Angiotensin‑converting enzyme 2 expression in human tumors: Implications for prognosis and therapy (Review)
- Authors:
- Published online on: June 25, 2025 https://doi.org/10.3892/or.2025.8934
- Article Number: 101
-
Copyright: © Rizopoulos et al. This is an open access article distributed under the terms of Creative Commons Attribution License.
Abstract
Introduction
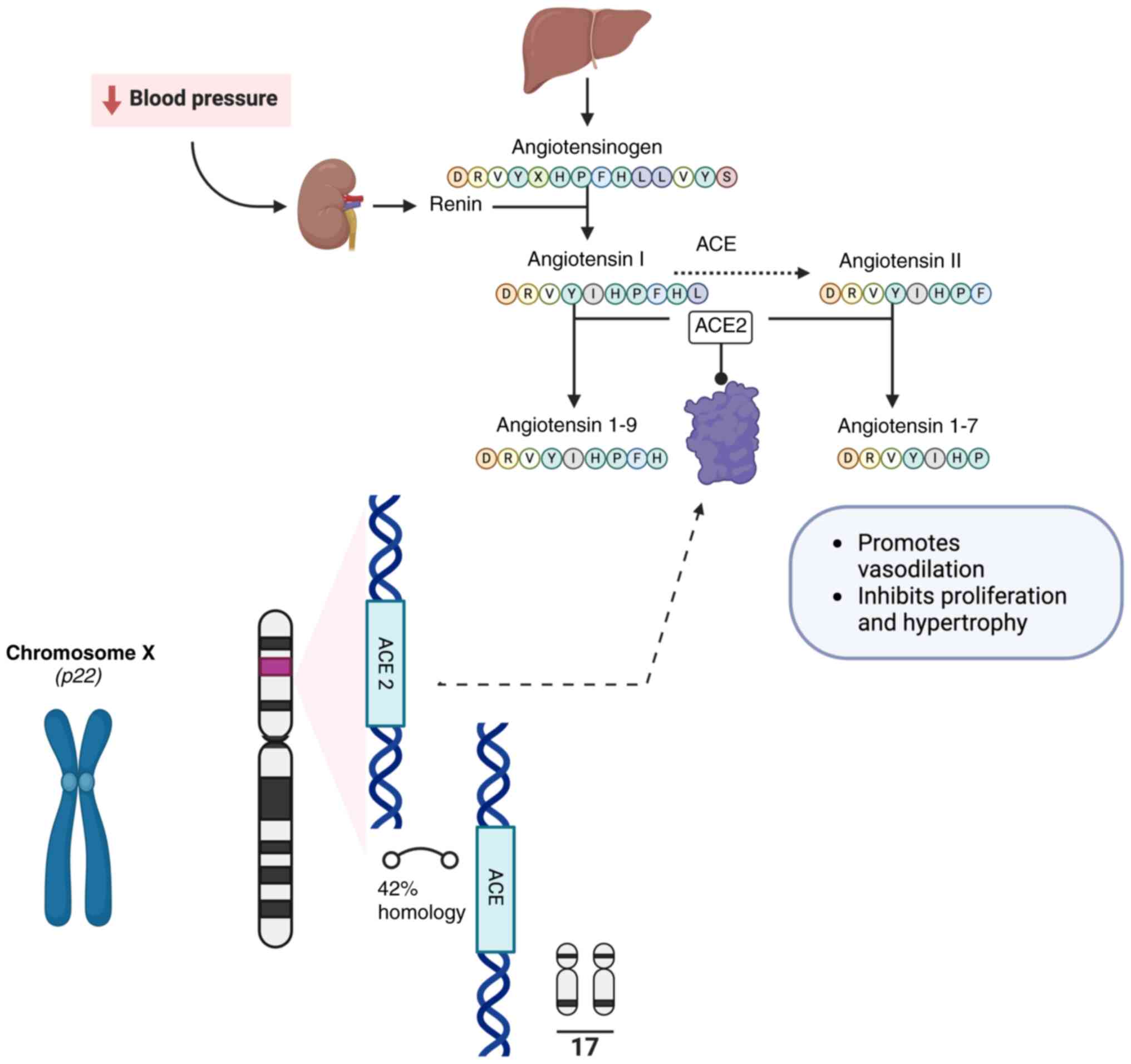
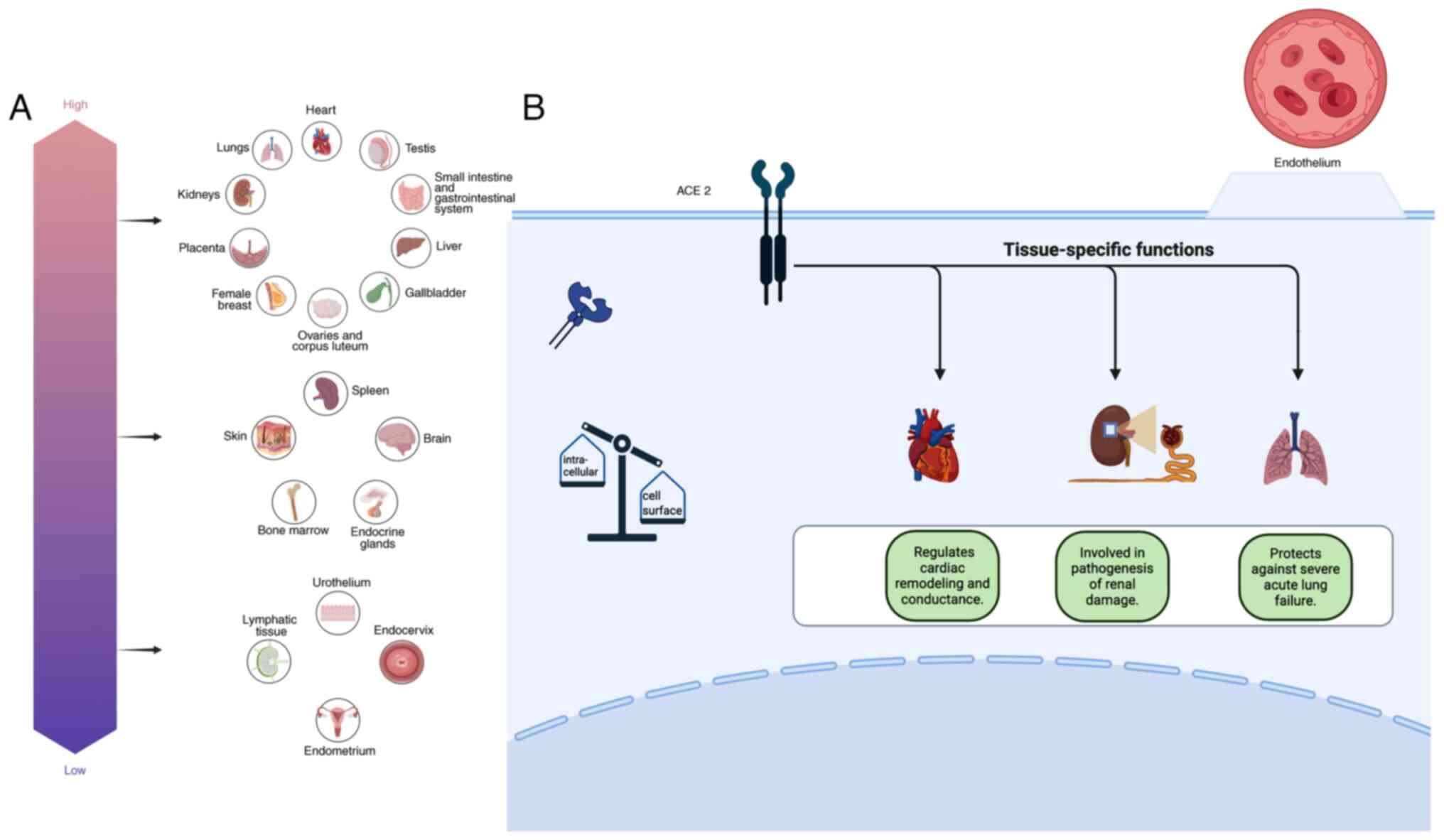
Human angiotensin-converting enzyme 2 (ACE2) regulates the renin-angiotensin (RAS) system by cleaving angiotensin (Ang) I to 1–9 and Ang II peptide to Ang 1–7 (1–3), reactions that promote vasodilation and lead to the inhibition of smooth muscle cell proliferation and hypertrophy (4,5) (Fig. 1). The emergence of the corona virus disease 2019 (COVID-19) augmented the interest in studies on ACE2, as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the β coronavirus that is responsible for the infection, uses ACE2 as functional receptor for cellular entry (1,2). The overall heritability of the ACE2 expression is ~16% among Europeans, of which 30% is linked to 10 protein quantitative trait loci (6). ACE2 is primarily expressed in endothelial cells as a cell-surface non-raft protein with little intracellular localization (7). High expression of ACE2 in the heart, kidney and lungs has been implicated in the pathophysiology of these organs (8–13). Moreover, ACE2 has been detected in a wide range of organs and tissue (14–23) (Fig. 2). In addition to organ-regulated ACE2 expression, demographic data have shown that the expression and activity of ACE2 are both sex- and age-regulated (6,24–27). In addition, androgens are associated with ACE2 upregulation; blocking androgens has the effect of decreasing ACE2 levels (28,29). Exercising has also been shown to affect ACE2 levels (30). Furthermore, high plasma ACE2 levels are observed in patients with type 2 diabetes mellitus, in male smokers, and patients diagnosed with hypertension, atherosclerosis, cardiopathy, respiratory system disorder, pulmonary arterial hypertension, cirrhosis, non-alcoholic steatohepatitis, chronic kidney and inflammatory bowel disease (6,24,31–40). Moreover, hypoxia, cell stress and treatment with IL-1 increase ACE2 levels (41). ACE2 levels are positively associated with cathepsin L1, body mass index, triglycerides and usage of calcium channel blockers (6).
The differential expression of ACE2 in tumors compared with controls has been revealed by epidemiological studies using microarray analysis and bioinformatics (14,18–20,27,33,42–47). Moreover, methylation disturbance and microsatellite instability are associated with ACE2 levels in different types of tumors (18,19). Additionally, ACE2 expression, being positively associated with immune cell infiltration in tumor tissue, may affect tumor prognosis through cancer immunity (19,43,47). Previous studies have also suggested that the ACE2/Ang-(1–7)/Mas receptor axis exerts a pivotal role in tumorigenesis (48,49). The possible antitumor effects of RAS have already been documented in breast and colorectal cancer, although these findings require further validation (50,51). Upregulation of ACE2 is associated with favorable anti-programmed cell death protein 1/programmed-death ligand-1/cytotoxic T lymphocyte-associated protein 4 immunotherapy responses, and inversely associated with tumor proliferation and epithelial-mesenchymal transition tumor properties (52). In addition, angiotensin I-converting enzyme inhibitors (ACEIs) and angiotensin receptor blockers improve disease-free survival rates in patients with urinary tract, colorectal, pancreatic and prostate cancer, with subsequent decreases in cancer mortality and recurrence (53). The present review discusses ACE2 expression in human tissue and their tumor counterparts. Furthermore, ACE2 prognostic and predictive value in human tumors is summarized (Table I).
ACE2 expression in central nervous system tumors
ACE2 expression has been detected in the human brain, wherein low levels of ACE2 mRNA have been reported (54). ACE2 expression in the human brain is localized in neurons, astrocytes, oligodendrocytes and brain endothelial cells (55,56). Furthermore, ACE2 expression is not age-dependent in astrocytoma and brain endothelial cells (26). Human-induced pluripotent stem cell-derived neural stem/progenitor cells exhibit ACE2 expression (57). The amygdala, the cerebral cortex and brainstem are rich in ACE2, although the highest ACE2 expression levels are identified in the pons and medulla oblongata, mainly in the medullary cardiorespiratory centers (58,59). ACE2 expression is also detected in the cerebellum (55,60,61). ACE2 was found to be expressed in vascular smooth muscle cells (62) and pericytes of the brain (63). Moreover, in mice with neurospecific expression of ACE2, ACE2 may protect the brain against ischemic injury by regulating NADPH oxidase levels (64).
ACE2 expression has been demonstrated in the neuroblastoma cell lines SH-SY5Y and SK-N-BE, as well as in the glioblastoma cell lines U87 and U-373 (65–67). High levels of ACE2 are associated with poor prognosis in patients with neuroblastoma (68). Similarly, ACE2 expression is upregulated in glioblastoma multiforme compared with normal brain, and this upregulation decreases survival rates (19,69). Moreover, ACE2 expression is upregulated in low-grade glioma of the brain, and this differential expression is associated with poor prognosis (19,47). In addition, bioinformatics analysis revealed that ACE2 and cathepsin B, which, along with cathepsin L, act as the entry-associated proteases of SARS-CoV-2, both predicted poor prognosis in brain low-grade glioma (45). In a different bioinformatics study (60), ACE2 expression in glioblastoma multiforme compared with normal brain was upregulated, although differences in the expression pattern were not identified by immunohistochemical analysis, potentially due to the small sample size. In the same study, ACE2 levels in glioblastoma multiforme tissue were shown to be associated with monocyte immune infiltration rates, which were increased in patients aged <60 years (60). However, in a transcriptomic analysis, ACE2 gene expression was found not to be significantly different between brain tumors (glioblastoma multiforme and lower-grade glioma) and normal brain (33). ACE2 co-localization with CD31, CD73, and nestin is detected in endothelial cells, bone marrow mesenchymal and neural precursor cells from glioblastoma multiforme samples (70). In a study by Lei et al (71), significantly higher immunohistochemical expression of ACE2 was reported in glioblastoma multiforme tissue (adjacent, peritumor and core) from a patient with COVID-19 compared with glioblastoma tissues from a patient without COVID-19 history. Additionally, ACE2, along with transmembrane serine protease 2 (TMPRSS2) and neuropilin-1 (NRP1) factors, has been detected in glioblastoma multiforme tissue (72).
ACE2 expression in endocrine system tumors
To the best of our knowledge, in the endocrine system, ACE2 has not been investigated in detail. A study on patients with SARS have revealed that ACE2 is expressed in the pituitary gland, thyroid, parathyroid, endocrine pancreas, adrenal glands and testis (73). Low expression of ACE is detected in pituitary neuroendocrine tumors, whereas higher expression of ACE2 is identified in gonadotropic adenomas compared with other pituitary tumors and normal pituitary specimens (74). By contrast, transcriptomic analysis revealed that ACE2 gene expression is similar in adrenocortical carcinomas and normal pituitary tissue (33). High ACE2 levels are associated with an improved prognosis of adrenocortical carcinoma (19).
ACE2 expression is low in the thyroid gland (22). In a transcriptomic analysis (33), ACE2 levels significantly differed in thyroid tissue carcinomas compared with controls. Cytoplasmic expression of ACE2 is significantly increased in papillary thyroid carcinoma and follicular adenomas and thyroid carcinomas as compared with goiters. Furthermore, in a study by Narayan et al (75), ACE2 expression was increased according to tumor size enlargement and the presence of node or remote metastasis, suggesting that ACE2 expression may serve as a biomarker for thyroid carcinoma. In the same study, ACE2 levels were found to be lower in well-differentiated thyroid carcinoma and in samples from patients aged >50 years. Furthermore, downregulation of ACE2 expression in thyroid carcinoma has been reported in a pan-cancer analysis (19). ACE2-positive staining in small vessels is associated with earlier tumor stages in papillary thyroid carcinomas (14). Similarly, ACE2 expression in capillaries is associated with lower tumor staging in low-grade neuroendocrine neoplasms (14). Cox regression analyses using data from The Cancer Genome Atlas revealed that ACE2 may affect the survival rates of patients with thyroid carcinoma (69).
ACE2 is expressed at low levels in the cytoplasm of α-, β- and δ-pancreatic cells, whereas ACE2 staining is stronger in islets, vasculature and duct system cells (14,76–81). In patients with COVID-19, ACE2 was expressed in ~70% of the pancreatic vasculature and in 30% of β-cells (82,83). Pericytes around the exocrine ducts and endocrine islets express ACE2 (63,80). The transcription factor hepatocyte nuclear factor-1 α (HNF1A) regulates ACE2 expression in pancreatic tissue (84). According to bioinformatics studies, ACE2 gene expression is higher in pancreatic adenocarcinoma compared with normal tissue (19,33,44,76), and ACE2 downregulation is associated with tumor progression (85). Moreover, these tumors present with decreased DNA methylation levels of ACE2 (44). However, immunohistochemical expression of ACE2 is not associated with the survival of patients with pancreatic cancer (76). ACE2 levels have been positively associated with immune cell infiltration in pancreatic adenocarcinoma (86). Previous data have provided evidence for ACE2 as a promising candidate biomarker for pancreatic cancer treatment (87). Restoration of the levels of ACE2 suppress the cell proliferative and migratory properties of BxPC3 and SW1990 pancreatic adenocarcinoma cell lines; moreover, the sensitivity to gemcitabine in a pancreatic cancer mouse model is increased with ACE2 overexpression (87). Lau and Leung (88) demonstrated that Ang II type 1 receptor (AT1R) blockers (ARBs) and ACEIs may exert therapeutic properties against pancreatic cancer. Finally, analyses of transcriptomic datasets have shown that the gene expression of ACE2 is similar in adrenocortical carcinoma, pheochromocytoma and paraganglioma compared with normal tissue (33), although the thymus gland contains no ACE2-expressing cells (22).
ACE2 expression in heart and respiratory tract tumors
ACE2 is highly expressed in the heart, and its expression is further increased under stress conditions, such as myocardial infarction (89,90). An enhanced expression of ACE2 is implicated in the pathogenesis of heart failure, atrial fibrillation, coronary artery disease and thoracic aneurysm formation (91–97). Single-cell RNA sequencing (scRNA-seq) suggested that myocardial cells express ACE2 (98) independently of the age of the patients (26). Additionally, cardiofibroblasts, cardiovascular progenitor and endothelial cells, as well as epicardial adipose cells, express ACE2 (21,22,34). Nearly 30% of pericytes in the heart express ACE2 (97,99).
In the upper respiratory tract, ACE2 is detected in the olfactory and respiratory epithelium cells (100,101). According to a single-cell transcriptomic study (102), nasal secretory cells express ACE2. However, ACE2 expression has not been detected in secretory goblet cells of the airway (103). The proportion of nasal epithelial cells express ACE2 was 8.4% (22). Olfactory receptor neurons do not exhibit ACE2 expression, even though the olfactory bulb is ACE2-immunopositive (100). In addition, expression of ACE2 increases with age in the olfactory epithelium of the experimental mice (104). Epithelial cells of the tongue and lymphocytes within the normal oral mucosa are rich in ACE2 (105). ACE2 protein and mRNA expression levels are similar comparing among normal and oral dysplastic and squamous cell carcinoma (106,107). By contrast, a previous study suggested that ACE and ACE2 are promising targets for oral squamous cell carcinoma therapy (108). As Ang-(1–7) may have an important role in oral carcinogenesis, Ang-(1–7) and AT1R antagonists have already been introduced as therapeutic agents for head and neck squamous cell carcinoma (109). Moreover, treatment with the Ang-(1–7) complex decreases the progression of human nasopharyngeal carcinoma via autophagy, cell proliferation and angiogenesis inhibition, illustrating a possible role for ACE2 in the treatment of nasopharyngeal carcinoma (110,111).
High levels of ACE2 are associated with an improved prognosis in patients with operable laryngeal cancer (112). A total of ~2% of epithelial cells of the lower respiratory tract express ACE2 (98), whereas the bronchus and trachea express low levels of ACE2 (22,98). In terms of subcellular localization, ACE2 has been shown to be concentrated in the cilia organelle (103). Human alveolar type II (ATII) cells exhibit high ACE2 expression (23,113,114). According to previous findings based on scRNA-seq expression profiling analysis, ATII cells express ACE2 at low levels (22). ACE2 has also been detected in ATII progenitor and in alveolar stem-like cells (115). By contrast, ACE2 is expressed at lower levels in ATI and bronchial epithelial cells, fibroblasts, pericytes, endothelial cells and macrophages of the respiratory system (63,113). ACE2 is co-expressed with key elements of the bradykinin, angiotensin and coagulation systems in alveolar cells, and is associated with the differentiation dynamics of these systems (116). ACE2 expression is also associated with androgen expression in lung epithelial cells (117). Smoking was associated with higher ACE2 protein levels in alveolar and bronchial lung epithelial cells (117), and this association is independent of age and sex (118). ACE2 was found to be expressed in goblet cells in smokers (119,120); however, in a single-cell sequencing study, smoking did not affect ACE2 expression levels in lung cells (121). Similarly, in a bioinformatics study, the ACE2 levels of bronchial epithelial cells were similar between smokers and non-smokers (122). By contrast, recent data have shown that hypercapnia increases ACE2 expression in human bronchial epithelial cells (123). These conflicting data concerning smoking and ACE2 expression may reflect differences in the susceptibility of patients to inflammation, and structural changes in the lung parenchyma leading to the development of chronic obstructive pulmonary disease.
ACE2 is strongly expressed in lung adenocarcinoma and squamous cell carcinoma compared with normal tissues according to database analysis studies (19,44,124). In the study by Chai et al (44), decreased levels of ACE2 DNA methylation are associated with high expression of ACE2 in lung adenocarcinomas Similarly, in a bioinformatics study by Zhang et al (125), the expression of ACE2 was shown to be significantly increased in lung adenocarcinoma and squamous cell carcinoma compared with normal tissue, without any variations being observed with respect to the stage of the tumor, whereas ACE2 upregulation is associated with DNA methylation. According to another study (126), ACE2 upregulation in patients with lung adenocarcinoma is age-dependent. Moreover, in a retrospective cohort study on the outcomes of patients with COVID-19 and lung cancer, ACE2 expression was higher at the resection margins of lung cancer survivors compared with the normal tissue of non-cancerous individuals (127). Furthermore, in a single-cell transcriptomic small-scale analysis (128), the expression of ACE2 was predominantly found in cancer and alveolar cells of lung adenocarcinoma, and no differentiation of expression was observed between lung adenocarcinoma and lung squamous cell carcinoma in the cancer cells, irrespective of smoke exposure. On the other hand, in a study that used gene expression analyses of data obtained from the Gene Expression Profiling Interactive Analysis 2 (GEPIA2), The Cancer Genome Atlas (TCGA) and Genotype-Tissue Expression (GTEx) public databases (129), the upregulation of ACE2 in lung adenocarcinoma was not confirmed. Furthermore, in a study by Kong et al (130) on lung squamous cell carcinoma, ACE2 expression was shown to be similar between normal lung and the classical, secretory and basal subtypes of lung squamous cell carcinoma whereas the primitive subtype demonstrated lower ACE2 gene expression compared with normal lung tissue. In addition to these findings, another study that employed gene expression analysis suggested that the gene expression of ACE2 does not change in lung tumors compared with normal tissue (33). By contrast, in the bioinformatics study of Cui et al (18), lower levels of ACE2 expression were reported in lung adenocarcinoma compared with normal tissue. However, the aforementioned bioinformatics gene expression studies did not provide the clinicopathological characteristics of the patients, and, to the best of our knowledge, only one dataset has been published containing information on smoke exposure of the patients (128); therefore, the results may not be considered comprehensive. Additional clinical data will need to be analyzed in the future to clarify the association between ACE2 and clinical characteristics of cancer, including exposure of the patients to pollutants, a previous diagnosis of chronic obstructive pulmonary disease and daily prescriptions. It is worth noting that ACE2 expression in lung metastases derived from different cancer subtypes is higher compared with organ metastases of other sites (126). Additionally, ACE2 is expressed at higher levels in lung metastases from colorectal cancer compared with normal lung tissue (131). Moreover, the proportion of ACE2-positive cells is higher in the pulmonary metastatic areas of patients with high-grade serous ovarian cancer compared with normal alveolar and bronchiolar areas. Notably, ACE2 is expressed in alveolar epithelial stem and stem-like cells adjacent to ovarian cancer in lung metastasis (132).
In a bioinformatics study by Feng et al (43), it was reported that high expression of ACE2 is beneficial in lung adenocarcinoma, although this has a detrimental effect on prognosis in lung squamous cell carcinoma. However, in the bioinformatics study by Zhang et al (125), although ACE2 expression was not associated with disease-free and overall survival for patients with lung adenocarcinoma, higher ACE2 expression was associated with prolonged disease-free survival in patients with lung squamous cell carcinoma, suggesting a complex role for ACE2 in lung cancer. By contrast, in the bioinformatics study by Samad et al (133), high expression of ACE2 mRNA, irrespective of the age and sex of the patients, was associated with shorter overall and relapse-free survival rates in patients with lung adenocarcinoma and squamous carcinoma. In a study by Lazar et al (134), ACE2 expression in either normal lung tissue or tumor tissue derived from non-small cell lung cancer (NSCLC) was not associated with disease-free survival of the patients. In the same study, higher expression levels of ACE2 were identified in advanced stages of lung cancer (134). Moreover, a bioinformatics study by Dai et al (135) revealed that ACE2 expression is not associated with the prognosis of patients with lung cancer compared with ACE2 expression from resected normal lung tissue. Furthermore, decreased ACE2 expression was associated with poor overall survival in mesothelioma (135).
In the study by Deben et al (136), wherein membranous and soluble ACE2 expression was investigated in patients with NSCLC and focus was made on standard-of-care therapies, no association between ACE2 levels and the patient clinicopathological data was observed. Moreover, higher frequency of ACE2 expression was reported in patients with epidermal growth factor receptor (EGFR) mutations, whereas no increased ACE2 expression was observed in KRAS-mutant patients, and prior therapy did not cause increases in soluble ACE2 levels (136). ACE2 levels are increased in the EGFR-mutant lung adenocarcinoma HCC827 cell line, and an anti-ACE2 antibody has been developed against tumors resistant to EGFR inhibitors, although further testing of this antibody is required (137). On the other hand, in vitro data have suggested that ACE2 overexpression exerts a protective effect by inhibiting cell proliferation and the production of vascular endothelial growth factor A (VEGFA) in NSCLC cell lines (138). Treatment with cisplatin and gemcitabine upregulate ACE2 expression in A549 NSCLC cancer cells, demonstrating that standard chemotherapy regimens affect ACE2 expression levels in lung cancer cells in vitro (139). Nevertheless, ACE2 overexpression may inhibit cell proliferation and VEGF production in NSCLC lines following the development of acquired platinum resistance (140). Moreover, the overexpression of ACE2 decreases both the invasive properties of A549 lung cancer cells and the production of matrix metalloproteinase (MMP)-2 and MMP-9, whereas it decreases tumor angiogenesis in vivo by lowering VEGFΑ levels (141). Τhe addition of DX600, which serves as an ACE2 inhibitor, to the A549 lung cancer cell line leads to a recovery of the sensitivity of lung cancer cells to TGF-β1-mediated induction of epithelial-mesenchymal transition, suggesting that ACE2 enhances lung cancer cell metastasis (142). Furthermore, patients with advanced lung cancer who receive ARBs or ACEIs in addition to platinum-based first-line treatment demonstrate a 3.1-month longer median survival rate compared with non-recipients (143). In addition, Ang-(1–7) peptides decrease tumor size in a lung cancer mouse model, wherein mice were injected with A549 human lung cancer cells, indicating a RAS-inhibitory effect in lung cancer cell proliferation (144). Nevertheless, the use of ACEIs is associated with an increased risk of lung cancer (145). A recent study highlighted the interaction of SARS-CoV-2 with ACE2 as a possible mechanism in lung tumor therapy (146). ACE2 has also been proposed as a postoperative prognostic factor for patients with NSCLC (147).
ACE2 expression in breast cancer
ACE2 expression is significantly decreased in breast cancer samples compared with normal tissue (18,148,149), with the exception of the basal-like subtype (148). In addition, decreased levels of ACE2 expression in the plasma of patients with breast cancer compared with healthy controls has been identified (150). DNA promoter methylation has been proposed as the primary mechanism underlying ACE2 downregulation in breast cancer (151). ACE2 expression is positively associated with immune cell infiltration (18). Jiang et al (148) suggested that ACE2 expression is associated with enhanced rates of neutrophil infiltration in basal-like breast cancer, whereas in the luminal subtype, ACE2 expression is associated with higher ratios of CD4+ and CD8+ T cells and neutrophil infiltration (47,148). ACE2 expression is also associated with both the inflammatory microenvironment of the tumors and estrogen receptor, progesterone receptor and HER2 negativity in breast cancer (149). Moreover, increased expression of ACE2 is associated with fewer disseminated foci, shorter metastatic distances, downregulation of VEGFA and suppression of the proliferation of MCF-7 cells in vitro (152).
In addition, bioinformatics studies have shown that patients with breast cancer with high ACE2 expression exhibit longer relapse-free survival rates and improved overall prognosis (151,152). Decreased expression of ACE2 are associated with poorer prognosis of luminal B breast cancer following SARS-CoV-2 infection (148). However, in breast invasive cancer, ACE2 expression is significantly higher compared with normal tissue (135), and the stronger ACE2 immunostaining was associated with high-tumor grade, HER2 overexpression and loss of estrogen and progesterone receptors (14). He et al (47) reported that high ACE2 expression is associated with poor prognosis of patients with breast invasive carcinoma. On the other hand, results obtained in vitro have demonstrated that ACE2 may inhibit the development of breast cancer (152), and downregulation of the ACE2/Ang-(1–7)/Mas axis increases breast cancer cell metastatic properties by enhancing calcium entry (153). Finally, in a bioinformatics study, ACE2 expression was positively associated with EGFR expression, whereas high levels of ACE2 are associated with decreased disease-free survival in the HER2-enriched subtype (154).
Regarding therapeutic intervention options, ACE2 levels, controlled by hypoxia-inducible factor-1 (HIF-1) and the concentration of reactive oxygen species (ROS), are elevated following chemotherapy in breast cancer cells (150). Moreover, increased ACE2 expression following chemotherapy is a poor prognostic factor for patients with breast cancer, as ACE2 upregulation is likely associated with the development of drug resistance in breast cancer (150); however, it has been reported that patients with high levels of ACE2 tend to be sensitive to a variety of therapeutic options, including immunotherapy and antiangiogenic therapy (149).
ACE2 expression in gastrointestinal tract tumors
Liver, pancreas, stomach, small intestine and colon tissue express ACE2, primarily in mesenchymal and capillary endothelial cells (61,76,86). The highest levels of ACE2 expression are found in the goblet cells (13,22,61). Furthermore, ACE2 is highly expressed in epithelial basal cells, cholangiocytes and colonocytes, ileum and rectum enterocytes and in the epithelial stomach cells, as well as in progenitor, stem and transient amplifying cells of the ileum (23). ACE2 has been shown to be expressed in 30% of ileal cells (98), whereas ACE2 expression in the terminal ileum is 25-fold higher compared with that in the colon (155). In the ileum and colon, ACE2 levels are regulated by the HNF-4α) transcription factor (156). Additionally, ACE2 is highly expressed in the epithelial cells of the tongue and salivary gland (17,23,157).
ACE2 levels are also increased in esophageal carcinoma (42,43,47). However, database analyses have revealed that ACE2 expression does not differ in esophageal carcinoma tumors compared with normal tissue (19,33). In a study that utilized data from an scRNA-seq analysis, ACE2 expression increased with progression from chronic gastritis to metaplasia, reaching its highest levels in early gastric cancer (158). Additionally, gene expression analyses have confirmed a significant upregulation of ACE2 expression in stomach cancer samples compared with that in adjacent non-tumor gastric tissue (44,76,129). By contrast, other bioinformatics studies have shown both that ACE2 levels are lower in stomach adenocarcinomas compared with the adjacent normal tissue, and that ACE2 expression either tends to increase in conjunction with the stages of tumor progression (19,47) or does not differ between stomach adenocarcinoma and normal tissues (33). However, the aforementioned studies did not include clinical information about the patients. ACE2 expression was found not associated with the prognosis of patients with stomach adenocarcinomas (19).
In normal liver tissue, ACE2 is expressed in the bile canaliculi of hepatocytes, biliary epithelium, sinusoidal and capillary endothelial cells (159). High ACE2 expression is observed at the apical surface of cholangiocytes (160). The hepatic expression of ACE2 is higher in adults compared with children, although it is augmented in pediatric patients with liver disease compared with normal subjects (160). ACE2 in hepatocytes limits fibrogenesis in mice, and ACEs are a negative glycolytic regulator for hepatocytes (161,162). ACE2 expression in hepatocytes is increased in inflammatory liver conditions and fibrotic/cirrhotic liver disease (163). ACE2 hepatic upregulation has been documented in a chronic liver injury rat model (164). In a different study (76), the immunohistochemical expression of ACE2 was found to be decreased in liver cancer tissue. A transcriptomic analysis by Facchiano et al (33) revealed no significant differences in ACE2 expression in liver hepatocellular carcinoma compared with normal liver. Moreover, increased mRNA and protein expression levels of ACE2 have been reported in well-to-moderately differentiated hepatocellular carcinoma tissue compared with poorly differentiated tumors (159). Furthermore, β-catenin mutations are associated with ACE2 DNA hypomethylation (159). In addition, higher levels of ACE2 are associated with improved overall and disease-free survival rates in patients with liver hepatocellular carcinoma (43,47,129,135), and ACE2 downregulation may serve as a predictor of poor prognosis in patients with hepatocellular cancer (162). An analysis using human using the Gene Expression Omnibus (GEO) and The Cancer Genome Atlas (TCGA) databases revealed that ACE2 mRNA levels are gradually decreased with an increasing grade of hepatic disease severity, suggesting that low ACE2 expression levels are a poor prognosis indicator for hepatocellular carcinomas (165). Infection with hepatitis C has been shown to cause increases in the mRNA and protein levels of ACE2 in Huh7.5 cells and human liver tissue (166). In a patient-derived xenograft model (162), ACE2 overexpression impedes tumor growth via a decrease in ROS-HIF1α activity. Therefore, the ACE2/Ang-(1–7)/MasR/ROS/HIF-1α axis may be a promising target for therapeutic intervention in hepatocellular cancer (162).
ACE2 is highly expressed in the gallbladder, and ACE2 levels differ in cholangiocarcinoma compared with normal tissue (33). ACE2 levels are higher than normal in gallbladder carcinoma and cholangiocarcinoma tumors (19). Negative ACE2 expression is an independent poor prognostic factor for squamous cell carcinoma/adenosquamous carcinoma and adenocarcinoma of the gallbladder, as it is associated with larger tumor size, higher TNM stage, lymph node metastasis, invasive properties and increased levels of carbohydrate antigen 19-9 (167). In vitro and in vivo data have shown that genetic replenishment of ACE2 and the use of angiotensin receptor blockers restore the expression of ACE2, thereby mitigating gallbladder cancer (168).
ACE2 is present in enterocytes in the small intestine and colonocytes, primarily in the surface epithelium (76,169). Moreover, these cells exhibit the highest proportion of cells co-expressing ACE2 and TMPRSS2, which are required for SARS-CoV-2 entry into mammalian cells (169). ACE2 expression is continuous across the surface epithelium of the small intestine, and has also been detected on the crypt epithelium, whereas ACE2 expression in the colon is patchy (170). ACE2 serves as a mediator in intestinal homeostasis (171,172). Moreover, ACE2 regulates the gut-blood barrier; in ACE2−/− Akita mice, the disrupted gut barrier was shown to be associated with lower levels of angiogenetic and hemopoietic cells and gut dysbiosis (34,173). ACE2 is a key player in the dopamine/trace amines metabolic pathway, as it supports the intestinal absorption of tyramine, tyrosine, phenylalanine, tryptophan and tryptamine (174). ACE2 knockdown induces the clinical and histological findings of dextran sodium sulfate-induced colitis in a mouse model (175). In addition, ACE2 expression is significantly decreased in the inflamed ileum of patients with Crohn's disease (155,156), whereas its expression is higher in the inflamed colon of patients with Crohn's disease or ulcerative colitis compared with control subjects (156). Furthermore, the mucosal expression of ACE2 is 50% higher in inflamed compared with non-inflamed colon sites in patients with ulcerative colitis (155). Serum ACE2 levels are also increased in patients with inflammatory bowel disease compared with controls (176). Finally, in a study by Potdar et al (177), decreased mRNA levels of ACE2 in the small bowel and increased levels of ACE2 in the colon were associated with demographic features, such as age and an elevated body mass index.
ACE2 expression is higher in rectum carcinomas (18,19,44) and colon cancer tissue compared with controls (18,19,33,42,47,76,129,178–182). The ACE2 expression increases moving from normal to adenoma samples, and colon cancer samples (183). ACE2 expression is associated with immune cell infiltration in both normal and cancerous stomach and colon tissue (87,184). Notably, colorectal tumors with upregulated ACE2 gene expression display decreased DNA methylation levels (44,179,184). In a study by Li et al (182), increased ACE2 gene mutation frequency, associated with decreased expression, was observed in the early-onset colorectal cancer group of patients compared with the late-onset group. However, according to bioinformatics studies, the upregulation of ACE2 expression is not associated with the survival outcomes of patients with colon adenocarcinoma (44,184). By contrast, higher expression levels of ACE2 are associated with a favorable prognosis and improved survival rates in patients with colon and colorectal adenocarcinoma, respectively (19,182). There are also data suggesting that decreased expression of ACE2 is associated with an unfavorable tumor phenotype in colorectal adenocarcinoma (14). Additionally, high mRNA levels of ACE2, along with high mRNA levels of bromodomain and extra terminal domain 4, are associated with reduced survival rates of patients with colorectal cancer (185).
ACE2 expression is downregulated in intestinal organoids following SARS-CoV-2 infection (186,187). Moreover, the radiological reduction of colon cancer metastasis in three patients following SARS-CoV-2 infection has been described; the potential mechanisms [a direct immune response, likely coordinated by natural killer (NK) cells and T lymphocytes, or cytokine release following SARS-CoV-2 infection in colorectal cells] merit further investigation as higher degranulation levels of NK cells has been documented in the presence of ACE-2/NRP-1-expressing cells (188,189). Ficin and bromelain, aqueous extracts derived from the fruits of Ficus carica and Ananas comosus plants, exert cytotoxic effects against human cholangiocarcinoma, breast, prostate, stomach and hypopharynx squamous carcinoma cells, respectively, inducing apoptosis and decreased ACE2 expression in Caco-2 cells (190). Higher ACE2 mRNA levels have also been associated with lower activity of the anti-cancer immune response in colorectal cancer (182).
ACE2 expression in genitourinary system tumors
ACE2 is highly expressed in proximal tubule cells and glomerular parietal epithelial cells of the kidney (14,23,46, 61,98,191–194). In addition, podocytes, endothelial and mesangial cells in the kidney also express ACE2 (22,61,195). ACE2 exerts renal protective properties against albuminuria and glomerular injury in diabetic mouse models (196,197). In a study by Vergara et al (198), the presence of ACE2 in the urine served as a prognostic indicator for acute kidney injury, and ACE2 tubular staining was 2-fold lower in the specimens of patients with COVID-19 compared with non-COVID-19 samples. ACE2 was not expressed in the ureter (22).
ACE2 promotes epithelial-mesenchymal transition in renal tubular cells (199). According to TCGA, the Genotype-Tissue Expression the Gene Expression Omnibus database analyses that did not categorize patient characteristics (42,86), the mRNA and protein expression levels of ACE2 were higher in kidney renal clear and papillary cell carcinoma compared with the normal kidney. In a retrospective cohort study, chromophobe renal cell carcinoma specimens exhibited negative immunostaining for ACE2 (200). The downregulation of ACE2, as determined by immunohistochemical expression and enzyme activity in tumors from the distal nephron (chromophobe renal cell carcinoma and renal oncocytoma) with respect to normal tissues, was also reported by Larrinaga et al (201) and Errarte et al (202). In addition, higher levels of immunohistochemical expression and serum activity of ACE2 are noted in kidney renal clear and papillary cell carcinoma compared with healthy individuals (202). Therefore, ACE2 may be useful for identification of the renal subtype, as its low rates of immunopositivity in chromophobe renal cancer cells compared with the high levels of ACE2 positivity in clear and papillary renal tumor cells may be useful for assessing undifferentiated tissues (14). However, in the study by Facchiano et al (33) using data retrieved from GEPIA2 and GENT2 databases, there was no difference in expression in kidney renal clear cell carcinoma compared with controls. Other bioinformatics studies (18,203,204) have reported contradictory data, namely a significant downregulation of ACE2 expression at the mRNA and protein levels in kidney renal clear cell carcinoma compared with normal samples. Furthermore, in the study by Yang et al (204), ACE2 immunohistochemical analysis of 54 patients revealed that a lower expression of ACE2 in kidney renal clear carcinoma compared with adjacent normal tissue was associated with malignant characteristics and metastasis of the tumors. As ACE2 expression in the human kidney differs in various clinicopathological conditions, including diabetes, acute kidney injury and chronic renal failure (196–198), the contradictory results regarding ACE2 expression in kidney tumors require further investigation.
ACE2 is positively associated with immune cell infiltration in kidney renal clear and papillary cell carcinoma (43,86,203–206). In addition, several ACE2-associated kinases, microRNAs and transcription factors involved in regulating genomic stability, cell cycle and ribosomal activity have been identified in kidney renal clear cell carcinoma (203). Furthermore, decreased levels of ACE2 gene methylation in kidney renal clear and papillary cell carcinomas have been identified (18,44,205). Early-stage tumors express ACE2 at higher levels compared with advanced tumors in kidney renal clear and papillary cell carcinomas (86,206), whereas ACE2 expression is significantly reduced in stage IV kidney renal papillary carcinoma (18). ACE2 staining has been found in cancer stem cells (CSCs) in a limited sample of kidney renal clear cell carcinoma tissues (207), and the presence of CSCs in renal clear cell carcinoma has also been identified (208).
As ACE2 is differentially expressed in different renal tumor subtypes, conflicting data regarding its impact on patient outcome have also been reported (14,18,19,43,44,69,86, 202–205). Retrospective studies have not identified any significant association between the immunohistochemical expression of ACE2 and the survival rates of patients with kidney renal clear cell carcinoma (202,207), whereas the majority of bioinformatics data report that ACE2 expression is increased in kidney renal clear and papillary cell carcinoma, and this increased expression is associated with increased survival rates and improved disease-specific survival rates for these patients (19,43,44,69,86,206,209). In another bioinformatics study however, ACE2 is downregulated in kidney renal clear cell carcinoma at the mRNA and protein level, whereas the upregulation of ACE2 is associated with a favorable prognosis and improved overall survival of the patients (203). Decreased ACE2 levels have been associated with worse pathological features such as advanced tumor stage, higher histological grade and pathological stage and metastasis (18,204). However, the low expression of ACE2 in kidney renal clear cell carcinoma may affect the prognosis of patients since the prognostic role of ACE2 is differentiated according to infiltration levels of different types of immune cell in these tumors (204). In the study by Tang et al (206), ACE2 downregulation was associated with poor overall survival in kidney renal clear and papillary cell carcinomas and metabolic homeostasis of tumor cells. In other studies (14,209), decreased expression of ACE2 and its activity in kidney renal clear cell carcinoma is associated with advanced pathological features, poorer overall survival and different immune cell subtypes. According to Cui et al (18), ACE2 downregulation is associated with higher clinical stage and worse survival rate, whereas hypermethylation of ACE2 is linked to higher survival in kidney renal clear cell carcinoma.
ACE2 overexpression was reported by Khanna et al (210) to exert inhibitory effects on tumor proliferation in kidney renal clear cell carcinoma. Additionally, treatment of clear cell renal cell carcinoma xenografts with VEGF receptor-tyrosine kinase inhibitor (VEGFR-TKI) decreases ACE2 expression, whereas combinatorial treatment with VEGFR-TKI and Ang-(1–7) leads to further suppression of tumor growth, thereby improving survival outcomes (210). ACE2 expression, along with MMP24, solute carrier family 44 member 4, complement C1r, chromosome 1 open reading frame 194 and ADAM metallopeptidase with thrombospondin type 1 motif 15, are associated with TKI resistance in kidney renal clear cell carcinoma, which limits the therapeutic opportunities and worsens the prognosis for patients with metastatic disease (211). Furthermore, lower ACE2 levels are associated with increased drug resistance in renal cancer cell lines (206). In addition, Kim et al (69) demonstrated that prioritizing ACEIs for blood pressure control over other antihypertensives may increase survival rates of patients with kidney renal clear cell carcinoma.
Testicular cells demonstrate high levels of ACE2 (22,46,61). ACE2 has been identified in both cytoplasmic and membranous regions of the testicular cells (13,192,212–214). Furthermore, male embryo primordial germ cells express ACE2 (215). In adulthood, ACE2 expression is negatively associated with age (215), and the age-associated expression of ACE2 in the testis is confirmed in other studies, with a peak occurring at ~30 years of age and the lowest expression occurring at ≥60 years (192,214,216). ACE2 in Leydig cells regulates steroidogenesis and vascular tone, and its expression is not affected by testosterone levels or downregulation of luteinizing hormone (212). Spermatogenesis is regulated by the RAS system (217). In particular, lower mRNA levels of ACE2 are found in testicular samples of patients diagnosed with non-obstructive azoospermia compared with patients with obstructive azoospermia (218). However, the activity of ACE2 is higher in infertile males compared with normal subjects following SARS-CoV-2 infection (216). Moreover, according to Fu et al (13), testis malignancy is associated with low expression rates of ACE2. In addition, a transcriptomic analysis revealed significantly higher levels of ACE2 in testicular germ cell tumors compared with healthy controls (33). Another bioinformatics study (86) identified lower levels of ACE2 in testicular germ cell tumors compared with the normal testis. In addition, Feng et al (43) showed that ACE2 may influence the tumor prognosis of testicular germ cell tumors via cancer immunity.
ACE2 is expressed in 0.32% of all prostate epithelial cells (219). Bioinformatics data show that ACE2 levels are lower in prostate adenocarcinomas compared with normal tissue (19,43,47). Contrary to these findings, however, results of studies that employed the same methodology (33,86) demonstrate ACE2 gene expression in prostate cancer tissue is not found significantly different from that of normal tissue. ACE2, as well as Ang-(1–7), has been shown to have a protective role against metastasis formation in prostate cancer (220,221). In addition, in the study by Huang et al (86), ACE2 was positively associated with immune cell infiltration in prostate cancer tissue. Since, as suggested by a study utilizing mice models, androgen inhibition augments ACE2 levels, ACE2 is a potential therapeutic target for patients with prostate cancer (222).
ACE2 is also expressed in the endometrium, although its expression is more pronounced in epithelial compared with stromal cells (223–226). The mRNA levels of ACE2 are increased in the endometrium of patients with polycystic ovaries (225). On the other hand, in another study (227), ACE2 was downregulated in the granulosa cells of patients with polycystic ovaries compared with controls. Other studies have demonstrated that ACE2 is expressed in syncytiotrophoblast, cytotrophoblast and trophoblastic cell lines, blastocysts, chorion cells of the placenta, endothelium and smooth muscle of the umbilical cord (12,226,228–230). High ACE2 mRNA levels have been confirmed in placental cells by Ren et al (61). ACE2 is expressed in the fallopian tube epithelium (22), although in primary oocytes, ACE2 expression is absent (230). However, ACE2 mRNA expression has been identified in tissue RNA from human ovarian samples (231). Moreover, high levels of ACE2 protein expression are identified using mass spectrometric analysis in ovarian cells, whereas immunohistochemical analyses did not detect the protein in either follicular or stromal ovarian cells (232).
The ACE2/Ang-(1–7)/Mas1 signaling axis may have both tumor-promoting and -inhibiting properties in ovarian cancer (233). ACE2 has been demonstrated to be a protective and independent prognostic factor in patients with ovarian cancer (19,43,47). High levels of ACE2 are associated with increased overall and disease-free survival rates in patients with ovarian cancer (19,43,47). High expression of ACE2 in patients with serous subtype ovarian cancer is associated with improved overall survival compared with the endometrioid subtype, and improved progression-free survival in the endometrioid subtype (47). High ACE2 expression is associated with poorer overall survival in patients with ovarian cancer without the TP53 mutation (47). In the study by Nagappan et al (234), ACE2 levels were increased in ovarian clear cell cancer carcinoma compared with normal tissue and this increase was associated with increased chemoresistance. According to the same study, ACE2 regulates caveolin-1-associated signaling pathways, thereby affecting the platinum-clearing enzyme cytochrome P450 3A4, and resistance to platinum-based drugs (234). Additionally, higher mRNA levels of ACE2 are detected in endometrium tumors compared with controls (235). ACE2 is upregulated in cervical squamous cell carcinoma, endocervical adenocarcinoma and uterine corpus endometrial carcinoma (19,42). In addition, higher expression of ACE2 in the uterine corpus and endometrial tumors is associated with both an enhanced infiltration of immune cells in the tumor environment and improved survival rates (205). By contrast, in the study by Facchiano et al (33), altered levels of ACE2 expression were not observed between ovarian serous cystadenomas and cervical squamous cell, endocervical and uterine carcinoma and normal tissue.
Low levels of ACE2 have been detected in bladder epithelial cells; 0.25 and 1.28% of intermediate and umbrella cells, respectively, express ACE2 (194). According to Zou et al (98), the proportion of ACE2-positive cells in bladder urothelial cells is ~2.4%; moreover, the levels of ACE2 were found not to differ in bladder urothelial cell carcinoma compared with normal tissue (19,33,86). However, ACE2 expression is associated with immune cell infiltration in bladder urothelial cell carcinoma and ACE2 gene mutations have been also observed (19). In addition, high expression of ACE2 is associated with improved prognosis for patients with uterine carcinosarcoma (19).
ACE2 expression in skin and bone tumors
A previous study reported expression of ACE2 in basal epidermal layers and in the sebaceous gland cells in normal skin, whereas non-melanoma skin cancer, such as basal and squamous cell carcinoma, does not express ACE2 (236). The transcriptomic analysis by Facchiano et al (33) identified similar levels of ACE2 gene expression in skin cutaneous melanoma and in normal skin. Similarly, in the same study, ACE2 expression levels did not differ between head and neck squamous cell carcinomas and controls (33). However, ACE2 expression is positively associated with immune cell infiltration in skin cutaneous melanoma (19). Negative ACE2 immunostaining was reported in all head and neck metastatic malignant melanoma tissue samples included in the study by Siljee et al (237), although ACE2 mRNA was detectable in these tumors. Higher ACE2 levels are associated with improved overall survival for patients with uveal melanoma (19). According to Facchiano et al (33), ACE2 gene expression is significantly altered in sarcoma tissues compared with normal tissues. Ender et al (238) demonstrated the osteosarcoma cell lines U-2 OS and MNNG-HOS express ACE2, and therefore activation of the ACE2/Ang-(1–7)/Mas axis may be a promising therapeutic option for osteosarcoma.
ACE2 expression in hematological malignancies
The RAS system in the bone marrow controls myelopoiesis, erythropoiesis and thrombopoiesis (239,240). ACE2 serves an important role in myelopoiesis (241). Ang peptides accelerate the rate of hematopoietic recovery following radiation exposure in mice (242,243). The RAS system is expressed in hematopoietic stem cells and ACE2 is detected in very small embryonic-like stem cells (239,240,244–247). Notably, almost no cells of the peripheral blood, bone marrow and spleen exhibit high expression of ACE2 (22,23). However, through data analysis, high mRNA expression levels of ACE2 have been detected in the bone marrow (61). mRNA expression levels of RAS components have been reported in patients with myeloma, implying that the local RAS is implicated in the pathology of the hematopoietic system (247). In a study by Gomez Casares et al (248), renin expression was evaluated in hematological malignancies and in certain acute myeloid human leukemia cell lines, and the results showed that renin expression may have a role in the development of acute myeloid leukemia, and may be used as an aberrant marker of leukemia. High ACE2 mRNA expression levels have also been reported in leukemia (47), and ACE2 mutations are observed in acute myeloid leukemia (19). In the study by Alshareef (249), ACE2 gene expression levels were significantly higher in patients with chronic myeloid leukemia but did not differ significantly when comparing myelodysplastic syndrome and acute myeloid leukemia with normal samples. In addition, ACE2 expression was shown to be significantly associated with microsatellite instability in diffuse large B cell lymphoma (19). According to the transcriptomic analysis by Facchiano et al (33), ACE2 gene expression is not associated with acute myeloid leukemia or diffuse large B cell lymphoma. Finally, telmisartan, an Ang receptor blocker that functions via peroxisome proliferator-activated receptor and caspase activation, induces apoptosis in adult T cell leukemia cells (250).
Discrepancies in ACE2 prognostic and predictive data
As aforementioned, contradictory data for ACE2 expression and its association with prognosis have emerged, mainly in lung and kidney tumors. Although ACE2 may serve as a prognostic and therapeutic target in human tumors, in the majority of studies, the patient clinicopathological features such as age and sex, medical history of inflammatory conditions, diabetes, metabolic comorbidities, heart failure, hormonal status and previous therapies, which alter ACE2 expression (6,24–27,31–40), were not taken in consideration. Moreover, ACE2 polymorphisms and epigenetic factors affect ACE2 expression (251).
In the respiratory system, smoking, hypercapnia and hyperoxia influence ACE2 levels (117–123,252). In addition, increased immunoexpression levels of ACE2 following SARS-CoV-2 infection are reported in the lung autopsy tissues of patients with COVID-19 (253). With regard to the COVID-19 treatment options, it has been demonstrated that dexamethasone increases ACE2 levels, whereas remdesivir decreases ACE2 levels, in bronchial and alveolar cell cultures (254). Furthermore, ACE2 levels decrease following acute lung inflammation, although it is not known how long this decrease lasts (255). Therefore, the role of ACE2 in lung cancer should be evaluated in the context of the lung pathophysiological conditions and recent infection status of patients.
Similarly, according to a recent study (256), ACE2 is not expressed in the thyroid tissue of patients without COVID-19 infection, although a high proportion of positive ACE2-immunostained cells in the thyroid tissues of deceased patients with COVID-19 is documented, suggesting that the conflicting data regarding ACE2 expression in thyroid cancer should be re-evaluated after the pandemic. In addition, Bronowicka-Szydełko et al (257) demonstrated the interaction between SARS-CoV-2 and thyroid cells which may explain how carcinogenesis is initiated in this gland. SARS-CoV-2 may also affect the viability and migratory properties of colorectal and prostate cancer cell lines (258), thereby providing knowledge of how SARS-CoV-2 infection modulates human tumor biology.
Differences in the expression of ACE2 across kidney tissue in acute kidney injury were described by Shirazi et al (259), whereas ACE2 exerts protective effects against aging and albuminuria in kidney tissue (166,197,260). Therefore, the conflicting results on ACE2 expression in human kidney tumors may reflect differences in the underlying renal pathophysiological mechanisms. In addition, the oncolytic effects of COVID-19 in renal cancer cells should be evaluated according to recent data describing the consequences of SARS-CoV-2 infection in the kidney tissue (261).
Singh Parmar et al (262) reported that patients with breast cancer are more susceptible to COVID-19 compared with their normal counterparts, and ACE2 inhibitors and ibuprofen therapy for COVID-19 treatment may aggravate the clinical condition of patients with breast cancer through chemoresistance and metastasis. On the other hand, the therapeutic properties of ACE2 in intestinal cancer were presented in a study that reported that treatment with bromelain and ficin decreases ACE2 protein levels, thereby decreasing the viability of human colon cancer cells (190). Since numerous drugs modify ACE2 levels (263), data about medication usage should be considered in evaluation of ACE2 expression in various types of tumors.
Finally, the aforementioned studies integrated information from multiple public bioinformatics databases, which do not include associations with clinicopathological characteristics of the patients; moreover, there are variations in the numbers of samples for each type of cancer. Furthermore, there were discrepancies between databases regarding the comparisons made between tumor tissue and control samples; certain databases include matched healthy tissues whereas other databases include adjacent normal tissues. for control samples. Huang et al (86) reported that adjacent normal tissue expresses ACE2 in a different manner compared with healthy tissue; for example, both cancers and adjacent normal tissues were shown to express ACE2 at higher levels compared with healthy tissue in the colon, stomach, kidney and lung.
As conflicting results exist between databases, it is difficult to elucidate the role of ACE2 in the pathogenesis of cell-specific tumors. Further validation of the data in multicohort clinical analyses is warranted to identify the associations between ACE2 and the clinical characteristics of patients with cancer. Furthermore, additional functional studies, including in vivo and in vitro experiments, are required to clarify the role of ACE2 in oncogenesis, especially following the COVID-19 pandemic.
Conclusion
ACE2 expression may be implicated in tumor pathogenesis, and serve as a prognostic marker, despite the conflicting data for various types of cancer. The present study summarizes ACE2 expression in human tumors and the need for further investigations of the involvement of ACE2 in tumorigenesis. Furthermore, ACE2 expression should be evaluated with a multidisciplinary approach as the effects of SARS-CoV-2 on cancer cells may be cell type-dependent. Since the expression of ACE2 is regulated by chemical and mechanical signal transduction pathways (264) it is necessary to elucidate epigenetic profiling of ACE2 expression and its role in clinicopathological features of various types of tumors.
Acknowledgements
The authors would like to thank Dr Kyriakos Birmpas (School of Biomedical Sciences, University of Leeds, Leeds, UK) for assistance in creating figures.
Funding
The publication fees of this manuscript have been financed by the Research Counsil of the University of Patras.
Availability of data and materials
Not applicable.
Authors' contributions
TR and MA wrote and edited the manuscript. MA supervised the study. Data authentication is not applicable. All authors have read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
|
Shang J, Ye G, Shi K, Wan Y, Luo C, Aihara H, Geng Q, Auerbach A and Li F: Structural basis of receptor recognition by SARS-CoV-2. Nature. 581:221–224. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Gupta A, Madhavan MV, Sehgal K, Nair N, Mahajan S, Sehrawat TS, Bikdeli B, Ahluwalia N, Ausiello JC, Wan EY, et al: Extrapulmonary manifestations of COVID-19. Nat Med. 26:1017–1032. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Danilczyk U, Eriksson U, Crackower MA and Penninger JM: A story of two ACEs. J Mol Med (Berl). 81:227–234. 2003. View Article : Google Scholar : PubMed/NCBI | |
|
Santos RA, Campagnole-Santos MJ and Andrade SP: Angiotensin-(1–7): An update. Regul Pept. 91:45–62. 2000. View Article : Google Scholar : PubMed/NCBI | |
|
Ferrario CM, Trask AJ and Jessup JA: Advances in biochemical and functional roles of angiotensin-converting enzyme 2 and angiotensin-(1–7) in regulation of cardiovascular function. Am J Physiol Heart Circ Physiol. 289:H2281–2290. 2005. View Article : Google Scholar : PubMed/NCBI | |
|
Yang Z, Macdonald-Dunlop E, Chen J, Zhai R, Li T, Richmond A, Klarić L, Pirastu N, Ning Z, Zheng C, et al: Genetic landscape of the ACE2 coronavirus receptor. Circulation. 145:1398–1411. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Hamming I, Cooper ME, Haagmans BL, Hooper NM, Korstanje R, Osterhaus AD, Timens W, Turner AJ, Navis G and van Goor H: The emerging role of ACE2 in physiology and disease. J Pathol. 12:1–11. 2007. View Article : Google Scholar | |
|
Crackower MA, Sarao R, Oudit GY, Yagil C, Kozieradzki I, Scanga SE, Oliveira-dos-Santos AJ, da Costa J, Zhang L, Pei Y, et al: Angiotensin-converting enzyme 2 is an essential regulator of heart function. Nature. 417:822–828. 2002. View Article : Google Scholar : PubMed/NCBI | |
|
Donoghue M, Wakimoto H, Maguire CT, Acton S, Hales P, Stagliano N, Fairchild-Huntress V, Xu J, Lorenz JN, Kadambi V, et al: Heart block, ventricular tachycardia, and sudden death in ACE2 transgenic mice with downregulated connexins. J Mol Cell Cardiol. 35:1043–1053. 2003. View Article : Google Scholar : PubMed/NCBI | |
|
Chen QL, Li JQ, Xiang ZD, Lang Y, Guo GJ and Liu ZH: Localization of cell receptor-related genes of SARS-CoV-2 in the kidney through single-cell transcriptome analysis. Kidney Dis (Basel). 6:258–270. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Lely AT, Hamming I, van Goor H and Navis GJ: Renal ACE2 expression in human kidney disease. J Pathol. 204:587–593. 2004. View Article : Google Scholar : PubMed/NCBI | |
|
Imai Y, Kuba K, Rao S, Huan Y, Guo F, Guan B, Yang P, Sarao R, Wada T, Leong-Poi H, et al: Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature. 436:112–116. 2005. View Article : Google Scholar : PubMed/NCBI | |
|
Fu J, Zhou B, Zhang L, Balaji KS, Wei C, Liu X, Chen H, Peng J and Fu J: Expressions and significances of the angiotensin-converting enzyme 2 gene, the receptor of SARS-CoV-2 for COVID-19. Mol Biol Rep. 47:4383–4392. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Meiners J, Jansen K, Gorbokon N, Büscheck F, Luebke AM, Kluth M, Hube-Magg C, Höflmayer D, Weidemann S, Fraune C, et al: Angiotensin-converting enzyme 2 protein is overexpressed in a wide range of human tumour types: A systematic tissue microarray study on >15,000 tumours. Biomedicines. 9:18312021. View Article : Google Scholar : PubMed/NCBI | |
|
Mahalingam R, Dharmalingam P, Santhanam A, Kotla S, Davuluri G, Karmouty-Quintana H, Ashrith G and Thandavarayan RA: Single-cell RNA sequencing analysis of SARS-CoV-2 entry receptors in human organoids. J Cell Physiol. 236:2950–2958. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Hikmet F, Méar L, Edvinsson Å, Micke P, Uhlén M and Lindskog C: The protein expression profile of ACE2 in human tissues. Mol Syst Biol. 16:e96102020. View Article : Google Scholar : PubMed/NCBI | |
|
Han T, Kang J, Li G, Ge J and Gu J: Analysis of 2019-nCoV receptor ACE2 expression in different tissues and its significance study. Ann Transl Med. 8:10772020. View Article : Google Scholar : PubMed/NCBI | |
|
Cui Y, Chen F, Gao J, Lei M, Wang D, Jin X, Guo Y, Shan L and Chen X: Comprehensive landscape of the renin-angiotensin system in Pan-cancer: A potential downstream mediated mechanism of SARS-CoV-2. Int J Biol Sci. 7:3795–3817. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Song J, Han J, Liu F, Chen X, Qian S, Wang Y, Jia Z, Duan X, Zhang X and Zhu J: Systematic analysis of coronavirus disease 2019 (COVID-19) receptor ACE2 in malignant tumors: Pan-cancer analysis. Front Mol Biosci. 7:5694142020. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang L, Han X and Shi Y: Comparative analysis of SARS-CoV-2 receptor ACE2 expression in multiple solid tumors and matched non-diseased tissues. Infect Genet Evol. 85:1044282020. View Article : Google Scholar : PubMed/NCBI | |
|
Li MY, Li L, Zhang Y and Wang XS: Expression of the SARS-CoV-2 cell receptor gene ACE2 in a wide variety of human tissues. Infect Dis Poverty. 9:452020. View Article : Google Scholar : PubMed/NCBI | |
|
Qi J, Zhou Y, Hua J, Zhang L, Bian J, Liu B, Zhao Z and Jin S: The scRNA-seq expression profiling of the receptor ACE2 and the cellular protease TMPRSS2 reveals human organs susceptible to SARS-CoV-2 infection. Int J Environ Res Public Health. 18:2842021. View Article : Google Scholar : PubMed/NCBI | |
|
Qi F, Qian S, Zhang S and Zhang Z: Single cell RNA sequencing of 13 human tissues identify cell types and receptors of human coronaviruses. Biochem Biophys Res Commun. 526:135–140. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Li S, Han J, Zhang A, Han Y, Chen M, Liu Z, Shao M and Cao W: Exploring the demographics and clinical characteristics related to the expression of angiotensin-converting enzyme 2, a receptor of SARS-CoV-2. Front Med (Lausanne). 7:5302020. View Article : Google Scholar : PubMed/NCBI | |
|
Viveiros A, Gheblawi M, Aujla PK, Sosnowski DK, Seubert JM, Kassiri Z and Oudit GY: Sex- and age-specific regulation of ACE2: Insights into severe COVID-19 susceptibility. J Mol Cell Cardiol. 164:13–16. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Schurink B, Roos E, Vos W, Breur M, van der Valk P and Bugiani M: ACE2 protein expression during childhood, adolescence, and early adulthood. Pediatr Dev Pathol. 25:404–408. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Ravaioli S, Tebaldi M, Fonzi E, Angeli D, Mazza M, Nicolini F, Lucchesi A, Fanini F, Pirini F, Tumedei MM, et al: ACE2 and TMPRSS2 potential involvement in genetic susceptibility to SARS-COV-2 in cancer patients. Cell Transplant. 29:09636897209687492020. View Article : Google Scholar : PubMed/NCBI | |
|
Chakladar J, Shende N, Li WT, Rajasekaran M, Chang EY and Ongkeko WM: Smoking-mediated upregulation of the androgen pathway leads to increased SARS-CoV-2 susceptibility. Int J Mol Sci. 21:36272020. View Article : Google Scholar : PubMed/NCBI | |
|
Goren A, Wambier CG, Herrera S, McCoy J, Vaño-Galván S, Gioia F, Comeche B, Ron R, Serrano-Villar S, Ramos PM, et al: Anti-androgens may protect against severe COVID-19 outcomes: Results from a prospective cohort study of 77 hospitalized men. J Eur Acad Dermatol Venereol. 35:e13–e15. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Bhardwaj V, Dela Cruz M, Subramanyam D, Kumar R, Markan S, Parker B and Roy HK: Exercise-induced myokines downregulates the ACE2 level in bronchial epithelial cells: Implications for SARS-CoV-2 prevention. PLoS One. 17:e02713032022. View Article : Google Scholar : PubMed/NCBI | |
|
Xie J, Huang QF, Zhang Z, Dong Y, Xu H, Cao Y, Sheng CS, Li Y, Wang C, Wang X and Wang JG: Angiotensin-converting enzyme 2 in human plasma and lung tissue. Blood Press. 32:6–15. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Rao S, Lau A and So HC: Exploring diseases/traits and blood proteins causally related to expression of ACE2, the putative receptor of SARS-CoV-2: A mendelian randomization analysis highlights tentative relevance of diabetes-related traits. Diabetes Care. 43:1416–1426. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Facchiano A, Facchiano F and Facchiano A: An investigation into the molecular basis of cancer comorbidities in coronavirus infection. FEBS Open Bio. 10:2363–2374. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Gheblawi M, Wang K, Viveiros A, Nguyen Q, Zhong JC, Turner AJ, Raizada MK, Grant MB and Oudit GY: Angiotensin-converting enzyme 2: SARS-CoV-2 receptor and regulator of the renin-angiotensin system: Celebrating the 20th anniversary of the discovery of ACE2. Circ Res. 126:1456–1474. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Leung JM, Yang CX, Tam A, Shaipanich T, Hackett TL, Singhera GK, Dorscheid DR and Sin DD: ACE-2 expression in the small airway epithelia of smokers and COPD patients: Implications for COVID-19. Eur Respir J. 55:20006882020. View Article : Google Scholar : PubMed/NCBI | |
|
Kornilov SA, Lucas I, Jade K, Dai CL, Lovejoy JC and Magis AT: Plasma levels of soluble ACE2are associated with sex, Metabolic Syndrome, and its biomarkers in a large cohort, pointing to a possible mechanism for increased severity in COVID-19. Crit Care. 24:4522020. View Article : Google Scholar : PubMed/NCBI | |
|
Chirinos JA, Cohen JB, Zhao L, Hanff T, Sweitzer N, Fang J, Corrales-Medina V, Anmar R, Morley M, Zamani P, et al: Clinical and proteomic correlates of plasma ACE2 (Angiotensin-Converting Enzyme 2) in human heart failure. Hypertension. 76:1526–1536. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Radzikowska U, Ding M, Tan G, Zhakparov D, Peng Y, Wawrzyniak P, Wang M, Li S, Morita H, Altunbulakli C, et al: Distribution of ACE2, CD147, CD26, and other SARS-CoV-2 associated molecules in tissues and immune cells in health and in asthma, COPD, obesity, hypertension, and COVID-19 risk factors. Allergy. 75:2829–2845. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Dhawale VS, Amara VR, Karpe PA, Malek V, Patel D and Tikoo K: Activation of angiotensin-converting enzyme 2 (ACE2) attenuates allergic airway inflammation in rat asthma model. Toxicol Appl Pharmacol. 306:17–26. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Ye R and Liu Z: ACE2 exhibits protective effects against LPS-induced acute lung injury in mice by inhibiting the LPS-TLR4 pathway. Exp Mol Pathol. 113:1043502020. View Article : Google Scholar : PubMed/NCBI | |
|
Clarke NE, Belyaev ND, Lambert DW and Turner AJ: Epigenetic regulation of angiotensin-converting enzyme 2 (ACE2) by SIRT1 under conditions of cell energy stress. Clin Sci (Lond). 126:507–516. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Li Y, Xu Q, Ma L, Wu D, Gao J, Chen G and Li H: Systematic profiling of ACE2 expression in diverse physiological and pathological conditions for COVID-19/SARS-CoV-2. J Cell Mol Med. 24:9478–9482. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Feng H, Wei X, Pang L, Wu Y, Hu B, Ruan Y, Liu Z, Liu J and Wang T: Prognostic and immunological value of angiotensin-converting enzyme 2 in pan-cancer. Front Mol Biosci. 7:1892020. View Article : Google Scholar : PubMed/NCBI | |
|
Chai P, Yu J, Ge S, Jia R and Fan X: Genetic alteration, RNA expression, and DNA methylation profiling of coronavirus disease 2019 (COVID-19) receptor ACE2 in malignancies: A pan-cancer analysis. J Hematol Oncol. 13:432020. View Article : Google Scholar : PubMed/NCBI | |
|
Li H, Xie L, Chen L, Zhang L, Han Y, Yan Z and Guo X: Genomic, epigenomic, and immune subtype analysis of CTSL/B and SARS-CoV-2 receptor ACE2 in pan-cancer. Aging. 12:22370–22389. 2020.PubMed/NCBI | |
|
Zhao K, Zhang D, Xu X, Wang S, Liu Z, Ren X, Zhang X, Lu Z, Ren S and Qin C: Exploring the potential mechanisms of impairment on genitourinary system associated with coronavirus disease 2019 infection: Bioinformatics and molecular simulation analyses. Asian J Urol. 10:344–355. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
He J, Yang X and Wang H: Construction of a risk map to understand the vulnerability of various types of cancer patients to COVID-19 infection. MedComm (2020). 2:69–81. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
de Paula Gonzaga ALAC, Palmeira VA, Ribeiro TFS, Costa LB, de Sá, Rodrigues KE and Simões-E-Silva AC: ACE2/Angiotensin-(1–7)/Mas receptor axis in human cancer: Potential role for pediatric tumors. Curr Drug Targets. 21:892–901. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Xu J, Fan J, Wu F, Huang Q, Guo M, Lv Z, Han J, Duan L, Hu G, Chen L, et al: The ACE2/Angiotensin-(1–7)/Mas receptor axis: Pleiotropic roles in cancer. Front Physiol. 8:2762017. View Article : Google Scholar : PubMed/NCBI | |
|
Bujak-Gizycka B, Madej J, Bystrowska B, Toton-Zuranska J, Kus K, Kolton-Wroz M, Jawien J and Olszanecki R: Angiotensin 1–7 formation in breast tissue is attenuated in breast cancer-a study on the metabolism of angiotensinogen in breast cancer cell lines. J Physiol Pharmacol. 702019.doi: 10.26402/jpp.2019.4.02. | |
|
Bernardi S, Zennaro C, Palmisano S, Velkoska E, Sabato N, Toffoli B, Giacomel G, Buri L, Zanconati F, Bellini G, et al: Characterization and significance of ACE2 and Mas receptor in human colon adenocarcinoma. J Renin Angiotensin Aldosterone Syst. 13:202–209. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang Z, Li L, Li M and Wang X: The SARS-CoV-2 host cell receptor ACE2 correlates positively with immunotherapy response and is a potential protective factor for cancer progression. Comput Struct Biotechnol J. 18:2438–2444. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Song T, Choi CH, Kim MK, Kim ML, Yun BS and Seong SJ: The effect of angiotensin system inhibitors (angiotensin-converting enzyme inhibitors or angiotensin receptor blockers) on cancer recurrence and survival: A meta-analysis. Eur J Cancer Prev. 26:78–85. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Harmer D, Gilbert M, Borman R and Clark KL: Quantitative mRNA expression profiling of ACE 2, a novel homologue of angiotensin converting enzyme. FEBS Lett. 532:107–110. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Chen R, Wang K, Yu J, Howard D, French L, Chen Z, Wen C and Xu Z: The spatial and cell-type distribution of SARS-CoV-2 receptor ACE2 in the human and mouse brains. Front Neurol. 11:5730952021. View Article : Google Scholar : PubMed/NCBI | |
|
Xia H and Lazartigues E: Angiotensin-converting enzyme 2 in the brain: Properties and future directions. J Neurochem. 107:1482–1494. 2008. View Article : Google Scholar : PubMed/NCBI | |
|
Kase Y and Okano H: Expression of ACE2 and a viral virulence-regulating factor CCN family member 1 in human iPSC-derived neural cells: Implications for COVID-19-related CNS disorders. Inflamm Regen. 40:322020. View Article : Google Scholar : PubMed/NCBI | |
|
Lukiw WJ, Pogue A and Hill JM: SARS-CoV-2 infectivity and neurological targets in the brain. Cell Mol Neurobiol. 42:217–224. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Doobay MF, Talman LS, Obr TD, Tian X, Davisson RL and Lazartigues E: Differential expression of neuronal ACE2 in transgenic mice with overexpression of the brain renin-angiotensin system. Am J Physiol Regul Integr Comp Physiol. 292:R373–R381. 2007. View Article : Google Scholar : PubMed/NCBI | |
|
Chen A, Zhao W, Li X, Sun G, Ma Z, Peng L, Shi Z, Li X and Yan J: Comprehensive oncogenic features of coronavirus receptors in glioblastoma multiforme. Front Immunol. 13:8407852022. View Article : Google Scholar : PubMed/NCBI | |
|
Ren X, Wang S, Chen X, Wei X, Li G, Ren S, Zhang T, Zhang X, Lu Z, You Z, et al: Multiple expression assessments of ACE2 and TMPRSS2 SARS-CoV-2 entry molecules in the urinary tract and their associations with clinical manifestations of COVID-19. Infect Drug Resist. 13:3977–3990. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Hamming I, Timens W, Bulthuis ML, Lely AT, Navis G and van Goor H: Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 203:631–637. 2004. View Article : Google Scholar : PubMed/NCBI | |
|
Muhl L, He L, Sun Y, Andaloussi Mäe M, Pietilä R, Liu J, Genové G, Zhang L, Xie Y, Leptidis S, et al: The SARS-CoV-2 receptor ACE2 is expressed in mouse pericytes but not endothelial cells: Implications for COVID-19 vascular research. Stem Cell Reports. 17:1089–1104. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Chen J, Zhao Y, Chen S, Wang J, Xiao X, Ma X, Penchikala M, Xia H, Lazartigues E, Zhao B and Chen Y: Neuronal over-expression of ACE2 protects brain from ischemia-induced damage. Neuropharmacology. 79:550–558. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Qiao J, Li W, Bao J, Peng Q, Wen D, Wang J and Sun B: The expression of SARS-CoV-2 receptor ACE2 and CD147, and protease TMPRSS2 in human and mouse brain cells and mouse brain tissues. Biochem Biophys Res Commun. 533:867–871. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Bielarz V, Willemart K, Avalosse N, De Swert K, Lotfi R, Lejeune N, Poulain F, Ninanne N, Gilloteaux J, Gillet N and Nicaise C: Susceptibility of neuroblastoma and glioblastoma cell lines to SARS-CoV-2 infection. Brain Res. 1758:1473442021. View Article : Google Scholar : PubMed/NCBI | |
|
Vanhulle E, Stroobants J, Provinciael B, Camps A, Noppen S, Maes P and Vermeire K: SARS-CoV-2 Permissive glioblastoma cell line for high throughput antiviral screening. Antiviral Res. 203:1053422022. View Article : Google Scholar : PubMed/NCBI | |
|
Bergsneider B, Bailey E, Ahmed Y, Gogineni N, Huntley D and Montano X: Analysis of SARS-CoV-2 infection associated cell entry proteins ACE2, CD147, PPIA, and PPIB in datasets from non SARS-CoV-2 infected neuroblastoma patients, as potential prognostic and infection biomarkers in neuroblastoma. Biochem Biophys Rep. 27:1010812021.PubMed/NCBI | |
|
Kim K, Ko Y, Ko DS and Kim YH: Prognostic significance of COVID-19 receptor ACE2 and recommendation for antihypertensive drug in renal cell carcinoma. Biomed Res Int. 2020:20543762020. View Article : Google Scholar : PubMed/NCBI | |
|
Wu B, Wang W, Wang H, Zou Q, Hu B, Ye L, Hu Y, Xie Y, Huang N, Lan Q, et al: Single-cell sequencing of glioblastoma reveals central nervous system susceptibility to SARS-CoV-2. Front Oncol. 10:5665992020. View Article : Google Scholar : PubMed/NCBI | |
|
Lei J, Liu Y, Xie T, Yao G, Wang G, Diao B and Song J: Evidence for residual SARS-CoV-2 in glioblastoma tissue of a convalescent patient. Neuroreport. 32:771–775. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Suarez-Meade P, Watanabe F, Ruiz-Garcia H, Rafferty SB, Moniz-Garcia D, Schiapparelli PV, Jentoft ME, Imitola J and Quinones-Hinojosa A: SARS-CoV2 entry factors are expressed in primary human glioblastoma and recapitulated in cerebral organoid models. Neurooncol. 161:67–76. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Parolin M, Parisotto M, Zanchetta F, Sartorato P and De Menis E: Coronaviruses and endocrine system: A systematic review on evidences and shadows. Endocr Metab Immune Disord Drug Targets. 21:1242–1251. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Gu WT, Zhou F, Xie WQ, Wang S, Yao H, Liu YT, Gao L and Wu ZB: A potential impact of SARS-CoV-2 on pituitary glands and pituitary neuroendocrine tumors. Endocrine. 72:340–348. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Narayan SS, Lorenz K, Ukkat J, Hoang-Vu C and Trojanowicz B: Angiotensin converting enzymes ACE and ACE2 in thyroid cancer progression. Neoplasma. 67:402–409. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
An X, Lin W, Liu H, Zhong W, Zhang X, Zhu Y, Wang X, Li J and Sheng Q: SARS-CoV-2 host receptor ACE2 protein expression atlas in human gastrointestinal tract. Front Cell Dev Biol. 9:6598092021. View Article : Google Scholar : PubMed/NCBI | |
|
Wu CT, Lidsky PV, Xiao Y, Lee IT, Cheng R, Nakayama T, Jiang S, Demeter J, Bevacqua RJ, Chang CA, et al: SARS-CoV-2 infects human pancreatic β cells and elicits β cell impairment. Cell Metab. 33:1565–1576.e5. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Müller JA, Groß R, Conzelmann C, Krüger J, Merle U, Steinhart J, Weil T, Koepke L, Bozzo CP, Read C, et al: SARS-CoV-2 infects and replicates in cells of the human endocrine and exocrine pancreas. Nat Metab. 3:149–165. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Fignani D, Licata G, Brusco N, Nigi L, Grieco GE, Marselli L, Overbergh L, Gysemans C, Colli ML, Marchetti P, et al: SARS-CoV-2 receptor angiotensin I-converting enzyme type 2 (ACE2) is expressed in human pancreatic β-cells and in the human pancreas microvasculature. Front Endocrinol (Lausanne). 11:5968982020. View Article : Google Scholar : PubMed/NCBI | |
|
Kusmartseva I, Wu W, Syed F, Van Der Heide V, Jorgensen M, Joseph P, Tang X, Candelario-Jalil E, Yang C, Nick H, et al: Expression of SARS-CoV-2 entry factors in the pancreas of normal organ donors and individuals with COVID-19. Cell Metab. 32:1041–1051.e6. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Coate KC, Cha J, Shrestha S, Wang W, Gonçalves LM, Almaça J, Kapp ME, Fasolino M, Morgan A, Dai C, et al: ARS-CoV-2 cell entry factors ACE2 and TMPRSS2 are expressed in the microvasculature and ducts of human pancreas but are not enriched in β cells. Cell Metab. 32:1028–1040.e4. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Steenblock C, Richter S, Berger I, Barovic M, Schmid J, Schubert U, Jarzebska N, von Mässenhausen A, Linkermann A, Schürmann A, et al: Viral infiltration of pancreatic islets in patients with COVID-19. Nat Commun. 12:35342021. View Article : Google Scholar : PubMed/NCBI | |
|
Qadir MMF, Bhondeley M, Beatty W, Gaupp DD, Doyle-Meyers LA, Fischer T, Bandyopadhyay I, Blair RV, Bohm R, Rappaport J, et al: SARS-CoV-2 infection of the pancreas promotes thrombofibrosis and is associated with new-onset diabetes. JCI Insight. 6:e1515512021. View Article : Google Scholar : PubMed/NCBI | |
|
Pedersen KB, Chhabra KH, Nguyen VK, Xia H and Lazartigues E: The transcription factor HNF1α induces expression of angiotensin-converting enzyme 2 (ACE2) in pancreatic islets from evolutionarily conserved promoter motifs. Biochim Biophys Acta. 1829:1225–1235. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Zhou L, Zhang R, Yao W, Wang J, Qian A, Qiao M, Zhang Y and Yuan Y: Decreased expression of angiotensin-converting enzyme 2 in pancreatic ductal adenocarcinoma is associated with tumor progression. Tohoku J Exp Med. 217:123–131. 2009. View Article : Google Scholar : PubMed/NCBI | |
|
Huang X, He C, Hua X, Kan A, Sun S, Wang J and Li S: Bioinformatic analysis of correlation between immune infiltration and COVID-19 in cancer patients. Int J Biol Sci. 16:2464–2476. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Zhou L, Zhang R, Zhang L, Yao W, Li J and Yuan Y: Angiotensin-converting enzyme 2 acts as a potential molecular target for pancreatic cancer therapy. Cancer Lett. 307:18–25. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
Lau ST and Leung PS: Role of the RAS in pancreatic cancer. Curr Cancer Drug Targets. 11:412–420. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
Donoghue M, Hsieh F, Baronas E, Godbout K, Gosselin M, Stagliano N, Donovan M, Woolf B, Robison K, Jeyaseelan R, et al: A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1–9. Circ Res. 87:E1–E9. 2000. View Article : Google Scholar : PubMed/NCBI | |
|
Burrell LM, Risvanis J, Kubota E, Dean RG, MacDonald PS, Lu S, Tikellis C, Grant SL, Lew RA, Smith AI, et al: Myocardial infarction increases ACE2 expression in rat and humans. Eur Heart J. 26:369–375. 2005. View Article : Google Scholar : PubMed/NCBI | |
|
Patel VB, Zhong JC, Grant MB and Oudit GY: Role of the ACE2/Angiotensin 1–7 axis of the renin-angiotensin system in heart failure. Circ Res. 118:1313–1326. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Basu R, Poglitsch M, Yogasundaram H, Thomas J, Rowe BH and Oudit GY: Roles of angiotensin peptides and recombinant human ACE2 in heart failure. J Am Coll Cardiol. 69:805–819. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Shen M, Hu M, Fedak PWM, Oudit GY and Kassiri Z: Cell-specific functions of ADAM17 regulate the progression of thoracic aortic aneurysm. Circ Res. 123:372–388. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Epelman S, Tang WH, Chen SY, Van Lente F, Francis GS and Sen S: Detection of soluble angiotensin-converting enzyme 2 in heart failure: Insights into the endogenous counter-regulatory pathway of the renin-angiotensin-aldosterone system. J Am Coll Cardiol. 52:750–754. 2008. View Article : Google Scholar : PubMed/NCBI | |
|
Madjid M, Safavi-Naeini P, Solomon SD and Vardeny O: Potential effects of coronaviruses on the cardiovascular system: A review. JAMA Cardiol. 5:831–840. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Chung MK, Zidar DA, Bristow MR, Cameron SJ, Chan T, Harding CV III, Kwon DH, Singh T, Tilton JC, Tsai EJ, et al: COVID-19 and cardiovascular disease: From bench to bedside. Circ Res. 128:1214–1236. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Brumback BD, Dmytrenko O, Robinson AN, Bailey AL, Ma P, Liu J, Hicks SC, Ng S, Li G, Zhang DM, et al: Human cardiac pericytes are susceptible to SARS-CoV-2 infection. JACC Basic Transl Sci. 8:109–120. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Zou X, Chen K, Zou J, Han P, Hao J and Han Z: Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front Med. 14:185–192. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Zhou Y, Huang Y, Song X, Guo X, Pang J, Wang J, Zhang S and Wang C: Single-cell transcriptional profile of ACE2 in healthy and failing human hearts. Sci China Life Sci. 64:652–655. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Klingenstein M, Klingenstein S, Neckel PH, Mack AF, Wagner AP, Kleger A, Liebau S and Milazzo A: Evidence of SARS-CoV2 entry protein ACE2 in the human nose and olfactory bulb. Cells Tissues Organs. 209:155–164. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Deprez M, Zaragosi LE, Truchi M, Becavin C, Ruiz García S, Arguel MJ, Plaisant M, Magnone V, Lebrigand K, Abelanet S, et al: A Single-cell atlas of the human healthy airways. Am J Respir Crit Care Med. 202:1636–1645. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Sungnak W, Huang N, Bécavin C, Berg M, Queen R, Litvinukova M, Talavera-López C, Maatz H, Reichart D, Sampaziotis F, et al: HCA lung biological network. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat Med. 26:681–687. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Lee IT, Nakayama T, Wu CT, Goltsev Y, Jiang S, Gall PA, Liao CK, Shih LC, Schürch CM, McIlwain DR, et al: ACE2 localizes to the respiratory cilia and is not increased by ACE inhibitors or ARBs. Nat Commun. 11:54532020. View Article : Google Scholar : PubMed/NCBI | |
|
Bilinska K, Jakubowska P, Von Bartheld CS and Butowt R: Expression of the SARS-CoV-2 entry proteins, ACE2 and TMPRSS2, in cells of the olfactory epithelium: Identification of cell types and trends with age. ACS Chem Neurosci. 11:1555–1562. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Xu H, Zhong L, Deng J, Peng J, Dan H, Zeng X, Li T and Chen Q: High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int J Oral Sci. 12:82020. View Article : Google Scholar : PubMed/NCBI | |
|
Sapkota D, Sharma S, Søland TM, Braz-Silva PH and Teh MT: Expression profile of SARS-CoV-2 cellular entry proteins in normal oral mucosa and oral squamous cell carcinoma. Clin Exp Dent Res. 8:117–122. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Sivasakthivel S, Ramani P and Poothakulath Krishnan R: Systematic review and Meta-analysis on angiotensin converting enzyme 2 in head and neck region. Cureus. 15:e336732023.PubMed/NCBI | |
|
de Carvalho Fraga CA, Farias LC, Jones KM, Batista de Paula AM and Guimaraes ALS: Angiotensin-converting enzymes (ACE and ACE2) as potential targets for malignant epithelial neoplasia: Review and bioinformatics analyses focused in oral squamous cell carcinoma. Protein Pept Lett. 24:784–792. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Hinsley EE, de Oliveira CE, Hunt S, Coletta RD and Lambert DW: Angiotensin 1–7 inhibits angiotensin II-stimulated head and neck cancer progression. Eur J Oral Sci. 125:247–257. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Lin YT, Wang HC, Chuang HC, Hsu YC, Yang MY and Chien CY: Pre-treatment with angiotensin-(1–7) inhibits tumor growth via autophagy by downregulating PI3K/Akt/mTOR signaling in human nasopharyngeal carcinoma xenografts. J Mol Med (Berl). 96:1407–1418. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Pei N, Wan R, Chen X, Li A, Zhang Y, Li J, Du H, Chen B, Wei W, Qi Y, et al: Angiotensin-(1–7) decreases cell growth and angiogenesis of human nasopharyngeal carcinoma xenografts. Mol Cancer Ther. 15:37–47. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Fountzilas E, Kotoula V, Angouridakis N, Karasmanis I, Wirtz RM, Eleftheraki AG, Veltrup E, Markou K, Nikolaou A, Pectasides D and Fountzilas G: Identification and validation of a multigene predictor of recurrence in primary laryngeal cancer. PLoS One. 8:e704292013. View Article : Google Scholar : PubMed/NCBI | |
|
Zhao Y, Zhao Z, Wang Y, Zhou Y, Ma Y and Zuo W: Single-cell RNA expression profiling of ACE2, the receptor of SARS-CoV-2. Am J Respir Crit Care Med. 202:756–759. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Ciechanowicz AK, Lay WX, Prado Paulino J, Suchocki E, Leszczak S, Leszczak C and Kucia M: Angiotensin 1–7 stimulates proliferation of lung bronchoalveolar progenitors-implications for SARS-CoV-2 infection. Cells. 11:21022022. View Article : Google Scholar : PubMed/NCBI | |
|
Salahudeen AA, Choi SS, Rustagi A, Zhu J, van Unen V, de la O SM, Flynn RA, Margalef-Català M, Santos AJM, Ju J, et al: Progenitor identification and SARS-CoV-2 infection in human distal lung organoids. Nature. 588:670–675. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Sidarta-Oliveira D, Jara CP, Ferruzzi AJ, Skaf MS, Velander WH, Araujo EP and Velloso LA: SARS-CoV-2 receptor is co-expressed with elements of the kinin-kallikrein, renin-angiotensin and coagulation systems in alveolar cells. Sci Rep. 10:195222020. View Article : Google Scholar : PubMed/NCBI | |
|
Qiao Y, Wang XM, Mannan R, Pitchiaya S, Zhang Y, Wotring JW, Xiao L, Robinson DR, Wu YM, Tien JC, et al: Targeting transcriptional regulation of SARS-CoV-2 entry factors ACE2 and TMPRSS2. Proc Natl Acad Sci USA. 118:e20214501182020. View Article : Google Scholar : PubMed/NCBI | |
|
Liu A, Zhang X, Li R, Zheng M, Yang S, Dai L, Wu A, Hu C, Huang Y, Xie M and Chen Q: Overexpression of the SARS-CoV-2 receptor ACE2 is induced by cigarette smoke in bronchial and alveolar epithelia. J Pathol. 253:17–30. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Cai G: Bulk and single-cell transcriptomics identify tobacco-use disparity in lung gene expression of ACE2. medRxiv. Feb 28–2020.doi: 10.1101/2020.02.05.20020107. | |
|
Cai G, Bosse Y, Xiao F, Kheradmand F and Amos CI: Tobacco smoking increases the lung gene expression of ACE2, the receptor of SARS-CoV-2. Am J Respir Crit Care Med. 201:1557–1559. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Lukassen S, Chua RL, Trefzer T, Kahn NC, Schneider MA, Muley T, Winter H, Meister M, Veith C, Boots AW, et al: SARS-CoV-2 receptor ACE2 and TMPRSS2 are primarily expressed in bronchial transient secretory cells. EMBO J. 39:e1051142020. View Article : Google Scholar : PubMed/NCBI | |
|
Voinsky I and Gurwitz D: Smoking and COVID-19: Similar bronchial ACE2 and TMPRSS2 expression and higher TMPRSS4 expression in current versus never smokers. Drug Dev Res. 81:1073–1080. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Chen F, Matsuda A, Budinger GRS, Sporn PHS and Casalino-Matsuda SM: Hypercapnia increases ACE2 expression and pseudo–SARS–CoV–2 entry in bronchial epithelial cells by augmenting cellular cholesterol. Front Immunol. 14:12511202023. View Article : Google Scholar : PubMed/NCBI | |
|
Ilikci Sagkan R and Akin-Bali DF: Structural variations and expression profiles of the SARS-CoV-2 host invasion genes in lung cancer. J Med Virol. 92:2637–2647. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang H, Quek K, Chen R, Chen J and Chen B: Expression of the SAR2-Cov-2 receptor ACE2 reveals the susceptibility of COVID-19 in non-small cell lung cancer. J Cancer. 11:5289–5292. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Subbarayan K, Ulagappan K, Wickenhauser C and Seliger B: xpression and Clinical Significance of SARS-CoV-2 human targets in neoplastic and non-neoplastic lung tissues. Curr Cancer Drug Targets. 21:428–442. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Wang Q, Li L, Qu T, Li J, Wu L, Li K, Wang Z, Zhu M, Huang B, Wu W, et al: High expression of ACE2 and TMPRSS2 at the resection margin makes lung cancer survivors susceptible to SARS-CoV-2 with unfavorable prognosis. Front Oncol. 11:6445752021. View Article : Google Scholar : PubMed/NCBI | |
|
Liu Z, Gu X, Li Z, Shan S, Wu F and Ren T: Heterogeneous expression of ACE2, TMPRSS2, and FURIN at single-cell resolution in advanced non-small cell lung cancer. J Cancer Res Clin Oncol. 149:3563–3573. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Hoang T, Nguyen TQ and Tran TTA: Genetic susceptibility of ACE2 and TMPRSS2 in six common cancers and possible impacts on COVID-19. Cancer Res Treat. 53:650–656. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Kong Q, Xiang Z, Wu Y, Gu Y, Guo J and Geng F: Analysis of the susceptibility of lung cancer patients to SARS-CoV-2 infection. Mol Cancer. 19:802020. View Article : Google Scholar : PubMed/NCBI | |
|
He C, Hua X, Sun S, Li S, Wang J and Huang X: Integrated bioinformatic analysis of SARS-CoV-2 infection related genes ACE2, BSG and TMPRSS2 in aerodigestive cancers. J Inflamm Res. 14:791–802. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Hayashi T, Sano K and Konishi I: Possibility of SARS-CoV-2 infection in the metastatic microenvironment of cancer. Curr Issues Mol Biol. 44:233–241. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Samad A, Jafar T and Rafi JH: Identification of angiotensin-converting enzyme 2 (ACE2) protein as the potential biomarker in SARS-CoV-2 infection-related lung cancer using computational analyses. Genomics. 112:4912–4923. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Lazar V, Raynaud J, Magidi S, Bresson C, Martini JF, Galbraith S, Wunder F, Onn A, Batist G, Girard N, et al: Comorbidity between lung cancer and COVID-19 pneumonia: Role of immunoregulatory gene transcripts in high ACE2-expressing normal lung. Ther Adv Med Oncol. 14:175883592211338932022. View Article : Google Scholar : PubMed/NCBI | |
|
Dai YJ, Hu F, Li H, Huang HY, Wang DW and Liang Y: A profiling analysis on the receptor ACE2 expression reveals the potential risk of different type of cancers vulnerable to SARS-CoV-2 infection. Ann Transl Med. 8:4812020. View Article : Google Scholar : PubMed/NCBI | |
|
Deben C, Le Compte M, Siozopoulou V, Lambrechts H, Hermans C, Lau HW, Huizing M, Lamote K, Hendriks JMH, Van Dam P, et al: Expression of SARS-CoV-2-Related surface proteins in non-small-cell lung cancer patients and the influence of standard of care therapy. Cancers (Basel). 14:40742022. View Article : Google Scholar : PubMed/NCBI | |
|
Yamaguchi M, Hirai S, Sumi T, Tanaka Y, Tada M, Nishii Y, Hasegawa T, Uchida H, Yamada G, Watanabe A, et al: Angiotensin-converting enzyme 2 is a potential therapeutic target for EGFR-mutant lung adenocarcinoma. Biochem Biophys Res Commun. 487:613–618. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Feng Y, Wan H, Liu J, Zhang R, Ma Q, Han B, Xiang Y, Che J, Cao H, Fei X and Qiu W: The angiotensin-converting enzyme 2 in tumor growth and tumor-associated angiogenesis in non-small cell lung cancer. Oncol Rep. 23:941–948. 2010.PubMed/NCBI | |
|
Teng JP, Yang ZY, Zhu YM, Ni D, Zhu ZJ and Li XQ: Gemcitabine and cisplatin for treatment of lung cancer in vitro and vivo. Eur Rev Med Pharmacol Sci. 22:3819–3825. 2018.PubMed/NCBI | |
|
Cheng Q, Zhou L, Zhou J, Wan H, Li Q and Feng Y: ACE2 overexpression inhibits acquired platinum resistance-induced tumor angiogenesis in NSCLC. Oncol Rep. 36:1403–1410. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Feng Y, Ni L, Wan H, Fan L, Fei X, Ma Q, Gao B, Xiang Y, Che J and Li Q: Overexpression of ACE2 produces antitumor effects via inhibition of angiogenesis and tumor cell invasion in vivo and in vitro. Oncol Rep. 26:1157–1164. 2011.PubMed/NCBI | |
|
Qian YR, Guo Y, Wan HY, Fan L, Feng Y, Ni L, Xiang Y and Li QY: Angiotensin-converting enzyme 2 attenuates the metastasis of non-small cell lung cancer through inhibition of epithelial-mesenchymal transition. Oncol Rep. 29:2408–2414. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Wilop S, von Hobe S, Crysandt M, Esser A, Osieka R and Jost E: Impact of angiotensin I converting enzyme inhibitors and angiotensin II type 1 receptor blockers on survival in patients with advanced non-small-cell lung cancer undergoing first-line platinum-based chemotherapy. J Cancer Res Clin Oncol. 135:1429–1435. 2009. View Article : Google Scholar : PubMed/NCBI | |
|
Menon J, Soto-Pantoja DR, Callahan MF, Cline JM, Ferrario CM, Tallant EA and Gallagher PE: Angiotensin-(1–7) inhibits growth of human lung adenocarcinoma xenografts in nude mice through a reduction in cyclooxygenase-2. Cancer Res. 67:2809–2815. 2007. View Article : Google Scholar : PubMed/NCBI | |
|
Hicks BM, Filion KB, Yin H, Sakr L, Udell JA and Azoulay L: Angiotensin converting enzyme inhibitors and risk of lung cancer: Population based cohort study. BMJ. 363:k42092018. View Article : Google Scholar : PubMed/NCBI | |
|
Sheinin M, Jeong B, Paidi RK and Pahan K: Regression of lung cancer in mice by intranasal administration of SARS-CoV-2 spike S1. Cancers (Basel). 14:56482022. View Article : Google Scholar : PubMed/NCBI | |
|
Li X, Zhou C and Hu W: Association between serum angiotensin-converting enzyme 2 level with postoperative morbidity and mortality after major pulmonary resection in non-small cell lung cancer patients. Heart Lung Circ. 23:661–666. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Jiang Y, Chen L, Shen J, Mei X, Yao J, Chen T and Zhou Y: The potential role of abnormal angiotensin-converting enzyme 2 expression correlated with immune infiltration after SARS-CoV-2 infection in the prognosis of breast cancer. Aging. 13:20886–20895. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Mei J, Cai Y, Xu R, Yu X, Han X, Weng M, Chen L, Ma T, Gao T, Gao F, et al: Angiotensin-converting enzyme 2 identifies immune-hot tumors suggesting angiotensin-(1–7) as a sensitizer for chemotherapy and immunotherapy in breast cancer. Biol Proced Online. 24:152022. View Article : Google Scholar : PubMed/NCBI | |
|
Zuo X, Ren S, Zhang H, Tian J, Tian R, Han B, Liu H, Dong Q, Wang Z, Cui Y, et al: Chemotherapy induces ACE2 expression in breast cancer via the ROS-AKT-HIF-1α signaling pathway: A potential prognostic marker for breast cancer patients receiving chemotherapy. J Transl Med. 20:5092022. View Article : Google Scholar : PubMed/NCBI | |
|
Ling J, Peng N and Luo L: ACE2 maybe serve as a prognostic biomarker in breast invasive carcinoma. J Clin Lab Anal. 36:e243622022. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang Q, Lu S, Li T, Yu L, Zhang Y, Zeng H, Qian X, Bi J and Lin Y: ACE2 inhibits breast cancer angiogenesis via suppressing the VEGFa/VEGFR2/ERK pathway. J Exp Clin Cancer Res. 38:1732019. View Article : Google Scholar : PubMed/NCBI | |
|
Yu C, Tang W, Wang Y, Shen Q, Wang B, Cai C, Meng X and Zou F: Downregulation of ACE2/Ang-(1–7)/Mas axis promotes breast cancer metastasis by enhancing store-operated calcium entry. Cancer Lett. 376:268–277. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Nair MG, Prabhu JS and Ts S: High expression of ACE2 in HER2 subtype of breast cancer is a marker of poor prognosis. Cancer Treat Res Commun. 27:1003212021. View Article : Google Scholar : PubMed/NCBI | |
|
Nowak JK, Lindstrøm JC, Kalla R, Ricanek P, Halfvarson J and Satsangi J: Age, inflammation, and disease location are critical determinants of intestinal expression of SARS-CoV-2 receptor ACE2 and TMPRSS2 in inflammatory bowel disease. Gastroenterology. 159:1151–1154. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Verstockt B, Verstockt S, Abdu Rahiman S, Ke BJ, Arnauts K, Cleynen I, Sabino J, Ferrante M, Matteoli G and Vermeire S: Intestinal receptor of SARS-CoV-2 in Inflamed IBD tissue seems downregulated by HNF4A in ileum and upregulated by interferon regulating factors in colon. J Crohns Colitis. 15:485–498. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Brandão TB, Gueiros LA, Melo TS, Prado-Ribeiro AC, Nesrallah ACFA, Prado GVB, Santos-Silva AR and Migliorati CA: Oral lesions in patients with SARS-CoV-2 infection: Could the oral cavity be a target organ? Oral Surg Oral Med Oral Pathol Oral Radiol. 31:45–51. 2021. View Article : Google Scholar | |
|
Xu J, Chu M, Zhong F, Tan X, Tang G, Mai J, Lai N, Guan C, Liang Y and Liao G: Digestive symptoms of COVID-19 and expression of ACE2 in digestive tract organs. Cell Death Discov. 6:762020. View Article : Google Scholar : PubMed/NCBI | |
|
Desquilles L, Cano L, Ghukasyan G, Mouchet N, Landreau C, Corlu A, Clément B, Turlin B, Désert R and Musso O: Well-differentiated liver cancers reveal the potential link between ACE2 dysfunction and metabolic breakdown. Sci Rep. 12:18592022. View Article : Google Scholar : PubMed/NCBI | |
|
Stevens JP, Kolachala VL, Joshi GN, Nagpal S, Gibson G and Gupta NA: Angiotensin-converting enzyme-2 (ACE2) expression in pediatric liver disease. Appl Immunohistochem Mol Morphol. 30:647–653. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Osterreicher CH, Taura K, De Minicis S, Seki E, Penz-Osterreicher M, Kodama Y, Kluwe J, Schuster M, Oudit GY, Penninger JM and Brenner DA: Angiotensin-converting-enzyme 2 inhibits liver fibrosis in mice. Hepatology. 50:929–938. 2009. View Article : Google Scholar : PubMed/NCBI | |
|
Dong F, Li H, Liu L, Yao LL, Wang J, Xiang D, Ma J, Zhang G, Zhang S, Li J, et al: ACE2 negatively regulates the Warburg effect and suppresses hepatocellular carcinoma progression via reducing ROS-HIF1α activity. Int J Biol Sci. 19:2613–2629. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Paizis G, Tikellis C, Cooper ME, Schembri JM, Lew RA, Smith AI, Shaw T, Warner FJ, Zuilli A, Burrell LM and Angus PW: Chronic liver injury in rats and humans upregulates the novel enzyme angiotensin converting enzyme 2. Gut. 54:1790–1796. 2005. View Article : Google Scholar : PubMed/NCBI | |
|
Herath CB, Warner FJ, Lubel JS, Dean RG, Jia Z, Lew RA, Smith AI, Burrell LM and Angus PW: Upregulation of hepatic angiotensin-converting enzyme 2 (ACE2) and angiotensin-(1–7) levels in experimental biliary fibrosis. J Hepatol. 47:387–395. 2007. View Article : Google Scholar : PubMed/NCBI | |
|
Ye G, Qin Y, Lu X, Xu X, Xu S, Wu C, Wang X, Wang S and Pan D: The association of renin-angiotensin system genes with the progression of hepatocellular carcinoma. Biochem Biophys Res Commun. 459:18–23. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Domovitz T, Ayoub S, Werbner M, Alter J, Izhaki Tavor L, Yahalom-Ronen Y, Tikhonov E, Meirson T, Maman Y, Paran N, et al: HCV infection increases the expression of ACE2 receptor, leading to enhanced entry of both HCV and SARS-CoV-2 into hepatocytes and a coinfection state. Microbiol Spectr. 10:e01150222022. View Article : Google Scholar : PubMed/NCBI | |
|
Li J, Yang ZL, Ren X, Zou Q, Yuan Y, Liang L, Chen M and Chen S: ACE2 and FZD1 are prognosis markers in squamous cell/adenosquamous carcinoma and adenocarcinoma of gallbladder. J Mol Histol. 45:47–57. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Zong H, Yin B, Zhou H, Cai D, Ma B and Xiang Y: Loss of angiotensin-converting enzyme 2 promotes growth of gallbladder cancer. Tumour Biol. 36:5171–5177. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Lee JJ, Kopetz S, Vilar E, Shen JP, Chen K and Maitra A: Relative abundance of SARS-CoV-2 Entry genes in the enterocytes of the lower gastrointestinal tract. Genes (Basel). 11:6452020. View Article : Google Scholar : PubMed/NCBI | |
|
Suárez-Fariñas M, Tokuyama M, Wei G, Huang R, Livanos A, Jha D, Levescot A, Irizar H, Kosoy R, Cording S, et al: Intestinal inflammation modulates the expression of ACE2 and TMPRSS2 and potentially overlaps with the pathogenesis of SARS-CoV-2-related disease. Gastroenterology. 160:287–301.e20. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Hashimoto T, Perlot T, Rehman A, Trichereau J, Ishiguro H, Paolino M, Sigl V, Hanada T, Hanada R, Lipinski S, et al: ACE2 links amino acid malnutrition to microbial ecology and intestinal inflammation. Nature. 487:477–481. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Bao R, Hernandez K, Huang L and Luke JJ: ACE2 and TMPRSS2 expression by clinical, HLA, immune, and microbial correlates across 34 human cancers and matched normal tissues: Implications for SARS-CoV-2 COVID-19. J Immunother Cancer. 8:e0010202020. View Article : Google Scholar : PubMed/NCBI | |
|
Duan Y, Prasad R, Feng D, Beli E, Li Calzi S, Longhini ALF, Lamendella R, Floyd JL, Dupont M, Noothi SK, et al: Bone Marrow-derived cells restore functional integrity of the gut epithelial and vascular barriers in a model of diabetes and ACE2 deficiency. Circ Res. 125:969–988. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Nataf S and Pays L: Molecular Insights into SARS-CoV2-induced alterations of the Gut/Brain axis. Int J Mol Sci. 22:104402021. View Article : Google Scholar : PubMed/NCBI | |
|
Liu C, Xiao L, Li F, Zhang H, Li Q, Liu H, Fu S, Li C, Zhang X, Wang J, et al: Generation of outbred Ace2 knockout mice by RNA transfection of TALENs displaying colitis reminiscent pathophysiology and inflammation. Transgenic Res. 24:433–46. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Shahrokh S, Baradaran Ghavami S, Asadzadeh Aghdaei H, Parigi TL, Farmani M, Danese S, Ebrahimi Daryani N, Vossoughinia H, Balaii H, Alborzi F, et al: High prevalence of SARS-Coronavirus-2 in patients with inflammatory bowel disease and the role of soluble angiotensin converting enzyme 2. Arch Physiol Biochem. 130:325–332. 2024. View Article : Google Scholar : PubMed/NCBI | |
|
Potdar AA, Dube S, Naito T, Li K, Botwin G, Haritunians T, Li D, Casero D, Yang S, Bilsborough J, et al: Altered intestinal ACE2 levels are associated with inflammation, severe disease, and response to Anti-cytokine therapy in inflammatory bowel disease. Gastroenterology. 160:809–822. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Yang K and Wang Y: Dandelion root extracts and taraxasterol inhibit LPS-induced colorectal cancer cell viability by blocking TLR4-NFκB-driven ACE2 and TMPRSS2 pathways. Exp Ther Med. 27:2562024. View Article : Google Scholar : PubMed/NCBI | |
|
Wang H and Yang J: Colorectal cancer that highly express both ACE2 and TMPRSS2, suggesting severe symptoms to SARS-CoV-2 infection. Pathol Oncol Res. 27:6129692021. View Article : Google Scholar : PubMed/NCBI | |
|
Liu C, Wang K, Zhang M, Hu X, Hu T, Liu Y, Hu Q, Wu S and Yue J: High expression of ACE2 and TMPRSS2 and clinical characteristics of COVID-19 in colorectal cancer patients. NPJ Precis Oncol. 5:12021. View Article : Google Scholar : PubMed/NCBI | |
|
Chen Y, Gong W, Wei H, Dai W and Xu S: 2019-nCoV may create complications in colon cancer patients with ACE2 expression. Int J Clin Exp Pathol. 13:2305–2311. 2020.PubMed/NCBI | |
|
Li S, Chen S, Zhu Q, Ben S, Gao F, Xin J, Du M, Chu H, Gu D, Zhang Z and Wang M: The impact of ACE2 and co-factors on SARS-CoV-2 infection in colorectal cancer. Clin Transl Med. 12:e9672022. View Article : Google Scholar : PubMed/NCBI | |
|
Chen H, Zou TH, Xuan B, Yan Y, Yan T, Shen C, Zhao G, Chen YX, Xiao X, Hong J and Fang JY: Single cell transcriptome revealed SARS-CoV-2 entry genes enriched in colon tissues and associated with coronavirus infection and cytokine production. Signal Transduct Target Ther. 5:1212020. View Article : Google Scholar : PubMed/NCBI | |
|
Ahmadi M, Pashangzadeh S, Mousavi P, Saffarzadeh N, Amin Habibi M, Hajiesmaeili F and Rezaei N: ACE2 correlates with immune infiltrates in colon adenocarcinoma: Implication for COVID-19. Int Immunopharmacol. 95:1075682021. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang S, Kapoor S, Tripathi C, Perez JT, Mohan N, Dashwood WM, Zhang K, Rajendran P and Dashwood R: Targeting ACE2-BRD4 crosstalk in colorectal cancer and the deregulation of DNA repair and apoptosis. NPJ Precis Oncol. 7:202023. View Article : Google Scholar : PubMed/NCBI | |
|
Triana S, Metz-Zumaran C, Ramirez C, Kee C, Doldan P, Shahraz M, Schraivogel D, Gschwind AR, Sharma AK, Steinmetz LM, et al: Single-cell analyses reveal SARS-CoV-2 interference with intrinsic immune response in the human gut. Mol Syst Biol. 17:e102322021. View Article : Google Scholar : PubMed/NCBI | |
|
Lamers MM, Beumer J, van der Vaart J, Knoops K, Puschhof J, Breugem TI, Ravelli RBG, Paul van Schayck J, Mykytyn AZ, Duimel HQ, et al: SARS-CoV-2 productively infects human gut enterocytes. Science. 369:50–54. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Ottaiano A, Scala S, D'Alterio C, D'Alterio C, Trotta A, Bello A, Rea G, Picone C, Santorsola M, Petrillo A and Nasti G: Unexpected tumor reduction in metastatic colorectal cancer patients during SARS-Cov-2 infection. Ther J Med Oncol. 13:175883592110114552021. View Article : Google Scholar : PubMed/NCBI | |
|
Cappello F, Burgio S, Conway de Macario E and Macario AJL: Unexpected tumor reduction in metastatic colorectal cancer patients during SARS-Cov-2 infection: Effect of ACE-2 expression on tumor cells or molecular mimicry phenomena? Two not mutually exclusive hypotheses. Ther Adv Med Oncol. 13:175883592110278252021. View Article : Google Scholar : PubMed/NCBI | |
|
Pakbin B, Dibazar SP, Allahyari S, Shariatifar H, Brück WM and Farasat A: ACE2-inhibitory effects of bromelain and ficin in colon cancer cells. Medicina (Kaunas). 59:3012023. View Article : Google Scholar : PubMed/NCBI | |
|
Li N, Zimpelmann J, Cheng K, Wilkins JA and Burns KD: The role of angiotensin converting enzyme 2 in the generation of angiotensin 1–7 by rat proximal tubules. Am J Physiol Renal Physiol. 288:F353–F362. 2005. View Article : Google Scholar : PubMed/NCBI | |
|
Fan C, Lu W, Li K, Ding Y and Wang J: ACE2 expression in kidney and testis may cause kidney and testis infection in COVID-19 patients. Front Med (Lausanne). 13:5638932021. View Article : Google Scholar | |
|
He Q, Mok TN, Yun L, He C, Li J and Pan J: Single-cell RNA sequencing analysis of human kidney reveals the presence of ACE2 receptor: A potential pathway of COVID-19 infection. Mol Genet Genomic Med. 8:e14422020. View Article : Google Scholar : PubMed/NCBI | |
|
Lin W, Fan J, Hu LF, Zhang Y, Ooi JD, Meng T, Jin P, Ding X, Peng LK, Song L, et al: Single-cell analysis of angiotensin-converting enzyme II expression in human kidneys and bladders reveals a potential route of 2019 novel coronavirus infection. Chin Med J (Engl). 134:935–943. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Pan XW, Xu D, Zhang H, Zhou W, Wang LH and Cui XG: Identification of a potential mechanism of acute kidney injury during the COVID-19 outbreak: A study based on single-cell transcriptome analysis. Intensive Care Med. 46:1114–1116. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Tikellis C, Johnston CI, Forbes JM, Burns WC, Burrell LM, Risvanis J and Cooper ME: Characterization of renal angiotensin-converting enzyme 2 in diabetic nephropathy. Hypertension. 41:392–397. 2003. View Article : Google Scholar : PubMed/NCBI | |
|
Soler MJ, Wysocki J, Ye M, Lloveras J, Kanwar Y and Batlle D: ACE2 inhibition worsens glomerular injury in association with increased ACE expression in streptozotocin-induced diabetic mice. Kidney Int. 72:614–623. 2007. View Article : Google Scholar : PubMed/NCBI | |
|
Vergara A, Wang K, Colombo D, Gheblawi M, Rasmuson J, Mandal R, Del Nonno F, Chiu B, Scholey JW, Soler MJ, et al: Urinary angiotensin-converting enzyme 2 and metabolomics in COVID-19-mediated kidney injury. Clin Kidney J. 16:272–284. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Burns WC, Velkoska E, Dean R, Burrell LM and Thomas MC: Angiotensin II mediates epithelial-to-mesenchymal transformation in tubular cells by ANG 1–7/MAS-1-dependent pathways. Am J Physiol Renal Physiol. 299:F585–F593. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Choong OK, Jakobsson R, Bergdahl AG, Brunet S, Kärmander A, Waldenström J, Arvidsson Y, Altiparmak G, Nilsson JA, Karlsson J, et al: SARS-CoV-2 replicates and displays oncolytic properties in clear cell and papillary renal cell carcinoma. PLoS One. 18:e02795782023. View Article : Google Scholar : PubMed/NCBI | |
|
Larrinaga G, Pérez I, Sanz B, Blanco L, López JI, Cándenas ML, Pinto FM, Gil J, Irazusta J and Varona A: Angiotensin-converting enzymes (ACE and ACE2) are downregulated in renal tumors. Regul Pept. 165:218–23. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Errarte P, Beitia M, Perez I, Manterola L, Lawrie CH, Solano-Iturri JD, Calvete-Candenas J, Unda M, López JI and Larrinaga G: Expression and activity of angiotensin-regulating enzymes is associated with prognostic outcome in clear cell renal cell carcinoma patients. PLoS One. 12:e01817112017. View Article : Google Scholar : PubMed/NCBI | |
|
Niu X, Zhu Z, Shao E and Bao J: ACE2 is a prognostic biomarker and associated with immune infiltration in kidney renal clear cell carcinoma: Implication for COVID-19. J Oncol. 2021:88473072021. View Article : Google Scholar : PubMed/NCBI | |
|
Yang W, Li L, Zhang K, Ma K, Xie H, Gong Y, Zhou J and Gong K: ACE2 correlated with immune infiltration serves as a novel prognostic biomarker in clear cell renal cell carcinoma: Implication for COVID-19. Int J Biol Sci. 17:20–31. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Yang J, Li H, Hu S and Zhou Y: ACE2 correlated with immune infiltration serves as a prognostic biomarker in endometrial carcinoma and renal papillary cell carcinoma: Implication for COVID-19. Aging (Albany NY). 12:6518–6535. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Tang Q, Wang Y, Ou L, Li J, Zheng K, Zhan H, Gu J, Zhou G, Xie S, Zhang J, et al: Downregulation of ACE2 expression by SARS-CoV-2 worsens the prognosis of KIRC and KIRP patients via metabolism and immunoregulation. Int J Biol Sci. 17:1925–1939. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Siljee S, Milne B, Brasch HD, Bockett N, Patel J, Davis PF, Kennedy-Smith A, Itinteang T and Tan ST: Expression of components of the Renin-angiotensin system by cancer stem cells in renal clear cell carcinoma. Biomolecules. 11:5372021. View Article : Google Scholar : PubMed/NCBI | |
|
Cane R, Kennedy-Smith A, Brasch HD, Savage S, Marsh RW and Itinteang T, Tan ST and Itinteang T: Characterization of cancer stem cells in renal clear cell carcinoma. J Stem Cell Regen Biol. 5:6–17. 2019. | |
|
Wang T, Xie F, Li YH and Liang B: Downregulation of ACE2 is associated with advanced pathological features and poor prognosis in clear cell renal cell carcinoma. Future Oncol. 17:5033–5044. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Khanna P, Soh HJ, Chen CH, Saxena R, Amin S, Naughton M, Joslin PN, Moore A, Bakouny Z, O'Callaghan C, et al: ACE2 abrogates tumor resistance to VEGFR inhibitors suggesting angiotensin-(1–7) as a therapy for clear cell renal cell carcinoma. Sci Transl Med. 13:eabc01702021. View Article : Google Scholar : PubMed/NCBI | |
|
Li Q, Yang W, Lu M and Zhang R: Identification of a 6-Gene signature associated with resistance to tyrosine kinase inhibitors: Prognosis for clear cell renal cell carcinoma. Med Sci Monit. 26:e9270782020. View Article : Google Scholar : PubMed/NCBI | |
|
Douglas GC, O'Bryan MK, Hedger MP, Lee DK, Yarski MA, Smith AI and Lew RA: The novel angiotensin-converting enzyme (ACE) homolog, ACE2, is selectively expressed by adult Leydig cells of the testis. Endocrinology. 145:4703–4711. 2004. View Article : Google Scholar : PubMed/NCBI | |
|
Wang Z and Xu X: scRNA-seq profiling of human testes reveals the presence of the ACE2 receptor, a target for SARS-CoV-2 infection in spermatogonia, leydig and sertoli cells. Cells. 9:9202020. View Article : Google Scholar : PubMed/NCBI | |
|
Ribeiro MR, Calado AM, Alves Â, Pereira R, Sousa M and Sá R: Spatial distribution of SARS-CoV-2 receptors and proteases in testicular cells. J Histochem Cytochem. 71:169–197. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Liu X, Chen Y, Tang W, Zhang L, Chen W, Yan Z, Yuan P, Yang M, Kong S, Yan L and Qiao J: Single-cell transcriptome analysis of the novel coronavirus (SARS-CoV-2) associated gene ACE2 expression in normal and non-obstructive azoospermia (NOA) human male testes. Sci China Life Sci. 63:1006–1015. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Shen Q, Xiao X, Aierken A, Yue W, Wu X, Liao M and Hua J: The ACE2 expression in Sertoli cells and germ cells may cause male reproductive disorder after SARS-CoV-2 infection. J Cell Mol Med. 24:9472–9477. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Gianzo M and Subirán N: Regulation of male fertility by the Renin-angiotensin system. Int J Mol Sci. 21:79432020. View Article : Google Scholar : PubMed/NCBI | |
|
Reis AB, Araújo FC, Pereira VM, Dos Reis AM, Santos RA and Reis FM: Angiotensin (1–7) and its receptor Mas are expressed in the human testis: Implications for male infertility. J Mol Histol. 41:75–80. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Song H, Seddighzadeh B, Cooperberg MR and Huang FW: Expression of ACE2, the SARS-CoV-2 Receptor, and TMPRSS2 in prostate epithelial cells. Eur Urol. 78:296–298. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Krishnan B, Smith TL, Dubey P, Zapadka ME, Torti FM, Willingham MC, Tallant EA and Gallagher PE: Angiotensin-(1–7) attenuates metastatic prostate cancer and reduces osteoclastogenesis. Prostate. 73:71–82. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Krishnan B, Torti FM, Gallagher PE and Tallant EA: Angiotensin-(1–7) reduces proliferation and angiogenesis of human prostate cancer xenografts with a decrease in angiogenic factors and an increase in sFlt-1. Prostate. 73:60–70. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Bhowmick NA, Oft J, Dorff T, Pal S, Agarwal N, Figlin RA, Posadas EM, Freedland SJ and Gong J: COVID-19 and androgen-targeted therapy for prostate cancer patients. Endocr Relat Cancer. 27:R281–R292. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Vaz-Silva J, Carneiro MM, Ferreira MC, Pinheiro SV, Silva DA, Silva-Filho AL, Witz CA, Reis AM, Santos RA and Reis FM: The vasoactive peptide angiotensin-(1–7), its receptor Mas and the angiotensin-converting enzyme type 2 are expressed in the human endometrium. Reprod Sci. 16:247–256. 2009. View Article : Google Scholar : PubMed/NCBI | |
|
Chadchan SB, Popli P, Maurya VK and Kommagani R: The SARS-CoV-2 receptor, angiotensin-converting enzyme 2, is required for human endometrial stromal cell decidualization. Biol Reprod. 104:336–343. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Qin S, Zhou YJ, Liu Y, Shen HM, Li XD, Yan X and Tang HJ: Expression and significance of ACE2-Ang-(1–7)-Mas axis in the endometrium of patients with polycystic ovary syndrome. Zhonghua Yi Xue Za Zhi. 93:1989–1992. 2013.(In Chinese). PubMed/NCBI | |
|
Haouzi D, Entezami F, Tuaillon E, Gala A, Ferrières-Hoa A, Brouillet S, Thierry AR and Hamamah S: SARS-CoV-2 and implantation window: Gene expression mapping of human endometrium and preimplantation embryo. Life (Basel). 11:13782021.PubMed/NCBI | |
|
Naigaonkar A, Patil K, Joseph S, Hinduja I and Mukherjee S: Ovarian granulosa cells from women with PCOS express low levels of SARS-CoV-2 receptors and co-factors. Arch Gynecol Obstet. 306:547–555. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Guo X, Semerci N, De Assis V, Kayisli UA, Schatz F, Steffensen TS, Guzeloglu-Kayisli O and Lockwood CJ: Regulation of proinflammatory molecules and tissue factor by SARS-CoV-2 spike protein in human placental cells: Implications for SARS-CoV-2 pathogenesis in pregnant women. Front Immunol. 13:8765552022. View Article : Google Scholar : PubMed/NCBI | |
|
Valdés G, Neves LA, Anton L, Corthorn J, Chacón C, Germain AM, Merrill DC, Ferrario CM, Sarao R, Penninger J and Brosnihan KB: Distribution of angiotensin-(1–7) and ACE2 in human placentas of normal and pathological pregnancies. Placenta. 27:200–207. 2006. View Article : Google Scholar : PubMed/NCBI | |
|
Essahib W, Verheyen G, Tournaye H and Van de Velde H: SARS-CoV-2 host receptors ACE2 and CD147 (BSG) are present on human oocytes and blastocysts. J Assist Reprod Genet. 37:2657–2660. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Reis FM, Bouissou DR, Pereira VM, Camargos AF, dos Reis AM and Santos RA: Angiotensin-(1–7), its receptor Mas, and the angiotensin-converting enzyme type 2 are expressed in the human ovary. Fertil Steril. 95:176–181. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
Stanley KE, Thomas E, Leaver M and Wells D: Coronavirus disease-19 and fertility: Viral host entry protein expression in male and female reproductive tissues. Fertil Steril. 114:33–43. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
Domińska K: Involvement of ACE2/Ang-(1–7)/MAS1 axis in the regulation of ovarian function in mammals. Int J Mol Sci. 21:45722020. View Article : Google Scholar : PubMed/NCBI | |
|
Nagappan A, Kim KH and Moon Y: Caveolin-1-ACE2 axis modulates xenobiotic metabolism-linked chemoresistance in ovarian clear cell carcinoma. Cell Biol Toxicol. 39:1181–1201. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Delforce SJ, Lumbers ER, Corbisier de Meaultsart C, Wang Y, Proietto A, Otton G, Scurry J, Verrills NM, Scott RJ and Pringle KG: Expression of renin-angiotensin system (RAS) components in endometrial cancer. Endocr Connect. 6:9–19. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Grzegrzolka J, Swiatko K, Pula B, Zamirska A, Olbromski M, Bieniek A, Szepietowski J, Rys J, Dziegiel P and Podhorska-Okolow M: ACE and ACE2 expression in normal and malignant skin lesions. Folia Histochem Cytobiol. 51:232–238. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Siljee S, Pilkington T, Brasch HD, Bockett N, Patel J, Paterson E, Davis PF and Tan ST: Cancer stem cells in head and neck metastatic malignant melanoma express components of the renin-angiotensin system. Life (Basel). 10:2682020.PubMed/NCBI | |
|
Ender SA, Dallmer A, Lässig F, Lendeckel U and Wolke C: Expression and function of the ACE2/angiotensin(1–7)/Mas axis in osteosarcoma cell lines U-2 OS and MNNG-HOS. Mol Med Rep. 10:804–810. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Nehme A, Cerutti C, Dhaouadi N, Gustin MP, Courand PY, Zibara K and Bricca G: Atlas of tissue renin-angiotensin-aldosterone system in human: A transcriptomic meta-analysis. Sci Rep. 5:100352015. View Article : Google Scholar : PubMed/NCBI | |
|
Haznedaroglu IC and Malkan UY: Local bone marrow renin-angiotensin system in the genesis of leukemia and other malignancies. Eur Rev Med Pharmacol Sci. 20:4089–4111. 2016.PubMed/NCBI | |
|
Lin C, Datta V, Okwan-Duodu D, Chen X, Fuchs S, Alsabeh R, Billet S, Bernstein KE and Shen XZ: Angiotensin-converting enzyme is required for normal myelopoiesis. FASEB J. 25:1145–1155. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
Rodgers KE, Xiong S and diZerega GS: Accelerated recovery from irradiation injury by angiotensin peptides. Cancer Chemother Pharmacol. 49:403–411. 2002. View Article : Google Scholar : PubMed/NCBI | |
|
Rodgers KE, Espinoza T, Roda N, Meeks CJ, Hill C, Louie SG and Dizerega GS: Accelerated hematopoietic recovery with angiotensin-(1–7) after total body radiation. Int J Radiat Biol. 88:466–476. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Ratajczak MZ, Bujko K, Ciechanowicz A, Sielatycka K, Cymer M, Marlicz W and Kucia M: SARS-CoV-2 Entry receptor ACE2 is expressed on very small CD45-precursors of hematopoietic and endothelial cells and in response to virus spike protein activates the Nlrp3 inflammasome. Stem Cell Rev Rep. 17:266–277. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Park TS and Zambidis ET: A role for the renin-angiotensin system in hematopoiesis. Haematologica. 94:745–747. 2009. View Article : Google Scholar : PubMed/NCBI | |
|
Zambidis ET, Park TS, Yu W, Tam A, Levine M, Yuan X, Pryzhkova M and Péault B: Expression of angiotensin-converting enzyme (CD143) identifies and regulates primitive hemangioblasts derived from human pluripotent stem cells. Blood. 112:3601–3614. 2008. View Article : Google Scholar : PubMed/NCBI | |
|
Uz B, Tatonyan SC, Sayitoglu M, Erbilgin Y, Ng OH, Buyukasik Y, Sayinalp N, Aksu S, Goker H, Ozcebe OI, et al: Local hematopoietic renin-angiotensin system in myeloid versus lymphoid hematological neoplastic disorders. J Renin Angiotensin Aldosterone Syst. 14:308–314. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Teresa Gomez Casares M, de la Iglesia S, Perera M, Lemes A, Campo C, Gonzalez San Miguel JD, Bosch JM, Suarez A, Guerra L, Rodriguez-Peréz JC and Molero T: Renin expression in hematological malignancies and its role in the regulation of hematopoiesis. Leuk Lymphoma. 43:2377–2381. 2002. View Article : Google Scholar : PubMed/NCBI | |
|
Alshareef A: Effect of SARS-CoV-2 entry factors on myeloid cancers. J Nippon Med Sch. 89:95–101. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Kozako T and Soeda S, Yoshimitsu M, Arima N, Kuroki A, Hirata S, Tanaka H, Imakyure O, Tone N, Honda S and Soeda S: Angiotensin II type 1 receptor blocker telmisartan induces apoptosis and autophagy in adult T-cell leukemia cells. FEBS Open Bio. 6:442–460. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Lima RS, Carvalho Rocha LP and Rocha Moreira P: Genetic and epigenetic control of ACE2 expression and its possible role in COVID-19. Cell Biochem Funct. 39:713–726. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Dowell J, Bice Z, Yan K, Girija G and Konduri GG: Hyperoxia-induced airflow restriction and Renin-Angiotensin system expression in a bronchopulmonary dysplasia mouse model. Physiol Rep. 12:e158952024. View Article : Google Scholar : PubMed/NCBI | |
|
Miroslavova Pencheva M and Nikolaeva Genova S: SARS-CoV-2 induced changes in the lungs based on autopsy cases. Indian J Pathol Microbiol. 66:19–23. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Ulrich–Merzenich GS, Shcherbakova A, Pizarro C and Dirk Skowasch D: Dexamethasone, remdesivir and azithromycin modulate ACE2 and IL-6 in lung epithelial cells. Pneumologie. 79:134–140. 2025. View Article : Google Scholar : PubMed/NCBI | |
|
Miura Y, Ohkubo H, Nakano A, Bourke JE and Kanazawa S: Pathophysiological conditions induced by SARS-CoV-2 infection reduce ACE2 expression in the lung. Front Immunol. 13:10286132022. View Article : Google Scholar : PubMed/NCBI | |
|
Koehler VF, Knösel T, Hasmann SE, Scherer C, Hellmuth JC, Muenchhoff M, Munker SM, Hoster E, Ladurner R and Spitzweg C: Thyroidal Angiotensin-Converting enzyme 2 protein expression and thyroid function tests in patients with COVID-19: Results from a retrospective case series and a prospective cohort study. Thyroid. 33:177–185. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Bronowicka–Szydełko A, Rabczyński M, Dumas I, Fiodorenko-Dumas Z, Wojtczak B, Kotyra L, Kustrzeba–Wójcicka I, Lewandowski L, Ponikowska B, Kuzan A, et al: State of knowledge about thyroid cancers in the era of COVID-19-A narrative review. Biomedicines. 12:28292024. View Article : Google Scholar : PubMed/NCBI | |
|
Serwaa A, Oyawoye F, Amoakoh Owusu I, Dosoo D, Adom Manu A, Kojo Sobo A, Fosu K, Ochieng Olwal C, Kojo Quashie P and Rosebud Aikins A: In vitro analysis suggests that SARS-CoV-2 infection differentially modulates cancer-like phenotypes and cytokine expression in colorectal and prostate cancer cells. Sci Rep. 14:246252024. View Article : Google Scholar : PubMed/NCBI | |
|
Shirazi M, Cianfarini C, Ismail A, Wysocki J, Wang JJ, Ye M, Zhang ZJ and Batlle D: Altered kidney distribution and loss of ACE2 into the urine in acute kidney injury. Am J Physiol Renal Physiol. 327:F412–F425. 2024. View Article : Google Scholar : PubMed/NCBI | |
|
Sanad AM, Qadri F, Popova E, Rodrigues AF, Heinbokel T, Quach S, Schulz A, Bachmann S, Kreutz R, Alenina N and Bader M: Transgenic angiotensin-converting enzyme 2 overexpression in the rat vasculature protects kidneys from ageing-induced injury. Kidney Int. 104:293–304. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Rago V, Bossio S, Lofaro D, Perri A and Di Agostino S: New insights into the link between SARS-CoV-2 infection and renal cancer. Life (Basel). 14:522023.PubMed/NCBI | |
|
Singh Parmar H, Nayak A, Kumar Gavel P, Chandra Jha H, Bhagwat S and Sharma R: Cross talk between COVID-19 and breast cancer. Curr Cancer Drug Targets. 21:575–600. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Sinha S, Cheng K, Schäffer AA, Aldape K, Schiff E and Ruppin E: In vitro and in vivo identification of clinically approved drugs that modify ACE2 expression. Mol Syst Biol. 16:e96282020. View Article : Google Scholar : PubMed/NCBI | |
|
Beacon TH, Delcuve GP and Davie JR: Epigenetic regulation of ACE2, the receptor of the SARS-CoV-2 virus. Genome. 64:386–399. 2021. View Article : Google Scholar : PubMed/NCBI |