Antibacterial and antiviral properties of punicalagin (Review)
- Authors:
- Published online on: August 26, 2025 https://doi.org/10.3892/mi.2025.264
- Article Number: 65
-
Copyright : © Song et al. This is an open access article distributed under the terms of Creative Commons Attribution License [CC BY 4.0].
Abstract
1. Introduction
Recently, the increasing prevalence of drug-resistant pathogens has created a strong demand for new antibiotics, further spurring the active exploration of natural plant compounds as alternatives to synthetic antimicrobial agents (1-3). Plants provide an abundance of antimicrobial materials, including tannins (4), flavonoids (5), phenolic acids (6), essential oils (7), saponins (8), alkaloids, terpenoids (9), coumarins (10) and lignans (11). Among these, polyphenols including punicalagin have garnered significant attention due to their diverse chemical structures and multiple mechanisms of action.
Punicalagin [2,3-(S)-hexahydroxydiphenoyl-4,6-(S,S)-gallagyl-D-glucose] is one of the principal active tannins isolated from pomegranate peel. Its molecular formula is C48H28O30, with a molecular weight of 1084.72(12). It is soluble in polar solvents, such as water and ethanol. In addition, the compound contains multiple phenolic hydroxyl groups, the presence of which renders punicalagin susceptible to degradation in alkaline environments. This results in the generation of compounds, such as andrographolide and ellagic acid, which possess the physicochemical properties of hydrolysable tannins. Additionally, the variety of ring structures (e.g., benzene rings) and chemical bonds in its structure imparts flexibility and conformational adaptability to the molecule, which may influence its biological activity.
Punicalagin exhibits a wide range of biological and pharmacological activities (13-20). Compared to other polyphenols, punicalagin exhibits unique advantages, such as its diverse mechanisms of action against both bacteria and viruses, and it has the potential to disrupt biofilm formation and quorum sensing, rendering it a promising candidate for developing new antimicrobial agents (21-23). Previous studies have mainly focused on its antioxidant (24,25), anticancer, anti-inflammatory and chronic-disease-relieving properties (26-28). In addition, punicalagin has been shown to have the ability to combat both bacterial and viral pathogens (29-32). While recent in vivo research confirms its therapeutic potential against bacterial infections (33), clinical evidence remains limited. Thus far, to the best of our knowledge, no comprehensive review has been published on this topic. Accordingly, the present review discusses the antibacterial and antiviral efficacy of punicalagin. The present review aimed to provide a comprehensive summary of the potential opportunities and challenges encountered in developing punicalagin as an effective therapeutic agent.
2. Antibacterial effects of punicalagin
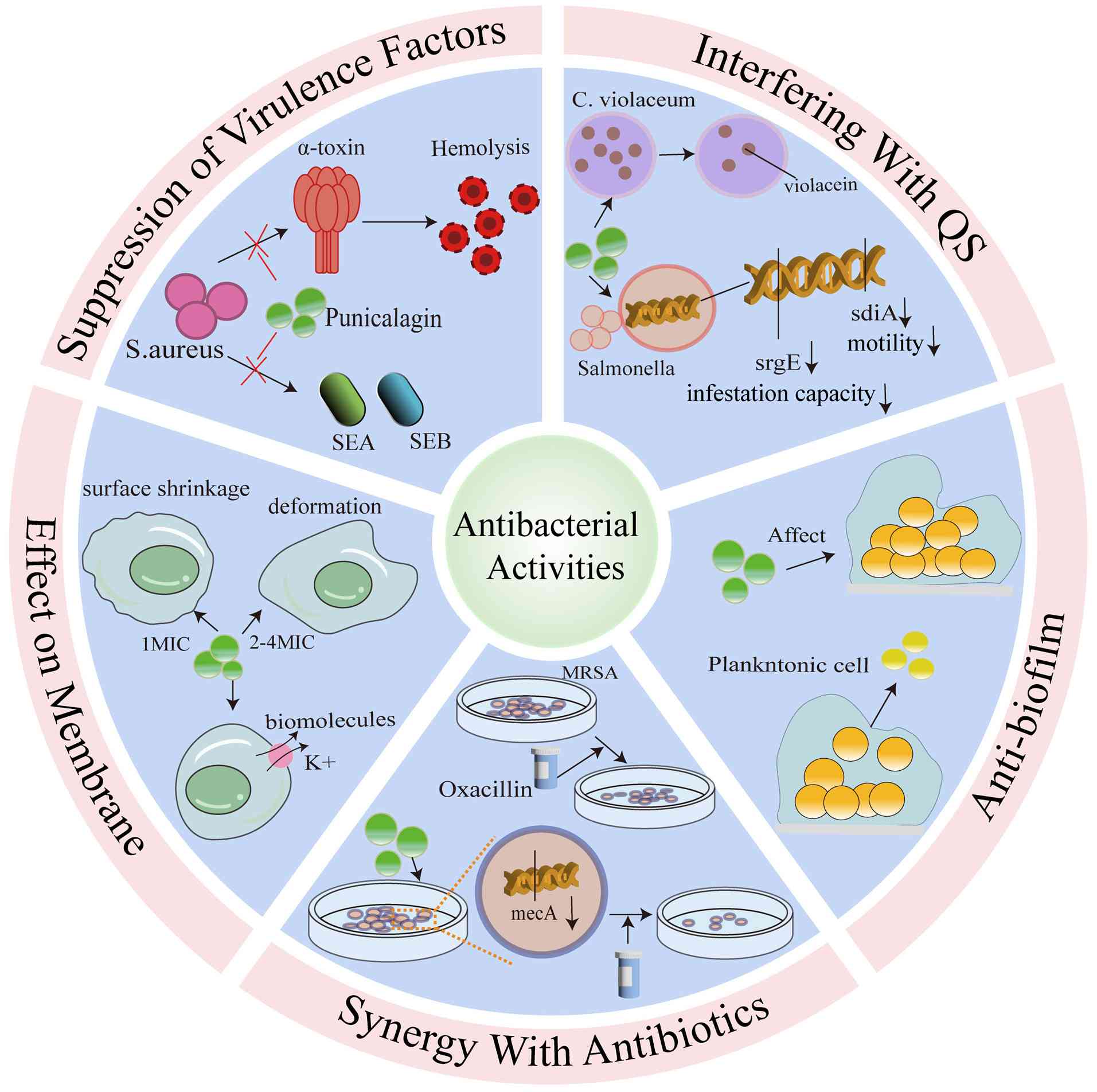
As illustrated in Fig. 1, the mechanisms of action of punicalagin against bacteria include targeting bacterial cell membranes and biofilms, quorum sensing, suppressing virulence factors and exerting synergistic effects with antibiotics.
Antibacterial activity
The inhibitory effects of punicalagin on bacterial growth have been extensively investigated, and its antimicrobial activity is usually measured using the minimum inhibitory concentration (MIC). A summary of the studies on the antibacterial effects of punicalagin against different bacteria is provided in Table I (34-38). Data from the literature reveal that MIC values for different bacterial strains vary significantly depending on the bacterial strains (34-38).
However, for the same strains, there were significant discrepancies in the reported MIC values. These variances are likely due to the following factors: i) Inherent differences between strains; ii) differences in culture conditions (e.g., Mueller-Hinton agar vs. tryptic soy broth); and/or iii) a discrepancy in the assay method (e.g., two-fold dilution method vs. agar dilution method). For instance, the MIC value of punicalagin against Escherichia coli ATCC 25922 was reported to be 1.2 µg/ml (35), while another study indicated an MIC value of 160,000 µg/ml for the same strain (36). Similarly, notable discrepancies were found in the MIC values for Staphylococcus aureus (S. aureus) ATCC 29213, with one study reporting an MIC value of 250 µg/ml (38) and another study reporting 20,000 µg/ml (36).
In addition, the antibacterial and bactericidal effects of punicalagin can be observed through bacterial growth curves. For instance, at MIC levels, punicalagin was previously shown to exert no significant effect on the growth of Salmonella typhimurium (S. typhimurium) within the first 2 h following exposure. However, by 24 h, viable cell counts were reduced by ~2 log units (39). For treatments at 2x and 4x MIC levels, the growth of S. typhimurium was completely inhibited within 24 h, indicating a bactericidal effect. Similarly, Xu et al (38) reported that at certain MIC levels, punicalagin decreased ~0.5 log of viable S. aureus cells following 2 h of exposure, and ~1 log of these cells was reduced following 24 h of incubation, indicating a bacteriostatic effect. When S. aureus was treated with 2x and 4x the MIC of punicalagin, the loss of viable cells was significantly more pronounced immediately after initial exposure (38).
Overall, punicalagin has demonstrated antibacterial effects against multiple bacterial strains in vitro; however, limited in vivo studies are available. Further studies are thus required to elucidate the differential susceptibility of various bacterial strains to punicalagin and to perform in vivo animal testing, which is crucial for the development of antibacterial drugs.
Inhibition of bacterial virulence factors of punicalagin
A key aspect of microbial pathogenesis is virulence, which is the ability of a pathogen to produce disease in a host, and virulence factors are the mechanisms through which pathogens cause damage to the host. These mechanisms include the secretion of toxins, adhesion to host surfaces, invasion of host tissues and the formation of biofilms (40). Bacterial pathogens can secrete diverse toxins with different structures and functions, which are crucial for the development of infectious diseases (41,42). Punicalagin is notable for its ability to directly target N-acyl-homoserine lactone-dependent quorum sensing (QS) systems, which are associated with virulence, invasiveness and pathogenicity. Staphylococcal protein A (SpA) is a key virulence factor of S. aureus, which binds to immunoglobulins through its Fcγ domain and the Fab heavy chain of VH3 family antibodies, thereby interfering with bacterial opsonophagocytosis and the development of adaptive B cell responses (43,44). Punicalagin reduces the fixation of SpA to the bacterial cell wall by inhibiting SrtA (33). Furthermore, punicalagin inhibits the expression of major virulence factors, such as hemolysin and enterotoxin in methicillin-resistant S. aureus (MRSA) (45). The main virulence factors of S. aureus cover a wide range of antigens, enzymes, cytotoxins and exotoxins. Among the virulence factors secreted by S. aureus, α-toxin is the main component of its hemolytic activity, while enterotoxin is one of the most prominent exotoxins of the bacterium. Enterotoxin is able to function as a superantigen to activate T-cells and induce them to release large amounts of pro-inflammatory cytokines (46). Therefore, targeting these virulence factors may provide an ideal approach for developing antibacterial therapeutics against S. aureus infections. Sub-inhibitory concentrations of punicalagin have been shown to reduce the production of α-toxin, staphylococcal enterotoxin A and staphylococcal enterotoxin B in an MRSA in a concentration-dependent manner and decrease the activity of the culture supernatant of S. aureus induced by tumor necrosis factor (45). Additionally, Li et al (37) found that punicalagin downregulated the expression of the majority of selected virulence genes in Salmonella and significantly reduced the invasion of Salmonella into colon cells, although it had no effect on adhesion. Moreover, sub-inhibitory concentrations of punicalagin have been shown to reduce the colony-forming ability and virulence factor expression of S. typhimurium (30). These findings strongly indicated that punicalagin might be used as a novel therapeutic agent in the treatment of bacterial infections.
Anti-biofilm properties of punicalagin
Bacteria can exist as planktonic cells, or they can attach to surfaces to form aggregates, which are termed biofilms. In biofilms, bacteria produce extracellular material in which cells are embedded (47). The formation of microbial biofilms enables individual planktonic cells to adopt a multicellular growth pattern (48). The benefit of bacterial biofilm formation is the creation of a more stable environment that allows bacteria to resist environmental threats, such as phagocytosis and antimicrobial agents (49). Thus, the inhibition of biofilm formation is viewed as a promising method for controlling bacterial infections (50). Punicalagin has been investigated for its ability to reduce biofilm formation and to affect the normal function of biofilms in various bacterial pathogens, and it exerts a significant inhibitory effect on the biofilm formation of S. aureus. In a previous study, the biomass of biofilm was reduced to 42.0% when the concentration of punicalagin was 1/64 MIC compared to the control, and it was significantly reduced to 8.1% when the concentration was further increased to 1/32 MIC (51). Similarly, the study by Xu et al (38) demonstrated that the biofilm formation of S. aureus was inhibited by 47% when it was treated with punicalagin at 1/64 MIC, while biofilm formation was inhibited by >90% when the concentration exceeded 1/32 MIC. Additionally, the study by Song et al (33) demonstrated that punicalagin effectively reduced biofilm formation by inhibiting sortase A activity on the cell surface of S. aureus.
Effects of punicalagin on the cytoplasmic membrane
The cytoplasmic membrane is a common target of action for a number of antimicrobial agents, and cell viability is usually closely linked to the integrity of the cytoplasmic membrane. It is generally recognized that the antimicrobial activity of numerous compounds is partly due to their ability to selectively disrupt the membrane structure and function of a variety of microorganisms. The significant loss of cytoplasmic components implies irreversible damage to the cytoplasmic membrane.
Potassium ions (K+) are the major cytoplasmic cations essential for bacterial growth, and they are involved in several key biological functions. Excessive K+ efflux can affect bacterial growth and can even result in mortality (52). The study by Liu et al (34) demonstrated that treatment with punicalagin induced significant potassium efflux from Vibrio parahaemolyticus (V. parahaemolyticus) bacterial cells in a concentration-dependent manner. In addition to ions, punicalagin increased the permeability to biomolecules such as nucleic acid. As the concentration of punicalagin treatment increased, the integrity of the V. parahaemolyticus cell membrane decreased and the severity of cellular damage increased in a concentration-dependent manner (34). This was mainly characterized by cell surface shrinkage under a 1x MIC treatment and extensive cellular deformation; the disruption of the cell membrane; and cytoplasmic leakage under 2x and 4x MIC treatments (44). Similarly, another study (38) demonstrated that punicalagin induced an immediate and massive efflux of K+ from S. aureus, suggesting that punicalagin affects the cytoplasmic membrane by increasing its permeability or interfering with the transmembrane proton gradient. Following treatment with punicalagin, S. aureus cells exhibited significant enlargement and a roughened surface, with a higher degree of deformation observed following treatment with high concentrations of punicalagin (38).
Moreover, it has been reported that punicalagin can exert an inhibitory effect on Salmonella by disrupting the cell membrane, as evidenced by potassium leakage, membrane depolarization, an increased pH gradient and microstructural alterations (39).
Punicalagin interference with QS
QS systems enable bacteria to respond to cell density and regulate gene expression through cell-to-cell communication (53). Bacteria with QS produce and release signaling molecules, termed autoinducers, that increase cell density (54). When the concentration of autoinducers reaches a certain threshold, the bacteria detect this signal and trigger changes in gene expression. In bacterial pathogens, QS systems play a crucial role in regulating virulence gene expression, allowing bacteria to launch coordinated attacks to overwhelm host defenses (55,56). QS systems have recently gained increasing attention as attractive targets for antimicrobial therapy (57). It has been suggested that the inactivation of the QS systems of a pathogen can result in a significant reduction in its virulence (58). In the study by Li et al (37), the findings from quantitative QS inhibition assays revealed that punicalagin inhibited the expression of Salmonella motility-related genes (e.g., sdiA and srgE), and punicalagin exhibited concentration-dependent anti-QS activity against Chromobacterium violaceum, suppressing violacein production to ~94.56% of the control at 1/64 MIC and markedly reducing it to 64.66% of the control at 1/32 MIC. However, research on the effects of punicalagin on bacterial QS systems remains limited, and further studies are warranted to fully elucidate its potential as an antimicrobial agent.
Synergistic effects of punicalagin with antibiotics against bacteria
Antibiotic adjuvants are used to achieve two primary objectives: i) Expand the antimicrobial spectrum of antibiotics; and ii) combat multidrug-resistant (MDR) bacterial infections through synergy with antibiotics. Therefore, the rational use of combination therapy with two or more drugs, such as antibiotics and natural compounds, can enhance antimicrobial efficacy by curbing the development of resistance in MDR bacteria (59-61). Punicalagin, in addition to its standalone antimicrobial activity, also exerts synergistic antimicrobial effects when combined with several common antibiotics. It is an effective enhancer, increasing the potency of cefotaxime and oxacillin against Gram-positive bacteria by interfering with bacterial transcription mechanisms and acting as a virulence inhibitor (33,62). In addition, the potential mechanism by which punicalagin enhances the potency of antibiotics against Gram-negative bacteria may involve membrane damage, allowing greater antibiotic absorption and toxicity. Additionally, impaired efflux pumps can lead to fatal interactions, rendering bacterial cells more sensitive to accelerated drug-induced cell death (39,63). The study by Song et al (33) demonstrated the synergistic effect of punicalagin with cefotaxime and ceftriaxone sodium, providing improved protection against MRSA-induced fatal pneumonia in mice, with inhibitory concentration fractional index values of 0.28125 and 0.3125, respectively. In another study, punicalagin enhanced the efficacy of oxacillin against MRSA by downregulating the transcription of mecA (a gene marker of methicillin resistance), resulting in a decrease in penicillin-binding protein 2a levels (62). In the presence of punicalagin, the MIC of oxacillin was reduced by 4- to 64-fold, clearly indicating that punicalagin enhanced the susceptibility of MRSA to oxacillin. This suggested that combination therapy with these two drugs could reverse β-lactam resistance in MRSA (62). Furthermore, it has also been demosntrated that punicalagin can enhance the sensitivity of MRSA to oxacillin (30).
In summary, punicalagin enhances the susceptibility of bacteria to some conventional antibiotics. However, whether punicalagin in combination with antibiotics enhances the antimicrobial effect on a broader range of bacteria remains to be studied.
3. Antiviral effects of punicalagin
The continued rise in the number of global viral infections has become a pressing issue for public health, and the development of new drugs is urgently required. In particular, coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has imposed an immense burden on global health, economies and societies (64-66). In this context, punicalagin has emerged as a promising antiviral agent against SARS-CoV-2. It has also been shown to inhibit a range of viruses, including human cytomegalovirus (HCMV), herpes simplex virus (HSV-1), hepatitis C virus (HCV), respiratory syncytial virus (RSV), measles virus (MV) and dengue virus (DENV), without exhibiting significant cytotoxicity at various concentrations (67).
Antiviral activity
The antiviral ability of punicalagin has been extensively studied by measuring the 50% inhibitory concentration (IC50), 50% effective concentration (EC50), 50% cytotoxic concentration (CC50), and selectivity index (SI). A summary of the data on the activity of punicalagin against various viral species is presented in Table II (68-77). The data obtained from the literature demonstrated that there are significant differences in IC50, EC50 and CC50 values, depending on the strain of viruses and substances (68-77).
Respiratory viruses
In recent years, respiratory infectious diseases have occurred more frequently worldwide, posing a serious threat to human health. Among these diseases, those caused by viral infections account for a large proportion. There are eight main viruses that cause acute respiratory infections in humans: RSV, influenza virus, coronavirus, rhinovirus, parainfluenza virus, adenovirus, boca virus and metapneumovirus (78). Research indicates that punicalagin can effectively eliminate infections caused by HCMV, HCV, DENV, MV and RSV at micromolar concentrations in a dose-dependent manner without significant cytotoxicity (67).
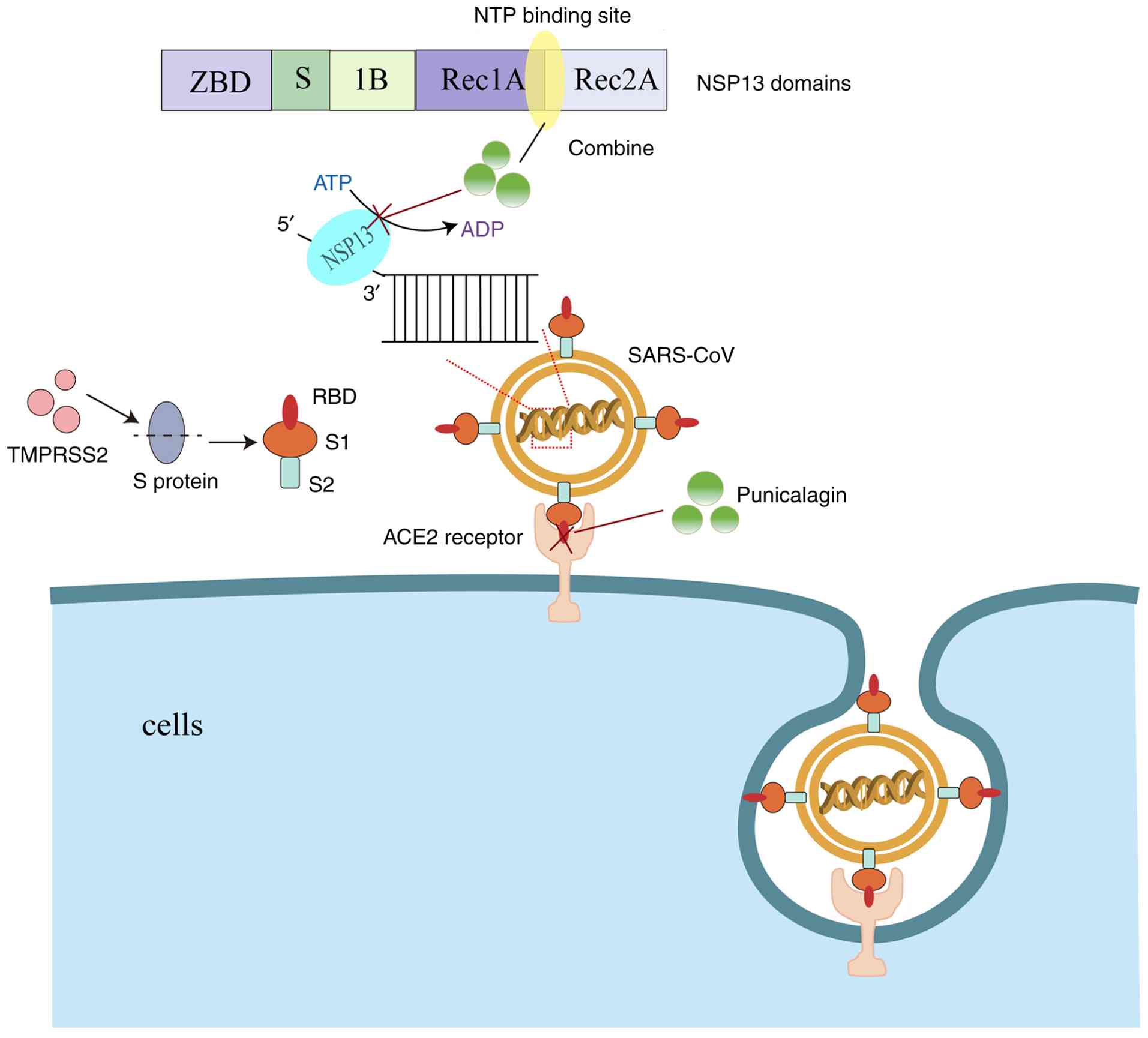
Infections with SARS-CoV-2 begin when the virus enters the cell through interactions between the viral spike (S) protein and host cell surface receptor angiotensin-converting enzyme 2 (ACE2) and transmembrane protease serine 2 (TMPRSS2), initiating the S protein (79). TMPRSS2 proteolytically processes the S protein into S1 and S2 fragments. The S1 fragment interacts with the ACE2 receptor via its receptor-binding domain (RBD), while the S2 fragment promotes the fusion of the virus with the host cell membrane (80). Punicalagin has been shown to disrupt the interaction between the spike glycoprotein RBD and the ACE2 receptor, with an IC50 value of 29 µM (68,81). SARS-CoV-2 NSP13 helicase is an essential enzyme for viral replication, and it has been identified as an ideal target for the development of antiviral drugs (82). Punicalagin binds NSP13 directly on the interface between the 1A and 2A domains and overlaps with the triphosphate binding site of the NTP, suggesting a direct competition mechanism. Punicalagin produces an antiviral effect by inhibiting the hydrolysis of ATP by NSP13 and the binding of NSP13 to DNA substrates, affecting viral replication (69). The aforementioned two mechanisms are illustrated in Fig. 2. Moreover, the main protease of SARS-CoV-2, also known as 3-chymotrypsin-like protease (3CLpro) or main protease (Mpro), is the only cysteine protease found in coronaviruses, and it is essential for viral replication (83,84). Published biological studies have demonstrated that small molecules that inhibit the activity of SARS-CoV-2 Mpro can lead to the inhibition of viral RNA replication, thereby exerting an antiviral effect (85-87). The IC50 value of punicalagin against 3CL protease has been found to be 6.192 µg/ml (70). In another similar experimental study, the results revealed that punicalagin reduced the activity of Mpro in a dose-dependent manner, with an IC50 value of 4.62±0.27 µM (71). Taken together, these studies suggest that punicalagin can exert anti-SARS-CoV-2 effects by the following mechanisms: i) Disrupting the interaction of spike glycoprotein RBD with ACE2 receptors; ii) inhibiting NSP13 helicase; and iii) Mpro activity.
Seasonal influenza is characterized by high morbidity and mortality rates, and it is mainly caused by the influenza A virus (IAV) or influenza B virus (IBV) (88). Due to antigenic mutations, adaptations, rearrangements and highly virulent strains, IAV can cause a devastating pandemic (89). In the search for natural products, punicalagin was previously shown to inhibit the replication of recombinant IAV PR8-PB2-Gluc (90,91). As previously demonstrated, punicalagin inhibited PR8-PB2-Gluc replication in a dose-responsive manner, with an IC50 value of 1.25±0.06 µM (75), and it induced a significant dose-dependent decrease in viral titers (76). In addition, the results from the virus release inhibition test revealed that punicalagin could block IAV release (75). Punicalagin also exhibited virucidal activity and inhibited the agglutination of the influenza virus to red blood cells (76). Punicalagin has been shown to inhibit the proliferation of IAV under both multi-cycle and single-cycle growth conditions (92).
In summary, some studies (as aforementioned) have shown that punicalagin inhibits a variety of respiratory viruses in vitro. These findings highlight the need for further clinical studies to explore the therapeutic and preventive potential of punicalagin in treating diseases caused by respiratory viruses (75).
Enterovirus
Globally, hand, foot and mouth disease (HFMD) is a common illness among young children and is a highly prevalent infectious disease in children <5 years of age (93). Coxsackievirus A16 (CVA16) and enterovirus 71 (EV71) are the two main pathogens of HFMD (94). Punicalagin has been shown to reduce the cytopathic effects caused by CAV16 infections in a dose-dependent manner. It inhibits infections by targeting the early entry phase of CVA16 via the direct inactivation of cell-free CVA16 viral particles and blocking viral attachment to the host cell surface (95). Previous studies have described punicalagin as a competitive inhibitor of glycosaminoglycans (GAGs), which are involved in viral entry. Punicalagin exerts broad-spectrum antiviral activity by preventing the entry of various enveloped viruses that utilize cell surface GAGs for infection (67), and the necessity of GAG involvement in CVA16 infections has also been demonstrated. The inhibitory effect of punicalagin on CVA16 is attributed to its ability to compete with cell surface GAGs for binding to viral particles, similarly to the action of soluble heparin (96). In addition to its effects on CVA16, punicalagin has also shown promise in combating EV71, another major pathogen associated with HFMD. Researchers have found that punicalagin significantly inhibits EV71 infections in rhabdomyosarcoma cells and reduces mortality in mice following a fatal EV71 challenge by inhibiting viral replication (97).
Therefore, given its demonstrated antiviral activity against both CVA16 and EV71, the two main causative agents of HFMD, punicalagin warrants further exploration and development as a potential therapeutic agent for the treatment of HFMD.
Hepatitis virus
The most common cause of viral hepatitis is HBV, which is the leading cause of end-stage liver disease worldwide (98). Covalently closed circular DNA (cccDNA) plays a crucial role in the life cycle of HBV. Thus, there is a pressing need to continuously develop new drugs for the treatment of HBV infections, in addition to developing drugs with the ability to eradicate cccDNA from HBV-infected hepatocytes (99,100). In a previous study, punicalagin significantly decreased the production of secreted HBeAg and cccDNA in a dose-dependent manner, without significant alterations in viral DNA replication. However, punicalagin exerted no significant effects on pronuclear/nuclear promoter activity, pgRNA transcription, core protein biosynthesis, or HBsAg secretion (101). Although vaccination is effective in interrupting vertical transmission and provides protection against HBV infections for 90% of the healthy population, there is a lack of 100% effective antiviral treatment options for patients with chronic hepatitis B (102). Thus, the anti-HBV infection effect of punicalagin is worth studying.
Other viruses
Feline herpesvirus type 1 (FHV-1), a key member of the α-Herpesviridae family, has triggered an extensive epidemiologic burden in feline populations worldwide, and its infection can result in acute upper respiratory syndrome, corneal ulcers, and lethal viral pneumonia; moreover, it now poses a threat to feline health worldwide (103). The complex glycoprotein system on the surface of the viral envelope is a key target for mediating host cell invasion and immune regulation, and 12 functional glycoproteins including glycoprotein B (gB), gC, gD, gE, gG, gH, gI, gJ, gK, gL, gM and gN have been identified (72,104). Among these, gB is an essential glycoprotein for herpesvirus-infected cells, and it plays critical roles in viral adsorption, mediating membrane fusion and in the infection of host cells by the virus (105). As an effective antiviral compound, punicalagin inhibits the early invasion process of the FHV-1 virus through a dual mechanism: i) It may function as a competitive inhibitor, blocking the binding of the viral gB protein to the host cell surface receptor; and ii) it durably inhibits its mediated membrane fusion by irreversibly inducing the closed conformation of the gB protein (72). Similarly, African swine fever (ASF) is an acute febrile infectious disease caused by the African swine fever virus (ASFV), which is characterized by extreme contagiousness and high lethality (106). Punicalagin has been found to act on the early stages of ASFV replication, including attachment and internalization, and it can directly inactivate the virus. In addition, punicalagin can regulate the NF-κB/STAT3/NLRP3 signaling pathway, thereby alleviating ASFV-induced inflammation (107).
Human immunodeficiency virus (HIV) primarily targets CD4+ T-lymphocytes and CD8+ T-lymphocytes, which are key components of the adaptive immune system; for this reason, HIV infections cause damage to the immune system. Damage to the body occurs at all stages of HIV infection, and patients with acquired immunodeficiency syndrome (AIDS) have a relatively high rate of disability (108,109). While antiretroviral therapy has significantly increased the life expectancy of patients with HIV, the disease remains a major global public health challenge (110). Punicalagin was previously found to inhibit HIV-1 reverse transcriptase-associated RNase H activity and integrase LEDGF-dependent activity, particularly targeting HIV-1 integrase LEDGF-dependent activity (73). This compound effectively suppressed HIV-1 replication in infected H9 lymphocytes while exhibiting low cytotoxicity (73).
Mayaro virus (MAYV) is an emerging arthropod-borne virus (Arbovirus). Mayaro fever (MF), caused by MAYV, typically presents as a non-specific febrile illness that can evolve into an arthritic condition that persists for months after the infection has resolved (111). An anti-MAYV study demonstrated that the ethanolic extract of Punica granatum exhibited a high selectivity index value of 49, with significant virucidal activity of about 98%; in contrast, it was observed that a partial selectivity index of 15 with punicalagin as the main component exhibited strong antiviral activity (74).
4. Conclusions and future perspectives
With the emergence and evolution of bacterial and viral drug resistance, the potential benefits and therapeutic value of punicalagin are of immense interest to scientists. The present review indicates that punicalagin exerts a broad inhibitory effect on a wide variety of bacteria and viruses. In terms of antibacterial effects, it is able to effectively inhibit the growth of Gram- and Gram-negative bacteria. The inhibitory effects of punicalagin on bacteria are mainly achieved by influencing virulence factors, interfering with the formation and normal activity of biofilms, and disrupting QS. In terms of antiviruses, punicalagin inhibits viral replication and spread by targeting the interaction of viral glycoproteins with host cells and preventing viruses from entering cells. For example, punicalagin exhibits good antiviral effects against both HSV-1 and EV71. In addition, punicalagin also exerts antioxidant and immunomodulatory effects, further enhancing its potential application value in disease prevention and treatment.
Possible interactions with different types of conventional antimicrobial agents also need to be considered in practical applications. Punicalagin, as an effective enhancer, can increase the potency of cefotaxime and oxacillin against Gram-positive bacteria and can function as a virulence inhibitor by interfering with bacterial transcriptional machinery. Therefore, future studies are warranted to explore the potential of using punicalagin as a synergist in combination with other treatments.
It is worth noting that while the present review highlights the multi-targeted antibacterial mechanisms of punicalagin, the risk of bacterial or viral resistance remains unaddressed. Unlike traditional antimicrobial drugs that target a single key pathway, pomegranate peel glycosides exhibit multifaceted pharmacological effects. For example, they inhibit the SARS-CoV-2 NSP13 helicase, 3CLpro protease, and the interactions between the spike protein and ACE2 receptor. These multifaceted effects may create greater evolutionary barriers to microbial escape. However, to draw definitive conclusions, future studies are required to characterize resistance mechanisms through mutation frequency analyses, antimicrobial screening experiments, and comparative risk assessments with traditional drugs. Additionally, the potential risks of punicalagin with respect to bacterial and viral resistance need to be evaluated. Furthermore, future studies are required to further uncover the mechanism of interactions between punicalagin and pathogens, particularly its specific targets at the cellular and molecular levels. On this basis, through chemical modification or nanotechnology and other means, the bioavailability and stability of punicalagin will be improved, its antimicrobial and antiviral efficacy in vivo will be enhanced, and new antibacterial and antiviral drug preparations based on punicalagin, such as oral preparations, injections, or topical drugs, will be developed. In addition, no clinical trials or regulatory filings for punicalagin as an antimicrobial have yet been reported. The primary challenges in translating preclinical research findings into clinical applications may include the need for further research to improve their bioavailability and stability, the necessity of conducting comprehensive pharmacokinetic and pharmacodynamic studies, and the requirement of thorough safety assessments to ensure their suitability for human use. It is also critical to conduct clinical trials in order to verify the safety and efficacy of punicalagin in the treatment of bacterial infections and viral infectious diseases and to explore the combined application of punicalagin with other drugs to produce synergistic antibacterial and antiviral effects and reduce the development of drug resistance.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by grants from the National Key Research and Development Project (grant no. 2023YFC2605603), the National Natural Science Foundation of China (grant no. 82273696) and the Program for Innovative Research Team (in Science and Technology) at the University of Henan Province (grant no. 25IRTSTHN038).
Availability of data and materials
Not applicable.
Authors' contributions
HY conceptualized the study. ZS, YW, PZ, YW, YL, JL and FL performed the literature search. ZS wrote the manuscript. All the authors reviewed and modified the final paper, and have read and approved the final manuscript. Data authentication is not applicable.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
|
Zhou YX, Cao XY and Peng C: Antimicrobial activity of natural products against MDR bacteria: A scientometric visualization analysis. Front Pharmacol. 13(1000974)2022.PubMed/NCBI View Article : Google Scholar | |
|
Arrigoni R, Ballini A, Topi S, Bottalico L, Jirillo E and Santacroce L: Antibiotic resistance to mycobacterium tuberculosis and potential use of natural and biological products as alternative anti-mycobacterial agents. Antibiotics (Basel). 11(1431)2022.PubMed/NCBI View Article : Google Scholar | |
|
Zhai X, Wu G, Tao X, Yang S, Lv L, Zhu Y, Dong D and Xiang H: Success stories of natural product-derived compounds from plants as multidrug resistance modulators in microorganisms. RSC Adv. 13:7798–7817. 2023.PubMed/NCBI View Article : Google Scholar | |
|
Ajebli M and Eddouks M: The promising role of plant tannins as bioactive antidiabetic agents. Curr Med Chem. 26:4852–4884. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Serafini M, Peluso I and Raguzzini A: Flavonoids as anti-inflammatory agents. Proc Nutr Soc. 69:273–278. 2010.PubMed/NCBI View Article : Google Scholar | |
|
Tessema FB, Gonfa YH, Asfaw TB, Tadesse TG, Tadesse MG, Bachheti A, Pandey DP, Wabaidur SM, Dahlous KA, Širić I, et al: Flavonoids and phenolic acids from aerial part of ajuga integrifolia (Buch.-Ham. Ex D. Don): Anti-shigellosis activity and in silico molecular docking studies. Molecules. 28(1111)2023.PubMed/NCBI View Article : Google Scholar | |
|
Aziz ZAA, Ahmad A, Setapar SHM, Karakucuk A, Azim MM, Lokhat D, Rafatullah M, Ganash M, Kamal MA and Ashraf GM: Essential oils: Extraction techniques, pharmaceutical and therapeutic potential-a review. Curr Drug Metab. 19:1100–1110. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Güçlü-Ustündağ O and Mazza G: Saponins: Properties, applications and processing. Crit Rev Food Sci Nutr. 47:231–258. 2007.PubMed/NCBI View Article : Google Scholar | |
|
Bergman ME, Davis B and Phillips MA: Medically useful plant terpenoids: Biosynthesis, occurrence, and mechanism of action. Molecules. 24(3961)2019.PubMed/NCBI View Article : Google Scholar | |
|
Bhattarai N, Kumbhar AA, Pokharel YR and Yadav PN: Anticancer potential of coumarin and its derivatives. Mini Rev Med Chem. 21:2996–3029. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Tchegnitegni Toussie B, Nguengang RT, Mawabo IK, Teponno RB, Kezetas Bankeu JJ, Chouna JR, Nkenfou CN, Tapondjou LA, Sewald N and Lenta BN: Bioactive arylnaphthalide lignans from justicia depauperata. J Nat Prod. 85:2731–2739. 2022.PubMed/NCBI View Article : Google Scholar | |
|
Barbieri M and Heard CM: Isolation of punicalagin from Punica granatum rind extract using mass-directed semi-preparative ESI-AP single quadrupole LC-MS. J Pharm Biomed Anal. 166:90–94. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Hassan MHU, Shahbaz M, Momal U, Naeem H, Imran M, Abdelgawad MA, Ghoneim MM, Mostafa EM, El-Ghorab AH, Alsagaby SA, et al: Exploring punicalagin potential against cancers: A comprehensive review. Food Sci Nutr. 13(e70072)2025.PubMed/NCBI View Article : Google Scholar | |
|
Abu-Elfotuh K, Abbas AN, Najm MAA, Qasim QA, Hamdan AME, Abdelrehim AB, Gowifel AMH, Al-Najjar AH, Atwa AM, Kozman MR, et al: Neuroprotective effects of punicalagin and/or micronized zeolite clinoptilolite on manganese-induced Parkinson's disease in a rat model: Involvement of multiple pathways. CNS Neurosci Ther. 30(e70008)2024.PubMed/NCBI View Article : Google Scholar | |
|
Zoofeen U, Shah M, Sultan S, Ehtesham E, Shah I, Sharif N, Khan M and Shah FA: Punicalagin improves inflammation and oxidative stress in rat model of pelvic inflammatory disease. Nat Prod Res. 39:2780–2786. 2025.PubMed/NCBI View Article : Google Scholar | |
|
Siddiqui N, Saifi A, Chaudhary A, Tripathi PN, Chaudhary A and Sharma A: Multifaceted neuroprotective role of punicalagin: A review. Neurochem Res. 49:1427–1436. 2024.PubMed/NCBI View Article : Google Scholar | |
|
Alalawi S, Albalawi F and Ramji DP: The role of punicalagin and its metabolites in atherosclerosis and risk factors associated with the disease. Int J Mol Sci. 24(8476)2023.PubMed/NCBI View Article : Google Scholar | |
|
Venusova E, Kolesarova A, Horky P and Slama P: Physiological and immune functions of punicalagin. Nutrients. 13(2150)2021.PubMed/NCBI View Article : Google Scholar | |
|
Salem HA, Abu-Elfotuh K, Alzahrani S, Rizk NI, Ali HS, Elsherbiny N, Aljohani A, Hamdan AME, Chellasamy P, Abdou NS, et al: Punicalagin's protective effects on Parkinson's progression in socially isolated and socialized rats: insights into multifaceted pathway. Pharmaceutics. 15(2420)2023.PubMed/NCBI View Article : Google Scholar | |
|
Al-Khawalde AAA, Abukhalil MH, Jghef MM, Alfwuaires MA, Alaryani FS, Aladaileh SH, Algefare AI, Karimulla S, Alasmari F, Aldal'in HK, et al: Punicalagin protects against the development of methotrexate-induced hepatotoxicity in mice via activating Nrf2 signaling and decreasing oxidative stress, inflammation, and cell death. Int J Mol Sci. 23(12334)2022.PubMed/NCBI View Article : Google Scholar | |
|
Xu W, Zhang T, Wang Z, Liu T, Liu Y, Cao Z and Sui Z: Two potent cytochrome P450 2D6 inhibitors found in Rhodiola rosea. Pharmazie. 68:974–976. 2013.PubMed/NCBI | |
|
Liu F, Smith AD, Wang TTY, Pham Q, Yang H and Li RW: Multi-omics analysis detected multiple pathways by which pomegranate punicalagin exerts its biological effects in modulating host-microbiota interactions in murine colitis models. Food Funct. 14:3824–3837. 2023.PubMed/NCBI View Article : Google Scholar | |
|
Liu F, Smith AD, Wang TTY, Pham Q, Yang H and Li RW: Ellagitannin punicalagin disrupts the pathways related to bacterial growth and affects multiple pattern recognition receptor signaling by acting as a selective histone deacetylase inhibitor. J Agric Food Chem. 71:5016–5026. 2023.PubMed/NCBI View Article : Google Scholar | |
|
Yaidikar L and Thakur S: Punicalagin attenuated cerebral ischemia-reperfusion insult via inhibition of proinflammatory cytokines, up-regulation of Bcl-2, down-regulation of Bax, and caspase-3. Mol Cell Biochem. 402:141–148. 2015.PubMed/NCBI View Article : Google Scholar | |
|
Kim JH, Kwack MH and Lee WJ: Effects of antioxidants on skin hydration, inflammatory cytokines, and keratinocyte differentiation markers in a PM(10)-exposed skin barrier-disrupted mouse model. Int J Immunopathol Pharmacol. 38(3946320241303860)2024.PubMed/NCBI View Article : Google Scholar | |
|
An X, Zhang Y, Cao Y, Chen J, Qin H and Yang L: Punicalagin protects diabetic nephropathy by inhibiting pyroptosis based on TXNIP/NLRP3 pathway. Nutrients. 12(1516)2020.PubMed/NCBI View Article : Google Scholar | |
|
Berdowska I, Matusiewicz M and Fecka I: Punicalagin in cancer prevention-via signaling pathways targeting. Nutrients. 13(2733)2021.PubMed/NCBI View Article : Google Scholar | |
|
Xu J, Cao K, Liu X, Zhao L, Feng Z and Liu J: Punicalagin regulates signaling pathways in inflammation-associated chronic diseases. Antioxidants (Basel). 11(29)2021.PubMed/NCBI View Article : Google Scholar | |
|
da Silva RA, Ishikiriama BLC, Ribeiro Lopes MM, de Castro RD, Garcia CR, Porto VC, Santos CF, Neppelenbroek KH and Lara VS: Antifungal activity of Punicalagin-nystatin combinations against Candida albicans. Oral Dis. 26:1810–1819. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Kiran S, Tariq A, Iqbal S, Naseem Z, Siddique W, Jabeen S, Bashir R, Hussain A, Rahman M, Habib FE, et al: Punicalagin, a pomegranate polyphenol sensitizes the activity of antibiotics against three MDR pathogens of the Enterobacteriaceae. BMC Complement Med Ther. 24(93)2024.PubMed/NCBI View Article : Google Scholar | |
|
Mandal A and Hazra B: Medicinal plant molecules against hepatitis C virus: Current status and future prospect. Phytother Res. 37:4353–4374. 2023.PubMed/NCBI View Article : Google Scholar | |
|
Ismat F, Tariq A, Shaheen A, Ullah R, Raheem K, Muddassar M, Mahboob S, Abbas W, Iqbal M and Rahman M: Inhibition of NS2B-NS3 protease from all four serotypes of dengue virus by punicalagin, punicalin and ellagic acid identified from Punica granatum. J Biomol Struct Dyn: Feb 19, 2024 (Epub ahead of print). | |
|
Song W, Wang L, Jin M, Guo X, Wang X, Guan J and Zhao Y: Punicalagin, an inhibitor of sortase a, is a promising therapeutic drug to combat methicillin-resistant staphylococcus aureus infections. Antimicrob Agents Chemother. 66(e0022422)2022.PubMed/NCBI View Article : Google Scholar | |
|
Liu H, Zhu W, Zou Y and Xia X: Antimicrobial activity and mechanisms of punicalagin against Vibrio parahaemolyticus. Foods. 13(1366)2024.PubMed/NCBI View Article : Google Scholar | |
|
Gosset-Erard C, Zhao M, Lordel-Madeleine S and Ennahar S: Identification of punicalagin as the bioactive compound behind the antimicrobial activity of pomegranate (Punica granatum L.) peels. Food Chem. 352(129396)2021.PubMed/NCBI View Article : Google Scholar | |
|
Taguri T, Tanaka T and Kouno I: Antimicrobial activity of 10 different plant polyphenols against bacteria causing food-borne disease. Biol Pharm Bull. 27:1965–1969. 2004.PubMed/NCBI View Article : Google Scholar | |
|
Li G, Yan C, Xu Y, Feng Y, Wu Q, Lv X, Yang B, Wang X and Xia X: Punicalagin inhibits Salmonella virulence factors and has anti-quorum-sensing potential. Appl Environ Microbiol. 80:6204–6211. 2014.PubMed/NCBI View Article : Google Scholar | |
|
Xu Y, Shi C, Wu Q, Zheng Z, Liu P, Li G, Peng X and Xia X: Antimicrobial activity of punicalagin against staphylococcus aureus and its effect on biofilm formation. Foodborne Pathog Dis. 14:282–287. 2017.PubMed/NCBI View Article : Google Scholar | |
|
Li G, Xu Y, Pan L and Xia X: Punicalagin damages the membrane of salmonella typhimurium. J Food Prot. 83:2102–2106. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Cai X, Zheng W and Li Z: High-throughput screening strategies for the development of anti-virulence inhibitors against staphylococcus aureus. Curr Med Chem. 26:2297–2312. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Mühlen S and Dersch P: Anti-virulence strategies to target bacterial infections. Curr Top Microbiol Immunol. 398:147–183. 2016.PubMed/NCBI View Article : Google Scholar | |
|
Rasko DA and Sperandio V: Anti-virulence strategies to combat bacteria-mediated disease. Nat Rev Drug Discov. 9:117–128. 2010.PubMed/NCBI View Article : Google Scholar | |
|
Goodyear CS and Silverman GJ: Death by a B cell superantigen: In vivo VH-targeted apoptotic supraclonal B cell deletion by a Staphylococcal Toxin. J Exp Med. 197:1125–1139. 2003.PubMed/NCBI View Article : Google Scholar | |
|
Falugi F, Kim HK, Missiakas DM and Schneewind O: Role of protein A in the evasion of host adaptive immune responses by Staphylococcus aureus. mBio. 4:e00575–00513. 2013.PubMed/NCBI View Article : Google Scholar | |
|
Mun SH, Kong R, Seo YS, Zhou T, Kang OH, Shin DW and Kwon DY: Subinhibitory concentrations of punicalagin reduces expression of virulence-related exoproteins by Staphylococcus aureus. FEMS Microbiol Lett. 363(fnw253)2016.PubMed/NCBI View Article : Google Scholar | |
|
Qiu J, Wang J, Luo H, Du X, Li H, Luo M, Dong J, Chen Z and Deng X: The effects of subinhibitory concentrations of costus oil on virulence factor production in Staphylococcus aureus. J Appl Microbiol. 110:333–340. 2011.PubMed/NCBI View Article : Google Scholar | |
|
Flemming HC and Wingender J: The biofilm matrix. Nat Rev Microbiol. 8:623–633. 2010.PubMed/NCBI View Article : Google Scholar | |
|
Xu S, Kang A, Tian Y, Li X, Qin S, Yang R and Guo Y: Plant flavonoids with antimicrobial activity against methicillin-resistant staphylococcus aureus (MRSA). ACS Infect Dis. 10:3086–3097. 2024.PubMed/NCBI View Article : Google Scholar | |
|
Singh S, Singh SK, Chowdhury I and Singh R: Understanding the mechanism of bacterial biofilms resistance to antimicrobial agents. Open Microbiol J. 11:53–62. 2017.PubMed/NCBI View Article : Google Scholar | |
|
Rumbaugh KP and Sauer K: Biofilm dispersion. Nat Rev Microbiol. 18:571–586. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Xu Y, Guo W, Luo D, Li P, Xiang J, Chen J, Xia X and Xie Q: Antibiofilm effects of punicalagin against Staphylococcus aureus in vitro. Front Microbiol. 14(1175912)2023.PubMed/NCBI View Article : Google Scholar | |
|
Ultee A, Kets EP and Smid EJ: Mechanisms of action of carvacrol on the food-borne pathogen Bacillus cereus. Appl Environ Microbiol. 65:4606–4610. 1999.PubMed/NCBI View Article : Google Scholar | |
|
Azimi S, Klementiev AD, Whiteley M and Diggle SP: Bacterial quorum sensing during infection. Annu Rev Microbiol. 74:201–219. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Garg N, Manchanda G and Kumar A: Bacterial quorum sensing: Circuits and applications. Antonie Van Leeuwenhoek. 105:289–305. 2014.PubMed/NCBI View Article : Google Scholar | |
|
Abisado RG, Benomar S, Klaus JR, Dandekar AA and Chandler JR: Bacterial quorum sensing and microbial community interactions. mBio. 9:e02331–17. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Miller MB and Bassler BL: Quorum sensing in bacteria. Annu Rev Microbiol. 55:165–199. 2001.PubMed/NCBI View Article : Google Scholar | |
|
Finch RG, Pritchard DI, Bycroft BW, Williams P and Stewart GS: Quorum sensing: a novel target for anti-infective therapy. J Antimicrob Chemother. 42:569–571. 1998.PubMed/NCBI View Article : Google Scholar | |
|
Hentzer M and Givskov M: Pharmacological inhibition of quorum sensing for the treatment of chronic bacterial infections. J Clin Invest. 112:1300–1307. 2003.PubMed/NCBI View Article : Google Scholar | |
|
Rudkin JK, Laabei M, Edwards AM, Joo HS, Otto M, Lennon KL, O'Gara JP, Waterfield NR and Massey RC: Oxacillin alters the toxin expression profile of community-associated methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 58:1100–1107. 2014.PubMed/NCBI View Article : Google Scholar | |
|
Gonzales PR, Pesesky MW, Bouley R, Ballard A, Biddy BA, Suckow MA, Wolter WR, Schroeder VA, Burnham CA, Mobashery S, et al: Synergistic, collaterally sensitive β-lactam combinations suppress resistance in MRSA. Nat Chem Biol. 11:855–861. 2015.PubMed/NCBI View Article : Google Scholar | |
|
Taylor PW: Alternative natural sources for a new generation of antibacterial agents. Int J Antimicrob Agents. 42:195–201. 2013.PubMed/NCBI View Article : Google Scholar | |
|
Mun SH, Kang OH, Kong R, Zhou T, Kim SA, Shin DW and Kwon DY: Punicalagin suppresses methicillin resistance of Staphylococcus aureus to oxacillin. J Pharmacol Sci. 137:317–323. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Chusri S, Villanueva I, Voravuthikunchai SP and Davies J: Enhancing antibiotic activity: A strategy to control Acinetobacter infections. J Antimicrob Chemother. 64:1203–1211. 2009.PubMed/NCBI View Article : Google Scholar | |
|
Chan JF, Yuan S, Kok KH, To KK, Chu H, Yang J, Xing F, Liu J, Yip CC, Poon RW, et al: A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: A study of a family cluster. Lancet. 395:514–523. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Tan W, Zhao X, Ma X, Wang W, Niu P, Xu W, Gao GF and Wu G: A novel coronavirus genome identified in a cluster of pneumonia cases - Wuhan, China 2019-2020. China CDC Wkly. 2:61–62. 2020.PubMed/NCBI | |
|
Wu F, Zhao S, Yu B, Chen YM, Wang W, Song ZG, Hu Y, Tao ZW, Tian JH, Pei YY, et al: A new coronavirus associated with human respiratory disease in China. Nature. 579:265–269. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Lin LT, Chen TY, Lin SC, Chung CY, Lin TC, Wang GH, Anderson R, Lin CC and Richardson CD: Broad-spectrum antiviral activity of chebulagic acid and punicalagin against viruses that use glycosaminoglycans for entry. BMC Microbiol. 13(187)2013.PubMed/NCBI View Article : Google Scholar | |
|
Chen HF, Wang WJ, Chen CY, Chang WC, Hsueh PR, Peng SL, Wu CS, Chen Y, Huang HY, Shen WJ, et al: The natural tannins oligomeric proanthocyanidins and punicalagin are potent inhibitors of infection by SARS-CoV-2. Elife. 12(e84899)2023.PubMed/NCBI View Article : Google Scholar | |
|
Lu L, Peng Y, Yao H, Wang Y, Li J, Yang Y and Lin Z: Punicalagin as an allosteric NSP13 helicase inhibitor potently suppresses SARS-CoV-2 replication in vitro. Antiviral Res. 206(105389)2022.PubMed/NCBI View Article : Google Scholar | |
|
Saadh MJ, Almaaytah AM, Alaraj M, Dababneh MF, Sa'adeh I, Aldalaen SM, Kharshid AM, Alboghdadly A, Hailat M, Khaleel A, et al: Punicalagin and zinc (II) ions inhibit the activity of SARS-CoV-2 3CL-protease in vitro. Eur Rev Med Pharmacol Sci. 25:3908–3913. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Du R, Cooper L, Chen Z, Lee H, Rong L and Cui Q: Discovery of chebulagic acid and punicalagin as novel allosteric inhibitors of SARS-CoV-2 3CL(pro). Antiviral Res. 190(105075)2021.PubMed/NCBI View Article : Google Scholar | |
|
Liu B, Jiao XQ, Dong XF, Guo P, Wang SB and Qin ZH: Saikosaponin B2, punicalin, and punicalagin in vitro block cellular entry of feline herpesvirus-1. Viruses. 16(231)2024.PubMed/NCBI View Article : Google Scholar | |
|
Sanna C, Marengo A, Acquadro S, Caredda A, Lai R, Corona A, Tramontano E, Rubiolo P and Esposito F: In Vitro Anti-HIV-1 reverse transcriptase and integrase properties of punica granatum L. Leaves, bark, and peel extracts and their main compounds. Plants (Basel). 10(2124)2021.PubMed/NCBI View Article : Google Scholar | |
|
Salles TS, Meneses MDF, Caldas LA, Sá-Guimarães TE, de Oliveira DM, Ventura JA, Azevedo RC, Kuster RM, Soares MR and Ferreira DF: Virucidal and antiviral activities of pomegranate (Punica granatum) extract against the mosquito-borne Mayaro virus. Parasit Vectors. 14(443)2021.PubMed/NCBI View Article : Google Scholar | |
|
Li P, Du R, Chen Z, Wang Y, Zhan P, Liu X, Kang D, Chen Z, Zhao X, Wang L, et al: Punicalagin is a neuraminidase inhibitor of influenza viruses. J Med Virol. 93:3465–3472. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Javadi-Farsani F, Karimi A, Razavi Nikoo H, Moradi MT and Tabarraei A: An in vitro antiviral evaluation of punicalagin toward influenza A virus. Avicenna J Phytomed. 14:496–504. 2024.PubMed/NCBI View Article : Google Scholar | |
|
Lin LT, Chen TY, Chung CY, Noyce RS, Grindley TB, McCormick C, Lin TC, Wang GH, Lin CC and Richardson CD: Hydrolyzable tannins (chebulagic acid and punicalagin) target viral glycoprotein-glycosaminoglycan interactions to inhibit herpes simplex virus 1 entry and cell-to-cell spread. J Virol. 85:4386–4398. 2011.PubMed/NCBI View Article : Google Scholar | |
|
Li ZJ, Zhang HY, Ren LL, Lu QB, Ren X, Zhang CH, Wang YF, Lin SH, Zhang XA, Li J, et al: Etiological and epidemiological features of acute respiratory infections in China. Nat Commun. 12(5026)2021.PubMed/NCBI View Article : Google Scholar | |
|
Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, et al: SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 181:271–280.e8. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Wang X, Xia S, Wang Q, Xu W, Li W, Lu L and Jiang S: Broad-spectrum coronavirus fusion inhibitors to combat COVID-19 and other emerging coronavirus diseases. Int J Mol Sci. 21(3843)2020.PubMed/NCBI View Article : Google Scholar | |
|
Invernizzi L, Moyo P, Cassel J, Isaacs FJ, Salvino JM, Montaner LJ, Tietjen I and Maharaj V: Use of hyphenated analytical techniques to identify the bioactive constituents of Gunnera perpensa L., a South African medicinal plant, which potently inhibit SARS-CoV-2 spike glycoprotein-host ACE2 binding. Anal Bioanal Chem. 414:3971–3985. 2022.PubMed/NCBI View Article : Google Scholar | |
|
Inniss NL, Rzhetskaya M, Ling-Hu T, Lorenzo-Redondo R, Bachta KE, Satchell KJF and Hultquist JF: Activity and inhibition of the SARS-CoV-2 Omicron nsp13 R392C variant using RNA duplex unwinding assays. SLAS Discov. 29(100145)2024.PubMed/NCBI View Article : Google Scholar | |
|
Hsu MF, Kuo CJ, Chang KT, Chang HC, Chou CC, Ko TP, Shr HL, Chang GG, Wang AH and Liang PH: Mechanism of the maturation process of SARS-CoV 3CL protease. J Biol Chem. 280:31257–31266. 2005.PubMed/NCBI View Article : Google Scholar | |
|
Kim Y, Mandadapu SR, Groutas WC and Chang KO: Potent inhibition of feline coronaviruses with peptidyl compounds targeting coronavirus 3C-like protease. Antiviral Res. 97:161–168. 2013.PubMed/NCBI View Article : Google Scholar | |
|
Qiao J, Li YS, Zeng R, Liu FL, Luo RH, Huang C, Wang YF, Zhang J, Quan B, Shen C, et al: SARS-CoV-2 M(pro) inhibitors with antiviral activity in a transgenic mouse model. Science. 371:1374–1378. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Rut W, Groborz K, Zhang L, Sun X, Zmudzinski M, Pawlik B, Wang X, Jochmans D, Neyts J, Młynarski W, et al: SARS-CoV-2 M(pro) inhibitors and activity-based probes for patient-sample imaging. Nat Chem Biol. 17:222–228. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Jin Z, Zhao Y, Sun Y, Zhang B, Wang H, Wu Y, Zhu Y, Zhu C, Hu T, Du X, et al: Structural basis for the inhibition of SARS-CoV-2 main protease by antineoplastic drug carmofur. Nat Struct Mol Biol. 27:529–532. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Li P, Cui Q, Wang L, Zhao X, Zhang Y, Manicassamy B, Yang Y, Rong L and Du R: A simple and robust approach for evaluation of antivirals using a recombinant influenza virus expressing gaussia luciferase. Viruses. 10(325)2018.PubMed/NCBI View Article : Google Scholar | |
|
Shao W, Li X, Goraya MU, Wang S and Chen JL: Evolution of influenza a virus by mutation and re-assortment. Int J Mol Sci. 18(1650)2017.PubMed/NCBI View Article : Google Scholar | |
|
Li P, Du R, Wang Y, Hou X, Wang L, Zhao X, Zhan P, Liu X, Rong L and Cui Q: Identification of chebulinic acid and chebulagic acid as novel influenza viral neuraminidase inhibitors. Front Microbiol. 11(182)2020.PubMed/NCBI View Article : Google Scholar | |
|
Zhao X, Wang Y, Cui Q, Li P, Wang L, Chen Z, Rong L and Du R: A parallel phenotypic versus target-based screening strategy for RNA-Dependent RNA polymerase inhibitors of the influenza a virus. Viruses. 11(826)2019.PubMed/NCBI View Article : Google Scholar | |
|
Haidari M, Ali M, Ward Casscells S III and Madjid M: Pomegranate (Punica granatum) purified polyphenol extract inhibits influenza virus and has a synergistic effect with oseltamivir. Phytomedicine. 16:1127–1136. 2009.PubMed/NCBI View Article : Google Scholar | |
|
Ooi MH, Wong SC, Lewthwaite P, Cardosa MJ and Solomon T: Clinical features, diagnosis, and management of enterovirus 71. Lancet Neurol. 9:1097–1105. 2010.PubMed/NCBI View Article : Google Scholar | |
|
Huang WC, Huang LM, Lu CY, Cheng AL and Chang LY: Atypical hand-foot-mouth disease in children: A hospital-based prospective cohort study. Virol J. 10(209)2013.PubMed/NCBI View Article : Google Scholar | |
|
Lin CJ, Liu CH, Wang JY, Lin CC, Li YF, Richardson CD and Lin LT: Small molecules targeting coxsackievirus A16 capsid inactivate viral particles and prevent viral binding. Emerg Microbes Infect. 7(162)2018.PubMed/NCBI View Article : Google Scholar | |
|
Liu CH, Kuo YT, Lin CJ and Lin LT: Involvement of cell surface glycosaminoglycans in chebulagic acid's and punicalagin's antiviral activities against Coxsackievirus A16 infection. Phytomedicine. 120(155047)2023.PubMed/NCBI View Article : Google Scholar | |
|
Yang Y, Xiu J, Zhang L, Qin C and Liu J: Antiviral activity of punicalagin toward human enterovirus 71 in vitro and in vivo. Phytomedicine. 20:67–70. 2012.PubMed/NCBI View Article : Google Scholar | |
|
Asandem DA, Segbefia SP, Kusi KA and Bonney JHK: Hepatitis B virus infection: A mini review. Viruses. 16(724)2024.PubMed/NCBI View Article : Google Scholar | |
|
Zoulim F and Durantel D: Antiviral therapies and prospects for a cure of chronic hepatitis B. Cold Spring Harb Perspect Med. 5(a021501)2015.PubMed/NCBI View Article : Google Scholar | |
|
Guo JT and Guo H: Metabolism and function of hepatitis B virus cccDNA: Implications for the development of cccDNA-targeting antiviral therapeutics. Antiviral Res. 122:91–100. 2015.PubMed/NCBI View Article : Google Scholar | |
|
Liu C, Cai D, Zhang L, Tang W, Yan R, Guo H and Chen X: Identification of hydrolyzable tannins (punicalagin, punicalin and geraniin) as novel inhibitors of hepatitis B virus covalently closed circular DNA. Antiviral Res. 134:97–107. 2016.PubMed/NCBI View Article : Google Scholar | |
|
Shepard CW, Simard EP, Finelli L, Fiore AE and Bell BP: Hepatitis B virus infection: Epidemiology and vaccination. Epidemiol Rev. 28:112–125. 2006.PubMed/NCBI View Article : Google Scholar | |
|
Gaskell R, Dawson S, Radford A and Thiry E: Feline herpesvirus. Vet Res. 38:337–354. 2007.PubMed/NCBI View Article : Google Scholar | |
|
Synowiec A, Dąbrowska A, Pachota M, Baouche M, Owczarek K, Niżański W and Pyrc K: Feline herpesvirus 1 (FHV-1) enters the cell by receptor-mediated endocytosis. J Virol. 97(e0068123)2023.PubMed/NCBI View Article : Google Scholar | |
|
Hilterbrand AT, Daly RE and Heldwein EE: Contributions of the four essential entry glycoproteins to HSV-1 tropism and the selection of entry routes. mBio. 12:e00143–21. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Gaudreault NN, Madden DW, Wilson WC, Trujillo JD and Richt JA: African swine fever virus: An emerging DNA arbovirus. Front Vet Sci. 7(215)2020.PubMed/NCBI View Article : Google Scholar | |
|
Geng R, Yin D, Liu Y, Lv H, Zhou X, Bao C, Gong L, Shao H, Qian K, Chen H and Qin A: Punicalagin inhibits african swine fever virus replication by targeting early viral stages and modulating inflammatory pathways. Vet Sci. 11(440)2024.PubMed/NCBI View Article : Google Scholar | |
|
Landovitz RJ, Scott H and Deeks SG: Prevention, treatment and cure of HIV infection. Nat Rev Microbiol. 21:657–670. 2023.PubMed/NCBI View Article : Google Scholar | |
|
Vidya Vijayan KK, Karthigeyan KP, Tripathi SP and Hanna LE: Pathophysiology of CD4+ T-Cell depletion in HIV-1 and HIV-2 infections. Front Immunol. 8(580)2017.PubMed/NCBI View Article : Google Scholar | |
|
Goguen RP, Chen MJ, Dunkley ORS, Gatignol A and Scarborough RJ: Gene therapy to cure HIV infection. Virologie (Montrouge). 27:63–84. 2023.PubMed/NCBI View Article : Google Scholar | |
|
de Carvalho AC, Dias CSB, Coimbra LD, Rocha RPF, Borin A, Fontoura MA, Carvalho M, Proost P, Nogueira ML, Consonni SR, et al: Characterization of systemic disease development and paw inflammation in a susceptible mouse model of mayaro virus infection and validation using x-ray synchrotron microtomography. Int J Mol Sci. 24(4799)2023.PubMed/NCBI View Article : Google Scholar |