Effect of deubiquitinases in head and neck squamous cell carcinoma (Review)
- Authors:
- Published online on: April 23, 2025 https://doi.org/10.3892/ol.2025.15053
- Article Number: 307
-
Copyright: © Wang et al. This is an open access article distributed under the terms of Creative Commons Attribution License.
Abstract
Introduction
Head and neck squamous cell carcinoma (HNSCC) is the sixth most common cancer type worldwide with a consistently increasing incidence (1). HNSCC includes nasopharyngeal, laryngeal and oral cancers (2). HNSCC pathogenesis is influenced by various factors, such as viral infection, cigarette smoking, alcohol consumption, environmental factors and genetic factors (2). The treatment of HNSCC involves a variety of approaches, including surgery, radiotherapy, chemotherapy and immunotherapy. Despite multiple therapeutic approaches, due to the insidious and highly invasive nature of HNSCC, more than half of patients have a poor prognosis and experience local recurrence or distal metastasis of the tumor, and patients continue to have poor survival and quality of life (3–5). Therefore, further research is needed to explore more efficient and tolerable targeted therapies to improve the clinical prognosis of patients with HNSCC.
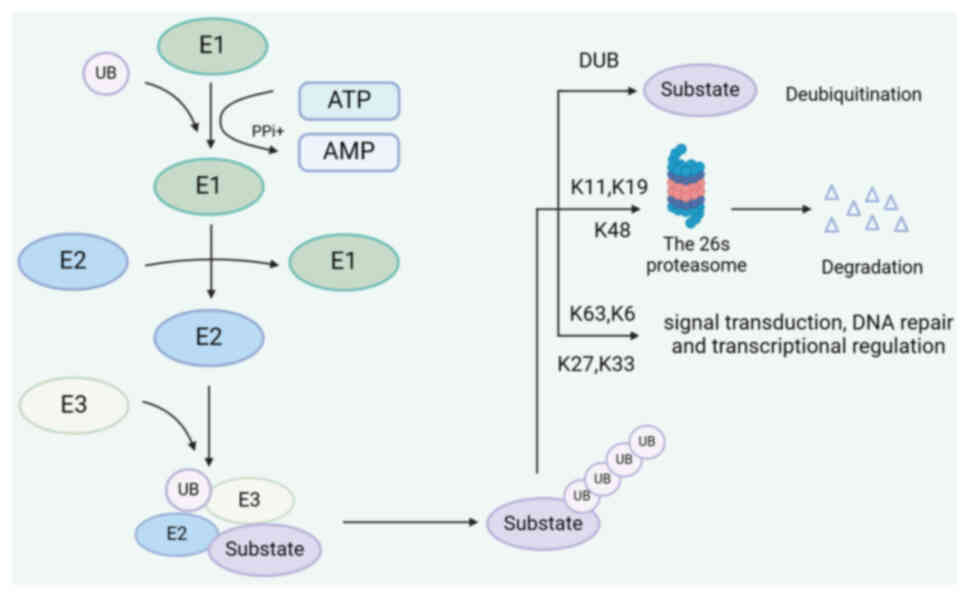
The ubiquitin (Ub)-proteasome system (UPS) is an important in vivo multicomponent system that is involved in cellular processes related to protein homeostasis and quality control (6,7). Mutations or aberrant expression of the UPS are frequently observed in various malignant tumors, including HNSCC. The UPS is a multistep process that involves several different proteins, including Ub, ub-activating enzymes (E1s), ub-conjugating enzymes (E2s), ub ligases (E3s), proteasomes and deubiquitinating enzymes (DUBs) (8–10). In the UPS pathway, the E1-E2-E3 catalytic cascade modifies the substrate and promotes its degradation. In brief, E1s utilize adenosine triphosphate (ATP) hydrolysis to activate and recruit Ub molecules. Activated Ub binds to E2 by forming a thioester bond and interacts with a specific Ub ligase, E3. Finally, it covalently binds to the target protein via E3. This complex is recognized and degraded, thereby regulating steady-state protein (9,11–13). In addition, the seven acceptor lysines (K6, K11, K27, K29, K33, K48 and K63) of the Ub molecule can participate in sequential conjugation, resulting in various Ub chain links, ultimately determining the various fates and activities of the target proteins. K63, K6, K27 and K33 are involved in numerous key biological processes, including signal transduction, DNA damage and mitochondrial homeostasis (10). K48, K11 and K29 primarily participate in substrate degradation via the 26s proteasome pathway (14,15) (Fig. 1).
Ubiquitination is a highly reversible, dynamic process. DUBs can remove Ub molecules from proteins, thus maintaining the balance of protein functions (11,16,17). So far, ~100 different deubiquitinating enzymes have been identified in humans, and depending on their structure, they can be classified into six families: ubiquitin-specific proteases (USPs), ubiquitin carboxy-terminal hydrolases (UCHs), ovarian tumor proteases (OTUs), Machado-Joseph disease protein domain proteases (MJDs), JAMM/MPN domain-associated metallopeptidases (JAMMs) and the monocyte chemotactic protein-induced proteases family (MINDYs) (18–20). Among them, the USP family, which consists of finger, palm and thumb domains, is the most extensively studied family of small-molecule inhibitors (21). The UCH family is a group of small molecular-weight cysteine proteases that promote ubiquitin recycling (16). Similar to the UCH family, the OTU family has three subgroups: OTU, OTUB and A20-like OTU, each having a distinct specificity for certain types of ubiquitin chains. There are four MJD family members: ataxin-3 (ATXN3), ATXN3L, Josephin domain containing 1 (JOSD1) and JOSD2. One of the most intensively studied enzymes is ATXN3, a cysteine protease (22,23). The JAMM/MPN family of metalloproteinases can bind ubiquitinated proteins to Ub molecules (24,25). The MINDY family remains largely elusive and specializes in cutting long Ub chains, such as the K48 Ub chain (26).
Recent advances in clinical research, targeted therapy and immunotherapy have shown promising efficacy in HNSCC. In addition, the widespread use of genetic testing has led to the discovery of an increasing number of affected genes in HNSCC, providing guidelines for the selection of targeted drugs (27). Of note, mutations or dysregulation in ubiquitination modifications can trigger a wide range of serious human diseases, including cancer. The present review focused on the different signaling dysregulations associated with DUBs in HNSCC to identify potential therapeutic targets and improve the therapeutic efficacy of HNSCC.
DUBs in signaling pathways associated with HNSCC
DUBs have been reported to act as key regulators of the development and progression of HNSCC. In addition, DUBs regulate various HNSCC-related signaling pathways, including the p53 signaling pathway, NF-κB signaling pathway, rat sarcoma (RAS)/rapidly accelerated fibrosarcoma (RAF)/MAPK kinase (MEK)/extracellular signal-regulated kinase (ERK), PI3K signaling pathway, TGF-β signaling pathway, hypoxia-inducible factor (HIF)-1α, Wnt/β-catenin, MYC signaling pathway and Hippo pathways.
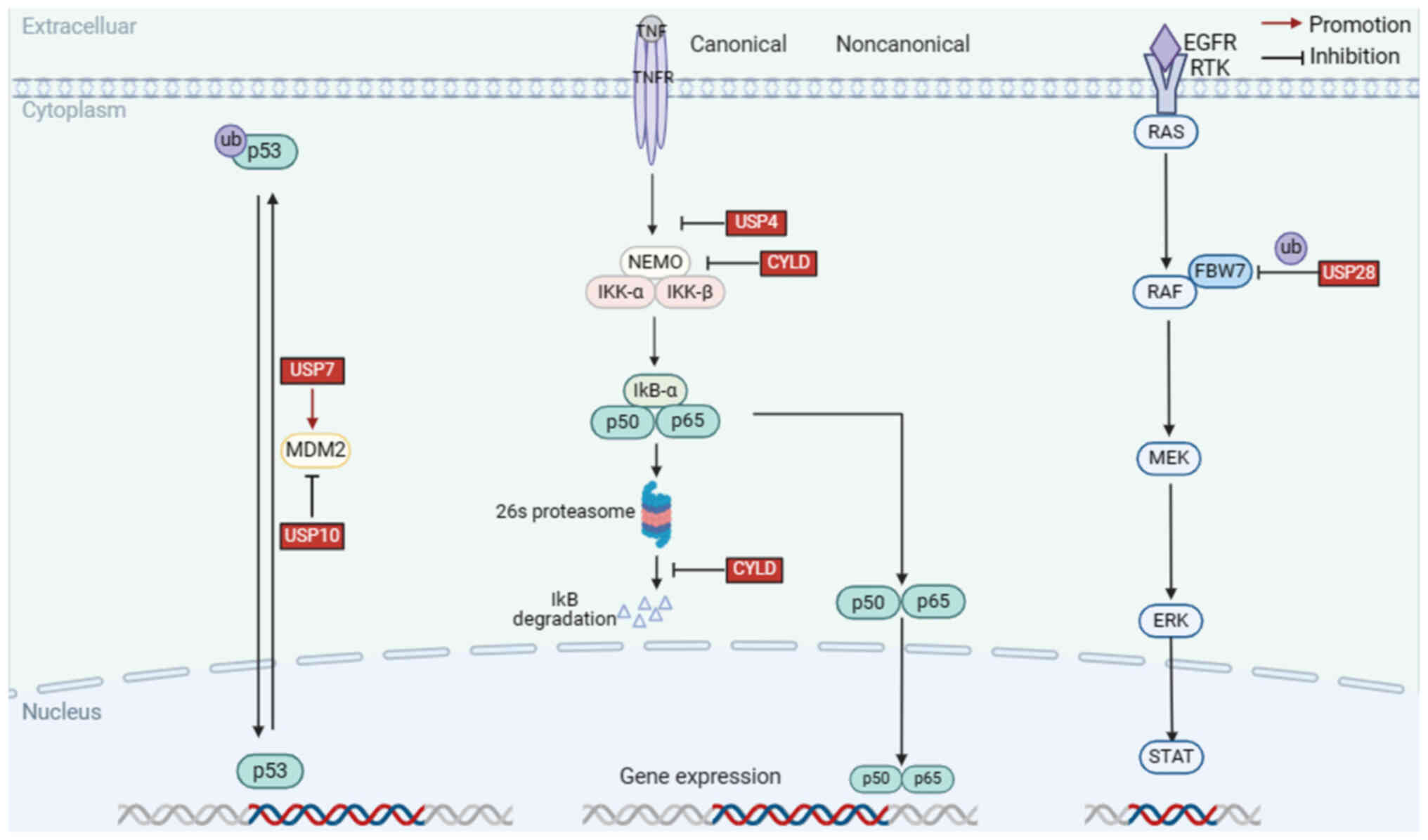
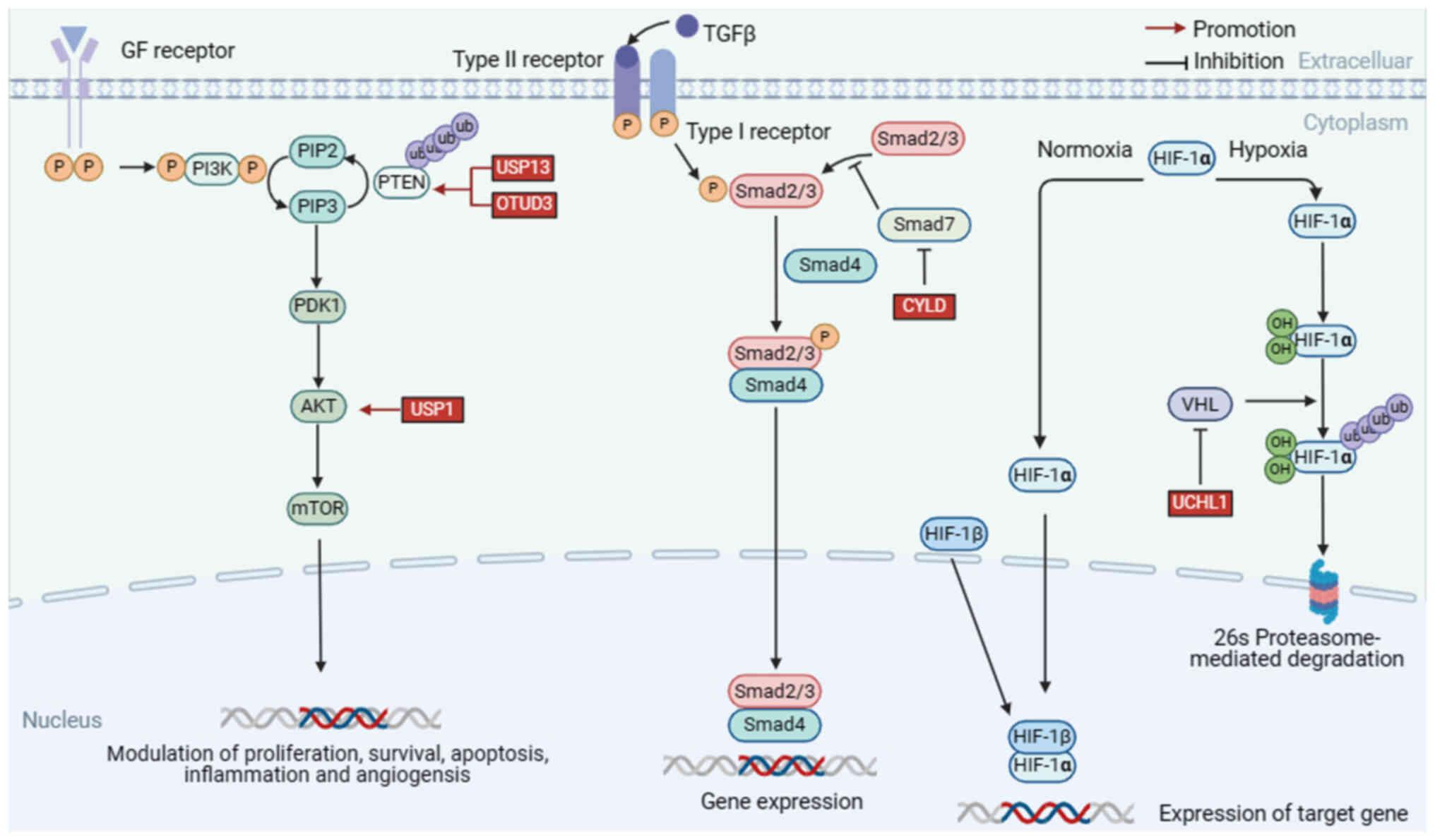
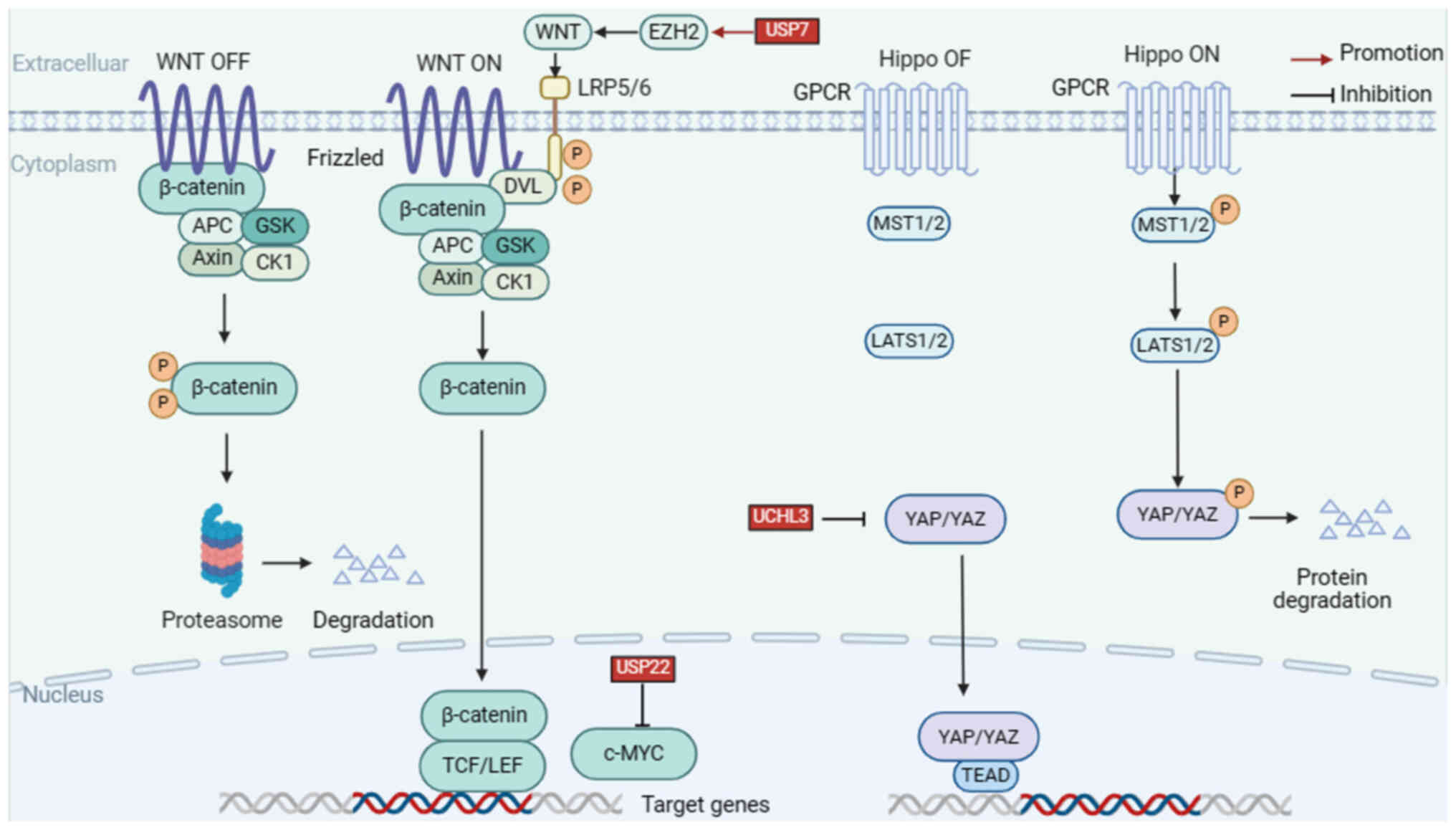
In this chapter, the DUBs implicated in HNSCC were summarized and the mechanisms underlying abnormal DUBs expression in signaling pathways were discussed. For instance, USP7 can promote cancer development by downregulating p53 levels through deubiquitination of mouse double minute 2 (MDM2); lysine 63 deubiquitinase (CYLD), which is frequently mutated in nasopharyngeal carcinoma, inhibits cell invasion and metastasis by interfering with the NF-κB signaling pathway; USP28 is an oncogenic factor that deubiquitinates F-box and WD repeat domain containing 7 (FBXW7), thereby enhancing the stability of RAF family members and inhibiting activation of the MEEK pathway. In addition, recent studies were reviewed and agonists and inhibitors of DUBs were summarized to identify more effective therapeutic strategies (Table I, Fig. 2, Fig. 3, Fig. 4).
p53 Pathway
p53 has a critical role in the cell cycle and is considered an important tumor suppressor. When stimulated by cellular stress or DNA damage, a sequence of phosphorylation events activates p53 (28). Notably, p53 is frequently mutated and aberrant stability of p53 has been observed in HNSCC (29,30). Emerging evidence has shown that various E3 ligases mediate the ubiquitination and stability of p53 (31). For instance, the E3 ligase MdM2 can maintain p53 at normal levels in vivo by accelerating its degradation of p53 under normal conditions (32). Furthermore, p53 is readjusted by deubiquitination via deubiquitinating enzymes that can eliminate Ub molecules from p53. p53 is important in genome stabilization and is the most common mutation gene in human papillomavirus (HPV)-negative patients with HNSCC (33). Compared to patients with normal p53 expression, patients with mutations have an increased oncogenic potential, poor sensitivity to radiotherapy and an increased rate of tumor recurrence, eventually leading to reduced survival (34). A summary of the different DUBs involved in the regulation of the p53 signaling pathway may provide new insights for understanding the occurrence and progression of HNSCC (35,36).
USP7
USP7 was first identified to interact with herpes simplex viruses; thus, it is also known as a herpesvirus-associated Ub-specific graphic protease (37). Functionally, USP7 is considered an oncogenic protein that removes Ub and prevents substrate degradation. Studies have found that USP7 is associated with a wide range of pathologies, including neurological lesions, inflammatory responses and tumors (38). USP7 comprises three structural domains: The tumor necrosis factor receptor-associated factor (TRAF) domain, a highly conserved USP domain, and five ubiquitin-like domains 1–5 (39,40). It is known that the p53 protein can be degraded by MDM2, and the TRAF-like domain of USP7 can mediate the deubiquitination of MDM2, increasing intracellular levels of MDM2, which in turn downregulates p53 (41). Therefore, inhibition of USP7 activity can stabilize p53 levels, thus generating anti-apoptotic functions (42). Recently, Niu et al (43) found that high expression of USP7 reduces radiosensitivity in laryngeal squamous cell carcinoma (LSCC) with p53 mutations, and clinical application of USP7 inhibitors in the future may improve the effects of radiotherapy for patients. Therefore, to further explore the expression of USP7 in HNSCC, The Cancer Genome Atlas (https://portal.gdc.cancer.gov/) database was analyzed, indicating that the expression level of USP7 mRNA in HNSCC tissues was higher than that in normal tissues (Table SI). Furthermore, an analysis of the Clinical Proteomic Tumor Analysis Consortium (https://proteomics.cancer.gov/) database suggested that USP7 protein expression was significantly upregulated in HNSCC (Table SII).
NF-κB signaling pathway
NF-κB is a nuclear transcription factor, and dysregulation of NF-κB activity can cause inflammation-related diseases and cancers (44). In the canonical pathway, the IKK complex containing IKK-α, IKK-β and NF-κB essential modifier (NEMO) is activated and induces the phosphorylation of IKK-β, which then phosphorylates IkB-α, causing ubiquitination and degradation by the 26S proteasome (45). Next, the p65/p50 dimer is released and enters the nucleus to activate downstream target genes (45,46). Accumulating evidence has demonstrated that the NF-κB signaling pathway is commonly activated during the occurrence and progression of HNSCC to regulate cell proliferation, invasion, angiogenesis and metastasis (47,48). It has been reported that DUBs can regulate the activation of the NF-κB pathway by removing polyubiquitin chains, suggesting that it is a potential therapeutic target (49).
CYLD
As a putative tumor suppressor gene, CYLD encodes an evolutionarily conserved protein of ~120 kDa (956aa) (50). CYLD contains a deubiquitinating enzyme catalytic structural domain and three CAP-Gly structural domains (51). TRAF2 is a component of a complex that can activate NEMO; mechanistically, CYLD can negatively regulate TRAF2-mediated IKK activation by removing the k63-polyubiquitin chain on TRAF2, thereby interfering with the NF-κB signaling pathway and inhibiting cell growth (52). Bcl-3 is an atypical member of the IκB protein family and a regulator of NF-κB, which activates the NF-κB pathway through the deubiquitination of Bcl-3 (53). CYLD mutations have also been reported in patients with HNSCC; CYLD is one of the most mutated genes in nasopharyngeal carcinoma, and according to functional assays, CYLD negatively regulates NF-κB by preventing nuclear translocation of p65, thereby inhibiting cell invasion and metastasis, enhancing the surveillance of patients with HNSCC (54,55).
USP4
USP4, a ubiquitous nuclear protein (UNP), contains a catalytic domain and a non-catalytic domain, capable of removing monoubiquitinated and polyubiquitinated chains (56). USP4 is involved in various pathological conditions, including hypertrophic cardiomyopathy and malignancy. USP4 is abnormally expressed in brain, breast and lung cancer (56,57). USP4 has also been reported to play a tumor-suppressive role in HNSCC. Hou et al (58) found that USP4 expression levels are significantly higher in HNSCC tissues in normal tissues. Receptor-interacting protein 1 (RIP1) is an upstream signaling molecule of NF-κB signaling that is essential for the regulation of apoptosis. Further research has demonstrated that USP4 suppresses tumor growth by interacting with RIP1, deubiquitinating K63-linked RIP1 and inhibiting NF-κB activation, resulting in tumor suppressor effects (58).
RAS/RAF/MEK/ERK pathway
The RAS/RAF/MEK/ERK pathway is one of the most important components of all MAPK signal transduction pathways, which are essential for the regulation of basic intercellular and intracellular functions, such as growth, survival and differentiation, thereby promoting the growth and metastasis of tumor cells (59). Activation of the MAPK signaling pathway is present in nearly one-fifth of HNSCC cases, leading to the expression of downstream genes, including Bcl-2, HIF-1α and vascular endothelial growth factor (VEGF), which affect the invasive, hypoxic, angiogenic and inflammatory processes in HNSCC (60–62).
Numerous stimulating factors, such as epidermal growth factor (EGF), receptor tyrosine kinases, TNF and Src family members, can activate an upstream activating protein known as RAS, subsequently triggers many downstream proteins and recruits the RAF kinase to the plasma membrane for activation (63,64). Activated RAF then activates downstream MEK and MAPK/ERK, eventually delivering cell proliferation and differentiation signals to the nucleus by regulating the activity of various transcriptional regulators that contribute to gene expression (60,64).
Aberrant activation of the RAS/RAF/MEK/ERK signaling pathway was reported to be associated with the development and progression of HNSCC (60).
USP28
USP28 is a functional protein belonging to the USP family that plays a key role in various biological processes, including DNA damage repair, transcription factor regulation and carcinogenesis (65,66). It's also found that USP28, which is located on chromosome 11q23, is frequently mutated in HNSCC (67). FBXW7 is considered a tumor suppressor, which is a component of the Ub ligase complex and can regulate the protein stability of B-RAF, a member of the RAF family (68). Certain studies have suggested that FBXW7 is anomalously expressed in various cancers, such as oral squamous cell carcinoma (OSCC) and esophageal squamous cell carcinoma (69,70). USP28 deubiquitinates and stabilizes FBW7, allowing the WD40 repeat sequence in FBW7 to bind and degrade substrates containing Cdc4 phosphorylation motifs, and it was found that the Cdc4 phosphorylation structural domains are widely present in human RAF subtypes. In conclusion, USP28 can destabilize RAF family members by indirectly increasing ubiquitination and degradation of B-RAF via FBW7 (71). Notably, RAF is an important target in the MAPK signaling pathway, and several inhibitors have been advanced to improve patient survival (72).
PI3K/AKT signaling pathway
Aberrant activation of the PI3K/AKT signaling pathway commonly occurs in cancer. As a downstream molecule of PI3K, AKT is a major effector of PI3K in cancers (73). Activated PI3K catalyzes phosphatidylinositol 4,5-biphosphate (PIP2) to generate phosphatidylinositol-3,4,5-trisphosphate (PIP3), which serves as a second messenger and recruits 3-phosphoinositide-dependent protein kinase 1 (PDK1) and AKT to the plasma membrane. Subsequently, PDK1 phosphorylates and activates AKT, which further activates the downstream pathways that regulate metastasis, metabolism and cell survival (74,75).
The PI3K/AKT signaling pathway is upregulated in >90% of HNSCC and is more evident in HPV-positive patients (76). Studies have shown that upregulation of the PI3K/AKT signaling pathway enhances resistance to radiotherapy and drugs by modulating cellular distribution during the cell cycle (77). Therefore, the development of selective inhibitors offers potential therapeutic opportunities for patients.
As a tumor suppressor gene, phosphatase and tensin homolog (PTEN) is frequently lost or mutated in tumors, reducing PI3K activity by converting PIP3 back to PIP2 (78). Loss of PTEN causes consistent activation of the PI3K pathway, leading to tumorigenesis (79,80).
Notably, several deubiquitinating enzymes, including USP13, OTU domain-containing protein 3 (OTUD3) and USP1, can deubiquitinate PTEN in cancer-specific contexts, thereby maintaining PTEN levels and preventing its degradation (81).
USP13
Structurally, USP13 consists of 863 amino acids and contains highly conserved zinc finger structural domains and USP catalytic structural domains (82). USP13 is the first PTEN deubiquitinating enzyme discovered, which binds to the phosphatase domain of PTEN and stabilizes PTEN levels through deubiquitination, inhibiting tumor development and glycolysis. Studies have shown that inhibition of PTEN by deletion of USP13 leads to aberrant phosphorylation of AKT, which affects downstream genes associated with cell survival, metabolism and growth (83). Meanwhile, Qu et al (84) found that the interaction between USP13 and PTEN ultimately inhibited AKT phosphorylation. The expression of USP13 is frequently reduced in OSCC tissues as indicated by immunohistochemical staining, suggesting that USP13 may act as a tumor suppressive role in OSCC (84–86).
OTUD3
OTUD3 is a member of the OTU family that participates in the regulation of multiple physiological processes, and the aberrant expression of OTUD3 triggers tumorigenesis in esophageal cancer, breast cancer and papillary thyroid carcinoma (87,88). Several studies have demonstrated that OTUD3 interacts with PTEN and induces its deubiquitination, increasing PTEN protein stability and inhibiting carcinogenic PI3K/AKT signaling (89). Mechanistically, OTUD3 binds to the PTEN C2 domain, a region that has been demonstrated to play a critical role in PTEN stability control and regulation of cell migration, and preferentially removes the K6, K11, K27 and K48 linkages of PTEN ubiquitination (89,90). Notably, Zhao et al (90) discovered that in thyroid carcinoma, OTUD3 deubiquitinates PTEN, thereby increasing PTEN protein stability and inhibiting the activation of PI3K/AKT signaling.
USP1
USP1 is a key deubiquitinating enzyme in the USP family. Structurally, it can be divided into a catalytically active C-terminal domain and a regulatory N-terminal domain (91). USP1 is expressed in a wide range of human tissues and participates in the regulation of DNA damage repair, cell cycle regulation, and tumor progression (92–95). Furthermore, the expression and function of USP1 have been reported to be associated with multiple cancers, including breast cancer, lung cancer, hepatocellular carcinoma, and HNSCC (96–98). Xu et al (92) found that USP1 is involved in the PI3K/AKT signaling pathway by removing K63-linked polyubiquitin chains from AKT. They used a PI3K inhibitor (S2793) to reverse the effects of USP1 and found that USP1 overexpression activated the PI3K/AKT signaling pathway, causing tumor cell migration and invasion in HNSCC (92,99).
TGF-β signaling pathway
TGF-β is a family of structurally related proteins. TGF-β ligands bind to TGF-βRII, recruiting and phosphorylating TGF-βRI (100,101). SMA and MAD-related family protein (SMAD)2/3 may be phosphorylated by activated TGF-βRI and form heteromeric complexes with SMAD4 (102). Finally, SMAD2/3-SMAD4 complexes are transported into the nucleus where they activate multiple downstream signaling pathways and regulate the expression of various genes (102,103). TGF-β upregulation may be involved in the recurrence and metastasis of HNSCC (104). Specifically, TGF-β upregulation regulates the expression of various genes, including the process of inducing epithelial-mesenchymal transition (EMT) and the regulation of VEGF, which drives cancer cell migration and angiogenesis (104,105).
SMAD7 is a key negative regulator of TGF-β signaling, which bind to TGF-βRI and competitively inhibit the phosphorylation of SMAD2/3, blocking the interaction between SMAD2/3 and the receptor complex, thereby inhibiting the downstream TGF-β signaling pathway (106). In certain instances, E3 ubiquitin ligases were observed to repress TGF-β signaling by interacting with SMAD7. This section focuses on the role of DUBs, which counteract the activity of E3 ubiquitin ligases and maintain TGF-β signaling pathways.
CYLD
Multiple studies have shown that CYLD plays an important role in the NF-κB signaling pathway, and it has also been proven that CYLD plays a role in regulating the TGF-β signaling pathway (107). Decreased CYLD expression promotes the invasion of OSCC cells. A study found that the loss of CYLD stabilized TGF-βRI by removing the k63-linked polyubiquitin chain on SMAD7, and consequently promoted TGF-β signaling in OSCC cells (108).
HIF-1α pathway
As an oxygen-dependent transcriptional activator, HIF-1α is widespread in mammalian and human cells, and is composed of an α subunit HIF-1α and a β subunit HIF-1β (109). In addition, experimental data have demonstrated that HIF-1α is one of the key regulators of >100 genes downstream (110).
Under aerobic conditions, the activity of prolyl hydroxylase (PHD) is regulated by oxygen, leading to hydroxylation of HIF-1α. Next, von Hippel Lindau (VHL) incorporates the hydroxylated subunit, leading to rapid and sustained degradation of HIF-1α via the proteasome pathway (111,112). However, under hypoxic conditions, the activities of PHD and VHL are inhibited by low oxygen levels, leading to the stabilization and activation of HIF-1α (111). Subsequently, it escapes degradation and is transported into the cell nucleus, where it regulates numerous downstream genes implicated in cancer progression (111). It then avoids destruction and enters the cell nucleus, where it upregulates numerous downstream genes implicated in the development of cancer (113). Studies have shown that overexpression of HIF-1α is associated with poor overall survival in nasopharyngeal, oropharyngeal and oral carcinomas, but no link has been found in laryngeal carcinoma (114).
UCHL1
UCHL1 is a DUB enzyme that belongs to the UCHs family (115). It controls diverse cellular processes such as metabolism, protein aggregation and autophagy by deubiquitinating multiple key substrate proteins (116). Furthermore, the expression of UCHL1 has been demonstrated to be dysregulated in several tumor types, including pancreatic, colorectal and breast cancers, where it can enhance cancer invasion and metastasis (117,118). Zhang et al (119) found that UCHL1 is highly expressed in HNSCC tissues and contributes to the proliferation and metastasis of HNSCC.
Furthermore, Li et al (120) found that cell migration is markedly enhanced by UCHL1 expression. In 3D spheroid culture models, certain malignancy-related factors, including solidity, volume and viable cell number, were considerably increased by the ectopic expression of UCHL1 in an HIF-1α-dependent manner. By contrast, these malignancy-related factors are downregulated when the HIF-1α pathway were inhibited and UCHL1-mediated cell invasion and proliferation were eliminated (120,121). As a novel upstream activator of HIF-1α, UCHL1 inhibits VHL-mediated ubiquitination of HIF-1α. These findings suggest that, as a deubiquitinating enzyme for HIF-1α, UCHL1 may be a prognostic indicator and therapeutic target for cancers because it promotes metastases (122).
Wnt/β-catenin pathway
The Wnt/β-catenin pathway is a complex network of protein actions. When there is no Wnt signal in the cell, a destructive complex consisting of adenomatous polyposis coli (APC) protein, Axin, casein kinase 1a (CK1a), glycogen synthase kinase-3 and β-catenin can degrade cytoplasmic β-catenin (123). Wnt signaling is activated when Wnt proteins bind to the structural domains of the Frizzled family receptors, which form complexes by binding to the Frizzled receptor and protein 5/6. After complex formation, scaffold proteins are recruited to the receptor, which is phosphorylated and inhibits the degradation of the β-linker proteins. This allows β-catenin proteins to accumulate and transpose into the nucleus, where they bind to TCF/LEF transcription factors, thereby activating the transcription of Wnt target genes and inducing subsequent cellular responses (124,125).
β-catenin is an important regulator of Wnt/β-catenin signaling. Yang et al (126) observed that the level of β-catenin was positively correlated with the cell invasion potential in HNSCC. Further experiments showed that activation of Wnt/β-catenin signaling not only promotes cell invasion, but also stimulates lymph node metastasis in HNSCC, and these results suggest that blocking the Wnt/β-catenin signaling may be a therapeutic strategy for lymph node metastasis and tumor recurrence in HNSCC (127).
USP7
USP7 functions as a tumor suppressor and stabilizes p53. Zheng et al (128) discovered the mechanism by which USP7 acts on the substrate EZH2, an interaction between the two was found by immunoprecipitation, and further experiments showed that overexpression of USP7 led to a decrease in ubiquitination of EZH2. EZH2 is able to inhibit multiple Wnt genes, thereby silencing Wnt/β-catenin signaling. Experimental results showed that USP7 overexpression suppressed the expression of several pathway-related genes, including Wnt, promoting cellular EMT and inhibiting cellular senescence and differentiation (128–130). One study suggested that USP7 and EZH2 are upregulated in LSCC tissues, which affects LSCC evolution and serves as an independent prognostic predictor. Thus, USP7 may serve as a novel therapeutic target (131).
MYC signaling pathway
It is well known that MYC is a proto-oncogene expressed in various cancers, including HNSCC (132). MYC is an important transcription factor that plays a key role in regulating cellular metabolic processes (133). As a target gene of the Wnt/β-catenin pathway, MYC is activated by the nuclear translocation of β-catenin and maintains intracellular homeostasis and cell proliferation (134). The stability of the MYC protein is primarily regulated by the UPS. For instance, MYC is converted to ubiquitin by SCFFBW7 and several other E3 ubiquitin ligases (135). However, little is known about MYC deubiquitination. The following section focuses on the process of MYC-associated deubiquitination.
USP22
USP22 is located on chromosome 17 and is composed of 14 exons. USP22 regulates the transcriptional activation of numerous genes by histone ubiquitination (H2A and H2B) and affects chromatin structures (136). It was found that USP22 positively regulates MYC protein levels and that USP22 promotes cancer progression by targeting k48-linked polyubiquitin chains to deubiquitinate MYC (137). Several studies have shown that USP22 is overexpressed in HNSCC and closely associated with the growth and proliferation of tumor cells (20). For instance, USP22 expression is enhanced in laryngeal carcinoma, contributing to invasion, metastasis and poor prognosis (138).
Hippo pathway
The Hippo pathway is a tumor suppressor pathway and dysregulation of its effector Yes-associated protein (YAP) and PDZ-binding motif (TAZ) is an important factor in the progression of HNSCC (139). Hyperactivation of YAP/TAZ is associated with the expansion of HNSCC stem cells and treatment resistance (140,141). Physiologically, the Hippo pathway is normally active (Hippo ON), and LATS1/2 kinases are phosphorylated and activated by macrophage stimulating 1 (MST1) and MST2, serine/threonine protein kinases (142). Subsequently, LATS1/2 kinases phosphorylate the transcriptional coactivator YAP and TAZ, ultimately leading to nuclear exclusion of YAP/TAZ, causing apoptosis and limiting excessive organ growth (142,143). YAP and TAZ translocate to the nucleus when the core Hippo kinases are inactive (Hippo OFF) and bind to the TEA domain transcription factor family to promote cell growth and proliferation or interact with other transcription factors or signaling molecules (144). Hyperactivation of YAP and TAZ may contribute to the development of multiple types of tumors (145). In this section, the role of ubiquitination modifications in regulating the Hippo pathway were summarized.
UCHL3
UCHL3, a member of the USPs family, plays an important role in protein degradation (146). It has been reported that UCHL3 is involved in several biological processes, including DNA repair, fertilization, the development of preimplantation embryos, osteoblast differentiation, and tumor progression (147–151). Recently, Tang et al (152) discovered that UCHL3, a novel YAP deubiquitinating enzyme, regulates YAP deubiquitination and stabilization in anaplastic thyroid cancer. Another study showed that UCHL3 stabilizes and maintains YAP by interacting with the WW domain of YAP to delete K11- and K48-linked ubiquitin chains (152). Collectively, UCHL3 can promote cancer progression and metastasis, indicating that UCHL3-specific inhibitors may have great therapeutic potential in HNSCC (152).
Other DUBs
USP14USP14, a member of the USPs family, also serves as a novel biomarker in HNSCC and participates in various canonical cellular signaling pathways, including the NF-κB and Wnt/β-catenin signaling pathways (153,154). In multiple malignant tumors, USP14 expression is anomalous, including colorectal, ovarian, prostate, lung and liver cancer (155–159). Wang et al (153) discovered that USP14 overexpression is strongly associated with cancer metastasis and tumor stage in patients with HNSCC and found that USP14 physically interacts with heat shock transcription factor 1 (HSF1) and stabilizes HSF1 through its deubiquitination. HNSCC is often exposed to the body's internal environment and lifestyle factors such as consuming betel nuts, smoking or drinking alcohol, which causes a heat shock response and stabilizes tumor cells, promoting the metastasis, invasion, migration and proliferation of tumor cells (153). As an oncogenic protein, HSF1 is an important transcription factor that maintains protein homeostasis in HNSCC (160,161). Wang et al (153) suggested that USP14 controls HSF1 stabilization by deubiquitinating it and increasing its downstream proteins, thereby leading to the proliferation of cancer cells and poor prognosis in patients with HNSCC. In summary, these findings consistently indicate that USP14 is a potential therapeutic target in HNSCC.
USP9X
The programmed death-1 (PD-1) PD-1/programmed death ligand 1 (PD-L1) pathway acts as an important regulator of tumor formation, and activation of this pathway may inhibit the activation and proliferation of T cells and promote the production of regulatory T cells, allowing tumor cells to escape recognition and killing by the immune system (162,163). A recent study demonstrated that the PD-1/PD-L1 pathway is aberrantly activated in HNSCC tissues, leading to tumor immunosuppression (164,165).
USP9X is a member of the largest family of USPs that regulate several signaling pathway components and various biological processes, including cell apoptosis, adhesion, proliferation, growth and migration (166–168). Furthermore, a series of studies have shown that USP9X has an important role in breast cancer, lung cancer, hepatocellular carcinoma and HNSCC (169–172). Recently, Wu et al (173) suggested that USP9X was associated with OSCC cell growth. Mechanistically, they found that USP9X deubiquitinated PD-L1 and stabilized its protein expression, thereby promoting immune escape of tumor cells in OSCC using immunohistochemistry, immunoprecipitation, western blotting, liquid chromatography-mass spectrometry and a T-cell-mediated tumor cell killing assay.
Treatment
Traditional treatment of HNSCC
The treatment of patients with HNSCC is complex and primary treatment modalities include surgery, radiotherapy and chemotherapy (27). Surgery is considered an effective treatment for oral cavity and early laryngeal cancers, while radiotherapy or concurrent chemoradiation is considered the standard treatment for other head and neck cancers (174,175).
Cetuximab, a monoclonal antibody targeting EGFR, was approved for combined radiotherapy for the treatment of patients with locally advanced HNSCC by the Food and Drug Administration in 2006 (4). For patients with recurrent or metastatic HNSCC, the extreme protocol, cetuximab, cisplatin or carboplatin, and 5-fluorouracil is recognized as the first-line therapy (2). Although cetuximab combined with chemotherapy/radiotherapy can lead to better efficacy in patients with HNSCC, improvements in the magnitude and duration of the clinical benefits are still needed (4,176).
Pembrolizumab (Keytruda) is an anti-PD-1 immune checkpoint inhibitor that has been widely used to treat a variety of malignancies (2). Pembrolizumab blocks the expression of PD-1 on tumor cells, thereby preventing tumor cells from evading antitumor immunity (4). In 2019, pembrolizumab was approved for the first-line treatment for HNSCC (177,178).
The PI3K pathway is one of the most frequently mutated pathways in HNSCC and is associated with tumorigenic processes, making it an attractive target for cancer therapy (179). Currently, several drugs that target members of the PI3K pathway are in clinical trials. Buparisib is a class I PI3K inhibitor that can inhibit the activity of various PI3K isoforms, and the combination of buparisib and cetuximab has a synergistic antitumor effect (180). In addition, rapamycin, Copanlisib and Everolimus have also been reported (181–183). In the future, the PI3K pathway may be a promising target for the treatment of HNSCC.
Targeting deubiquitinase for HNSCC therapy
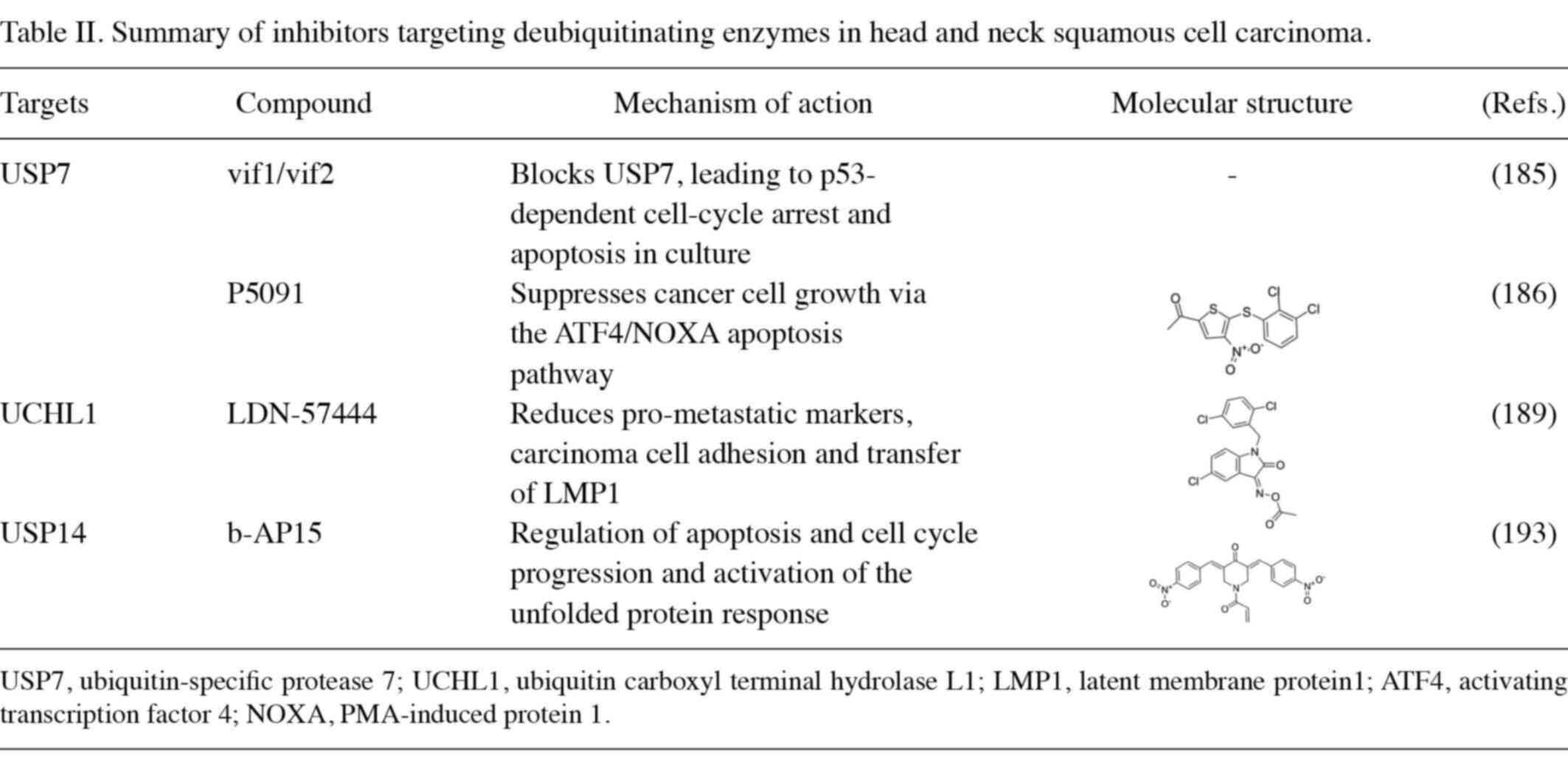
Previous studies have shown that members of the DUB family are aberrantly expressed in various malignant tumors (184). Numerous DUBs control apoptotic and proliferative processes in cells, and compared to proteasome inhibition, therapeutic targeting of DUBs has lower cytotoxicity and better therapeutic efficacy (18). Hence, it is a potential target for cancer therapies. Vif1 and vif2 are USP7 inhibitors that have the potential to be molecularly targeted agents for laryngeal cancer (185). Mechanistically, the vif1 peptide blocked the binding of cellular substrates to TRAF, while the vif2 peptide bound to the active catalytic sites of the TRAF and DUB structural domains of USP7, thereby inhibiting USP7 activity (185). Hu et al (186) discovered that P5091 is a selective and efficient USP7 inhibitor that can effectively suppress esophageal cancer cell growth via the ATF4/NOXA apoptotic pathway.
HIF-1α is considered a well-established pro-metastatic factor that can be activated by the viral oncogene latent membrane protein 1 (LMP1) and transferred to nasopharyngeal carcinoma-associated LMP1-positive exosomes (187,188). LDN-57444 is a small-molecule inhibitor of UCHL1. Kobayashi et al (189) found that LDN-57444 has anti-metastatic effects in cell lines of OSCC and nasopharyngeal carcinoma and that LDN-57444 inhibits exosomes and levels of pre-metastatic factors by inhibiting UCHL1 activity. Taken together, LDN-57444 could reduce the level of metastatic markers, inhibit the metastasis of LMP1 and decrease the adhesion of cancer cells, and may provide promising therapeutic potential for Epstein-Barr virus-positive malignancies (189).
b-AP15 is a new small-molecule USP14 inhibitor. Treatment with b-AP15 inhibits tumorigenesis in multiple cancers, including colorectal cancer, lymphoma, lung cancer and breast cancer (190–193). Tian et al (193) discovered that b-AP15 inhibits cell proliferation and induces endogenous apoptosis and cell cycle arrest by promoting the accumulation of high-molecular-weight polyubiquitin proteins. In tongue cancer, b-AP15 targets DUBs to overcome bortezomib resistance (Table II).
DUBTAC
The use of Proteolysis-Targeting Chimeras to target the degradation of numerous disease-causing proteins in cells offers a potentially promising therapeutic strategy for cancer treatment and several projects are currently in clinical development (194). However, the degradation of aberrant proteins is also a causative mechanism in certain diseases, therefore targeting deubiquitinase chimeras (DUBTAC) to mediate the recruitment of DUB and avoid the degradation of target proteins has become a new therapeutic strategy (195).
DUBTAC belongs to a family of targeted covalent inhibitors, which consists of DUB recruiters and protein of interest ligands (196). In a recent study, Henning et al (196) identified two distinct targets: The cystic fibrosis transmembrane conductance regulator (CFTR) and the WEE1 kinase. Transcoding mutations in CFTR (DF508) are the most common cause of cystic fibrosis and the authors used lumacaftor, a drug that has an affinity with DF508-CFTR, conjugated to the OTUB1 recruiter, EN523, and inhibited CFTR degradation, partially restoring the function of CFTR. In numerous cancers, WEE1 is degraded to promote cancer cell proliferation. The second DUBTAC consists of the WEE1 inhibitors AZD1775 and EN523. Studies have shown that this DUBTAC stabilizes the levels of WEE1 kinase in cancer cells, thus promising to stop tumor growth (196,197).
However, DUBTAC technology is still in the early stages of development, and the safety of drugs developed based on DUBTAC remains to be determined. In addition to the two diseases mentioned above, there are many other diseases that could be stabilized by DUBTAC to gain a therapeutic benefit.
Conclusions and prospects
HNSCC is a complex disease, and due to the insidious nature of its development, the majority of patients are found to be in a locally advanced stage with poor therapeutic outcomes. With the development of precision medicine, targeted therapies are gaining attention and may bring more options for patients with HNSCC. The aberrant expression of DUBs may be involved in tumorigenesis, progression and eventually metastasis, including HNSCC, by affecting the transduction of cellular signaling pathways. Current studies have shown that DUBs can modulate the levels of proteins insensitive to conventionally targeted therapies, so they have been considered a promising anti-tumor target. This review summarized DUBs in multiple pathways linked to the development of HNSCC, including the p53 signaling pathway, NF-κB signaling pathway and PI3K signaling pathway, aiming to providing new ideas for the clinical treatment of HNSCC in the future.
Compared with previous proteasome inhibitors, DUB inhibitors are able to target specific DUBs to selectively block protein degradation, thereby reducing drug resistance and toxicity. However, DUB inhibitors are still in the developmental stage and are generally in preclinical studies, and more than half of them are multi-targeted inhibitors, with the biological mechanisms of DUBs related to tumors not yet fully clarified. Currently, the research on DUBs is progressing further, and it is esteemed that in the future, highly efficient and specific small-molecule DUB inhibitors will bring more therapeutic choices to patients.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This project was supported by the Ningbo Natural Science Foundation (grant nos. 2021 J017 and 2021 J065), The Fundamental Research Funds for the Provincial Universities of Zhejiang (grant no. SJLZ2022004) and The K.C. Wong Magna Fund of Ningbo University.
Availability of data and materials
Not applicable.
Authors' contributions
JW conceptualized the study, wrote the original draft, wrote the review and edited the manuscript. LW conceptualized the study, wrote the review and edited the manuscript. JC provided the funding and revised and edited the manuscript. ZT revised and edited the manuscript. Data authentication is not applicable. All authors have read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Glossary
Abbreviations
Abbreviations:
|
HNSCC |
head and neck squamous cell carcinoma |
|
Ub |
ubiquitin |
|
UPS |
ubiquitin-proteasome system |
|
E1s |
ubiquitin-activating enzymes |
|
E2s |
ubiquitin-conjugating enzymes |
|
E3s |
ubiquitin ligases |
|
DUBs |
deubiquitinating enzymes |
|
ATP |
adenosine triphosphate |
|
USPs |
ubiquitin-specific proteases |
|
UCHs |
ubiquitin carboxy-terminal hydrolases |
|
OTUs |
ovarian tumor proteases |
|
MJDs |
machado-Joseph disease protein domain proteases |
|
JAMMs |
JAMM/MPN domain-associated metallopeptidases |
|
MINDYs |
monocyte chemotactic protein-induced proteases family |
|
MdM2 |
mouse double minute 2 |
|
ATXN3 |
ataxin-3 |
|
JOSD |
Josephin domain containing |
|
RAS |
rat sarcoma |
|
RAF |
rapidly accelerated fibrosarcoma |
|
MEK |
MAPK kinase |
|
ERK |
extracellular signal-regulated kinase |
|
HIF |
hypoxia-inducible factor |
|
CYLD |
lysine 63 deubiquitinase |
|
FBXW7 |
F-box and WD repeat domain containing 7 |
|
HPV |
human papillomavirus |
|
TRAF |
tumor necrosis factor receptor-associated factor |
|
LSCC |
laryngeal squamous cell carcinoma |
|
NEMO |
NF-κB essential modifier |
|
UNP |
ubiquitous nuclear protein |
|
RIP1 |
receptor-interacting protein 1 |
|
VEGF |
vascular endothelial growth factor |
|
EGF |
epidermal growth factor |
|
RTKs |
receptor tyrosine kinases |
|
OSCC |
oral squamous cell carcinoma |
|
PIP2 |
phosphatidylinositol 4,5-biphosphate |
|
PIP3 |
phosphatidylinositol-3,4,5-trisphosphate |
|
PDK1 |
3-phosphoinositide-dependent protein kinase 1 |
|
AKT |
protein kinase B |
|
PTEN |
phosphatase and tensin homolog |
|
OTUD3 |
OTU domain-containing protein 3 |
|
SMAD |
SMA and MAD-related protein |
|
EMT |
epithelial-mesenchymal transition |
|
PHD |
prolyl hydroxylase |
|
VHL |
von Hippel-Lindau |
|
APC |
adenomatous polyposis coli |
|
CK1a |
casein kinase 1a |
|
GSK3 |
glycogen synthase kinase-3 |
|
TCF/LEF |
T-cell factor/lymphoid enhancer-binding factor |
|
MST |
Mammalian sterile 20-like kinase |
|
EZH2 |
enhancer of zeste homolog 2 |
|
YAP |
yes-associated protein |
|
TAZ |
PDZ-binding motif |
|
TEAD |
TEA domain transcription factor |
|
DVL |
disheveled segment polarity protein |
|
GPCR |
G protein-coupled receptors |
|
MST |
macrophage stimulating |
|
HSF1 |
heat shock transcription factor 1 |
|
PD-1 |
programmed death-1 |
|
LMP1 |
latent membrane protein 1 |
|
DUBTAC |
deubiquitinase chimeras |
|
CFTR |
cystic fibrosis transmembrane conductance regulator |
References
|
Parmar K, Mohamed A, Vaish E, Thawani R, Cetnar J and Thein KZ: Immunotherapy in head and neck squamous cell carcinoma: An updated review. Cancer Treat Res Commun. 33:1006492022. View Article : Google Scholar : PubMed/NCBI | |
|
Johnson DE, Burtness B, Leemans CR, Lui VWY, Bauman JE and Grandis JR: Head and neck squamous cell carcinoma. Nat Rev Dis Primers. 6:922020. View Article : Google Scholar : PubMed/NCBI | |
|
McDermott JD and Bowles DW: Epidemiology of head and neck squamous cell carcinomas: Impact on staging and prevention strategies. Curr Treat Options Oncol. 20:432019. View Article : Google Scholar : PubMed/NCBI | |
|
Bhatia A and Burtness B: Treating head and neck cancer in the age of immunotherapy: A 2023 update. Drugs. 83:217–248. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Daste A, Larroquette M, Gibson N, Lasserre M and Domblides C: Immunotherapy for head and neck squamous cell carcinoma: Current status and perspectives. Immunotherapy. 16:187–197. 2024. View Article : Google Scholar : PubMed/NCBI | |
|
Kim YJ, Lee Y, Shin H, Hwang S, Park J and Song EJ: Ubiquitin-proteasome system as a target for anticancer treatment-an update. Arch Pharm Res. 46:573–597. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Wang J, Xiang Y, Fan M, Fang S and Hua Q: The ubiquitin-proteasome system in tumor metabolism. Cancers (Basel). 15:23852023. View Article : Google Scholar : PubMed/NCBI | |
|
Schulman BA and Harper JW: Ubiquitin-like protein activation by E1 enzymes: The apex for downstream signalling pathways. Nat Rev Mol Cell Biol. 10:319–331. 2009. View Article : Google Scholar : PubMed/NCBI | |
|
Zhai F, Wang J, Yang W, Ye M and Jin X: The E3 ligases in cervical cancer and endometrial cancer. Cancers (Basel). 14:53542022. View Article : Google Scholar : PubMed/NCBI | |
|
Kong L and Jin X: Dysregulation of deubiquitination in breast cancer. Gene. 902:1481752024. View Article : Google Scholar : PubMed/NCBI | |
|
Park J, Cho J and Song EJ: Ubiquitin-proteasome system (UPS) as a target for anticancer treatment. Arch Pharm Res. 43:1144–1161. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Hochstrasser M: Ubiquitin, proteasomes, and the regulation of intracellular protein degradation. Curr Opin Cell Biol. 7:215–223. 1995. View Article : Google Scholar : PubMed/NCBI | |
|
Spano D and Catara G: Targeting the ubiquitin-proteasome system and recent advances in cancer therapy. Cells. 13:292023. View Article : Google Scholar : PubMed/NCBI | |
|
Bennett EJ and Harper JW: DNA damage: Ubiquitin marks the spot. Nat Struct Mol Biol. 15:20–22. 2008. View Article : Google Scholar : PubMed/NCBI | |
|
Pickart CM and Eddins MJ: Ubiquitin: Structures, functions, mechanisms. Biochim Biophys Acta. 1695:55–72. 2004. View Article : Google Scholar : PubMed/NCBI | |
|
Komander D, Clague MJ and Urbé S: Breaking the chains: Structure and function of the deubiquitinases. Nat Rev Mol Cell Biol. 10:550–563. 2009. View Article : Google Scholar : PubMed/NCBI | |
|
Dewson G, Eichhorn PJA and Komander D: Deubiquitinases in cancer. Nat Rev Cancer. 23:842–862. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
D'Arcy P, Wang X and Linder S: Deubiquitinase inhibition as a cancer therapeutic strategy. Pharmacol Ther. 147:32–54. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Abdul Rehman SA, Kristariyanto YA, Choi SY, Nkosi PJ, Weidlich S, Labib K, Hofmann K and Kulathu Y: MINDY-1 is a member of an evolutionarily conserved and structurally distinct new family of deubiquitinating enzymes. Mol Cell. 63:146–155. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Jin S, Kudo Y and Horiguchi T: The role of deubiquitinating enzyme in head and neck squamous cell carcinoma. Int J Mol Sci. 24:5522022. View Article : Google Scholar : PubMed/NCBI | |
|
Hu M, Li P, Li M, Li W, Yao T, Wu JW, Gu W, Cohen RE and Shi Y: Crystal structure of a UBP-family deubiquitinating enzyme in isolation and in complex with ubiquitin aldehyde. Cell. 111:1041–1054. 2002. View Article : Google Scholar : PubMed/NCBI | |
|
Kuhlbrodt K, Janiesch PC, Kevei É, Segref A, Barikbin R and Hoppe T: The Machado-Joseph disease deubiquitylase ATX-3 couples longevity and proteostasis. Nat Cell Biol. 13:273–281. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
Nicastro G, Menon RP, Masino L, Knowles PP, McDonald NQ and Pastore A: The solution structure of the Josephin domain of ataxin-3: Structural determinants for molecular recognition. Proc Natl Acad Sci USA. 102:10493–10498. 2005. View Article : Google Scholar : PubMed/NCBI | |
|
Hurley JH and Stenmark H: Molecular mechanisms of ubiquitin-dependent membrane traffic. Annu Rev Biophys. 40:119–142. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
Davies CW, Paul LN, Kim MI and Das C: Structural and thermodynamic comparison of the catalytic domain of AMSH and AMSH-LP: Nearly identical fold but different stability. J Mol Biol. 413:416–429. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
Liang J, Saad Y, Lei T, Wang J, Qi D, Yang Q, Kolattukudy PE and Fu M: MCP-induced protein 1 deubiquitinates TRAF proteins and negatively regulates JNK and NF-kappaB signaling. J Exp Med. 207:2959–2973. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Chen SMY, Krinsky AL, Woolaver RA, Wang X, Chen Z and Wang JH: Tumor immune microenvironment in head and neck cancers. Mol Carcinog. 59:766–774. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Gulve N, Su C, Deng Z, Soldan SS, Vladimirova O, Wickramasinghe J, Zheng H, Kossenkov AV and Lieberman PM: DAXX-ATRX regulation of p53 chromatin binding and DNA damage response. Nat Commun. 13:50332022. View Article : Google Scholar : PubMed/NCBI | |
|
Li J, Shang L, Zhou F, Wang S, Liu N, Zhou M, Lin Q, Zhang M, Cai Y, Chen G and Yang S: Herba patriniae and its component isovitexin show anti-colorectal cancer effects by inducing apoptosis and cell-cycle arrest via p53 activation. Biomed Pharmacother. 168:1156902023. View Article : Google Scholar : PubMed/NCBI | |
|
Wang H, Guo M, Wei H and Chen Y: Targeting p53 pathways: mechanisms, structures, and advances in therapy. Signal Transduct Target Ther. 8:922023. View Article : Google Scholar : PubMed/NCBI | |
|
Hassin O and Oren M: Drugging p53 in cancer: One protein, many targets. Nat Rev Drug Discov. 22:127–144. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Brummer T and Zeiser R: The role of the MDM2/p53 axis in antitumor immune responses. Blood. 143:2701–2709. 2024. View Article : Google Scholar : PubMed/NCBI | |
|
Bradford CR, Zhu S, Poore J, Fisher SG, Beals TF, Thoraval D, Hanash SM, Carey TE and Wolf GT: p53 mutation as a prognostic marker in advanced laryngeal carcinoma. Department of veterans affairs laryngeal cancer cooperative study group. Arch Otolaryngol Head Neck Surg. 123:605–609. 1997. View Article : Google Scholar : PubMed/NCBI | |
|
Nathan CA, Khandelwal AR, Wolf GT, Rodrigo JP, Mäkitie AA, Saba NF, Forastiere AA, Bradford CR and Ferlito A: TP53 mutations in head and neck cancer. Mol Carcinog. 61:385–391. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Koo N, Sharma AK and Narayan S: Therapeutics targeting p53-MDM2 interaction to induce cancer cell death. Int J Mol Sci. 23:50052022. View Article : Google Scholar : PubMed/NCBI | |
|
Kwon SK, Saindane M and Baek KH: p53 stability is regulated by diverse deubiquitinating enzymes. Biochim Biophys Acta Rev Cancer. 1868:404–411. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Saha G, Roy S, Basu M and Ghosh MK: USP7-a crucial regulator of cancer hallmarks. Biochim Biophys Acta Rev Cancer. 1878:1889032023. View Article : Google Scholar : PubMed/NCBI | |
|
Shin SC, Park J, Kim KH, Yoon JM, Cho J, Ha BH, Oh Y, Choo H, Song EJ and Kim EE: Structural and functional characterization of USP47 reveals a hot spot for inhibitor design. Commun Biol. 6:9702023. View Article : Google Scholar : PubMed/NCBI | |
|
Nininahazwe L, Liu B, He C, Zhang H and Chen ZS: The emerging nature of Ubiquitin-specific protease 7 (USP7): A new target in cancer therapy. Drug Discov Today. 26:490–502. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Pozhidaeva A and Bezsonova I: USP7: Structure, substrate specificity, and inhibition. DNA Repair (Amst). 76:30–39. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Brooks CL, Li M, Hu M, Shi Y and Gu W: The p53-Mdm2-HAUSP complex is involved in p53 stabilization by HAUSP. Oncogene. 26:7262–7266. 2007. View Article : Google Scholar : PubMed/NCBI | |
|
Sacco JJ, Coulson JM, Clague MJ and Urbé S: Emerging roles of deubiquitinases in cancer-associated pathways. IUBMB Life. 62:140–157. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Niu H, Zhu Y, Wang J, Wang T, Wang X and Yan L: Effects of USP7 on radiation sensitivity through p53 pathway in laryngeal squamous cell carcinoma. Transl Oncol. 22:1014662022. View Article : Google Scholar : PubMed/NCBI | |
|
Guo Q, Jin Y, Chen X, Ye X, Shen X, Lin M, Zeng C, Zhou T and Zhang J: NF-κB in biology and targeted therapy: New insights and translational implications. Signal Transduct Target Ther. 9:532024. View Article : Google Scholar : PubMed/NCBI | |
|
Yu H, Lin L, Zhang Z, Zhang H and Hu H: Targeting NF-κB pathway for the therapy of diseases: Mechanism and clinical study. Signal Transduct Target Ther. 5:2092020. View Article : Google Scholar : PubMed/NCBI | |
|
Tan Y, Sun R, Liu L, Yang D, Xiang Q, Li L, Tang J, Qiu Z, Peng W, Wang Y, et al: Tumor suppressor DRD2 facilitates M1 macrophages and restricts NF-κB signaling to trigger pyroptosis in breast cancer. Theranostics. 11:5214–5231. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Schrank TP, Prince AC, Sathe T, Wang X, Liu X, Alzhanov DT, Burtness B, Baldwin AS, Yarbrough WG and Issaeva N: NF-κB over-activation portends improved outcomes in HPV-associated head and neck cancer. Oncotarget. 13:707–722. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Jackson-Bernitsas DG, Ichikawa H, Takada Y, Myers JN, Lin XL, Darnay BG, Chaturvedi MM and Aggarwal BB: Evidence that TNF-TNFR1-TRADD-TRAF2-RIP-TAK1-IKK pathway mediates constitutive NF-kappaB activation and proliferation in human head and neck squamous cell carcinoma. Oncogene. 26:1385–1397. 2007. View Article : Google Scholar : PubMed/NCBI | |
|
Napetschnig J and Wu H: Molecular basis of NF-κB signaling. Annu Rev Biophys. 42:443–468. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Courtois G: Tumor suppressor CYLD: Negative regulation of NF-kappaB signaling and more. Cell Mol Life Sci. 65:1123–1132. 2008. View Article : Google Scholar : PubMed/NCBI | |
|
Yang Y and Zhou J: CYLD-a deubiquitylase that acts to fine-tune microtubule properties and functions. J Cell Sci. 129:2289–2295. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Cui Z, Kang H, Grandis JR and Johnson DE: CYLD alterations in the tumorigenesis and progression of human papillomavirus-associated head and neck cancers. Mol Cancer Res. 19:14–24. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Massoumi R: CYLD: A deubiquitination enzyme with multiple roles in cancer. Future Oncol. 7:285–297. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
Verhoeft KR, Ngan HL and Lui VWY: The cylindromatosis (CYLD) gene and head and neck tumorigenesis. Cancers Head Neck. 1:102016. View Article : Google Scholar : PubMed/NCBI | |
|
Deng M, Dai W, Yu VZ, Tao L and Lung ML: Cylindromatosis lysine 63 deubiquitinase (CYLD) regulates NF-kB signaling pathway and modulates fibroblast and endothelial cells recruitment in nasopharyngeal carcinoma. Cancers (Basel). 12:19242020. View Article : Google Scholar : PubMed/NCBI | |
|
Hu B, Zhang D, Zhao K, Wang Y, Pei L, Fu Q and Ma X: Spotlight on USP4: Structure, function, and regulation. Front Cell Dev Biol. 9:5951592021. View Article : Google Scholar : PubMed/NCBI | |
|
Tao Y and You W: The deubiquitinating enzyme USP4 functions as an oncoprotein in gastric cancer and mediates NF-κB signaling by regulating PRL-3 expression. Front Biosci (Landmark Ed). 27:2862022. View Article : Google Scholar : PubMed/NCBI | |
|
Hou X, Wang L, Zhang L, Pan X and Zhao W: Ubiquitin-specific protease 4 promotes TNF-α-induced apoptosis by deubiquitination of RIP1 in head and neck squamous cell carcinoma. FEBS Lett. 587:311–316. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Shi A, Liu L, Li S and Qi B: Natural products targeting the MAPK-signaling pathway in cancer: Overview. J Cancer Res Clin Oncol. 150:62024. View Article : Google Scholar : PubMed/NCBI | |
|
Cheng Y, Chen J, Shi Y, Fang X and Tang Z: MAPK signaling pathway in oral squamous cell carcinoma: Biological function and targeted therapy. Cancers (Basel). 14:46252022. View Article : Google Scholar : PubMed/NCBI | |
|
Roskoski R Jr: ERK1/2 MAP kinases: Structure, function, and regulation. Pharmacol Res. 66:105–143. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Ngan HL, Law CH, Choi YCY, Chan JY and Lui VWY: Precision drugging of the MAPK pathway in head and neck cancer. NPJ Genom Med. 7:202022. View Article : Google Scholar : PubMed/NCBI | |
|
Guo YJ, Pan WW, Liu SB, Shen ZF, Xu Y and Hu LL: ERK/MAPK signalling pathway and tumorigenesis. Exp Ther Med. 19:1997–2007. 2020.PubMed/NCBI | |
|
Wu PK, Becker A and Park JI: Growth inhibitory signaling of the Raf/MEK/ERK pathway. Int J Mol Sci. 21:54362020. View Article : Google Scholar : PubMed/NCBI | |
|
Shen J, Xie M, Xu Y, Qian Q, Qiu T, Shi W, Ren D, Ji J and Huang J: Identification of the deubiquitinase USP28 as a novel molecular therapeutic target of ovarian cancer. Biochem Biophys Res Commun. 638:184–191. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Li J, Peng J, Wu L, Shen X, Zhen X, Zhang Y, Ma H, Xu Y, Xiong Q, Zhu Q and Zhang P: The deubiquitinase USP28 maintains the expression of the transcription factor MYCN and is essential in neuroblastoma cells. J Biol Chem. 299:1048562023. View Article : Google Scholar : PubMed/NCBI | |
|
Prieto-Garcia C, Tomašković I, Shah VJ, Dikic I and Diefenbacher M: USP28: oncogene or tumor suppressor? A unifying paradigm for squamous cell carcinoma. Cells. 10:26522021. View Article : Google Scholar : PubMed/NCBI | |
|
Park HB and Baek KH: E3 ligases and deubiquitinating enzymes regulating the MAPK signaling pathway in cancers. Biochim Biophys Acta Rev Cancer. 1877:1887362022. View Article : Google Scholar : PubMed/NCBI | |
|
Arita H, Nagata M, Yoshida R, Matsuoka Y, Hirosue A, Kawahara K, Sakata J, Nakashima H, Kojima T, Toya R, et al: FBXW7 expression affects the response to chemoradiotherapy and overall survival among patients with oral squamous cell carcinoma: A single-center retrospective study. Tumour Biol. 39:10104283177317712017. View Article : Google Scholar : PubMed/NCBI | |
|
Yu H, Ling T, Shi R, Shu Q, Li Y and Tan Z: Expression of FBXW7 in esophageal squamous cell carcinoma and its clinical significance. Zhonghua Zhong Liu Za Zhi. 37:347–351. 2015.(In Chinese). PubMed/NCBI | |
|
Saei A, Palafox M, Benoukraf T, Kumari N, Jaynes PW, Iyengar PV, Muñoz-Couselo E, Nuciforo P, Cortés J, Nötzel C, et al: Loss of USP28-mediated BRAF degradation drives resistance to RAF cancer therapies. J Exp Med. 215:1913–1928. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Cheng Y and Tian H: Current development status of MEK inhibitors. Molecules. 22:15512017. View Article : Google Scholar : PubMed/NCBI | |
|
Glaviano A, Foo ASC, Lam HY, Yap KCH, Jacot W, Jones RH, Eng H, Nair MG, Makvandi P, Geoerger B, et al: PI3K/AKT/mTOR signaling transduction pathway and targeted therapies in cancer. Mol Cancer. 22:1382023. View Article : Google Scholar : PubMed/NCBI | |
|
Jin J, He J, Li X, Ni X and Jin X: The role of ubiquitination and deubiquitination in PI3K/AKT/mTOR pathway: A potential target for cancer therapy. Gene. 889:1478072023. View Article : Google Scholar : PubMed/NCBI | |
|
Chen M, Choi S, Wen T, Chen C, Thapa N, Lee JH, Cryns VL and Anderson RA: A p53-phosphoinositide signalosome regulates nuclear AKT activation. Nat Cell Biol. 24:1099–1113. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Marquard FE and Jücker M: PI3K/AKT/mTOR signaling as a molecular target in head and neck cancer. Biochem Pharmacol. 172:1137292020. View Article : Google Scholar : PubMed/NCBI | |
|
De Felice F and Guerrero Urbano T: New drug development in head and neck squamous cell carcinoma: The PI3-K inhibitors. Oral Oncol. 67:119–123. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Borgato GB, Borges GA, Souza AP, Squarize CH and Castilho RM: Loss of PTEN sensitizes head and neck squamous cell carcinoma to 5-AZA-2′-deoxycytidine. Oral Surg Oral Med Oral Pathol Oral Radiol. 130:181–190. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Psyrri A, Seiwert TY and Jimeno A: Molecular pathways in head and neck cancer: EGFR, PI3K, and more. Am Soc Clin Oncol Educ Book. 2013:246–255. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Tewari D, Patni P and Bishayee A, Sah AN and Bishayee A: Natural products targeting the PI3K-Akt-mTOR signaling pathway in cancer: A novel therapeutic strategy. Semin Cancer Biol. 80:1–17. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Vander Broek R, Mohan S, Eytan DF, Chen Z and Van Waes C: The PI3K/Akt/mTOR axis in head and neck cancer: Functions, aberrations, cross-talk, and therapies. Oral Dis. 21:815–825. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Li X, Yang G, Zhang W, Qin B, Ye Z, Shi H, Zhao X, Chen Y, Song B, Mei Z, et al: USP13: Multiple functions and target inhibition. Front Cell Dev Biol. 10:8751242022. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang J, Zhang P, Wei Y, Piao HL, Wang W, Maddika S, Wang M, Chen D, Sun Y, Hung MC, et al: Deubiquitylation and stabilization of PTEN by USP13. Nat Cell Biol. 15:1486–1494. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Qu Z, Zhang R, Su M and Liu W: USP13 serves as a tumor suppressor via the PTEN/AKT pathway in oral squamous cell carcinoma. Cancer Manag Res. 11:9175–9183. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Huang J, Ye Z, Wang J, Chen Q, Huang D and Liu H: USP13 mediates PTEN to ameliorate osteoarthritis by restraining oxidative stress, apoptosis and inflammation via AKT-dependent manner. Biomed Pharmacother. 133:1110892021. View Article : Google Scholar : PubMed/NCBI | |
|
Wang K, Liu J, Li YL, Li JP and Zhang R: Ubiquitination/de-ubiquitination: A promising therapeutic target for PTEN reactivation in cancer. Biochim Biophys Acta Rev Cancer. 1877:1887232022. View Article : Google Scholar : PubMed/NCBI | |
|
Wang M, Li Y, Xiao Y, Yang M, Chen J, Jian Y, Chen X, Shi D, Chen X, Ouyang Y, et al: Nicotine-mediated OTUD3 downregulation inhibits VEGF-C mRNA decay to promote lymphatic metastasis of human esophageal cancer. Nat Commun. 12:70062021. View Article : Google Scholar : PubMed/NCBI | |
|
Geng W, Song H, Zhao Q, Dong K, Pu Q, Gao H and Lv Y: miR-520h stimulates drug resistance to paclitaxel by targeting the OTUD3-PTEN axis in breast cancer. Biomed Res Int. 2020:95127932020. View Article : Google Scholar : PubMed/NCBI | |
|
Yuan L, Lv Y, Li H, Gao H, Song S, Zhang Y, Xing G, Kong X, Wang L, Li Y, et al: Deubiquitylase OTUD3 regulates PTEN stability and suppresses tumorigenesis. Nat Cell Biol. 17:1169–1181. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Zhao M, Yang F, Sang C, Yan C and Wang Z: BGL3 inhibits papillary thyroid carcinoma progression via regulating PTEN stability. J Endocrinol Invest. 44:2165–2174. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Antonenko S, Zavelevich M and Telegeev G: The role of USP1 deubiquitinase in the pathogenesis and therapy of cancer. Acta Biochim Pol. 70:219–231. 2023.PubMed/NCBI | |
|
Xu J, Li B, Song W, Cao L, Zhu C and Lin S: Tumor suppressor functions of miRNA-375 in nasopharyngeal carcinoma through inhibition of ubiquitin-specific protease 1 expression. Int J Biochem Cell Biol. 141:1060922021. View Article : Google Scholar : PubMed/NCBI | |
|
Vucic D, Dixit VM and Wertz IE: Ubiquitylation in apoptosis: A post-translational modification at the edge of life and death. Nat Rev Mol Cell. 12:439–452. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
Schaefer A, Nethe M and Hordijk PL: Ubiquitin links to cytoskeletal dynamics, cell adhesion and migration. Biochem J. 442:13–25. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Ulrich HD and Walden H: Ubiquitin signalling in DNA replication and repair. Nat Rev Mol Cell Biol. 11:479–489. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Ma A, Tang M, Zhang L, Wang B, Yang Z, Liu Y, Xu G, Wu L, Jing T, Xu X, et al: Correction to: USP1 inhibition destabilizes KPNA2 and suppresses breast cancer metastasis. Oncogene. 41:16732022. View Article : Google Scholar : PubMed/NCBI | |
|
Liu D, Li Q, Zang Y, Li X, Li Z, Zhang P, Feng C, Yang P, Cui J, Sun Y, et al: USP1 modulates hepatocellular carcinoma progression via the Hippo/TAZ axis. Cell Death Dis. 14:2642023. View Article : Google Scholar : PubMed/NCBI | |
|
Huang Z, Chen Y, Chen R, Zhou B, Wang Y, Hong L, Wang Y, Wang J, Xu X, Huang Z and Chen W: HPV Enhances HNSCC chemosensitization by inhibiting SERPINB3 expression to disrupt the fanconi anemia pathway. Adv Sci (Weinh). 10:e22024372022. View Article : Google Scholar : PubMed/NCBI | |
|
Goldbraikh D, Neufeld D, Eid-Mutlak Y, Lasry I, Gilda JE, Parnis A and Cohen S: USP1 deubiquitinates Akt to inhibit PI3K-Akt-FoxO signaling in muscle during prolonged starvation. EMBO Rep. 21:e487912020. View Article : Google Scholar : PubMed/NCBI | |
|
Shi Y and Massagué J: Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 113:685–700. 2003. View Article : Google Scholar : PubMed/NCBI | |
|
Heldin CH, Miyazono K and ten Dijke P: TGF-beta signalling from cell membrane to nucleus through SMAD proteins. Nature. 390:465–471. 1997. View Article : Google Scholar : PubMed/NCBI | |
|
Derynck R and Budi EH: Specificity, versatility, and control of TGF-β family signaling. Sci Signal. 12:eaav51832019. View Article : Google Scholar : PubMed/NCBI | |
|
Meulmeester E and Ten Dijke P: The dynamic roles of TGF-β in cancer. J Pathol. 223:205–218. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
Britton WR, Cioffi I, Stonebraker C, Spence M, Okolo O, Martin C, Henick B, Nakagawa H and Parikh AS: Advancements in TGF-β Targeting Therapies for Head and Neck Squamous Cell Carcinoma. Cancers (Basel). 16:30472024. View Article : Google Scholar : PubMed/NCBI | |
|
Ibi H, Takahashi K, Harada H, Watabe T and Podyma-Inoue KA: Transforming growth factor-β signals promote progression of squamous cell carcinoma by inducing epithelial-mesenchymal transition and angiogenesis. Biochem Biophys Res Commun. 714:1499652024. View Article : Google Scholar : PubMed/NCBI | |
|
Itoh S and ten Dijke P: Negative regulation of TGF-beta receptor/Smad signal transduction. Curr Opin Cell Biol. 19:176–184. 2007. View Article : Google Scholar : PubMed/NCBI | |
|
Shinriki S, Jono H, Maeshiro M, Nakamura T, Guo J, Li JD, Ueda M, Yoshida R, Shinohara M, Nakayama H, et al: Loss of CYLD promotes cell invasion via ALK5 stabilization in oral squamous cell carcinoma. J Pathol. 244:367–379. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Zhao Y, Thornton AM, Kinney MC, Ma CA, Spinner JJ, Fuss IJ, Shevach EM and Jain A: The deubiquitinase CYLD targets Smad7 protein to regulate transforming growth factor β (TGF-β) signaling and the development of regulatory T cells. J Biol Chem. 286:40520–40530. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
Zhao Y, Xing C, Deng Y, Ye C and Peng H: HIF-1α signaling: Essential roles in tumorigenesis and implications in targeted therapies. Genes Dis. 11:234–251. 2024. View Article : Google Scholar : PubMed/NCBI | |
|
Yu Z, Li H, Zhu J, Wang H and Jin X: The roles of E3 ligases in Hepatocellular carcinoma. Am J Cancer Res. 12:1179–1214. 2022.PubMed/NCBI | |
|
Semenza GL: Oxygen sensing, hypoxia-inducible factors, and disease pathophysiology. Annu Rev Pathol. 9:47–71. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Wenger RH, Stiehl DP and Camenisch G: Integration of oxygen signaling at the consensus HRE. Sci STKE. 2005:re122005. View Article : Google Scholar : PubMed/NCBI | |
|
Jaakkola P, Mole DR, Tian YM, Wilson MI, Gielbert J, Gaskell SJ, von Kriegsheim A, Hebestreit HF, Mukherji M, Schofield CJ, et al: Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science. 292:468–472. 2001. View Article : Google Scholar : PubMed/NCBI | |
|
Gong L, Zhang W, Zhou J, Lu J, Xiong H, Shi X and Chen J: Prognostic value of HIFs expression in head and neck cancer: A systematic review. PLoS One. 8:e750942013. View Article : Google Scholar : PubMed/NCBI | |
|
Grethe C, Schmidt M, Kipka GM, O'Dea R, Gallant K, Janning P and Gersch M: Structural basis for specific inhibition of the deubiquitinase UCHL1. Nat Commun. 13:59502022. View Article : Google Scholar : PubMed/NCBI | |
|
Bishop P, Rocca D and Henley JM: Ubiquitin C-terminal hydrolase L1 (UCH-L1): Structure, distribution and roles in brain function and dysfunction. Biochem J. 473:2453–2462. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Lee KC, Chen HH, Cheng KC, Liu TT, Lee KF, Teng CC, Huang CY, Hsieh MC and Kuo HC: Use of iTRAQ-based quantitative proteomic identification of CHGA and UCHL1 correlated with lymph node metastasis in colorectal carcinoma. J Cell Mol Med. 27:2004–2020. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Li J, Liang Y, Zhou S, Chen J and Wu C: UCHL1 contributes to insensitivity to endocrine therapy in triple-negative breast cancer by deubiquitinating and stabilizing KLF5. Breast Cancer Res. 26:442024. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang D, Fu Y, Tian G, Li J, Shang D and Zhou S: UCHL1 promotes proliferation and metastasis in head and neck squamous cell carcinoma and could be a potential therapeutic target. Oral Surg Oral Med Oral Pathol Oral Radiol. 133:684–697. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Li X, Hattori A, Takahashi S, Goto Y, Harada H and Kakeya H: Ubiquitin carboxyl-terminal hydrolase L1 promotes hypoxia-inducible factor 1-dependent tumor cell malignancy in spheroid models. Cancer Sci. 111:239–252. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Zanoni M, Piccinini F, Arienti C, Zamagni A, Santi S, Polico R, Bevilacqua A and Tesei A: 3D tumor spheroid models for in vitro therapeutic screening: A systematic approach to enhance the biological relevance of data obtained. Sci Rep. 6:191032016. View Article : Google Scholar : PubMed/NCBI | |
|
Günter J, Ruiz-Serrano A, Pickel C, Wenger RH and Scholz CC: The functional interplay between the HIF pathway and the ubiquitin system-more than a one-way road. Exp Cell Res. 356:152–159. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Tejeda-Muñoz N and Robles-Flores M: Glycogen synthase kinase 3 in Wnt signaling pathway and cancer. IUBMB Life. 67:914–922. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Gordon MD and Nusse R: Wnt signaling: Multiple pathways, multiple receptors, and multiple transcription factors. J Biol Chem. 281:22429–22433. 2006. View Article : Google Scholar : PubMed/NCBI | |
|
Xie J, Huang L, Lu YG and Zheng DL: Roles of the Wnt signaling pathway in head and neck squamous cell carcinoma. Front Mol Biosci. 7:5909122020. View Article : Google Scholar : PubMed/NCBI | |
|
Yang F, Zeng Q, Yu G, Li S and Wang CY: Wnt/beta-catenin signaling inhibits death receptor-mediated apoptosis and promotes invasive growth of HNSCC. Cell Signal. 18:679–687. 2006. View Article : Google Scholar : PubMed/NCBI | |
|
Moon JH, Lee SH and Lim YC: Wnt/β-catenin/Slug pathway contributes to tumor invasion and lymph node metastasis in head and neck squamous cell carcinoma. Clin Exp Metastasis. 38:163–174. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Zheng N, Chu M, Lin M, He Y and Wang Z: USP7 stabilizes EZH2 and enhances cancer malignant progression. Am J Cancer Res. 10:299–313. 2020.PubMed/NCBI | |
|
Yamagishi M and Uchimaru K: Targeting EZH2 in cancer therapy. Curr Opin Oncol. 29:375–381. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Wang L, Jin Q, Lee JE, Su IH and Ge K: Histone H3K27 methyltransferase Ezh2 represses Wnt genes to facilitate adipogenesis. Proc Natl Acad Sci USA. 107:7317–7322. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang MJ, Chen DS, Li H, Liu WW, Han GY and Han YF: Clinical significance of USP7 and EZH2 in predicting prognosis of laryngeal squamous cell carcinoma and their possible functional mechanism. Int J Clin Exp Pathol. 12:2184–2194. 2019.PubMed/NCBI | |
|
Liu S, Qin Z, Mao Y, Zhang W, Wang Y, Jia L and Peng X: Therapeutic targeting of MYC in head and neck squamous cell carcinoma. Oncoimmunology. 11:21305832022. View Article : Google Scholar : PubMed/NCBI | |
|
Llombart V and Mansour MR: Therapeutic targeting of ‘undruggable’ MYC. EBioMedicine. 75:1037562022. View Article : Google Scholar : PubMed/NCBI | |
|
Dejure FR and Eilers M: MYC and tumor metabolism: Chicken and egg. EMBO J. 36:3409–3420. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Wang L, Chen C, Song Z, Wang H, Ye M, Wang D, Kang W, Liu H and Qing G: EZH2 depletion potentiates MYC degradation inhibiting neuroblastoma and small cell carcinoma tumor formation. Nat Commun. 13:122022. View Article : Google Scholar : PubMed/NCBI | |
|
Dou Y, Lin J, Shu H and Jiang N: Role of ubiquitin-specific peptidase 22 in carcinogenesis of human pharyngeal squamous cell carcinoma. Mol Med Rep. 10:2973–2978. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Kim D, Hong A, Park HI, Shin WH, Yoo L, Jeon SJ and Chung KC: Deubiquitinating enzyme USP22 positively regulates c-Myc stability and tumorigenic activity in mammalian and breast cancer cells. J Cell Physiol. 232:3664–3676. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Li L, Wen S, Wang B, Gao W, Zhang W, Meng X, Yang L and Kong L: Expression of cancer stem cell marker USP22 in laryngeal squamous cell carcinoma. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 49:479–482. 2014.(In Chinese). PubMed/NCBI | |
|
Shin E and Kim J: The potential role of YAP in head and neck squamous cell carcinoma. Exp Mol Med. 52:1264–1274. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Faraji F, Ramirez SI, Anguiano Quiroz PY, Mendez-Molina AN and Gutkind JS: Genomic hippo pathway alterations and persistent YAP/TAZ activation: New hallmarks in head and neck cancer. Cells. 11:13702022. View Article : Google Scholar : PubMed/NCBI | |
|
Segrelles C, Paramio JM and Lorz C: The transcriptional co-activator YAP: A new player in head and neck cancer. Oral Oncol. 86:25–32. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Chan EH, Nousiainen M, Chalamalasetty RB, Schäfer A, Nigg EA and Silljé HH: The Ste20-like kinase Mst2 activates the human large tumor suppressor kinase Lats1. Oncogene. 24:2076–2086. 2005. View Article : Google Scholar : PubMed/NCBI | |
|
Kanai F, Marignani PA, Sarbassova D, Yagi R, Hall RA, Donowitz M, Hisaminato A, Fujiwara T, Ito Y, Cantley LC and Yaffe MB: TAZ: A novel transcriptional co-activator regulated by interactions with 14-3-3 and PDZ domain proteins. EMBO J. 19:6778–6791. 2000. View Article : Google Scholar : PubMed/NCBI | |
|
Zhao B, Ye X, Yu J, Li L, Li W, Li S, Yu J, Lin JD, Wang CY, Chinnaiyan AM, et al: TEAD mediates YAP-dependent gene induction and growth control. Genes Dev. 22:1962–1971. 2008. View Article : Google Scholar : PubMed/NCBI | |
|
Pocaterra A, Romani P and Dupont S: YAP/TAZ functions and their regulation at a glance. J Cell Sci. 133:jcs2304252020. View Article : Google Scholar : PubMed/NCBI | |
|
Fang Y, Fu D and Shen XZ: The potential role of ubiquitin c-terminal hydrolases in oncogenesis. Biochim Biophys Acta. 1806:1–6. 2010.PubMed/NCBI | |
|
Mtango NR, Sutovsky M, Susor A, Zhong Z, Latham KE and Sutovsky P: Essential role of maternal UCHL1 and UCHL3 in fertilization and preimplantation embryo development. J Cell Physiol. 227:1592–1603. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Suzuki M, Setsuie R and Wada K: Ubiquitin carboxyl-terminal hydrolase l3 promotes insulin signaling and adipogenesis. Endocrinology. 150:5230–5239. 2009. View Article : Google Scholar : PubMed/NCBI | |
|
Mtango NR, Sutovsky M, Vandevoort CA, Latham KE and Sutovsky P: Essential role of ubiquitin C-terminal hydrolases UCHL1 and UCHL3 in mammalian oocyte maturation. J Cell Physiol. 227:2022–2029. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Nishi R, Wijnhoven PWG, Kimura Y, Matsui M, Konietzny R, Wu Q, Nakamura K, Blundell TL and Kessler BM: The deubiquitylating enzyme UCHL3 regulates Ku80 retention at sites of DNA damage. Sci Rep. 8:178912018. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang X, Smits AH, van Tilburg GB, Jansen PW, Makowski MM, Ovaa H and Vermeulen M: An interaction landscape of ubiquitin signaling. Mol Cell. 65:941–955.e8. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Tang J, Yang Q, Mao C, Xiao D, Liu S, Xiao L, Zhou L, Wu G and Tao Y: The deubiquitinating enzyme UCHL3 promotes anaplastic thyroid cancer progression and metastasis through Hippo signaling pathway. Cell Death Differ. 30:1247–1259. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Wang J, Xiang Y, Xie Z, Fan M, Fang S, Wan H, Zhao R, Zeng F and Hua Q: USP14 positively modulates head and neck squamous carcinoma tumorigenesis and potentiates heat shock pathway through HSF1 Stabilization. Cancers (Basel). 15:43852023. View Article : Google Scholar : PubMed/NCBI | |
|
Wang F, Ning S, Yu B and Wang Y: USP14: Structure, function, and target inhibition. Front Pharmacol. 12:8013282022. View Article : Google Scholar : PubMed/NCBI | |
|
Shi D, Wu X, Jian Y, Wang J, Huang C, Mo S, Li Y, Li F, Zhang C, Zhang D, et al: USP14 promotes tryptophan metabolism and immune suppression by stabilizing IDO1 in colorectal cancer. Nat Commun. 13:56442022. View Article : Google Scholar : PubMed/NCBI | |
|
Ji J, Lv J, Lv M, Jing A, Xu M, Yuan Q, Ma X, Qian Q, Wang W, Geng T, et al: USP14 regulates heme metabolism and ovarian cancer invasion through BACH1 deubiquitination and stabilization. Biochem Biophys Res Commun. 667:186–193. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Tao L, Liu X, Jiang X, Zhang K, Wang Y, Li X, Jiang S and Han T: USP10 as a potential therapeutic target in human cancers. Genes (Basel). 13:8312022. View Article : Google Scholar : PubMed/NCBI | |
|
Xu Y, Pan J, Lin Y, Wu Y, Chen Y and Li H: Ceramide synthase 1 inhibits brain metastasis of non-small cell lung cancer by interacting with USP14 and downregulating the PI3K/AKT/mTOR signaling pathway. Cancers (Basel). 15:19942023. View Article : Google Scholar : PubMed/NCBI | |
|
Zhao C, Gong J, Bai Y, Yin T, Zhou M, Pan S, Liu Y, Gao Y, Zhang Z, Shi Y, et al: A self-amplifying USP14-TAZ loop drives the progression and liver metastasis of pancreatic ductal adenocarcinoma. Cell Death Differ. 30:1–15. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Scherz-Shouval R, Santagata S, Mendillo ML, Sholl LM, Ben-Aharon I, Beck AH, Dias-Santagata D, Koeva M, Stemmer SM, Whitesell L and Lindquist S: The reprogramming of tumor stroma by HSF1 is a potent enabler of malignancy. Cell. 158:564–578. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Carpenter RL and Gökmen-Polar Y: HSF1 as a cancer biomarker and therapeutic target. Curr Cancer Drug Targets. 19:515–524. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Tang Q, Chen Y, Li X, Long S, Shi Y, Yu Y, Wu W, Han L and Wang S: The role of PD-1/PD-L1 and application of immune-checkpoint inhibitors in human cancers. Front Immunol. 13:9644422022. View Article : Google Scholar : PubMed/NCBI | |
|
Yi M, Zheng X, Niu M, Zhu S, Ge H and Wu K: Combination strategies with PD-1/PD-L1 blockade: Current advances and future directions. Mol Cancer. 21:282022. View Article : Google Scholar : PubMed/NCBI | |
|
Fasano M, Corte CMD, Liello RD, Viscardi G, Sparano F, Iacovino ML, Paragliola F, Piccolo A, Napolitano S, Martini G, et al: Immunotherapy for head and neck cancer: Present and future. Crit Rev Oncol Hematol. 174:1036792022. View Article : Google Scholar : PubMed/NCBI | |
|
Jiang S, Li X, Huang L, Xu Z and Lin J: Prognostic value of PD-1, PD-L1 and PD-L2 deserves attention in head and neck cancer. Front Immunol. 13:9884162022. View Article : Google Scholar : PubMed/NCBI | |
|
Kapuria V, Peterson LF, Fang D, Bornmann WG, Talpaz M and Donato NJ: Deubiquitinase inhibition by small-molecule WP1130 triggers aggresome formation and tumor cell apoptosis. Cancer Res. 70:9265–9276. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Huang G, Liao J, Wang M, Huang Y, Tang M and Hao Y: USP9X increased tumor angiogenesis in mantle cell lymphoma by upregulation of CCND1-mediated SOX11. Mediterr J Hematol Infect Dis. 14:e20220482022. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang FK, Ni QZ, Wang K, Cao HJ, Guan DX, Zhang EB, Ma N, Wang YK, Zheng QW, Xu S, et al: Targeting USP9X-AMPK axis in ARID1A-deficient hepatocellular carcinoma. Cell Mol Gastroenterol Hepatol. 14:101–127. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Li X, Song N, Liu L, Liu X, Ding X, Song X, Yang S, Shan L, Zhou X, Su D, et al: USP9X regulates centrosome duplication and promotes breast carcinogenesis. Nat Commun. 8:148662017. View Article : Google Scholar : PubMed/NCBI | |
|
Wang Y, Liu Y, Yang B, Cao H, Yang CX, Ouyang W, Zhang SM, Yang GF, Zhou FX, Zhou YF and Xie CH: Elevated expression of USP9X correlates with poor prognosis in human non-small cell lung cancer. J Thorac Dis. 7:672–679. 2015.PubMed/NCBI | |
|
Nanayakkara DM, Nguyen MN and Wood SA: Deubiquitylating enzyme, USP9X, regulates proliferation of cells of head and neck cancer lines. Cell Prolif. 49:494–502. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Potu H, Peterson LF, Kandarpa M, Pal A, Sun H, Durham A, Harms PW, Hollenhorst PC, Eskiocak U, Talpaz M and Donato NJ: Usp9× regulates Ets-1 ubiquitination and stability to control NRAS expression and tumorigenicity in melanoma. Nat Commun. 8:144492017. View Article : Google Scholar : PubMed/NCBI | |
|
Wu J, Guo W, Wen D, Hou G, Zhou A and Wu W: Deubiquitination and stabilization of programmed cell death ligand 1 by ubiquitin-specific peptidase 9, X-linked in oral squamous cell carcinoma. Cancer Med. 7:4004–4011. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Pandruvada S, Kessler R and Thai A: Head and neck cancer treatment in the era of molecular medicine. Adv Cancer Res. 160:205–252. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Runnels J, Bloom JR, Hsieh K, Dickstein DR, Shi Y, Jones BM, Lehrer EJ and Bakst RL: Combining radiotherapy and immunotherapy in head and neck cancer. Biomedicines. 11:20972023. View Article : Google Scholar : PubMed/NCBI | |
|
Chung CH, Li J, Steuer CE, Bhateja P, Johnson M, Masannat J, Poole MI, Song F, Hernandez-Prera JC, Molina H, et al: Phase II multi-institutional clinical trial result of concurrent cetuximab and nivolumab in recurrent and/or metastatic head and neck squamous cell carcinoma. Clin Cancer Res. 28:2329–2338. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Kitamura N, Sento S, Yoshizawa Y, Sasabe E, Kudo Y and Yamamoto T: Current trends and future prospects of molecular targeted therapy in head and neck squamous cell carcinoma. Int J Mol Sci. 22:2402020. View Article : Google Scholar : PubMed/NCBI | |
|
Harrington KJ, Burtness B, Greil R, Soulières D, Tahara M, de Castro G Jr, Psyrri A, Brana I, Basté N, Neupane P, et al: Pembrolizumab with or without chemotherapy in recurrent or metastatic head and neck squamous cell carcinoma: Updated results of the phase III KEYNOTE-048 study. J Clin Oncol. 41:790–802. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Cai Y, Dodhia S and Su GH: Dysregulations in the PI3K pathway and targeted therapies for head and neck squamous cell carcinoma. Oncotarget. 8:22203–22217. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Bozec A, Ebran N, Radosevic-Robin N, Chamorey E, Yahia HB, Marcie S, Gautier M, Penault-Llorca F and Milano G: Combination of phosphotidylinositol-3-kinase targeting with cetuximab and irradiation: A preclinical study on an orthotopic xenograft model of head and neck cancer. Head Neck. 39:151–159. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Liao YM, Kim C and Yen Y: Mammalian target of rapamycin and head and neck squamous cell carcinoma. Head Neck Oncol. 3:222011. View Article : Google Scholar : PubMed/NCBI | |
|
Marret G, Isambert N, Rezai K, Gal J, Saada-Bouzid E, Rolland F, Chausson M, Borcoman E, Alt M, Klijanienko J, et al: Phase I trial of copanlisib, a selective PI3K inhibitor, in combination with cetuximab in patients with recurrent and/or metastatic head and neck squamous cell carcinoma. Invest New Drugs. 39:1641–1648. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Nathan CO, Hayes DN, Karrison T, Harismendy O, Flores JM, Moore-Medlin T, Vokes EE, Gutkind JS, Neupane P, Mills G, et al: A Randomized multi-institutional phase II trial of everolimus as adjuvant therapy in patients with locally advanced squamous cell cancer of the head and neck. Clin Cancer Res. 28:5040–5048. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Poondla N, Chandrasekaran AP, Kim KS and Ramakrishna S: Deubiquitinating enzymes as cancer biomarkers: New therapeutic opportunities? BMB Rep. 52:181–189. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Lee HR, Choi WC, Lee S, Hwang J, Hwang E, Guchhait K, Haas J, Toth Z, Jeon YH, Oh TK, et al: Bilateral inhibition of HAUSP deubiquitinase by a viral interferon regulatory factor protein. Nat Struct Mol Biol. 18:1336–1344. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
Hu T, Zhang J, Sha B, Li M, Wang L, Zhang Y, Liu X, Dong Z, Liu Z, Li P and Chen P: Targeting the overexpressed USP7 inhibits esophageal squamous cell carcinoma cell growth by inducing NOXA-mediated apoptosis. Mol Carcinog. 58:42–54. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Yoshizaki T, Kondo S, Endo K, Nakanishi Y, Aga M, Kobayashi E, Hirai N, Sugimoto H, Hatano M, Ueno T, et al: Modulation of the tumor microenvironment by Epstein-Barr virus latent membrane protein 1 in nasopharyngeal carcinoma. Cancer Sci. 109:272–278. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Kondo S, Seo SY, Yoshizaki T, Wakisaka N, Furukawa M, Joab I, Jang KL and Pagano JS: EBV latent membrane protein 1 up-regulates hypoxia-inducible factor 1alpha through Siah1-mediated down-regulation of prolyl hydroxylases 1 and 3 in nasopharyngeal epithelial cells. Cancer Res. 66:9870–9877. 2006. View Article : Google Scholar : PubMed/NCBI | |
|
Kobayashi E, Hwang D, Bheda-Malge A, Whitehurst CB, Kabanov AV, Kondo S, Aga M, Yoshizaki T, Pagano JS, Sokolsky M and Shakelford J: Inhibition of UCH-L1 deubiquitinating activity with two forms of LDN-57444 has anti-invasive effects in metastatic carcinoma cells. Int J Mol Sci. 20:37332019. View Article : Google Scholar : PubMed/NCBI | |
|
Ding W, Wang JX, Wu JZ, Liu AC, Jiang LL, Zhang HC, Meng Y, Liu BY, Peng GJ, Lou EZ, et al: Targeting proteasomal deubiquitinases USP14 and UCHL5 with b-AP15 reduces 5-fluorouracil resistance in colorectal cancer cells. Acta Pharmacol Sin. 44:2537–2548. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Kropp KN, Maurer S, Rothfelder K, Schmied BJ, Clar KL, Schmidt M, Strunz B, Kopp HG, Steinle A, Grünebach F, et al: The novel deubiquitinase inhibitor b-AP15 induces direct and NK cell-mediated antitumor effects in human mantle cell lymphoma. Cancer Immunol Immunother. 67:935–947. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Wang S, Wang T, Yang Q, Cheng S, Liu F, Yang G, Wang F, Wang R, Yang D, Zhou M, et al: Proteasomal deubiquitylase activity enhances cell surface recycling of the epidermal growth factor receptor in non-small cell lung cancer. Cell Oncol (Dordr). 45:951–965. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Tian Z, D'Arcy P, Wang X, Ray A, Tai YT, Hu Y, Carrasco RD, Richardson P, Linder S, Chauhan D and Anderson KC: A novel small molecule inhibitor of deubiquitylating enzyme USP14 and UCHL5 induces apoptosis in multiple myeloma and overcomes bortezomib resistance. Blood. 123:706–716. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Nalawansha DA and Crews CM: PROTACs: An emerging therapeutic modality in precision medicine. Cell Chem Biol. 27:998–1014. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Noblejas-López MDM, Tébar-García D, López-Rosa R, Alcaraz-Sanabria A, Cristóbal-Cueto P, Pinedo-Serrano A, Rivas-García L and Galán-Moya EM: TACkling cancer by targeting selective protein degradation. Pharmaceutics. 15:24422023. View Article : Google Scholar : PubMed/NCBI | |
|
Henning NJ, Boike L, Spradlin JN, Ward CC, Liu G, Zhang E, Belcher BP, Brittain SM, Hesse MJ, Dovala D, et al: Deubiquitinase-targeting chimeras for targeted protein stabilization. Nat Chem Biol. 18:412–421. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Chen Y, Xue H and Jin J: Applications of protein ubiquitylation and deubiquitylation in drug discovery. J Biol Chem. 300:1072642024. View Article : Google Scholar : PubMed/NCBI |