protein S-glutathionylation. Antioxid Redox Signal. 32:677–700. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Sevilla F, Martí MC, De Brasi-Velasco S and Jiménez A: Redox regulation, thioredoxins, and glutaredoxins in retrograde signalling and gene transcription. J Exp Bot. 74:5955–5969. 2023.PubMed/NCBI View Article : Google Scholar | |
|
Yang Y, Liao Z and Xiao Q: Metformin ameliorates skeletal muscle atrophy in Grx1 KO mice by regulating intramuscular lipid accumulation and glucose utilization. Biochem Biophys Res Commun. 533:1226–1232. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Li Y, Liang K, Yuan L, Gao J, Wei L and Zhao L: The role of thioredoxin and glutathione systems in arsenic-induced liver injury in rats under glutathione depletion. Int J Environ Health Res. 34:547–563. 2024.PubMed/NCBI View Article : Google Scholar | |
|
Chen X, Chen Y, Li C, Li J, Zhang S, Liang C, Deng Q, Guo Z, Guo C and Yan H: Glutaredoxin 2 protects lens epithelial cells from epithelial-mesenchymal transition by suppressing mitochondrial oxidative stress-related upregulation of integrin-linked kinase. Exp Eye Res. 234(109609)2023.PubMed/NCBI View Article : Google Scholar | |
|
Ai Y, Meng Y, Yan B, Zhou Q and Wang X: The biochemical pathways of apoptotic, necroptotic, pyroptotic, and ferroptotic cell death. Mol Cell. 84:170–179. 2024.PubMed/NCBI View Article : Google Scholar | |
|
Vignane T and Filipovic MR: Emerging chemical biology of protein persulfidation. Antioxid Redox Signal. 39:19–39. 2023.PubMed/NCBI View Article : Google Scholar | |
|
Benhar M: Oxidants, antioxidants and thiol redox switches in the control of regulated cell death pathways. Antioxidants (Basel). 9(309)2020.PubMed/NCBI View Article : Google Scholar | |
|
Liu T, Sun L, Zhang Y, Wang Y and Zheng J: Imbalanced GSH/ROS and sequential cell death. J Biochem Mol Toxicol. 36(e22942)2022.PubMed/NCBI View Article : Google Scholar | |
|
Liaghati A, Pileggi CA, Parmar G, Patten DA, Hadzimustafic N, Cuillerier A, Menzies KJ, Burelle Y and Harper ME: Grx2 regulates skeletal muscle mitochondrial structure and autophagy. Front Physiol. 12(604210)2021.PubMed/NCBI View Article : Google Scholar | |
|
D'Arcy MS: Cell death: A review of the major forms of apoptosis, necrosis and autophagy. Cell Biol Int. 43:582–592. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Nie C, Tian C, Zhao L, Petit PX, Mehrpour M and Chen Q: Cysteine 62 of Bax is critical for its conformational activation and its proapoptotic activity in response to H2O2-induced apoptosis. J Biol Chem. 283:15359–15369. 2008.PubMed/NCBI View Article : Google Scholar | |
|
Adluri RS, Thirunavukkarasu M, Zhan L, Dunna NR, Akita Y, Selvaraju V, Otani H, Sanchez JA, Ho YS and Maulik N: Glutaredoxin-1 overexpression enhances neovascularization and diminishes ventricular remodeling in chronic myocardial infarction. PLoS One. 7(e34790)2012.PubMed/NCBI View Article : Google Scholar | |
|
Corteselli E, Aboushousha R and Janssen-Heininger Y: S-glutathionylation-controlled apoptosis of lung epithelial cells; potential implications for lung fibrosis. Antioxidants (Basel). 11(1789)2022.PubMed/NCBI View Article : Google Scholar | |
|
Pan S and Berk BC: Glutathiolation regulates tumor necrosis factor-alpha-induced caspase-3 cleavage and apoptosis: Key role for glutaredoxin in the death pathway. Circ Res. 100:213–219. 2007.PubMed/NCBI View Article : Google Scholar | |
|
Sun X, Ye C, Deng Q, Chen J and Guo C: Contribution of glutaredoxin-1 to Fas s-glutathionylation and inflammation in ethanol-induced liver injury. Life Sci. 264(118678)2021.PubMed/NCBI View Article : Google Scholar | |
|
Zhang J, Guo J, Yang N, Huang Y, Hu T and Rao C: Endoplasmic reticulum stress-mediated cell death in liver injury. Cell Death Dis. 13(1051)2022.PubMed/NCBI View Article : Google Scholar | |
|
Ye ZW, Zhang J, Aslam M, Blumental-Perry A, Tew KD and Townsend DM: Protein disulfide isomerase family mediated redox regulation in cancer. Adv Cancer Res. 160:83–106. 2023.PubMed/NCBI View Article : Google Scholar | |
|
He M, Hu J, Fang T, Tang W, Lv B, Yang B and Xia J: Protein convertase subtilisin/Kexin type 9 inhibits hepatocellular carcinoma growth by interacting with GSTP1 and suppressing the JNK signaling pathway. Cancer Biol Med. 19:90–103. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Feng Y, Chen Y, Wu X, Chen J, Zhou Q, Liu B, Zhang L and Yi C: Interplay of energy metabolism and autophagy. Autophagy. 20:4–14. 2024.PubMed/NCBI View Article : Google Scholar | |
|
Jeong SJ and Oh GT: Unbalanced redox with autophagy in cardiovascular disease. J Lipid Atheroscler. 12:132–151. 2023.PubMed/NCBI View Article : Google Scholar | |
|
Li N, Wang J, Zang X, Wang Z, Zhang T, Zhao B, Miao J and Lin Z: H2S probe CPC inhibits autophagy and promotes apoptosis by inhibiting glutathionylation of Keap1 at Cys434. Apoptosis. 26:111–131. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Mancilla H, Maldonado R, Cereceda K, Villarroel-Espíndola F, Montes de Oca M, Angulo C, Castro MA, Slebe JC, Vera JC, Lavandero S and Concha II: Glutathione depletion induces spermatogonial cell autophagy. J Cell Biochem. 116:2283–2292. 2015.PubMed/NCBI View Article : Google Scholar | |
|
Cianfruglia L, Perrelli A, Fornelli C, Magini A, Gorbi S, Salzano AM, Antognelli C, Retta F, Benedetti V, Cassoni P, et al: KRIT1 loss-of-function associated with cerebral cavernous malformation disease leads to enhanced S-glutathionylation of distinct structural and regulatory proteins. Antioxidants (Basel). 8(27)2019.PubMed/NCBI View Article : Google Scholar | |
|
Armeni T, Ercolani L, Urbanelli L, Magini A, Magherini F, Pugnaloni A, Piva F, Modesti A, Emiliani C and Principato G: Cellular redox imbalance and changes of protein S-glutathionylation patterns are associated with senescence induced by oncogenic H-ras. PLoS One. 7(e52151)2012.PubMed/NCBI View Article : Google Scholar | |
|
Mallén-Ponce MJ and Pérez-Pérez ME: Redox-mediated activation of ATG3 promotes ATG8 lipidation and autophagy progression in Chlamydomonas reinhardtii. Plant Physiol. 194:359–375. 2023.PubMed/NCBI View Article : Google Scholar | |
|
Foerster EG, Mukherjee T, Cabral-Fernandes L, Rocha JDB, Girardin SE and Philpott DJ: How autophagy controls the intestinal epithelial barrier. Autophagy. 18:86–103. 2022.PubMed/NCBI View Article : Google Scholar | |
|
Lee SR, Yang KS, Kwon J, Lee C, Jeong W and Rhee SG: Reversible inactivation of the tumor suppressor PTEN by H2O2. J Biol Chem. 277:20336–20342. 2002.PubMed/NCBI View Article : Google Scholar | |
|
Zhang J, Tripathi DN, Jing J, Alexander A, Kim J, Powell RT, Dere R, Tait-Mulder J, Lee JH, Paull TT, et al: ATM functions at the peroxisome to induce pexophagy in response to ROS. Nat Cell Biol. 17:1259–1269. 2015.PubMed/NCBI View Article : Google Scholar | |
|
Wang H, Wang N, Xu D, Ma Q, Chen Y, Xu S, Xia Q, Zhang Y, Prehn JHM, Wang G and Ying Z: Oxidation of multiple MiT/TFE transcription factors links oxidative stress to transcriptional control of autophagy and lysosome biogenesis. Autophagy. 16:1683–1696. 2020.PubMed/NCBI View Article : Google Scholar | |
|
López-Grueso MJ, Lagal DJ, García-Jiménez ÁF, Tarradas RM, Carmona-Hidalgo B, Peinado J, Requejo-Aguilar R, Bárcena JA and Padilla CA: Knockout of PRDX6 induces mitochondrial dysfunction and cell cycle arrest at G2/M in HepG2 hepatocarcinoma cells. Redox Biol. 37(101737)2020.PubMed/NCBI View Article : Google Scholar | |
|
Murata H, Ihara Y, Nakamura H, Yodoi J, Sumikawa K and Kondo T: Glutaredoxin exerts an antiapoptotic effect by regulating the redox state of Akt. J Biol Chem. 278:50226–50233. 2003.PubMed/NCBI View Article : Google Scholar | |
|
López-Grueso MJ, González-Ojeda R, Requejo-Aguilar R, McDonagh B, Fuentes-Almagro CA, Muntané J, Bárcena JA and Padilla CA: Thioredoxin and glutaredoxin regulate metabolism through different multiplex thiol switches. Redox Biol. 21(101049)2019.PubMed/NCBI View Article : Google Scholar | |
|
Ye K, Chen Z and Xu Y: The double-edged functions of necroptosis. Cell Death Dis. 14(163)2023.PubMed/NCBI View Article : Google Scholar | |
|
Che L, Yang CL, Chen Y, Wu ZL, Du ZB, Wu JS, Gan CL, Yan SP, Huang J, Guo NJ, et al: Mitochondrial redox-driven mitofusin 2 S-glutathionylation promotes neuronal necroptosis via disrupting ER-mitochondria crosstalk in cadmium-induced neurotoxicity. Chemosphere. 262(127878)2021.PubMed/NCBI View Article : Google Scholar | |
|
Bozonet SM, Magon NJ, Schwartfeger AJ, Konigstorfer A, Heath SG, Vissers MCM, Morris VK, Göbl C, Murphy JM, Salvesen GS and Hampton MB: Oxidation of caspase-8 by hypothiocyanous acid enables TNF-mediated necroptosis. J Biol Chem. 299(104792)2023.PubMed/NCBI View Article : Google Scholar | |
|
Gorelenkova Miller O, Behring JB, Siedlak SL, Jiang S, Matsui R, Bachschmid MM, Zhu X and Mieyal JJ: Upregulation of glutaredoxin-1 activates microglia and promotes neurodegeneration: Implications for Parkinson's disease. Antioxid Redox Signal. 25:967–982. 2016.PubMed/NCBI View Article : Google Scholar | |
|
Thawkar BS and Kaur G: Inhibitors of NF-κB and P2X7/NLRP3/Caspase 1 pathway in microglia: Novel therapeutic opportunities in neuroinflammation induced early-stage Alzheimer's disease. J Neuroimmunol. 326:62–74. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Shao R, Lou X, Xue J, Yang Y, Ning D, Chen G and Jiang L: Thioredoxin-1 regulates IRE1α to ameliorate sepsis-induced NLRP3 inflammasome activation and oxidative stress in Raw 264.7 cell. Immunopharmacol Immunotoxicol. 45:277–286. 2023.PubMed/NCBI View Article : Google Scholar | |
|
Wang Y, Shi P, Chen Q, Huang Z, Zou D, Zhang J, Gao X and Lin Z: Mitochondrial ROS promote macrophage pyroptosis by inducing GSDMD oxidation. J Mol Cell Biol. 11:1069–1082. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Newton K, Strasser A, Kayagaki N and Dixit VM: Cell death. Cell. 187:235–256. 2024.PubMed/NCBI View Article : Google Scholar | |
|
Chen T, Liang L, Wang Y, Li X and Yang C: Ferroptosis and cuproptposis in kidney diseases: Dysfunction of cell metabolism. Apoptosis. 29:289–302. 2024.PubMed/NCBI View Article : Google Scholar | |
|
Lee J, You JH, Shin D and Roh JL: Inhibition of glutaredoxin 5 predisposes cisplatin-resistant head and neck cancer cells to Ferroptosis. Theranostics. 10:7775–7786. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Seco-Cervera M, González-Cabo P, Pallardó FV, Romá-Mateo C and García-Giménez JL: Thioredoxin and glutaredoxin systems as potential targets for the development of new treatments in Friedreich's ataxia. Antioxidants (Basel). 9(1257)2020.PubMed/NCBI View Article : Google Scholar | |
|
Liu X, Zhuang L and Gan B: Disulfidptosis: Disulfide stress-induced cell death. Trends Cell Biol. 34:327–337. 2024.PubMed/NCBI View Article : Google Scholar | |
|
Elftmaoui Z and Bignon E: Robust AMBER force field parameters for glutathionylated cysteines. Int J Mol Sci. 24(15022)2023.PubMed/NCBI View Article : Google Scholar | |
|
Guan L, Mao Z, Yang S, Wu G, Chen Y, Yin L, Qi Y, Han L and Xu L: Dioscin alleviates Alzheimer's disease through regulating RAGE/NOX4 mediated oxidative stress and inflammation. Biomed Pharmacother. 152(113248)2022.PubMed/NCBI View Article : Google Scholar | |
|
Rani P, Krishnan S and Rani Cathrine C: Study on analysis of peripheral biomarkers for Alzheimer's disease diagnosis. Front Neurol. 8(328)2017.PubMed/NCBI View Article : Google Scholar | |
|
Akterin S, Cowburn RF, Miranda-Vizuete A, Jiménez A, Bogdanovic N, Winblad B and Cedazo-Minguez A: Involvement of glutaredoxin-1 and thioredoxin-1 in beta-amyloid toxicity and Alzheimer's disease. Cell Death Differ. 13:1454–1465. 2006.PubMed/NCBI View Article : Google Scholar | |
|
Kommaddi RP, Tomar DS, Karunakaran S, Bapat D, Nanguneri S, Ray A, Schneider BL, Nair D and Ravindranath V: Glutaredoxin1 diminishes amyloid beta-mediated oxidation of F-actin and reverses cognitive deficits in an Alzheimer's disease mouse model. Antioxid Redox Signal. 31:1321–1338. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Dionísio PA, Amaral JD and Rodrigues CMP: Oxidative stress and regulated cell death in Parkinson's disease. Ageing Res Rev. 67(101263)2021.PubMed/NCBI View Article : Google Scholar | |
|
Johnson WM, Yao C, Siedlak SL, Wang W, Zhu X, Caldwell GA, Wilson-Delfosse AL, Mieyal JJ and Chen SG: Glutaredoxin deficiency exacerbates neurodegeneration in C. elegans models of Parkinson's disease. Hum Mol Genet. 24:1322–1335. 2015.PubMed/NCBI View Article : Google Scholar | |
|
Xu J, Kao SY, Lee FJ, Song W, Jin LW and Yankner BA: Dopamine-dependent neurotoxicity of alpha-synuclein: A mechanism for selective neurodegeneration in Parkinson disease. Nat Med. 8:600–606. 2002.PubMed/NCBI View Article : Google Scholar | |
|
Johnson WM, Golczak M, Choe K, Curran PL, Miller OG, Yao C, Wang W, Lin J, Milkovic NM, Ray A, et al: Regulation of DJ-1 by glutaredoxin 1 in vivo: Implications for Parkinson's disease. Biochemistry. 55:4519–4532. 2016.PubMed/NCBI View Article : Google Scholar | |
|
Tokarew JM, El-Kodsi DN, Lengacher NA, Fehr TK, Nguyen AP, Shutinoski B, O'Nuallain B, Jin M, Khan JM, Ng ACH, et al: Age-associated insolubility of parkin in human midbrain is linked to redox balance and sequestration of reactive dopamine metabolites. Acta Neuropathol. 141:725–754. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Mailloux RJ, Xuan JY, McBride S, Maharsy W, Thorn S, Holterman CE, Kennedy CR, Rippstein P, deKemp R, da Silva J, et al: Glutaredoxin-2 is required to control oxidative phosphorylation in cardiac muscle by mediating deglutathionylation reactions. J Biol Chem. 289:14812–14828. 2014.PubMed/NCBI View Article : Google Scholar | |
|
Zima AV and Mazurek SR: Functional impact of ryanodine receptor oxidation on intracellular calcium regulation in the heart. Rev Physiol Biochem Pharmacol. 171:39–62. 2016.PubMed/NCBI View Article : Google Scholar | |
|
Wegener JW, Wagdi A, Wagner E, Katschinski DM, Hasenfuss G, Bruegmann T and Lehnart SE: The RyR2-R2474S mutation sensitizes cardiomyocytes and hearts to catecholaminergic stress-induced oxidation of the mitochondrial glutathione pool. Front Physiol. 12(777770)2021.PubMed/NCBI View Article : Google Scholar | |
|
Rashdan NA, Shrestha B and Pattillo CB: S-glutathionylation, friend or foe in cardiovascular health and disease. Redox Biol. 37(101693)2020.PubMed/NCBI View Article : Google Scholar | |
|
Mizuno M, Matsuzaki T, Ozeki N, Katano H, Koga H, Takebe T, Yoshikawa HY and Sekiya I: Cell membrane fluidity and ROS resistance define DMSO tolerance of cryopreserved synovial MSCs and HUVECs. Stem Cell Res Ther. 13(177)2022.PubMed/NCBI View Article : Google Scholar | |
|
Duan R, Pan H, Li D, Liao S and Han B: Ergothioneine improves myocardial remodeling and heart function after acute myocardial infarction via S-glutathionylation through the NF-ĸB dependent Wnt5a-sFlt-1 pathway. Eur J Pharmacol. 950(175759)2023.PubMed/NCBI View Article : Google Scholar | |
|
Fan X, Dong T, Yan K, Ci X and Peng L: PM2.5 increases susceptibility to acute exacerbation of COPD via NOX4/Nrf2 redox imbalance-mediated mitophagy. Redox Biol. 59(102587)2023.PubMed/NCBI View Article : Google Scholar | |
|
Kuipers I, Guala AS, Aesif SW, Konings G, Bouwman FG, Mariman EC, Wouters EF, Janssen-Heininger YM and Reynaert NL: Cigarette smoke targets glutaredoxin 1, increasing s-glutathionylation and epithelial cell death. Am J Respir Cell Mol Biol. 45:931–937. 2011.PubMed/NCBI View Article : Google Scholar | |
|
Chia SB, Nolin JD, Aboushousha R, Erikson C, Irvin CG, Poynter ME, van der Velden J, Taatjes DJ, van der Vliet A, Anathy V, et al: Glutaredoxin deficiency promotes activation of the transforming growth factor beta pathway in airway epithelial cells, in association with fibrotic airway remodeling. Redox Biol. 37(101720)2020.PubMed/NCBI View Article : Google Scholar | |
|
Guo Y, Liu Y, Zhao S, Xu W, Li Y, Zhao P, Wang D, Cheng H, Ke Y and Zhang X: Oxidative stress-induced FABP5 S-glutathionylation protects against acute lung injury by suppressing inflammation in macrophages. Nat Commun. 12(7094)2021.PubMed/NCBI View Article : Google Scholar | |
|
Zhang D, Wang X, Chen S, Chen S, Yu W, Liu X, Yang G, Tao Y, Tang X, Bu D, et al: Endogenous hydrogen sulfide sulfhydrates IKKβ at cysteine 179 to control pulmonary artery endothelial cell inflammation. Clin Sci (Lond). 133:2045–2059. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Reynaert NL, van der Vliet A, Guala AS, McGovern T, Hristova M, Pantano C, Heintz NH, Heim J, Ho YS, Matthews DE, et al: Dynamic redox control of NF-kappaB through glutaredoxin-regulated S-glutathionylation of inhibitory kappaB kinase beta. Proc Natl Acad Sci USA. 103:13086–13091. 2006.PubMed/NCBI View Article : Google Scholar | |
|
Zhang X, Liu P, Zhang C, Chiewchengchol D, Zhao F, Yu H, Li J, Kambara H, Luo KY, Venkataraman A, et al: Positive regulation of interleukin-1β bioactivity by physiological ROS-mediated cysteine S-glutathionylation. Cell Rep. 20:224–235. 2017.PubMed/NCBI View Article : Google Scholar | |
|
Anathy V, Lahue KG, Chapman DG, Chia SB, Casey DT, Aboushousha R, van der Velden JLJ, Elko E, Hoffman SM, McMillan DH, et al: Reducing protein oxidation reverses lung fibrosis. Nat Med. 24:1128–1135. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Shahmarvand N, Nagy A, Shahryari J and Ohgami RS: Mutations in the signal transducer and activator of transcription family of genes in cancer. Cancer Sci. 109:926–933. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Anastasiou D, Poulogiannis G, Asara JM, Boxer MB, Jiang JK, Shen M, Bellinger G, Sasaki AT, Locasale JW, Auld DS, et al: Inhibition of pyruvate kinase M2 by reactive oxygen species contributes to cellular antioxidant responses. Science. 334:1278–1283. 2011.PubMed/NCBI View Article : Google Scholar | |
|
Li X, Ma Y, Wu J, Ni M, Chen A, Zhou Y, Dai W, Chen Z, Jiang R, Ling Y, et al: Thiol oxidative stress-dependent degradation of transglutaminase2 via protein S-glutathionylation sensitizes 5-fluorouracil therapy in 5-fluorouracil-resistant colorectal cancer cells. Drug Resist Updat. 67(100930)2023.PubMed/NCBI View Article : Google Scholar | |
|
Zhang L, Zhang J, Ye Z, Manevich Y, Townsend DM, Marshall DT and Tew KD: S-glutathionylated serine proteinase inhibitors as biomarkers for radiation exposure in prostate cancer patients. Sci Rep. 9(13792)2019.PubMed/NCBI View Article : Google Scholar | |
|
Zhang J, Ye ZW, Chen W, Culpepper J, Jiang H, Ball LE, Mehrotra S, Blumental-Perry A, Tew KD and Townsend DM: Altered redox regulation and S-glutathionylation of BiP contribute to bortezomib resistance in multiple myeloma. Free Radic Biol Med. 160:755–767. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Abo M and Weerapana E: Chemical probes for redox signaling and oxidative stress. Antioxid Redox Signal. 30:1369–1386. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Xiao H, Jedrychowski MP, Schweppe DK, Huttlin EL, Yu Q, Heppner DE, Li J, Long J, Mills EL, Szpyt J, et al: A quantitative tissue-specific landscape of protein redox regulation during aging. Cell. 180:968–983.e24. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Subramani J, Kundumani-Sridharan V and Das KC: Chaperone-mediated autophagy of eNOS in myocardial ischemia-reperfusion injury. Circ Res. 129:930–945. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Wu X, Liu L, Zheng Q, Ye H, Yang H, Hao H and Li P: Dihydrotanshinone I preconditions myocardium against ischemic injury via PKM2 glutathionylation sensitive to ROS. Acta Pharm Sin B. 13:113–127. 2023.PubMed/NCBI View Article : Google Scholar |