Risk score model with two immune infiltration‑related long non‑coding RNAs to predict prognosis in patients with osteosarcoma
- Authors:
- Published online on: July 15, 2025 https://doi.org/10.3892/ol.2025.15189
- Article Number: 443
-
Copyright: © Zeng et al. This is an open access article distributed under the terms of Creative Commons Attribution License.
Abstract
Introduction
Osteosarcoma (OS), a common primary bone tumor, primarily affects children and young adults (1,2). Notable progress in the prevention and treatment of OS has been attained via novel treatment approaches(such as lenvatinib with chemotherapy) and immunotherapy agents currently under clinical investigation) (3). However, the incidence and mortality rates of OS have continued to increase by ~1.4% every year (4). A previous study reported that ~80% of patients with OS exhibit high programmed cell death ligand 1 (PD-L1) mRNA expression (5). Although programmed cell death protein 1/PD-L1 cascade inhibitors have been assessed in the treatment of bone and soft tissue sarcomas in clinical trials (6,7), no notable response has been reported in patients with advanced OS (8). The success of immunotherapy is dependent on the level of immune cells in the host microenvironment (9,10). Therefore, assessment of the immunoreactivity of OS is key in making decisions regarding individualized treatment.
Long non-coding RNAs (lncRNAs) are ~200 nucleotides long and participate in numerous pathological processes, such as the occurrence and development of cancer (11,12). Previous studies have reported that lncRNAs also serve a key role in the modulation of genes involved in tumor immune escape (13–15). For example, lncRNA prostate cancer-associated transcript 6 induces polarization of M2 macrophages in cholangiocarcinoma (CCA) and regulates the immune response of macrophages to promote CCA progression (15). The expression of lncRNA small nucleolar RNA host gene 9 is associated with poor survival and immune infiltration in prostate cancer (14). Thus, the identification of lncRNA markers for cancer immunoreactivity may provide novel insights into OS treatment in the future.
The aim of the present study was to identify immune infiltration-related lncRNAs (IIRLs) in osteosarcoma, construct a clinical signature based on IIRLs for survival prognosis in OS and investigate the association between this IIRL signature and tumor immune infiltration in OS.
Materials and methods
Data abstraction
The workflow of the present study is presented in Fig. S1. All information was downloaded from the Therapeutically Applicable Research to Generate Effective Treatments (TARGET) database (https://www.cancer.gov/ccg/research/genome-sequencing/target). The cohort included 87 patients with OS, of which 47 were male and 40 were female, with an average age of 3.5–32.4 years old.
Determination of OS subtypes on the basis of immune gene sets
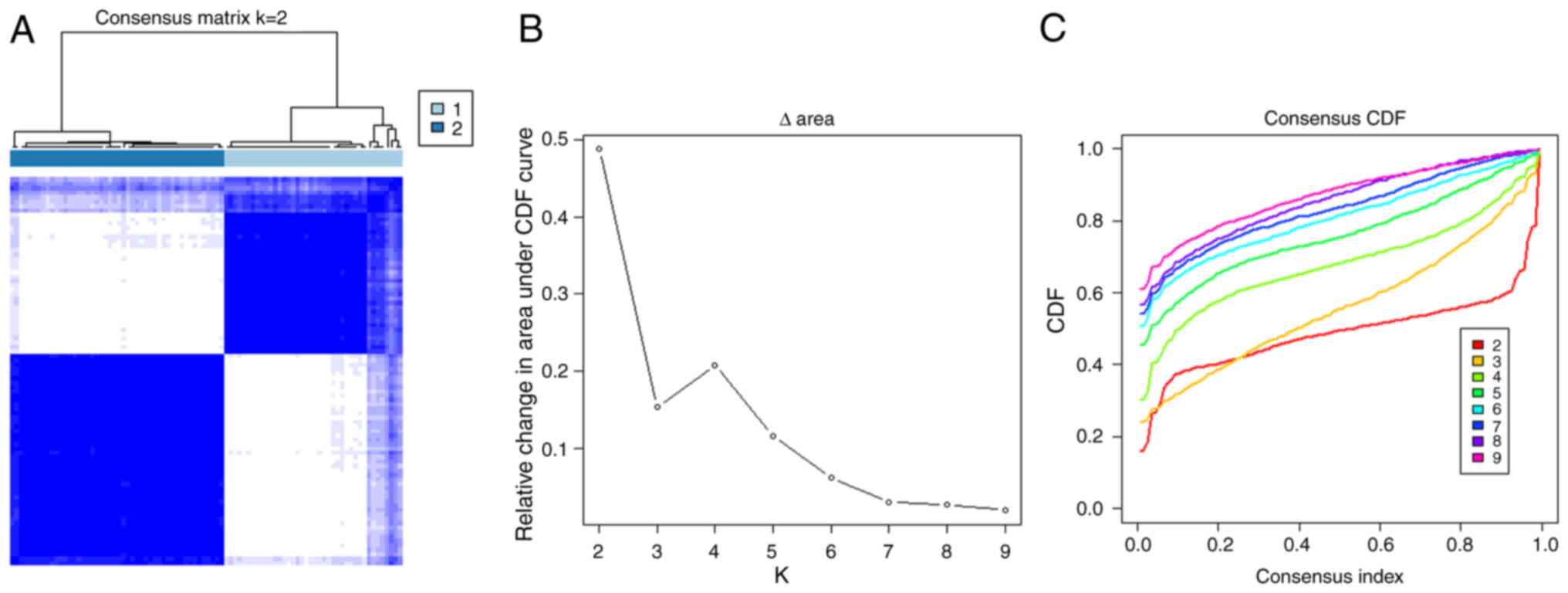
The 29 immune-linked gene sets represent types of immune cell, functions and cascades (16). Immune cell invasion levels in cancer were estimated using ssGSEA with the ‘gsva’ package (bioconductor.org/packages/release/bioc/html/GSVA.html) of R software (R 4.2.2 with Bioconductor 3.16.). ‘ESTIMATE’ R package [v1.0.13 Kosuke Yoshihara Lab (Academic)] was used to calculate tumor purity, immune and stromal scores. Consensus clustering and molecular subtype were implemented using the ‘ConsensusClusterPlus’ R package [bioconductor.org/packages/release/bioc/html/ConsensusClusterPlus.htmlv1.68.0 (Bioconductor release 3.19)] on the basis of the ssGSEA scores. In brief, K-means, spectral and PCA-K-means clustering were replicated 50 times each turn and the optimum clustering result was used.
Differential expression analysis
Differentially expressed lncRNAs (DELs) or differentially expressed genes (DEGs) between Clusters 1 and 2 samples were defined with ‘limma’ R package [https://bioconductor.org/packages/release/bioc/html/limma.html v3.60.0 (Bioconductor release 3.19)]. lncRNAs or genes that met the adjusted P<0.05 and fold-change >0.5 criteria were regarded as DELs or DEGs, respectively. The ‘pheatmap’ R package (https://cran.r-project.org/package=pheatmap v1.0.12 Raivo Kolde (Academic))was employed for plotting heatmaps, while ‘ggpubr’(cran.r-project.org/package=ggpubr v0.6.0 Alboukadel Kassambara) was employed for generating boxplots.
.cut-off value=43.2963
Development of a prognostic model based on lncRNAs
Prognosis-associated DELs were determined via a univariate Cox regression (UCR) model. Expression values of all the prognosis-associated DELs were integrated and the estimated regression coefficients were then employed to determine prognostic scores for all patients. The risk score for every patient was determined as follows: Risk score=β1×1 + β2×2 +…+ βixi, where × is predictor Variables/Features/Risk Factors): i (Index): i represents the total number of variables included.) Based on the median risk score cut-off value. (cut-off value=43.2963), the patients were stratified into high- (n=43 people) and low-risk (44 people) groups. The Kaplan-Meier analysis was performed to determine the survival rate. The risk scores were included in UCR and multivariate Cox regression (MCR) analysis to validate the independence of the risk model. Receiver operating characteristic (ROC) curves were used to assess the accuracy and specificity of the risk model.
Co-expression analysis, Gene Ontology (GO) annotation, pathway enrichment analysis and network construction
Pearson's correlation coefficient between the DEGs and two lncRNAs was calculated by using R package corr.test (https://cran.r-project.org/package=psych v2.4.3 William Revelle) function and the mRNA pairs were selected (|Pearson's correlation coefficient|>0.5 and P<0.001). RStudio software (version 4.2; Posit Software, PBC; rstudio.com/) was employed to conduct mRNA GO enrichment and Kyoto Encyclopedia of Genes and Genomes (KEGG; kegg.jp) functional analyses. P<0.05 was considered to indicate statistically significant enrichment. For the construction of the lncRNA-mRNA network, Cytoscape software (version 3.7.2) (17) was used.
CIBERSORT estimation. CIBERSORT provides an estimation of the abundance ratio on the basis of gene expression data of multiple cell types in a mixed cell population (18,19). The proportions of immune cells were quantified using the CIBERSORT algorithm [v2.0 (Newman Lab, Stanford University) https://cibersortx.stanford.edu]. Patients were divided into low- and high-risk groups.
GSEA. GSEA was used to analyze the differences in pathways between the high- and low-risk groups. The C2.cp.kegg.v7.0.symbols.gmt dataset (gsea-msigdb.org/gsea/msigdb)was obtained from the Molecular Signatures database(https://www.gsea-msigdb.org/gsea/msigdb). Nominal P<0.05, |normalized enrichment score|>1 and false discovery rate q<0.25 were considered to indicate a statistically significant difference.
Quantification of lncRNA expression by reverse transcription-quantitative PCR (RT-qPCR)
The normal human osteoblast cell line hFOB1.19 (iCell Bioscience Inc.) was maintained in DMEM/F12 (Gibco; Thermo Fisher Scientific, Inc.) at 34°C with 5% CO2 in a humidified atmosphere. The human OS cell lines MG-63 and 143B cells (iCell Bioscience Inc.) were cultured in MEM (Gibco; Thermo Fisher Scientific, Inc.), while U2OS cells (iCell Bioscience Inc.) were cultured in McCoy's 5A medium(cat. no. 16600082; Gibco; Thermo Fisher Scientific, Inc.). Both culture media were supplemented with 10% FBS (Gibco; Thermo Fisher Scientific, Inc.), 100 µg/ml streptomycin and 100 U/ml penicillin, and the cells were maintained at 37°C with 5% CO2 in a humidified atmosphere. Using TRIzol® (Thermo Fisher Scientific, Inc.) following the manufacturer's protocol, total RNA was obtained from hFOB1.19, MG-63, 143B and U2OS cells. Next, microRNA (miRNA or miR) 1st-Strand cDNA Synthesis kit (cat. no. MR101-01 Vazyme Biotech Co., Ltd.) was used to synthesize cDNA from miRNA according to the manufacturer's protocol, while the SuperScript IV First-Strand Synthesis System (Thermo Fisher Scientific, Inc.) was used to synthesize cDNA from lncRNA and mRNA according to the manufacturer's protocol. RT-qPCR was performed with SYBR™ Green Master Mix (Thermo Fisher Scientific, Inc.) according to the manufacturer's protocol. The thermocycling conditions were as follows: Initial denaturation at 95°C for 5 min, followed by 40 cycles of 95°C for 30 sec and 60°C for 45 sec. GAPDH served as an endogenous control for normalization. The relative levels of gene expression were calculated as ΔCq=Cq gene-Cq reference and the gene expression was calculated in fold-change according to the 2−ΔΔCq method (20), repeated independently in triplicate. The following primer sequences were used for RT-qPCR: LINC01094 forward, 5′-GCATGGCCAGGGGATCTTCA-3′ and reverse, 5′-CCAGCTCTTGGCAAGCAACAC-3′; RP11-15K2.2 forward, 5′-TCTGCTGTGGCCTGAACTGC-3′ and reverse, 5′-TTTCCCAACGCTGGGTGGAC-3′ and GAPDH forward, 5′-GACCTGACCTGCCGTCTA-3′ and reverse, 5′-AGGAGTGGGTGTCGCTGT-3′. Authentication testing of MG-63, 143B, U2OS and hFOB1.19 cell lines have been performed (Shanghai Biowing Applied Biotechnology Co., Ltd) via STR profiling. STR profiles match the standards recommended for MG-63, 143B, U2OS and hFOB1.19 cell lines authentication Based on previous research, GAPDH was selected as the internal reference (21,22).
Statistical analysis
Statistical analyses were performed using GraphPad Prism 8.0 (Dotmatics). Data are presented as the mean ± standard deviation of ≥3 independent experimental repeats. Comparisons between multiple groups were performed using one-way ANOVA followed by Tukey's post hoc test. Overall survival was determined using Kaplan-Meier survival curves along with the log-rank test. P<0.05 was considered to indicate a statistically significant difference. UCR analysis was performed with OS prognosis as the dependent variable and P<0.05. A total of six prognostic IIRLs were identified and fitted into a MCR model with OS as a dependent variable to determine their relative contribution to survival estimation and P<0.05.
Results
Identification of two immune subtypes in OS
A total of 87 patients with OS were included in the present study. ssGSEA was carried out on each sample with gene sets consisting of mRNA transcripts distinct for most subpopulations of innate and adaptive immune cells (B cell native, T cell CD8,etc). Tumor samples were classified into k subtypes (k=2-9). Based on the cumulative distribution function curve consensus score, k=2 was considered to be optimal (referred to as Cluster 1- 2; Fig. 1A-C).
Immune features of immune subtypes
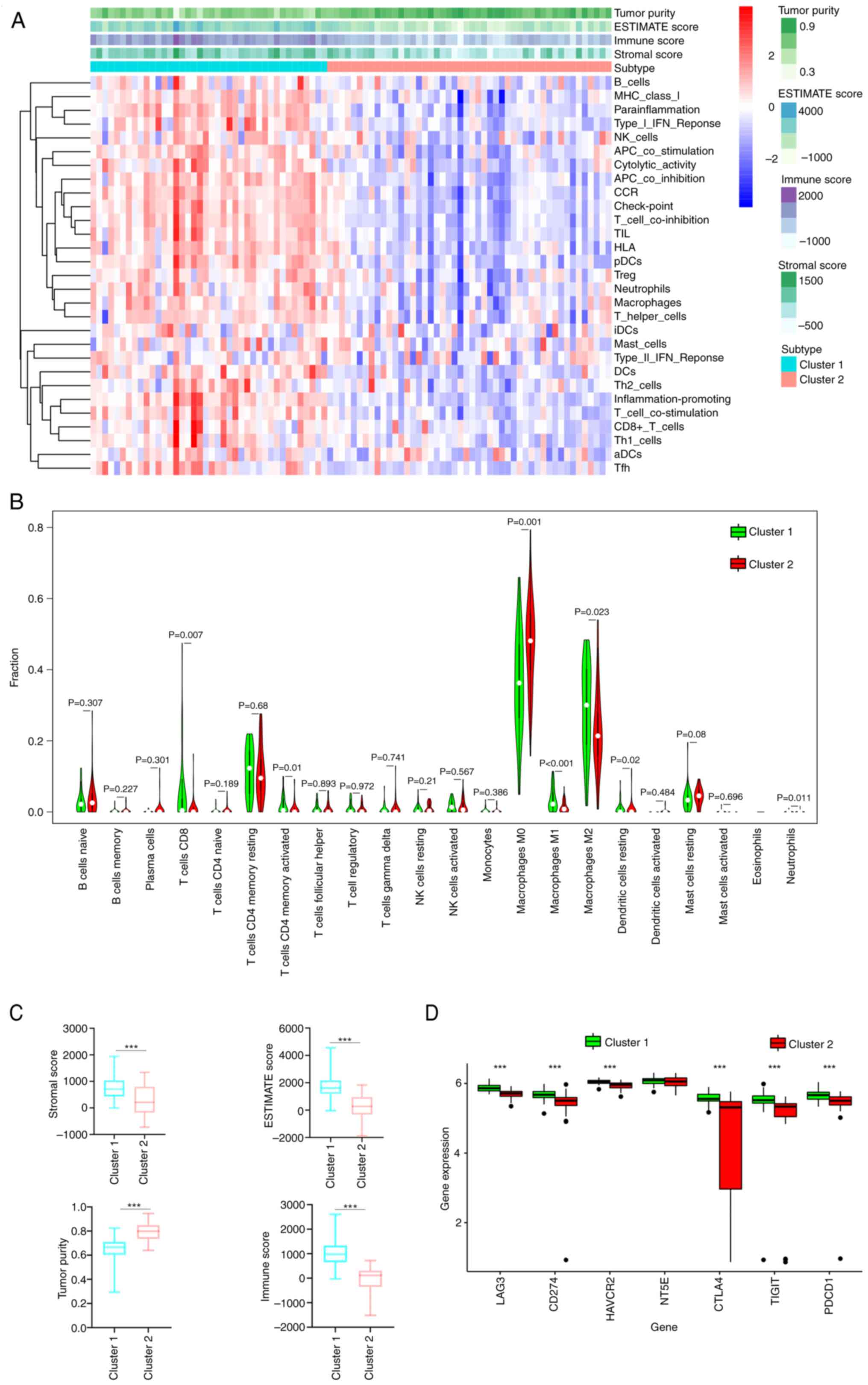
ssGSEA clustering analysis indicated that the extent of immune infiltration was greater in Cluster 1 than 2 (Fig. 2A). Compared with those OS cases with low immune infiltration, the OS cases who had high immune infiltration were significantly associated with lower tumor purity and higher ESTIMATE, stromal and immune scores (P<0.005; Fig. 2A and C). The two subtypes generated according to ssGSEA scores of the immune gene sets were used to explore the tumor-infiltrating immune cell abundance. Cluster 1 subtype exhibited higher levels of CD8+ (P=0.007) and activated memory CD4+ T cell (P=0.01) and M2 (P=0.023) and M1 macrophage (P<0.001) infiltration compared with that of Cluster 2. Cluster 1 demonstrated lower infiltration of M0 macrophages (P=0.001), resting dendritic cells (P=0.02) and neutrophils (P=0.011) compared with that of Cluster 2 (Fig. 2B). Furthermore, the expression of eight immune checkpoint genes, including V-set immunoregulatory receptor, lymphocyte activation gene (LAG)3, TIGIT (T cell Immunoreceptor with Ig and ITIM Domains), NT5E (cto-5′-Nucleotidase), CD274, hepatitis A virus cellular receptor 2 (HAVCR2), cytotoxic T lymphocyte antigen 4 (CTLA4) and programmed cell death protein 1, which are associated with immune escape, were explored. Expression levels of these genes were greater in Cluster 1 than in 2 (P<0.05; Fig. 2D).
Identification of DELs
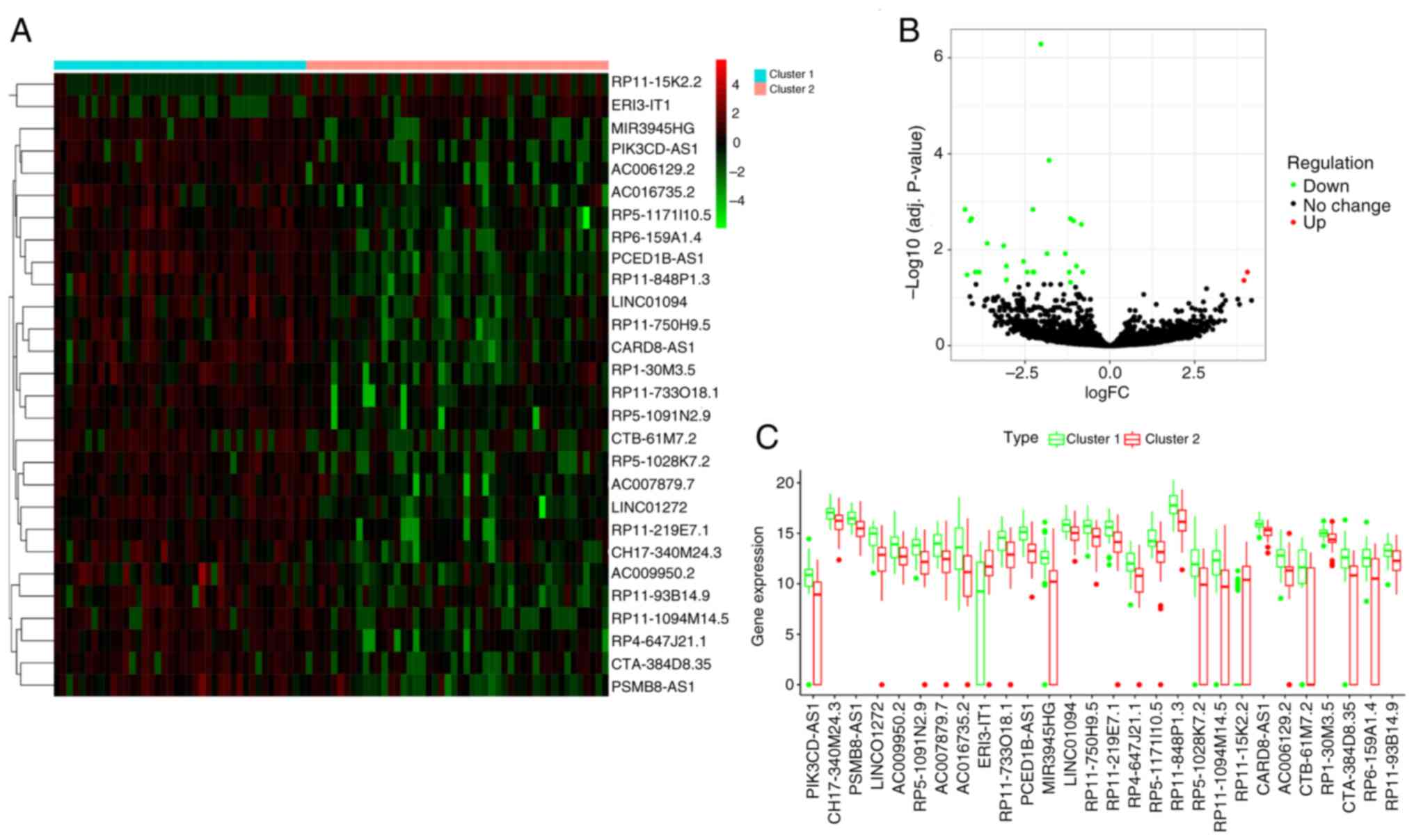
Cluster 1 was an immune-invasion type, while Cluster 2 was an ‘immune-desert phenotype’. A total of 29 differentially expressed immune infiltration-related lncRNAs (DEIIRLs) were obtained, of which, two were upregulated and 27 were downregulated (Table I; Fig. 3A-C).
Construction of a diagnostic and prognostic model based on two lncRNAs
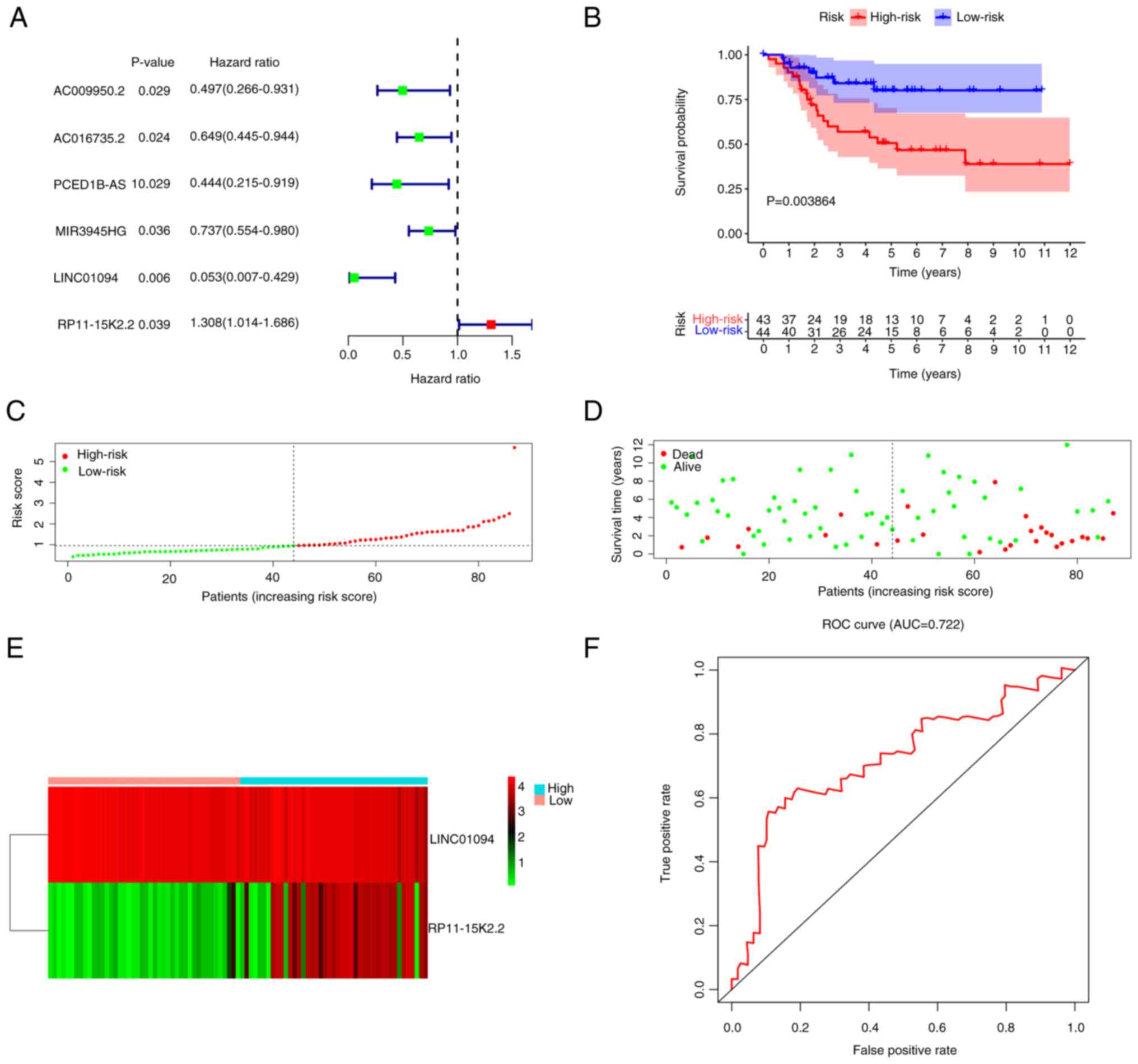
UCR analysis was performed with OS prognosis as the dependent variable and P<0.05. A total of six prognostic IIRLs, namely, AC009950.2, AC016735.2, PCED1B-AS1, MIR3945HG, LINC01094 and RP11-15K2.2, were identified (Fig. 4A). To establish a predictive model, these lncRNAs were fitted into a MCR model with OS as a dependent variable to determine their relative contribution to survival estimation. Through integration of the expression profiles of lncRNAs and matching the determined regression coefficient obtained from the aforementioned multivariate regression assessment, a prognostic signature was developed using the formula Risk score=(−2.5354 × LINC01094 expression value) + (0.2185 × RP11-15K2.2 expression value).
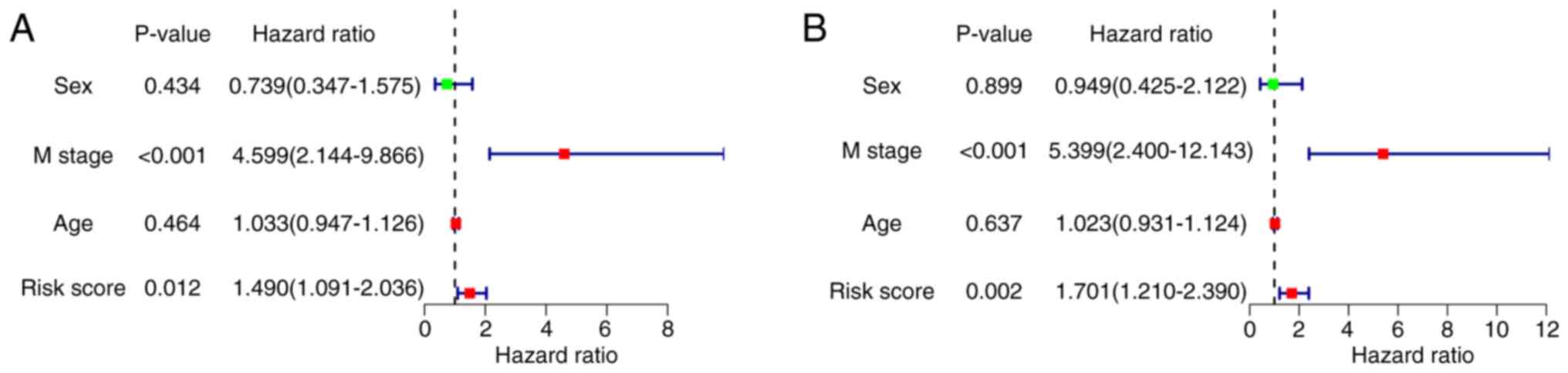
Patients were stratified into high- (n=43) and low- (n=44) risk groups according to the median risk score. The high-risk group exhibited notably poor prognosis compared with the low-risk group (P=3.864×10−3; Fig. 4B). The area under the curve (AUC) demonstrated that the Cox prediction model based on lncRNAs achieved improved accuracy in the monitoring of survival (AUC=0.722; Fig. 4F). The survival time, risk score and status plots are presented in Fig. 4C-E. Cox regression analyses were performed to verify the likelihood of the risk score of the present signature serving as an independent predictor of prognosis in patients with OS. The risk score of the present signature could be applied to estimate the prognosis of patients with OS, which eliminated the effect of clinical characteristics (sex and age; P<0.05; Fig. 5A and B).
A total of two lncRNAs are enriched in immune-linked functions and pathways
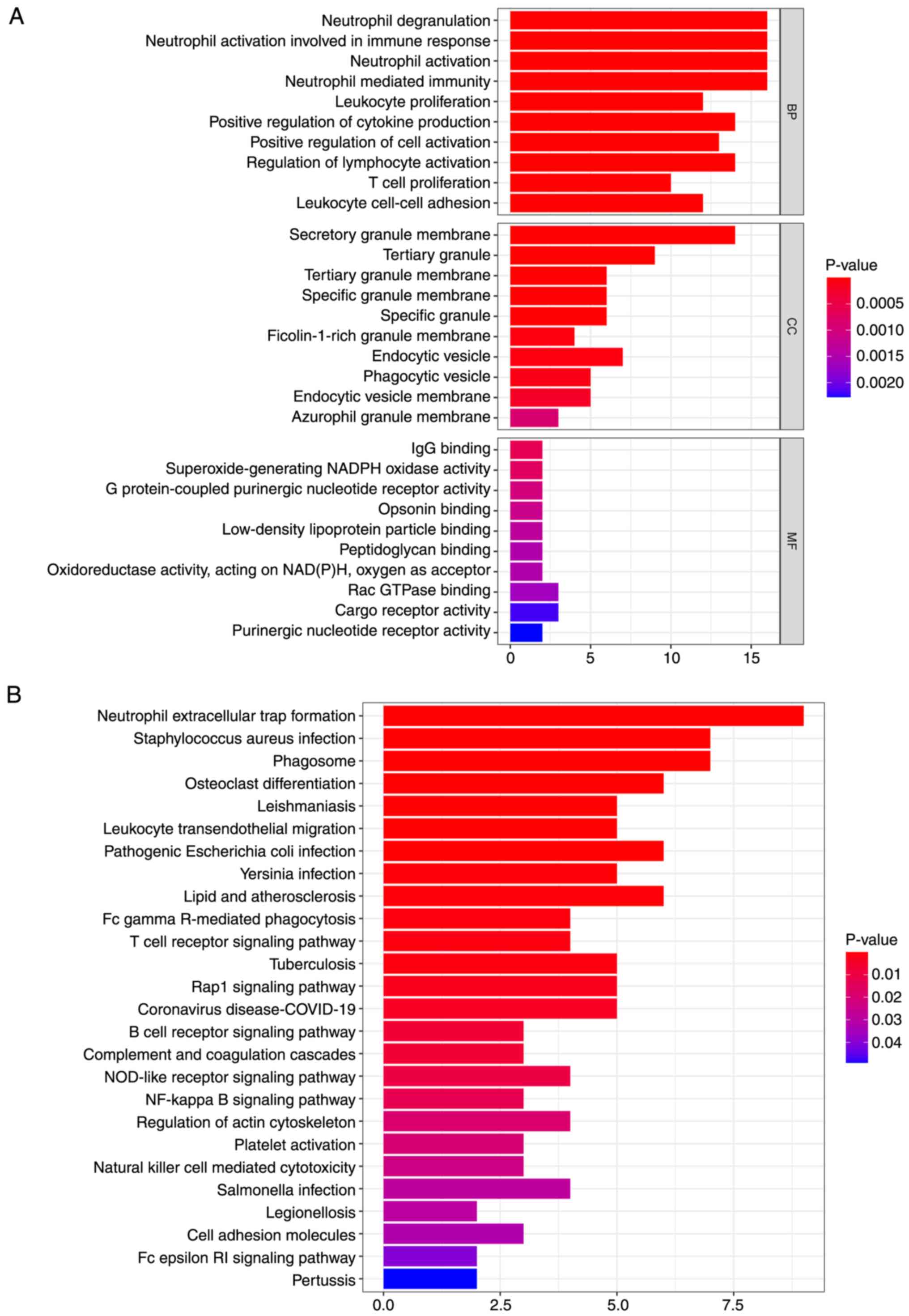
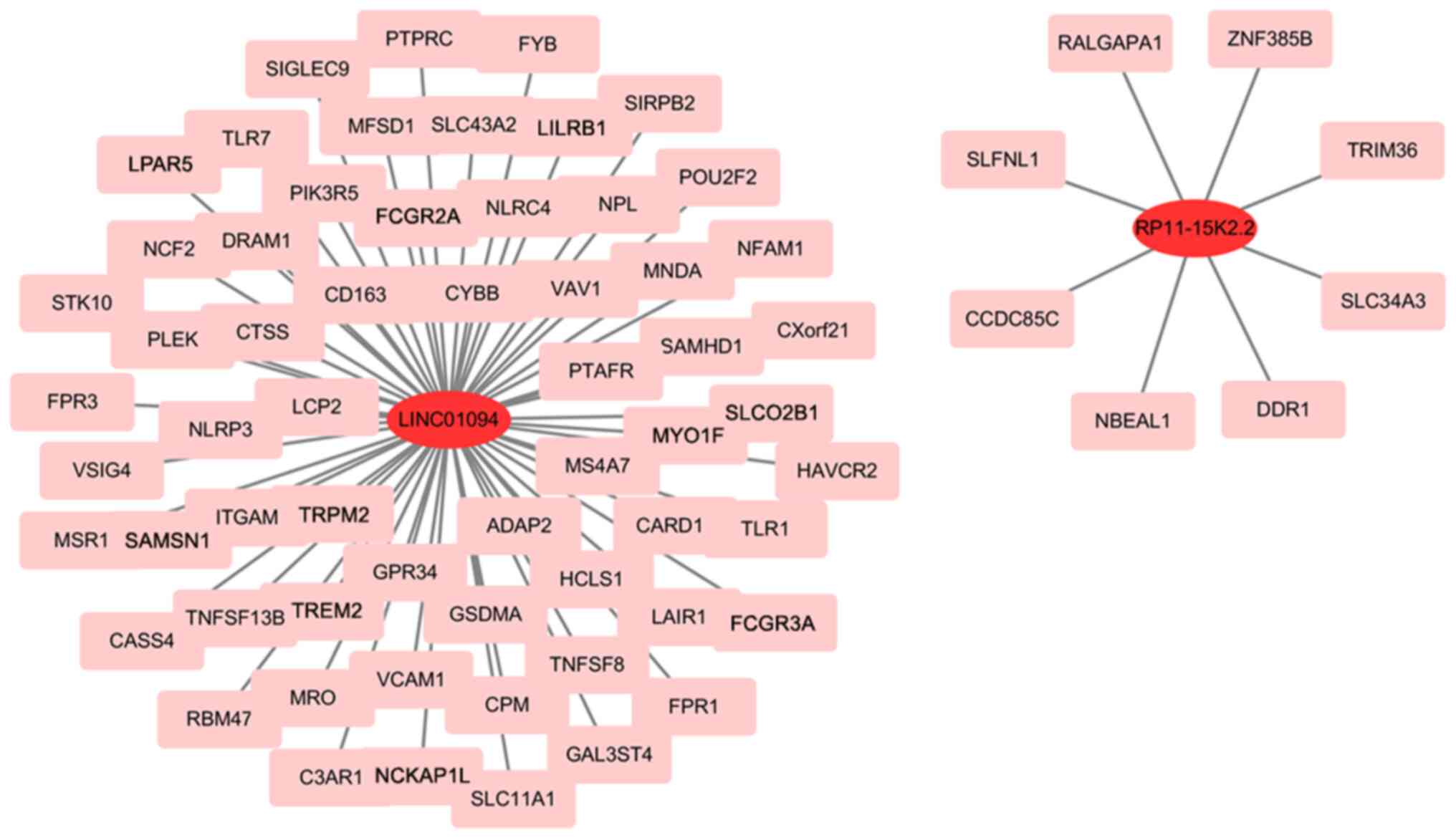
DEGs were obtained from Cluster 1 and 2. A total of 1,262 DEGs were obtained, of which, 448 were up- and 772 were downregulated. DEGs significantly associated with expression of two lncRNAs were screened (|Pearson correlation coefficient|>0.5 and P<0.001). A total of 69 mRNAs were screened (Table II). KEGG pathway and GO term analyses were employed to explore the biological roles of the lncRNAs. Overall, 69 mRNA were annotated to 617 functions with significant differences, including ‘neutrophil mediated immunity’, ‘neutrophil degranulation’, ‘T cell proliferation’, ‘positive regulation of cytokine production’, ‘secretory granule membrane’, ‘tertiary granule’, ‘G protein-coupled purinergic nucleotide receptor activity’ and ‘IgG binding’ (Fig. 6A). Those mRNA were annotated to 27 significant KEGG cascades, including ‘neutrophil extracellular trap formation’, ‘Staphylococcus aureus infection’, ‘T cell receptor signaling pathway’, ‘B cell receptor signaling pathway’, ‘NOD-like receptor signaling pathway’ and ‘NF-kappa B signaling pathway’ (Fig. 6B). An OS prognostic lncRNA-mRNA regulatory network composed of two lncRNAs and 69 DEGs was constructed (Fig. 7).
Survival is associated with percentage of tumor-infiltrating immune cell (TIIC) types in OS
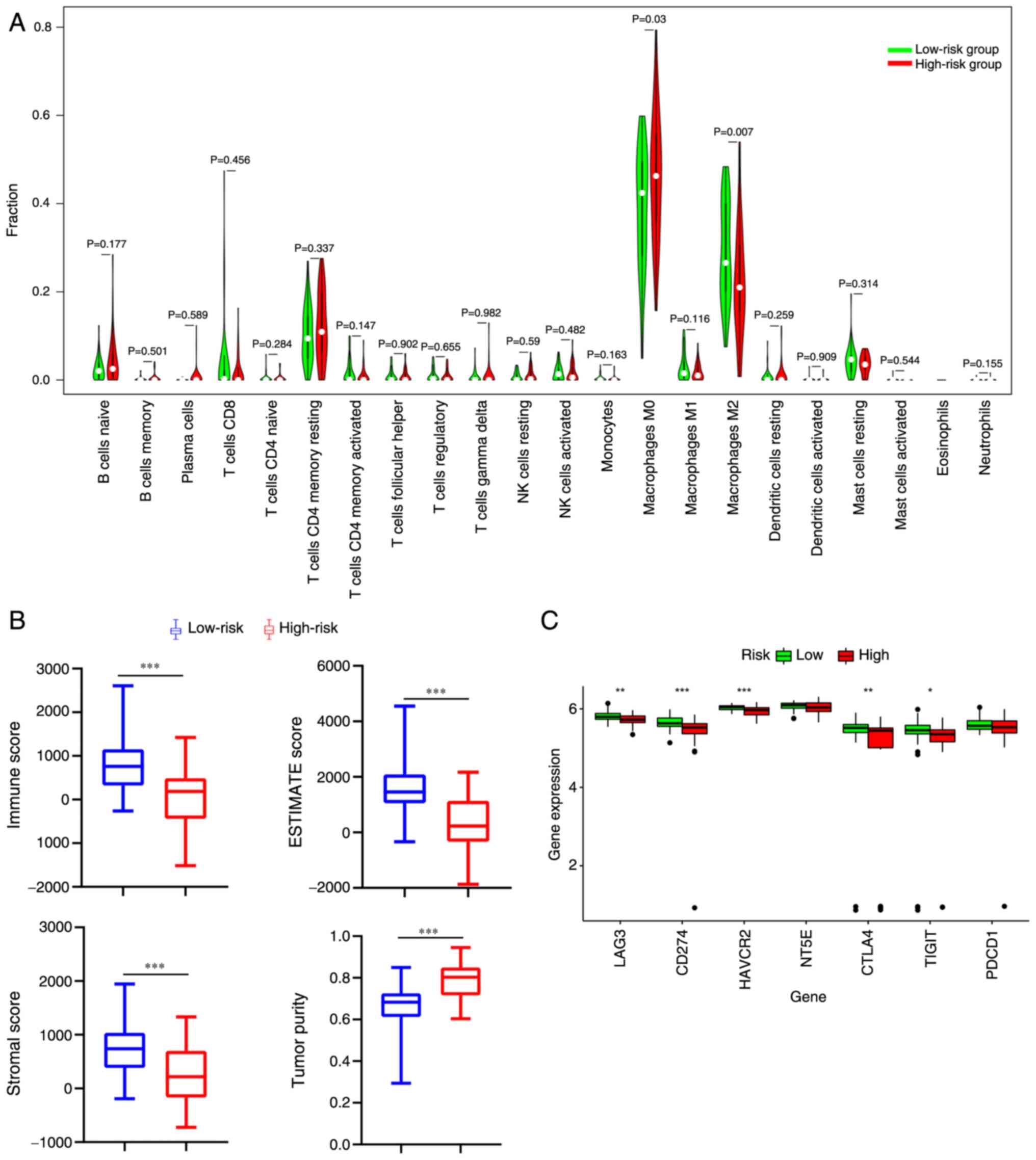
Using CIBERSORT, the immune infiltration percentage of each cell type was determined based on the gene matrix and the differences in TIIC profiles between the two risk groups were established. Patients in the high-risk group had elevated expression of M0 macrophages (P=0.03; Fig. 8A). By contrast, patients in the low-risk group had elevated expression of M2 macrophages (P=0.007; Fig. 8A). Furthermore, the expression levels of five immune checkpoint genes (LAG3, HAVCR2, CTLA4, TIGIT and CD274) in the low-risk group were significantly higher compared with those in the high-risk group (P<0.05; Fig. 8C). Compared with the high-risk group, the OS in the low-risk group was significantly associated with lower tumor purity and higher ESTIMATE, stromal and immune score (P<0.005; Fig. 8B).
High-risk group is involved in multiple immune-associated pathways
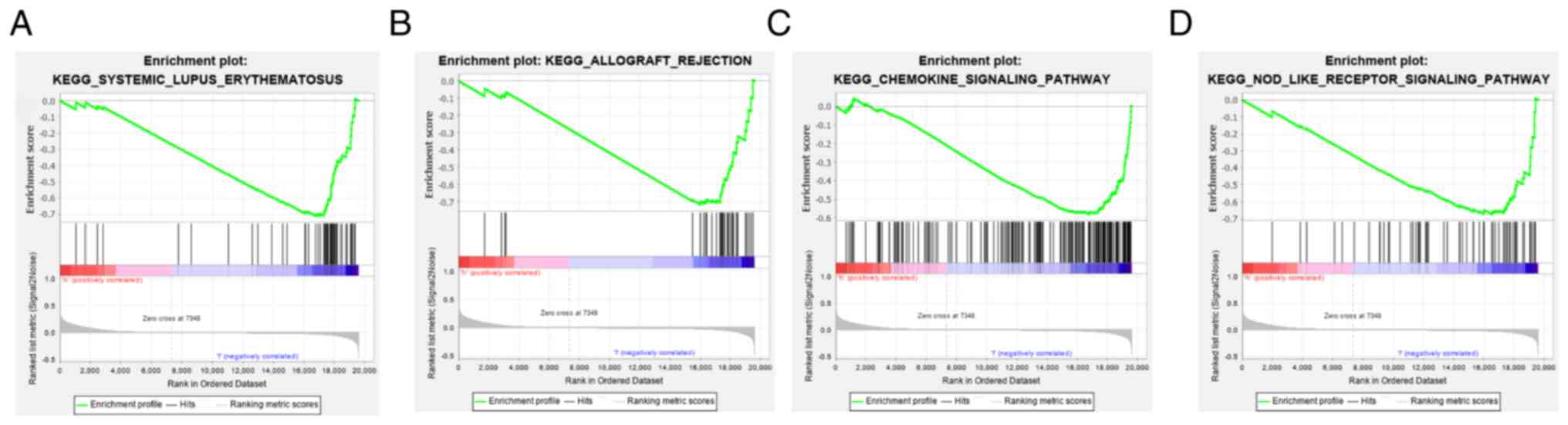
GSEA suggested a significant negative correlation (P<0.05) between the high-risk group and immune-associated pathways, including ‘chemokine signaling pathway’, ‘NOD-like receptor signaling pathway’, ‘allograft rejection’ and ‘systemic lupus erythematosus’ (Fig. 9).
Validation of the expression levels of IIRLs (LINC01094 and RP11-15K2.2) in hFOB1.19, MG-63, 143B and U2OS cells
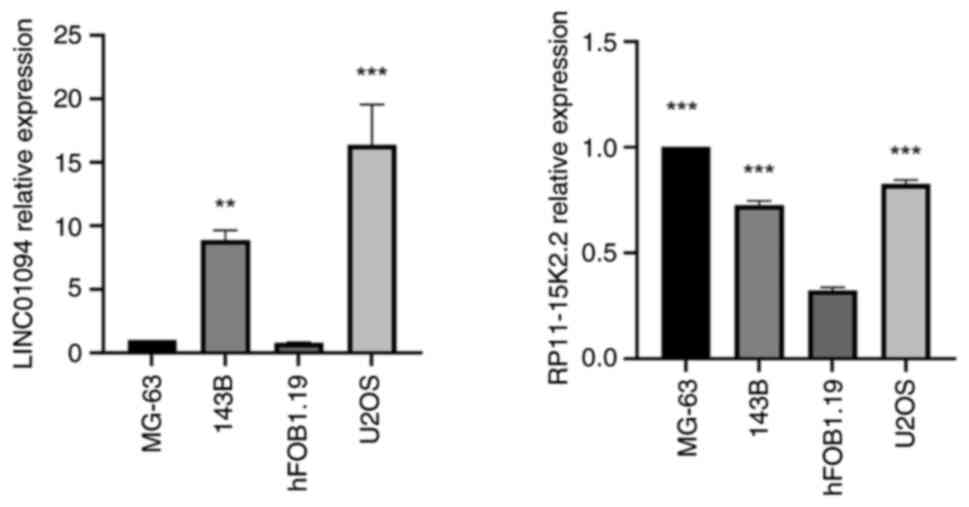
To verify that the aforementioned IIRLs are specifically expressed in OS cells, RT-qPCR was performed in the MG-63, 143B, U2OS and hFOB1.19 cell lines. . LINC01094 and RP11-15K2.2 were expressed in MG-63, 143B and U2OS cells (Fig. 10). The expression of LINC01094 in 143B and U2OS cells was significantly higher compared with that in hFOB1.19, and the expression of RP11-15K2.2 in MG-63, 143B and U2OS cells was significantly higher compared with that in hFOB1.19 cells (P<0.05). These results verified the reliability of the present bioinformatics analysis.
Discussion
Human OS, a primary malignant bone tumor, is prevalent among children and young adults (22). Due to developments in molecular biology technology, there is a notable interest in anticancer immunotherapy, including immune checkpoint inhibitors and modulators, and genetically modified T cells (23,24). Patients with OS lacking immune cell infiltration exhibit high rates of metastasis and poor clinical outcomes (25). According to a previous study, immune reconstitution suppresses the recurrence of OS and improve the survival of patients with metastatic OS (26).
In the present study, according to the immune signature score, the patients were separated into two clusters. Cluster 1 and 2 demonstrated significant differences in ESTIMATE, tumor purity, stromal and immune scores and expression levels of immune checkpoint markers. The levels of immune infiltration in patients form Cluster 1 were higher compared with those in Cluster 2. A two IIRL signature for overall survival was employed to develop a risk model to estimate the prognosis of patients with OS. By employing the risk score, patients were divided into a low- and high-risk group. As indicated by the Kaplan-Meier curve, survival status and score plot and ROC curve, the aforementioned IIRLs had a notable prognostic potential.
In the low-risk group, LINC01094 expression was significantly higher compared with that in the high-risk group, while RP11-15K2.2 expression was significantly higher in the high-compared with that in the low-risk group. Dysregulation of LINC01094 has similarly been documented in clear cell renal cell carcinoma (27), glioblastoma (28) and ovarian cancer (29). For example, LINC01094 expression is markedly upregulated in glioblastoma cells, colony formation and invasiveness are inhibited by silencing LINC01094 (28). Silencing LINC01094 blocks SLC2A3 expression by upregulating miR-184, which further inhibits the invasion, migration and proliferation of clear cell renal cell carcinoma cells, as well as tumor growth, but promotes apoptosis (27). LINC01094 activates the Wnt signaling pathway by adsorbing miR-577 and promotes the epithelial-mesenchymal transition (EMT) of OC cells (29). In the breast tumor microenvironment, immune infiltration enhancement is associated with EMT markers (insulin-like growth factor-1 receptor;SH2-containing-5′-inositol phosphatase-2) (30). A previous study reported that, in non-small-cell lung cancer, EMT status is associated with an inflammatory tumor microenvironment (31). The aforementioned study demonstrated that LINC01094 participates in tumor immunity through EMT. However, in the present study, LINC01094 expression was associated with improved survival in OS, which is contrary to the aforementioned study results; thus, further research is warranted to verify its clinical relevance. Mechanistically, LINC01094 acts as a competitive endogenous RNA in gene regulation, sequestering miRNAs and modulating downstream target genes. Key pathways, including the Wnt/β-catenin, PI3K/AKT and TGF-β signaling pathways, have been identified as targets of LINC01094 (32). To the best of our knowledge, there is no research on the role of RP11-15K2.2 expression in tumors and immunity or immune infiltration in OS. However, it may affect immune escape by regulating immune cell function or metabolic pathways in the tumor microenvironment (33–35).
Considering the key role of tumor-invading immune cells in the progression of cancer, the present study explored the differences in the composition of 22 infiltrating immune-cell types between low- and high-risk groups. In gastric cancer samples, one of the most enriched tumor invasion immune cell types is resting memory CD4+ T cells (36). Previous study have demonstrated that, in the tertiary lymphoid structures of numerous tumors, follicular helper T cells are found, which suggests that they contribute to effective and sustained antitumor immune responses (37). Macrophages exhibit antitumor M1 and protumor M2 phenotypes, and high density of M1 macrophages is associated with a favorable overall survival in patients with cancer (38). In the high-risk group, the levels of M0 macrophage were elevated, while the proportion of M2 macrophages was increased in the low-risk group. The immune checkpoint molecules CD274, CTLA4 and LAG3 participate in tumor immunology (39). The targeted blockade of CD274 and CTLA-4 results in a clinical benefit for individuals with diverse solid tumors (40,41). The present study demonstrated that, in the low-risk group, the expression of five immune checkpoint genes (LAG3, HAVCR2, CTLA4, TIGIT and CD274) was higher compared with that in the high-risk group. Furthermore, the present GSEA demonstrated that risk score was associated with ‘chemokine signaling pathway’, ‘NOD-like receptor signaling pathway’, ‘allograft rejection’ and ‘systemic lupus erythematosus’. In the tumor microenvironment, chemokines mediate the balance between antitumor and protumor responses by regulating cell migration and cell-cell interactions (42). The NOD-like receptor signaling pathway is a key regulator in immune responses. NOD-like receptor signaling increases major histocompatibility complex class I) expression and the presentation of tumor antigens to CD8+ T lymphocytes (43). Therefore, the NOD-like receptor signaling pathway may be exploited to elicit protective antitumor immunity during immunotherapy. Altogether, the present data demonstrated that the high-risk group may be affected by immune cell infiltration signatures.
In summary, a risk score model with two IIRLs was constructed to predict the prognosis of OS and the prognostic index was observed to be an independent index for OS prognosis. Furthermore, the prognostic index may affect immune-associated biological processes and TIIC. In conclusion, the IIRL signature developed in the present had an independent prognostic value for OS. The present findings may contributed to improved survival of patients with OS and provide prospective immunotherapy targets. While the present study provides insights into the prognostic and immune-associated roles of lncRNAs in OS, there are limitations. First, the sample size derived from the TARGET database was relatively small, which may restrict the statistical power and generalizability of the present findings. Second, the present computational predictions, although supported by preliminary RT-qPCR validation of LINC01094 and RP11-15K2.2 in OS cell lines, lack external validation in independent clinical cohorts or functional assays such as western blotting. Third, the mechanistic links between these lncRNAs and immune infiltration, particularly their regulatory effects on macrophage polarization or signaling pathways, remain speculative and require experimental confirmation. Finally, resource constraints, including limited funding and laboratory access, precluded further validation or mechanistic exploration. To address these limitations, future research can prioritize expanding the sample size by integrating additional databases (for example, Gene Expression Omnibus or International Cancer (ncbi.nlm.nih.gov/geo/) Genome Consortium (icgc.org) and validating the two IIRL model in prospective clinical cohorts; performing functional experiments, such as co-culture systems with macrophages, to elucidate how LINC01094 and RP11-15K2.2 regulate immune cell polarization and tumor microenvironment dynamics; mechanistic investigations, including pathway inhibition, RNA-protein interaction assays and luciferase reporter studies, to identify specific signaling pathways (for example, STAT3, NF-κB or TGF-β) by which these lncRNAs exert their effects and multi-omics integration to explore downstream targets and epigenetic modifications. These efforts may strengthen the translational relevance of the present study findings and provide deeper insight into lncRNA-driven immune evasion in OS.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the Hunan Provincial Key Laboratory of Pediatric Orthopedics (grant no. 2023TP1019), Science and Technology Project of Furong Laboratory (grant no. 2023SK2111) and Hunan Provincial Clinical Medical Research Center for Pediatric Limb Deformities (grant no. 2019SK4006).
Availability of data and materials
The data generated in the present study may be requested from the corresponding author
Authors' contributions
LRZ and GY conceived and designed the study. LRZ collected data and wrote the manuscript. GHZ and HBM analyzed data and constructed the figures and tables. GY reviewed and revised the manuscript, LRZ and GY confirm the authenticity of all the raw data. All authors have read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Glossary
Abbreviations
Abbreviations:
|
OS |
osteosarcoma |
|
IIRL |
immune infiltration-related lncRNA |
|
DEL |
differentially expressed lncRNA |
|
DEG |
differentially expressed gene |
|
UCR |
univariate Cox regression |
|
MCR |
multivariate Cox regression |
|
ssGSEA |
single-sample gene set enrichment analysis |
|
TARGET |
Therapeutically Applicable Research to Generate Effective Treatments |
|
ROC |
receiver operating characteristic |
|
KEGG |
Kyoto Encyclopedia of Genes and Genomes |
|
GO |
Gene Ontology |
|
TIIC |
tumor-infiltrating immune cell |
|
RT-q |
reverse transcriptionquantitative |
References
|
Rothzerg E, Xu J and Wood D: Different subtypes of Osteosarcoma: histopathological patterns and clinical behaviour. J Mol Pathol. 4:99–108. 2023. View Article : Google Scholar | |
|
Moukengue B, Lallier M, Marchandet L, Baud'huin M, Verrecchia F, Ory B and Lamoureux F: Origin and therapies of osteosarcoma. Cancers (Basel). 14:35032022. View Article : Google Scholar : PubMed/NCBI | |
|
Gaspar N, Hung GY, Strauss SJ, Campbell-Hewson Q, Dela Cruz FS, Glade Bender JL, Koh KN, Whittle SB, Chan GC, Gerber NU, et al: Lenvatinib plus ifosfamide and etoposide in children and young adults with relapsed osteosarcoma: A phase 2 randomized clinical trial. JAMA Oncol. 10:1645–1653. 2024. View Article : Google Scholar : PubMed/NCBI | |
|
Pei Y, Yao Q, Li Y, Zhang X and Xie B: microRNA-211 regulates cell proliferation, apoptosis and migration/invasion in human osteosarcoma via targeting EZRIN. Cell Mol Biol Lett. 24:482019. View Article : Google Scholar : PubMed/NCBI | |
|
Shen JK, Cote GM, Choy E, Yang P, Harmon D, Schwab J, Nielsen GP, Chebib I, Ferrone S, Wang X, et al: Programmed cell death ligand 1 expression in osteosarcoma. Cancer Immunol Res. 2:690–698. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
D'Angelo SP, Mahoney MR, Van Tine BA, Atkins J, Milhem MM, Jahagirdar BN, Antonescu CR, Horvath E, Tap WD, Schwartz GK and Streicher H: Nivolumab with or without ipilimumab treatment for metastatic sarcoma (Alliance A091401): Two open-label, non-comparative, randomised, phase 2 trials. Lancet Oncol. 19:416–426. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Xie L, Liang X, Xu J, Sun X, Liu K, Sun K, Li Y, Tang X, Li X, Zhan X, et al: Exploratory study of an anti-PD-L1/TGF-β antibody, TQB2858, in patients with refractory or recurrent osteosarcoma and alveolar soft part sarcoma: A report from Chinese sarcoma study group (TQB2858-Ib-02). BMC Cancer. 23:8682023. View Article : Google Scholar : PubMed/NCBI | |
|
Tawbi HA, Burgess M, Bolejack V, Van Tine B, Schuetze S, Hu J, D'Angelo S, Attia S, Riedel RF, Priebat DA, et al: Pembrolizumab in advanced soft-tissue sarcoma and bone sarcoma (SARC028): A multicentre, two-cohort, single-arm, open-label, phase 2 trial. Lancet Oncol. 18:1493–1501. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Pardoll DM: The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 12:252–264. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Daud AI, Wolchok JD, Robert C, Hwu WJ, Weber JS, Ribas A, Hodi FS, Joshua AM, Kefford R, Hersey P, et al: Programmed death-ligand 1 expression and response to the anti-programmed death 1 antibody pembrolizumab in melanoma. J Clin Oncol. 34:4102–4109. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Zhai X, Zhao J, Wang Y, Wei X, Li G, Yang Y, Chen Z, Bai Y, Wang Q, Chen X and Li M: Bibliometric analysis of global scientific research on lncRNA: A swiftly expanding trend. Biomed Res Int. 2018:76250782018. View Article : Google Scholar : PubMed/NCBI | |
|
Martens-Uzunova ES, Böttcher R, Croce CM, Jenster G, Visakorpi T and Calin GA: Long noncoding RNA in prostate, bladder, and kidney cancer. Eur Urol. 65:1140–1151. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Wang CJ, Zhu CC, Xu J, Wang M, Zhao WY, Liu Q, Zhao G and Zhang ZZ: The lncRNA UCA1 promotes proliferation, migration, immune escape and inhibits apoptosis in gastric cancer by sponging anti-tumor miRNAs. Mol Cancer. 18:1152019. View Article : Google Scholar : PubMed/NCBI | |
|
Li C, Hu J, Hu X, Zhao C, Mo M, Zu X and Li Y: LncRNA SNHG9 is a prognostic biomarker and correlated with immune infiltrates in prostate cancer. Transl Androl Urol. 10:215–226. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Tu J, Wu F, Chen L, Zheng L, Yang Y, Ying X, Song J, Chen C, Hu X, Zhao Z and Ji J: Long non-coding RNA PCAT6 induces M2 polarization of macrophages in cholangiocarcinoma via modulating miR-326 and RhoA-ROCK signaling pathway. Front Oncol. 10:6058772021. View Article : Google Scholar : PubMed/NCBI | |
|
Nguyen CB, Kumar S, Zucknick M, Kristensen VN, Gjerstad J, Nilsen H and Wyller VB: Associations between clinical symptoms, plasma norepinephrine and deregulated immune gene networks in subgroups of adolescent with chronic fatigue syndrome. Brain Behav Immun. 76:82–96. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Doncheva NT, Morris JH, Gorodkin J and Jensen LJ: Cytoscape stringApp: Network analysis and visualization of proteomics data. J Proteome Res. 18:623–632. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Gentles AJ, Newman AM, Liu CL, Bratman SV, Feng W, Kim D, Nair VS, Xu Y, Khuong A, Hoang CD, et al: The prognostic landscape of genes and infiltrating immune cells across human cancers. Nat Med. 21:938–945. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Newman AM, Liu CL, Green MR, Gentles AJ, Feng W, Xu Y, Hoang CD, Diehn M and Alizadeh AA: Robust enumeration of cell subsets from tissue expression profiles. Nat Methods. 12:453–457. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Livak KJ and Schmittgen TD: Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI | |
|
Jiang Z, Jiang J, Zhao B, Yang H, Wang Y, Guo S, Deng Y, Lu D, Ma T, Wang H and Wang J: CPNE1 silencing inhibits the proliferation, invasion and migration of human osteosarcoma cells. Oncol Rep. 39:643–650. 2018.PubMed/NCBI | |
|
Cai X, Liu Y, Yang W, Xia Y, Yang C, Yang S and Liu X: Long noncoding RNA MALAT1 as a potential therapeutic target in osteosarcoma. J Orthop Res. 34:932–941. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Wang SD, Li HY, Li BH, Xie T, Zhu T, Sun LL, Ren HY and Ye ZM: The role of CTLA-4 and PD-1 in anti-tumor immune response and their potential efficacy against osteosarcoma. Int Immunopharmacol. 38:81–89. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Tsukahara T, Emori M, Murata K, Mizushima E, Shibayama Y, Kubo T, Kanaseki T, Hirohashi Y, Yamashita T, Sato N and Torigoe T: The future of immunotherapy for sarcoma. Expert Opin Biol Ther. 16:1049–1057. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Scott MC, Temiz NA, Sarver AE, Larue RS, Rathe SK, Varshney J, Wolf NK, Moriarity BS, O'Brien TD, Spector LG, et al: Comparative transcriptome analysis quantifies immune cell transcript levels, metastatic progression and survival in osteosarcoma. Cancer Res. 78:326–337. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Merchant MS, Melchionda F, Sinha M, Khanna C, Helman L and Mackall CL: Immune reconstitution prevents metastatic recurrence of murine osteosarcoma. Cancer Immunol Immunother. 56:1037–1046. 2007. View Article : Google Scholar : PubMed/NCBI | |
|
Xu H, Wang X, Wu J, Ji H, Chen Z, Guo H and Hou J: Long non-coding RNA LINC01094 promotes the development of clear cell renal cell carcinoma by upregulating SLC2A3 via microRNA-184. Front Genet. 11:5629672020. View Article : Google Scholar : PubMed/NCBI | |
|
Li XX and Yu Q: Linc01094 accelerates the growth and metastatic-related traits of glioblastoma by sponging miR-126-5p. Onco Targets Ther. 13:9917–9928. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Xu J, Zhang P, Sun H and Liu Y: LINC01094/miR-577 axis regulates the progression of ovarian cancer. J Ovarian Res. 13:1222020. View Article : Google Scholar : PubMed/NCBI | |
|
Kotiyal S and Bhattacharya S: Breast cancer stem cells, EMT and therapeutic targets. Biochem Biophys Res Commun. 453:112–116. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Lou Y, Diao L, Cuentas ER, Denning WL, Chen L, Fan YH, Byers LA, Wang J, Papadimitrakopoulou VA, Behrens C, et al: Epithelial-mesenchymal transition is associated with a distinct tumor microenvironment including elevation of inflammatory signals and multiple immune checkpoints in lung adenocarcinoma. Clin Cancer Res. 22:3630–3642. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Yi Q, Zhu G, Zhu W, Wang J, Ouyang X, Yang K, Fan Y and Zhong J: LINC01094: A key long non-coding RNA in the regulation of cancer progression and therapeutic targets. Heliyon. 10:e375272024. View Article : Google Scholar : PubMed/NCBI | |
|
Yang F, Wang M, Shi J and Xu G: IncRNA MALAT1 regulates the proliferation, apoptosis, migration, and invasion of osteosarcoma cells by targeting miR-873-5p/ROCK1. Crit Rev Eukaryot Gene Expr. 33:67–79. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Liu M, Liu C, Li X and Li S: RP11-79H23.3 inhibits the proliferation and metastasis of non-small-cell lung cancer through promoting miR-29c. Biochem Genet. 61:506–520. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Liu Y, Lv H, Liu X, Xu L, Li T, Zhou H, Zhu H, Hao C, Lin C and Zhang Y: The RP11-417E7.1/THBS2 signaling pathway promotes colorectal cancer metastasis by activating the Wnt/β-catenin pathway and facilitating exosome-mediated M2 macrophage polarization. J Exp Clin Cancer Res. 43:1952024. View Article : Google Scholar : PubMed/NCBI | |
|
Li L, Ouyang Y, Wang W, Hou D and Zhu Y: The landscape and prognostic value of tumor-infiltrating immune cells in gastric cancer. PeerJ. 7:e79932019. View Article : Google Scholar : PubMed/NCBI | |
|
Gu-Trantien C, Loi S, Garaud S, Equeter C, Libin M, de Wind A, Ravoet M, Le Buanec H, Sibille C, Manfouo-Foutsop G, et al: CD4+ follicular helper T cell infiltration predicts breast cancer survival. J Clin Invest. 123:2873–2892. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Gambardella V, Castillo J, Tarazona N, Gimeno-Valiente F, Martínez-Ciarpaglini C, Cabeza-Segura M, Roselló S, Roda D, Huerta M, Cervantes A and Fleitas T: The role of tumor-associated macrophages in gastric cancer development and their potential as a therapeutic target. Cancer Treat Rev. 86:1020152020. View Article : Google Scholar : PubMed/NCBI | |
|
Harrington BK, Wheeler E, Hornbuckle K, Shana'ah AY, Youssef Y, Smith L, Hassan Q II, Klamer B, Zhang X, Long M, et al: Modulation of immune checkpoint molecule expression in mantle cell lymphoma. Leuk Lymphoma. 60:2498–2507. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, Segal NH, Ariyan CE, Gordon RA, Reed K, et al: Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 369:122–133. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Motzer RJ, Rini BI, Mcdermott DF, Redman BG, Kuzel TM, Harrison MR, Vaishampayan UN, Drabkin HA, George S, Logan TF, et al: Nivolumab for metastatic renal cell carcinoma: Results of a randomized phase II trial. J Clin Oncol. 33:1430–1437. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Goralski KB, Jackson AE, McKeown BT and Sinal CJ: More than an adipokine: The complex roles of chemerin signaling in cancer. Int J Mol Sci. 20:47782019. View Article : Google Scholar : PubMed/NCBI | |
|
Rodriguez GM, Bobbala D, Serrano D, Mayhue M, Champagne A, Saucier C, Steimle V, Kufer TA, Menendez A, Ramanathan S and Ilangumaran S: NLRC5 elicits antitumor immunity by enhancing processing and presentation of tumor antigens to CD8(+) T lymphocytes. Oncoimmunology. 5:e11515932016. View Article : Google Scholar : PubMed/NCBI |