Evaluation of HMGB1 as possible marker via breast organoid cultures research
- Authors:
- Published online on: July 1, 2025 https://doi.org/10.3892/or.2025.8939
- Article Number: 106
-
Copyright: © Ciaramella et al. This is an open access article distributed under the terms of Creative Commons Attribution License.
Abstract
Introduction
Breast cancer (BC) is one of the most prevalent cancers globally with inter-tumor and intra-tumor variability. Several risk factors are linked to BC, including age, family history and genetic mutations (1).
Additional factors that may contribute to the development of BC, include obesity and hormone therapies (2). The prevalence of BC has steadily increased over the past decade: Between 2010 and 2019, the incidence rate grew by 0.5% each year. In 2020, there were >2.3 million new BC cases diagnosed, resulting in ~685,000 deaths (3). The diagnosis of BC involves a physical exam, imaging techniques such as mammography, and a tissue biopsy (4). Overcoming therapy resistance necessitates exploring novel diagnostic proteins, such as high mobility group box 1 (HMGB1), in BC.
Among them, HMGB1 protein, previously known as amphoterin, was discovered by Marco Emilio Bianchi's group in 1989 (5). It is well known that HMGB1 is a structural, non-histone chromatin protein, a member of the High Mobility Group (HMG) protein family, specifically the HMGB subfamily, containing proteins with an HMG-box DNA-binding domain (6). This protein is abundant in the nucleus of all eukaryotic cells, where it plays an important role in chromatin remodeling. It is also a key mediator of inflammation, particularly in the case of necrosis (7). Furthermore, HMGB1 protein shows double function in cancer, serving both as an oncogene and a tumor suppressor, influencing cellular death and survival pathways, and it is involved in various stages of tumor progression, such as proliferation, invasion and metastasis (8).
The biological effects of HMGB1 are contingent upon its expression and subcellular location. When present within the nucleus, HMGB1 is involved in a multitude of DNA-related processes, including DNA repair, transcription, telomere maintenance and genome stability. Conversely, when situated outside the nucleus, it assumes a more intricate role, regulating cell proliferation, autophagy, inflammation and immunity (9). In the context of tumor development, HMGB1 has been identified as a protein with both pro- and antitumor properties. Its actions can either promote or suppress tumor growth, proliferation, angiogenesis, invasion and metastasis.
From a physiological perspective, it is evident that cell cycle progression and cell proliferation are meticulously and harmoniously governed. Conversely, the occurrence of uncontrolled cell proliferation resulting from aberrant cell cycle progression is an integral aspect of the complex process of cancer development and progression. It appears to be clear that understanding cell cycle progression and regulation is of great relevance for improving cancer treatments (10).
Recent findings suggest that HMGB1 may be released from activated innate immune cells or necrotic cells and plays a potential role in endotoxemia, sepsis, arthritis and local inflammation (11). Therapeutic agents that may inhibit HMGB1 release or action have shown promise in animal models, indicating a potential for clinical management of various inflammatory diseases.
Numerous studies demonstrated the importance of HMGB1 protein in BC diagnosis and therapy (12). Regarding the most promising inhibitors of this protein, Glycyrrhizin (GL), derived from rhizome extracts of liquorice (or ‘licorice’ in US English) (Glycyrrhiza glabra) binds directly to HMG1 box in HMGB1, thereby inhibiting the chemoattractant and mitogenic activity of HMGB1 (13).
GL exerts anticancer effects by regulating multiple signaling pathways, including those involving the phosphatase and tensin homolog/phosphatidylinositol 3-kinase/protein kinase B, mitogen-activated protein kinase, and mammalian target of rapamycin/signal transducer and activator of transcription 3 pathways. These pathways primarily govern cancer cell death, oxidative stress and inflammation (14). Moreover, Chang et al (15) demonstrated that GL effectively blocked the HMGB1-induced epithelial-to-mesenchymal transition, indicating its potential role as a diagnostic and therapeutic agent for cancer research. Because of the multiple functions in which HMGB1 protein is involved, in the present study, a BC-derived organoid culture was adopted as a model because they maintain the tumor microenvironment even after long-term expansion in culture under the same conditions. The goal of the present study was to further investigate the HMGB1 protein to identify new molecular mechanisms and biomarkers at an early stage for the management of BC, with the long-term goal of improving personalized therapy.
Materials and methods
Patient selection
Between June 2023 and January 2024, 14 female patients who had breast surgery at Clinica Villa Fiorita S.p.A. (Aversa, Italy) were included in the present study. On May 29, 2019, the study was authorized (approval no. 3/19) by the IRCCS Pascale Ethics Committee (Naples, Italy). Based on the Declaration of Helsinki (16), all human samples were taken only after each patient and healthy donor provided written informed consent (Table I). All the procedures listed below were carried out in compliance with the applicable rules and laws.
Generation of ex vivo 3D patient-derived organoids (PDOs) from patient samples
Ex-vivo 3D PDOs from samples of patients with BC were generated (17), by using a protocol approved by our local Ethics Committee. Moreover, all patients involved in the present study approved and signed a written informed consent form to authorize use of their tumor samples for research purpose. On the day of collection, all new tumor tissue samples need to be treated sterilely and stored on ice. Prior to being used in tissue transplantation, tumors are extracted from patients and physically dissected into tissue fragments. Tumor pieces were minced and digested enzymatically using collagenase and hyaluronidase while being shaken for 12–18 h at 37°C at a low to moderate speed (for example, 20 × g). The amount of tissue that is recovered during surgery and the amount that is set aside as fragments determines how much tissue is needed. Using two disposable scalpels, the tissue is cut into fine pieces in a crisscross pattern. Then, a cell lifter was used to move the tissue to a 50-ml conical container. The digestive process was observed using both visual inspection and light microscopy of the reactions.
If the tissue is resistant to enzymatic digestion, a 100-µm cell strainer was used to strain the digested tissue into a fresh tube, removing all debris and undigested pieces. The organoid fraction should be heavily enriched for refractile, solid clusters of tumor cells called ‘organoids’ and should contain few single cells (18,19). To preserve their three-dimensional structure, cells were implanted in Matrigel to create 3D cultures.
Ex vivo drug treatment
Glycyrrhizin (Glycyrrhizic Acid; cat. no. 167409; Selleck Chemicals) is a direct HMGB1 inhibitor that prevents oxidative stress and inflammatory molecule development that is dependent on HMGB1. Glycyrrhizin, a naturally occurring anti-inflammatory and antiviral triterpene, is employed in clinical settings to block HMGB1′s chemoattractant, mitogenic and intranuclear DNA-binding properties. Studies using NMR and fluorescence have shown that glycyrrhizin binds directly to HMGB1 [K(d) ~150 µM]. The two arms of both HMG boxes form two shallow concave surfaces with which it interacts (20). Glycyrrhizin 50 µM was administered for 72 h while examining samples from 14 PDOs in this pilot investigation.
Reverse transcription-quantitative PCR (RT-qPCR)
Total RNA was extracted using TRIzol reagent (Thermo Fisher Scientific, Inc.). Reverse transcription was performed to convert 1 µg of isolated RNA into cDNA using SensiFAST Reverse Transcriptase (Bioline), following the manufacturer's instructions. Expression levels of genes encoding for HMGB-1, Cyclin A, Cyclin B, Cyclin D and Cyclin E were analyzed using RT-qPCR. Applied Biosystems' PRIMER EXPRESS software was used to generate gene-specific primers. The SYBR Green PCR Master Mix (Applied Biosystems) was used for the amplifications. A denaturation step at 95°C for 10 min (stage 2), a starting step at 50°C for 2 min (stage 1), and 40 cycles at 95°C for 15 sec and 60°C for 1 min (stage 3) comprised the thermal cycling conditions. Every sample was performed twice in 25 µl reactions with an Applied Biosystems QuantStudio 7 Flex. The 2−ΔΔCq method was used to calculate relative expression values after normalizing to Actin, which was employed as an internal control gene (21). Non-specific signals generated by primer dimers were excluded from analysis using non-template controls. RT-PCR primer sequences were reported in Table SI.
Immunofluorescence
To characterize the PDOs, confocal microscopy analysis was conducted. Images were acquired three days post-formation. Cell nuclei were stained with Hoechst 33342 (cat. no. H3570; Thermo Fisher Scientific, Inc.) at a final concentration of 1 µg/ml for 1 h at 37°C. To characterize HMGB-1, β-Catenin and NF-κB expression levels in tumor and non-cancerous ex vivo PDOs, the organoids were incubated with antibodies against HMGB-1 (cat. no 3935S; Cell Signalling Technology, Inc.), β-Catenin (cat. no sc-7963; Santa Cruz Biotechnology, Inc.) and NF-κB (cat. no 3865015; Sony Biotechnology, Inc.) at a 1:100 dilution for 1 h at room temperature (RT). Subsequently, they were incubated with secondary antibodies Alexa Fluor™ 488 Rabbit (cat. no A21206; Invitrogen; Thermo Fisher Scientific, Inc.), Alexa Fluor® 594 Goat anti-mouse IgG (cat. no 405326; BioLegend, Inc.) and PE (cat. no 3D6C02; BioLegend, Inc.), respectively, diluted 1:200 for 1 h at RT in the dark. Maximal projection images were acquired using confocal microscopy (Mica; Leica Microsystems GmbH) at ×63 magnification and saved in TIFF format.
Apoptosis assay
Annexin V-FITC Kit-AAD Kit (cat. no IM3614; Beckman Coulter, Inc.) was utilized, according to manufacturer's instructions, to analyze the cell cycle of PDOs three days post-formation, with a minimum of 50,000 single-cell events recorded. Briefly, PDOs cells suspension with 1X ice-cold PBS was centrifuged for 5 min at 500 × g at 4°C. The pellet was resuspended in 100 µl 1X Binding Buffer solution, containing 10 µl of Annexin A5-FITC and 20 µl of 7-AAD Viability dye, and incubated for 15 min on ice in dark conditions. Subsequently, 400 µl of 1X Binding Buffer solution were added and the cell preparation was analyzed by flow cytometry using CytoFLEX (Beckman Coulter, Inc.). The percentages of cells in apoptosis were calculated using the Michael Fox algorithm. Later, apoptotic cell analysis was conducted using Kaluza Analysis Software 2.1 (Beckman Coulter, Inc.).
Cell cycle analysis
The DNA-Prep Reagents kit (cat. no 6607055; Beckman Coulter, Inc.) was utilized, according to manufacturer's instructions, to analyze the cell cycle of PDOs 3 days post-formation, with a minimum of 10,000 single-cell events recorded. Briefly, PDOs cells suspension was fixed and permeabilized with DNA PREP RPL for 5 min at RT. Then, 1 ml of DNA PREP STAIN was added for 1 h at RT. All the samples were analyzed using CytoFLEX (Beckman Coulter, Inc.). The percentages of cells in the G1, S, and G2/M phases were calculated using the Michael Fox algorithm. Subsequently, cell cycle analysis was conducted using Kaluza Analysis Software 2.1 (Beckman Coulter, Inc.).
Online data and statistical analysis
The Gene Expression Profiling Interactive Analysis (GEPIA; http://gepia2.cancer-pku.cn/#analysis) web server has been a valuable and highly cited resource for gene expression analysis based on tumor and normal samples from the TCGA and the GTEx databases. GEPIA2 is an updated and enhanced version to provide insights with higher resolution and more functionalities (22). A total of three separate experiments were conducted in triplicate, and the assay results were reported as the mean ± SD. GraphPad Prism software, version 9.0 (GraphPad Software Inc.; Dotmatics), was used to conduct statistical analysis. Tukey's test was used after the ordinary one-way ANOVA statistical test to compare the data. P<0.05 was considered to indicate a statistically significant difference.
Results
Expression analysis of HMGB1 in BC PDOs
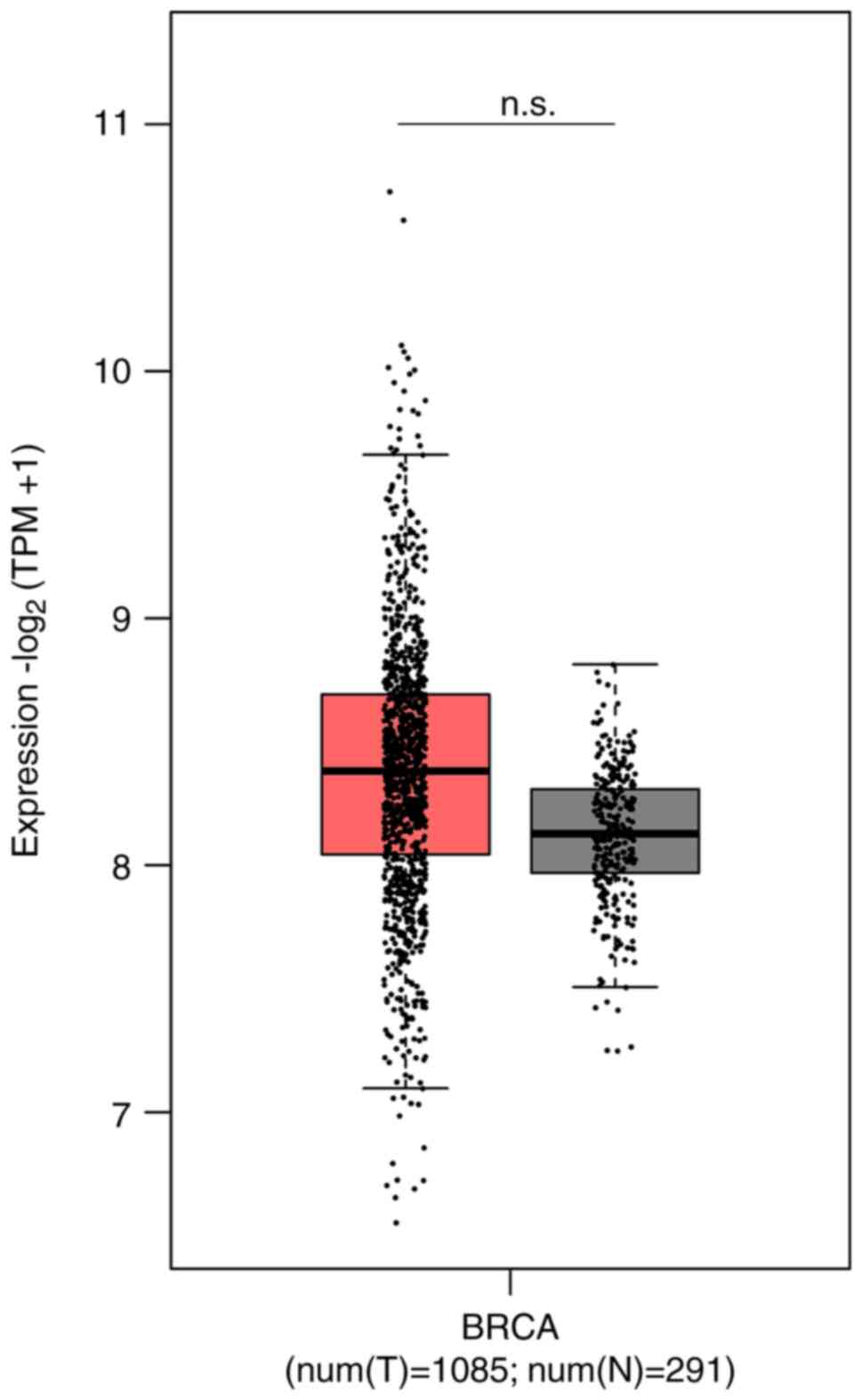
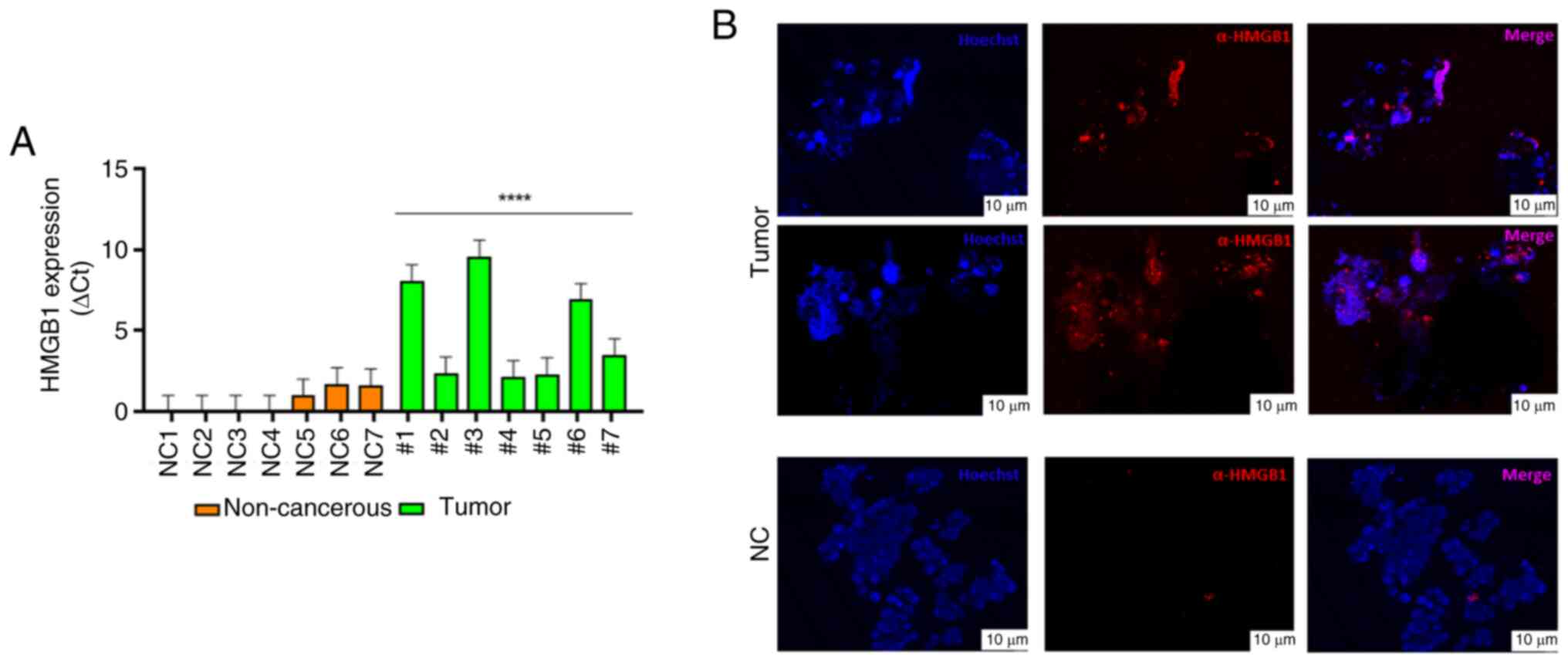
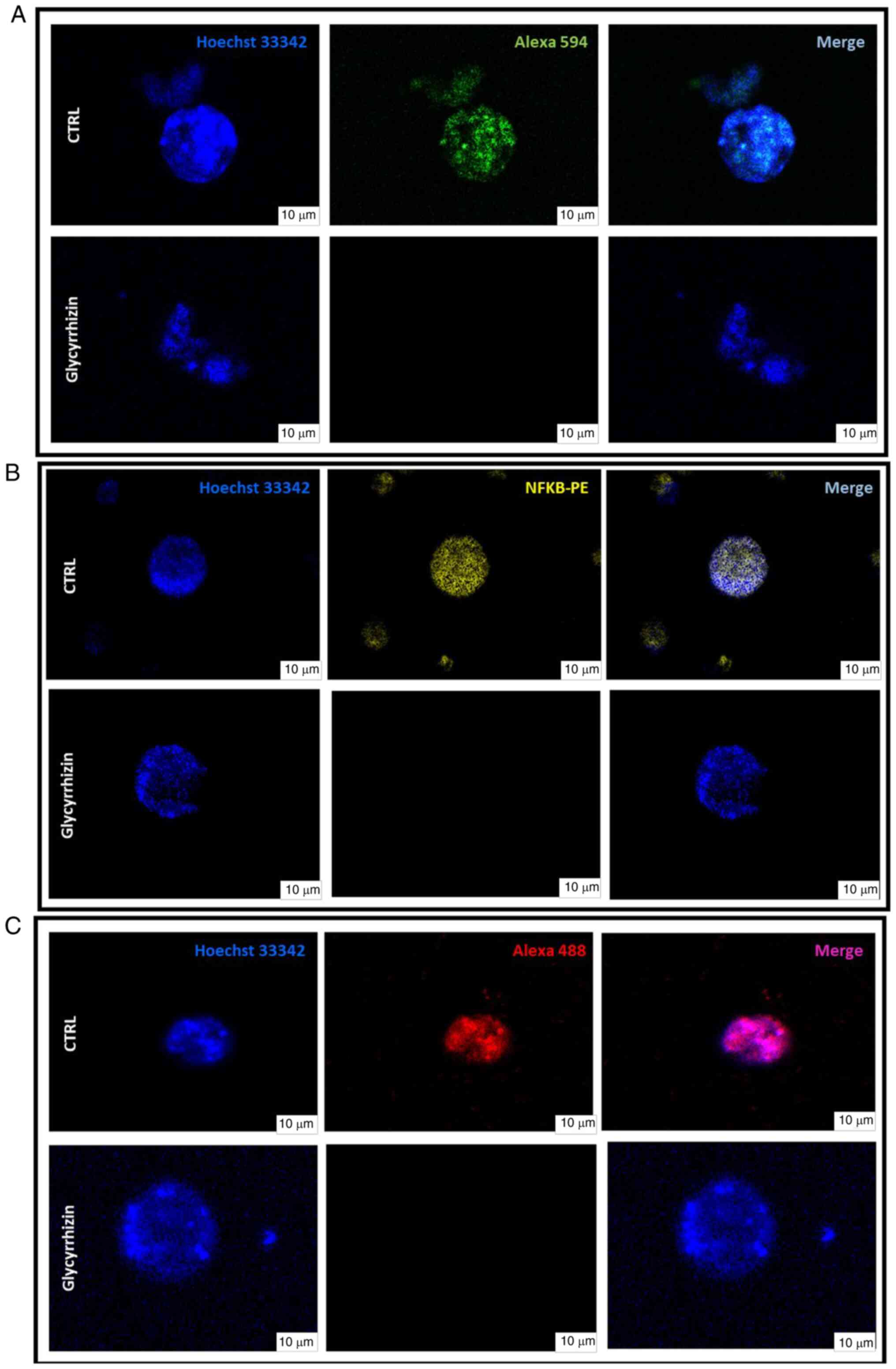
In a preliminary study, HMGB1 mRNA expression was analyzed in normal and breast tumor tissues using GEPIA2; although no significant difference was observed, the HMGB1 mRNA expression was higher in breast tumor tissues compared with normal (t=1085; n=291) (Fig. 1). In our laboratories, in parallel ex vivo 3D cultures obtained from BC surgical specimens were generated to study molecular mechanisms involving HMGB1 protein. Briefly, human breast tumor and non-tumor tissues were obtained from a limited number of patients undergoing surgery to obtain PDOs. Despite the difficulties related to the collection of samples from patients and the maintenance of the 3D ex vivo cultures, which represent the main limitations of organoid studies, 14 tissues obtained from human breast biopsy samples were collected to derive 14 ex vivo PDOs primary cell cultures and they were divided into two groups; fibroadenoma (n=7) and BC (n=7). RT-qPCR results revealed higher HMGB1 mRNA levels in tumor PDOs subgroups compared with the non-tumor PDO subgroups in which low levels of HMGB1 mRNA expression were recorded (Fig. 2A). This result was confirmed by the localization of HMGB1 through immunofluorescence labeling of PDOs in which the signal was higher specifically in tumor-PDOs compared with fibroadenoma PDOs (Fig. 2B).
Analysis of apoptosis and cell cycle after HMGB1 inhibition in PDOs
The processes of cell cycle progression and cell proliferation are subject to precise and orchestrated control in normal cells. However, uncontrolled cell proliferation resulting from aberrant cell cycle progression represents a pivotal characteristic of cancer. To understand the regulatory basis of these mechanisms and a possible involvement of HMGB1, treatment was performed with 50 µM of Glycyrrhizin, a licorice root extract known as selective HMGB1 inhibitor on PDOs samples for 72 h. Propidium iodide (PI) is a commonly used fluorescent dye in conjunction with annexin V to determine whether cells are viable, undergoing apoptosis, or necrotic. This is achieved through differences in plasma membrane integrity and permeability. The Annexin V/PI staining method is a common approach for detecting cells undergoing apoptosis.
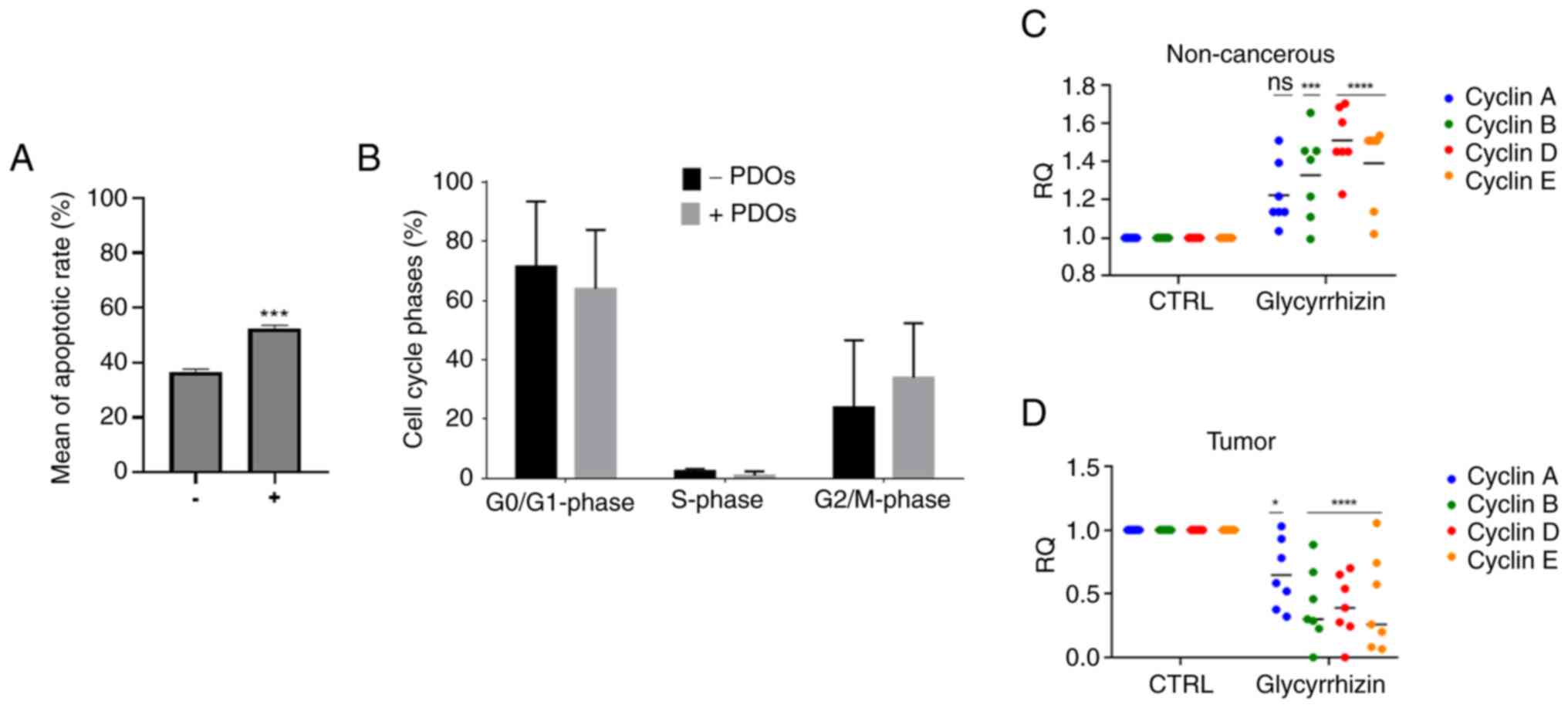
Following 72 h of Glycyrrhizn treatment, a pro-apoptotic effect induced by HMGB1 inhibition was found. In the treated tumor PDOs, a significant increase in apoptotic cells was observed with a mean of apoptotic rate increased to 30% than in the untreated control (Fig. 3A). The primary characteristics of apoptosis include DNA fragmentation and damage. To ascertain whether Glycyrrhizin-induced apoptotic cells affected cell cycle distribution, a further analysis of the cell cycle was conducted after 72 h of Glycyrrhizin treatment by flow cytometry. The PI fluorescent dye can enter cells with compromised membranes and bind stoichiometrically to DNA, thereby enabling the assessment of the proportion of cells at each stage based on the levels of DNA present.
By comparing the proportions of cells in each phase, the percentage of cells in the G2-M phase gradually increased in the treated PDOs samples while a clear reduction in the G0-G1 phase was observed, suggesting that HMGB1 inhibition may arrest the transition from G2 to M phase in tumor PDOs so that their proliferation capacity is weakened, and the cell has a reduced vitality (Figs. 3B, S1 and S2).
Tumor-associated cell cycle defects are often mediated by alterations in cyclin activity, in these cases inducing uncontrolled proliferation as well as genomic and chromosomal instability. Therefore, selective inhibition of the cyclin pathway may provide therapeutic benefit against some human malignancies. Considering this, we chose to investigate the gene expression trend of cyclins to also support the results obtained by cell cycle analysis. By blocking HMGB1, we observed an opposite trend of cyclins A, B, D and E between tumor and non-cancerous PDOs (Fig. 3C and D). Following glycyrrhizin therapy, our data showed a significant decrease in all cyclins examined in tumor samples; this decrease in expression is not seen in non-tumor samples that continue to be active. Emerging data suggests that the use of an HMGB1 inhibitor could be a therapeutic strategy that selectively targets tumor cells.
Effect of HMGB1 blockade on β-Catenin/NF-κB pathway
It has been reported that interactions between the Wnt/β-Catenin and PI3K/AKT/mTOR pathways are often hyperactivated in numerous solid tumors, such as breast and ovarian cancer and act downstream on NF-κB (23). To study a possible role of HMBG1 in Wnt/β-Catenin modulation on NF-κB also in BC, preliminary experiments were performed observing that inhibiting HMGB1 causes a blockade of both β-Catenin and NF-κB.
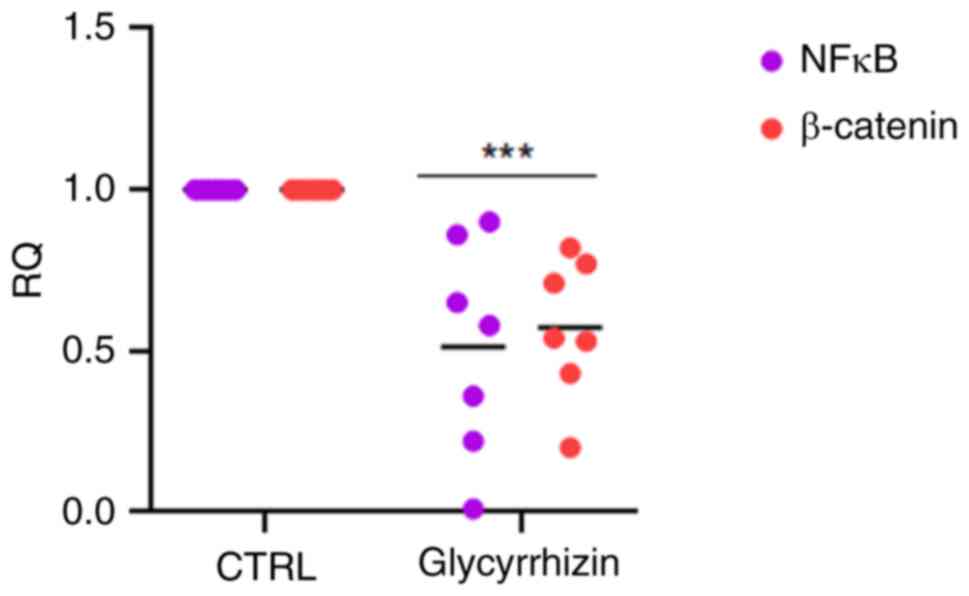
The transcription levels were investigated in this initial stage of the study because the number of organoids produced is dependent on the ex vivo proliferation index as well as the size of the starting tissue. Thus, HMBG1 was blocked and its downstream effects were investigated by administering glycyrrhizin for 72 h to BC PDO. Lowering of NF-κB and β-catenin expression levels had the most significant effect (Fig. 4).
Inhibition of HMGB1-induced signalling by switching off HMGB1 causes downregulation of the Wnt/β-Catenin/NF-κB pathway, key regulator of anti-apoptotic genes and consequently the initiation of apoptosis. Glycyrrhizin (50 µM) was administered to organoids in order to examine this aspect, and then immunofluorescence labeling with some targets related to the pathway under investigation was used. The results of the localization analysis demonstrated that the organoids treated with glycyrrhizin did not change morphologically, but rather function. Indeed, following treatment, β-catenin (Fig. 5A) and NF-κB (Fig. 5B) signals were lost; this effect suggests a direct and guided involvement of HMGB1, which is totally inactivated by glycyrrhizin (Fig. 5C).
Discussion
HMGB1 overexpression and/or elevated serum HMGB1 levels have been observed in several types of cancer and are often associated with a poor prognosis. This evidence indicated that HMGB1 plays a critical role in the invasion and metastasis of BC (24).
In the present study, PDOs were generated from breast tumors as a disease model investigating the role of HMGB1 as a possible prognostic marker. It has been demonstrated that irregular activation of this pathway is correlated with several key characteristics of BC, including cell proliferation, invasion behavior and drug resistance (24). Those data suggest that targeting the HMGB1 pathway may offer a potential early diagnosis method for BC.
Previously, Glycyrrhizin has shown tremendous therapeutic activities such as anti-inflammatory, antiviral, hepatoprotective and antitumor activity (25). Particularly, in vitro and in vivo studies on Glycyrrhizin have revealed that it enhances the antitumor effect of ‘Taxol’: Glycyrrhizin in combination with paclitaxel shows a multifunctional behavior by suppressing cell proliferation in a markedly higher way, almost 5-fold higher, compared with paclitaxel in MCF-7 BC cells (26). The present results confirm the Glycyrrhizin effect; for the first time to the best of our knowledge, a PDOs' model was studied where the involvement of HMGB1 in tumor development and progression was observed. After treatment, a significant increase in apoptosis and cell cycle inhibition was observed with consequent reduction in cyclin levels. The present results demonstrated a significant reduction of all cyclins analyzed in tumor samples after treatment with Glycyrrhizin suggesting a potential role in the block of the G2/M transition by inducing apoptosis and by reducing the cyclins' expression level (27).
In their study, Wang et al (28) demonstrated that glycyrrhizin was capable of inhibiting cell proliferation, adhesion, and migration. Additionally, they observed a notable reduction in the expression of β-catenin, Bcl-2, CyclinD1, and survivin.
The current findings support the Glycyrrhizin effect, but for the first time, PDO models in which HMGB1 is involved in tumor formation and progression, were investigated. Following therapy, a considerable rise in apoptosis and cell cycle inhibition was observed, with a subsequent decrease in cyclin levels. This aspect leads us to hypothesize that cyclin genes are linked to prognosis and/or medication tolerance in BC. BC is distinguished by weak tumor antigenicity and a relative lack of immune cells when compared with numerous other types of solid tumors.
In the present study, it was demonstrated that HMBG1 inhibition modulates the Wnt/β-catenin/NFκB signaling, which is involved in cell processes including multiplication, migration, stability and apoptosis. This pathway also maintains the pluripotent state of adult stem cells, which is linked to tumor generation and development.
HMGB1 and major inflammatory factors, such as IL-1, IL-6 and TNF-α, stimulate each other. Inflammatory factors such as NF-κB, VEGF, TNF-α, MCP-1, IL-8 and IL-1β, as well as RAGE end products, can play a role in these processes. The HMGB1 gene, which has functional NF-κB binding sites, phosphorylates p38 mitogen-activated protein kinase and activates NF-κB through RAGE. HMGB1 also stimulates the production of inflammatory cytokines.
While HMGB1 secretion is limited in activated cells by blocking the canonical NF-κB pathway, the NF-κB pathway plays a role in HMGB1 release. Even though the NF-κB targets the HMGB1 gene directly, it remains unclear who oversees this process. One scenario is that TNF, the traditional NF-κB target gene, could be engaged in the release of HMGB1 that is dependent on NF-κB (29).
Among numerous functions, the Wnt/β-Catenin/NF-κB pathway regulates tumor MHC I expression and T cell activation, important components for greater tumor antigenicity (30). A variety of immune and non-immune cells, including dendritic cells, monocytes, neutrophils, macrophages and natural killer cells, may produce HMGB1 extracellularly in response to various stimuli (31). Overall, further research is needed to increase our understanding of HMGB1′s function in cancer immunotherapy as well as the possible therapeutic results of HMGB1 blocking as a means of preventing the spread and metastasis of cancer. In future experiments, the authors will also further investigate the induction of apoptosis in ex vivo 3D organoids, their inhibitory genes, interactions with other proteins, and other physical parameters.
To summarize, HMGB1 is a complicated and diverse molecule that has wide-ranging implications in a variety of pathological diseases. In future research, the authors hope to elucidate these specific roles while also researching interactions in the immune response, translocation dynamics and inflammatory modulation to improve understanding of its role in tumor development and progression. Future study will investigate this factor in relation to immune-escape mechanisms and the tumor microenvironment, both of which are now mainstays of tumor treatment. An improved understanding of the role of HMGB1, as well as its synergy with cyclins and NF-κB, in experimental human-like organoid models could aid personalized medicine by allowing for targeted therapy based on the mutational status of the individual patient and the identification of specific early diagnostic markers.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by Italian Ministry of Health Project “Ricerca Corrente”.
Availability of data and materials
The data generated in the present study may be requested from the corresponding author.
Authors' contributions
VC and MS conceptualized the study. BC, LC, GM and MD developed methodology. VC and GS validated data. BC curated data. VC, LC and GS wrote the original draft. MS and AS supported interpretation of data. VC and GM confirm the authenticity of all the raw data. All authors read and approved the final version of the manuscript.
Ethics approval and consent to participate
The present study was approved (approval no. 3/19; approval date 29 May 2019) by the Ethics Committee of IRCCS Pascale (Naples, Italy). All human samples were collected after obtaining written informed consent from each patient and healthy donor.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
|
Zeng X, Liu C, Yao J, Wan H, Wan G, Li Y and Chen N: Breast cancer stem cells, heterogeneity, targeting therapies and therapeutic implications. Pharmacol Res. 163:1053202021. View Article : Google Scholar : PubMed/NCBI | |
|
Leone I, Santoro J, Soricelli A, Febbraro A, Santoriello A and Carrese B: Triple-negative breast cancer EVs modulate growth and migration of normal epithelial lung cells. Int J Mol Sci. 25:58642024. View Article : Google Scholar : PubMed/NCBI | |
|
Arnold M, Morgan E, Rumgay H, Mafra A, Singh D, Laversanne M, Vignat J, Gralow J, Cardoso F, Siesling S and Soerjomataram I: Current and future burden of breast cancer: Global statistics for 2020 and 2040. Breast. 66:15–23. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Obeagu E and Obeagu G: Breast cancer: A review of risk factors and diagnosis. Medicine (Baltimore). 103:e369052024. View Article : Google Scholar : PubMed/NCBI | |
|
Bianchi ME, Beltrame M and Paonessa G: Specific recognition of cruciform DNA by nuclear protein HMG1. Science. 243:1056–1059. 1989. View Article : Google Scholar : PubMed/NCBI | |
|
Starkova T, Polyanichko A, Artamonova T, Tsimokha A, Tomilin A and Chikhirzhina E: Structural characteristics of High-mobility group proteins HMGB1 and HMGB2 and their interaction with DNA. Int J Mol Sci. 24:35772023. View Article : Google Scholar : PubMed/NCBI | |
|
Chen R, Zou J, Kang R and Tang D: The redox protein High-Mobility group box 1 in cell death and cancer. Antioxid Redox Signal. 39:569–590. 2023. View Article : Google Scholar : PubMed/NCBI | |
|
Lv G, Yang M, Gai K, Jia Q, Wang Z, Wang B and Li X: Multiple functions of HMGB1 in cancer. Front Oncol. 14:13841092024. View Article : Google Scholar : PubMed/NCBI | |
|
He S, Cheng J, Feng X, Yu Y, Tian L and Huang Q: The dual role and therapeutic potential of High-mobility group box 1 in cancer. Oncotarget. 8:64534–64550. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Cai Z and Liu Q: Cell cycle regulation in treatment of breast cancer. Adv Exp Med Biol. 1026:251–270. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Chen G, Ward MF and Wang H: Extracellular HMGB1 as a proinflammatory cytokine. J Interferon Cytokine Res. 24:329–33. 2004. View Article : Google Scholar : PubMed/NCBI | |
|
Dong Y, Ming B and Dong L: The role of HMGB1 in rheumatic diseases. Front Immunol. 13:8152572022. View Article : Google Scholar : PubMed/NCBI | |
|
Oh H, Choi A, Seo N, Lim J, You J and Chung Y: Protective effect of glycyrrhizin, a direct HMGB1 inhibitor, on Post-contrast acute kidney injury. Sci Rep. 11:156252021. View Article : Google Scholar : PubMed/NCBI | |
|
Jain R, Hussein M, Pierce S, Martens C, Shahagadkar P and Munirathinam G: Oncopreventive and oncotherapeutic potential of licorice triterpenoid compound glycyrrhizin and its derivatives: Molecular insights. Pharmacol Res. 178:1061382022. View Article : Google Scholar : PubMed/NCBI | |
|
Chang H, Chen S, Wu C, Lu C and Yen G: Glycyrrhizin attenuates the process of Epithelial-to-Mesenchymal transition by modulating HMGB1 initiated novel signaling pathway in prostate cancer cells. J Agric Food Chem. 67:323–3332. 2019. View Article : Google Scholar | |
|
Mastroleo I: Post-trial obligations in the declaration of helsinki 2013: Classification, reconstruction and interpretation. Dev World Bioeth. 16:80–90. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Lee GY, Kenny PA, Lee EH and Bissell MJ: Three-dimensional culture models of normal and malignant breast epithelial cells. Nat Methods. 4:359–365. 2007. View Article : Google Scholar : PubMed/NCBI | |
|
Della Corte CM, Barra G, Ciaramella V, Di Liello R, Vicidomini G, Zappavigna S, Luce A, Abate M, Fiorelli A, Caraglia M, et al: Antitumor activity of dual blockade of PD-L1 and MEK in NSCLC patients derived three-dimensional spheroid cultures. J Exp Clin Cancer Res. 38:2532019. View Article : Google Scholar : PubMed/NCBI | |
|
DeRose YS, Gligorich KM, Wang G, Georgelas A, Bowman P, Courdy SJ, Welm AL and Welm BE: Patient-derived models of human breast cancer: Protocols for in vitro and in vivo applications in tumor biology and translational medicine. Curr Protoc Pharmacol. Chapter 14: Unit14. 232013.PubMed/NCBI | |
|
Mollica L, De Marchis F, Spitaleri A, Dallacosta C, Pennacchini D, Zamai M, Agresti A, Trisciuoglio L, Musco G and Bianchi ME: Glycyrrhizin binds to High-mobility group box 1 protein and inhibits its cytokine activities. Chem Biol. 14:431–441. 2007. View Article : Google Scholar : PubMed/NCBI | |
|
Livak KJ and Schmittgen TD: Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI | |
|
Tang Z, Kang B, Li C, Chen T and Zhang Z: GEPIA2: An enhanced web server for large-scale expression profiling and interactive analysis. Nucleic Acids Res. 47:W556–W560. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Pourbarkhordar V, Rahmani S, Roohbakhsh A, Hayes AW and Karimi G: Melatonin effect on breast and ovarian cancers by targeting the PI3K/Akt/mTOR pathway. IUBMB Life. 76:1035–1049. 2024. View Article : Google Scholar : PubMed/NCBI | |
|
Guangyao L, Yang M, Gai K, Jia Q, Wang Z, Wang B and Li X: Multiple functions of HMGB1 in cancer. Front Oncol. 14:13841092024. View Article : Google Scholar : PubMed/NCBI | |
|
Li C, Tang Z, Zhang W, Ye Z and Liu F: GEPIA2021: Integrating multiple deconvolution-based analysis into GEPIA. Nucleic Acids Res. 49:W242–W246. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Dudhatra G, Mody S, Awale M, Patel H, Modi C, Kumar A, Kamani D and Chauhan B: A Comprehensive review on pharmacotherapeutics of herbal bioenhancers. ScientificWorldJournal. 2012:6379532012. View Article : Google Scholar : PubMed/NCBI | |
|
Pucci B, Kasten M and Giordano A: Cell cycle and apoptosis. Neoplasia. 2:291–299. 2000. View Article : Google Scholar : PubMed/NCBI | |
|
Wang CM, Jiang M and Wang HJ: Effect of NF-κB inhibitor on high-mobility group protein B1 expression in a COPD rat model. Mol Med Rep. 7:499–502. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Kim M, Park S and Lee D: Glycyrrhizin as a nitric oxide regulator in cancer chemotherapy. Cancers (Basel). 13:57622021. View Article : Google Scholar : PubMed/NCBI | |
|
Dholakia J, Scalise CB, Katre AA, Goldsberry WN, Meza-Perez S, Randall TD, Norian LA, Novak L and Arend RC: Sequential modulation of the Wnt/β-catenin signaling pathway enhances Tumor-intrinsic MHC I expression and tumor clearance. Gynecol Oncol. 164:170–180. 2022. View Article : Google Scholar : PubMed/NCBI | |
|
Scaffidi P, Misteli T and Bianchi ME: Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 418:191–195. 2022. View Article : Google Scholar : PubMed/NCBI |