Chromosome 20q gene signature associated with colorectal cancer progression
- Authors:
- Published online on: July 18, 2025 https://doi.org/10.3892/or.2025.8954
- Article Number: 121
-
Copyright: © Jones et al. This is an open access article distributed under the terms of Creative Commons Attribution License.
Abstract
Introduction
Colorectal cancer (CRC) remains the third most common cancer type and cause of cancer-related deaths in the United States (1). Among all CRC cases, ~15% constitute microsatellite instability (MSI) subtype with defects in DNA mismatch repair pathways displaying higher somatic mutation burden and immune infiltration in the tumor microenvironment (TME) compared with the remaining majority patients with microsatellite stable (MSS) subtype (2). Incidence of distant metastasis is the single most significant factor influencing survival of patients with CRC with five-year survival dropping to <1% when distant metastases are detected (3). Advancing stage and grade of CRC was reported to correlate with increasing incidence of chromosome 20q amplification (4), with distant metastasis in 94% of metastatic lesions, 89% of colorectal primaries with liver metastases and 41% of primary tumors without metastases (5). Presence of shared chromosome 20q gain in high grade adenomas with matched carcinomas suggest that genetic alteration is uncoupled from histological changes associated with malignant progression (6) and elevated expression of critical genes in this region contribute to tumor progression. These studies together with those showing association of 20q amplification with poor clinical outcome for CRC (7–9), indicate important role for a set of genes on chromosome 20q in the development of aggressive disease. Interestingly, 20q amplification was reported to be the largest global change at both mRNA and protein levels in CRC (10). Since 20q amplification has also been associated with improved overall survival in a subset of patients (11), it is imperative that the critical genes on chromosome 20q amplicon determining poor prognosis be identified to stratify patients requiring appropriate clinical intervention in a timely manner.
In the current study, integrated genomic copy number and expression profiling approach were utilized, previously successfully used to identify functionally significant amplified-overexpressed genes in human cancers (12,13), to identify candidate CRC progression associated genes on chromosome 20q amplicon, utilizing two in vitro cell line models (KM12C/L4A/SM and SW480/SW620) of CRC progression. Microarray comparative genomic hybridization (aCGH) of these cells identified regional copy gains common to all the cell lines. Expression array analysis of the genes residing in the Minimal Common Regions (MCR) of amplification identified a four gene signature possibly contributing to malignant progression process and thus representing candidate biomarkers of aggressive disease. Findings were validated first in a training set of 23 colorectal adenocarcinoma (tumor) and 5 adenoma (polyp) samples followed by analyses of two separate test datasets comprising of a cohort of 195 patients with CRC investigated in the TCGA project, accessed through the cBioPortal for Cancer Genomics (14), and an independent, in-house annotated set of 182 colorectal tumor samples from MD Anderson Cancer Center (MDACC).
Materials and methods
Cell culture and nucleic acid isolation
The KM12 model cell lines, established by intrasplenic or subcutaneously injecting poorly metastatic KM12C cells derived from a Dukes' B stage colon carcinoma into nude mice to isolate variant lines KM12L4A and KM12SM, both with high liver metastatic potential (15), were obtained from Dr. Isaiah J. Fidler's laboratory at University of Texas MD Anderson Cancer Center, and cultured according to their published protocols (15). The highly metastatic variants are genetically related to the poorly metastatic parental cell line KM12C and gain of 20q has been identified in all the three lines (16). The SW480, SW620 and the HCT116 cells were obtained from the American Type Culture Collection (ATCC) and cultured according to the recommended protocols. The SW620 cells were derived from a metastatic lesion in the same patient whose primary tumor was the source of the SW480 cells unlike the KM cell line model in which the metastatic variants were isolated from mouse xenografts of primary tumor derived cells. The cell line SW480 was cultured from a Duke's Stage B colon carcinoma, while the cell line SW620 was from an abdominal lymph node in the same patient (17). Additionally, the HCT 116 cell line was derived from a poorly differentiated human colon carcinoma (18). Genomic DNA was isolated from these cells using the Qiagen DNeasy Blood and Tissue Kit (cat. no. 69504; Qiagen) according to the manufacturer's protocols and stored at −20°C. Total cellular RNA was isolated from the cells using TRIZOL reagent (cat. no. 15596026; Invitrogen; Thermo Fisher Scientific, Inc.) according to the manufacturer's protocol and further purified using the Qiagen RNeasy RNA Extraction Kit with an on-column DNase digestion (cat. no. 74104; Qiagen).
Primary colorectal tumor and polyp samples
Gene copy number analysis and reverse transcription-quantitative (RT-qPCR) were performed using DNA and RNA of 23 matched tumor-normal tissue samples that were snap-frozen or stored in RNAlater (cat. no. AM7020; Invitrogen; Thermo Fisher Scientific, Inc.), obtained from the laboratories of Drs. Stanley Hamilton and Marsha Frazier at UT MD Anderson Cancer Center. The five polyp samples with matched WBC and normal tissue were obtained from Dr. Stephen Meltzer's laboratory at the Johns Hopkins University School of Medicine. All samples including normal and tumor tissues were obtained from residual samples remaining after clinical diagnosis under IRB approved protocols with ethics approval and participant consent received at the time of admission approved by the Institutional Review Boards of Johns Hopkins University School of Medicine and UT MD Anderson Cancer Center (approval no. LAB90-018).
aCGH
DNA from KM12, SW, HCT116 cell lines, five tumors and four polyps that passed the QC were used for the arrayCGH analysis. Commercially available normal female genomic DNA (Promega Corporation; containing DNA from various anonymous donors) was used as the source of control DNA for the experiments. DNA from the samples was diluted to a concentration of 25 ng/ml and 1 ml was used in a Phi29 amplification step using the Qiagen Repli-g amplification kit (cat. no. 150023; Qiagen) according to Agilent-modified manufacturer's protocol, omitting the initial denaturing step. DNA labeling, hybridization, and washing were carried out according to manufacturer's protocol. Genomic arrays were scanned with the Molecular Devices GenePix 4000B Microarray scanner and fluorescence intensities, normalization and ratios obtained using the Agilent Feature Extraction Software 7.5.1 at the recommended settings. Dye-swap hybridizations were performed for each cell line, tumor and polyp samples, and the resulting data combined and further analyzed by statistical methods as mentioned below. Heat maps were generated using Agilent's CGH Analytics software Version 3.4 and Nexus Copy Number 7 software.
Expression array analysis
Total RNA from cell lines and commercially available normal (non-diseased) colonic epithelial RNA (Stratagene; Agilent) was labeled, hybridized and washed using manufacturer's protocol and reagents [Agilent Low RNA Input Labeling Kit (cat. no. 5184-3523), Agilent in situ Hybridization Kit (cat. no. 5190-0404), Agilent's Stabilization and Drying Solution cat. no. 5185-5979)]. Arrays were scanned on Axon 4000B scanner and Agilent DNA Microarray scanner. Dye-swap hybridizations were performed for each cell line and the resulting data combined. The initial fluorescence data was generated with GenePix Pro 5.1 software (Axon; Molecular Devices, LLC) for all images produced with the Axon scanner, and normalization, filtering and ratios calculated as described below.
RT-qPCR
Total RNA was used to produce cDNA with the Invitrogen First Strand cDNA synthesis kit according to manufacturer's protocol (cat no. 18091050). RT-qPCR was carried out on isolated total RNA in MD Anderson Cancer Center's DNA Core facility. RT-qPCR analysis was performed using ABI TaqMan Assays for the candidate genes, showing gain of expression, as well as for the internal control gene ACTB, with an ABI 7500HT Real-Time PCR System, according to manufacturer's recommendations. All assays were run in duplicate and the relative expression of the selective genes in terms of fold change in the cancer cell lines, tumor and polyp samples, compared with normal tissue samples was calculated using the comparative CT (2−ΔΔCq) method (19), using the endogenous control to normalize the data. Primers used are summarized in Table SI.
MSI status
MSI status was evaluated by fluorescence labeled microsatellite marker PCR followed by capillary electrophoresis fragment size analysis using an ABI 3130 sequencer and Genescan software (Applied Biosystems; Thermo Fisher Scientific, Inc.). Five markers (BAT25, BAT26, D2S123, D5S346, D17S250; of the National Cancer Institute panel) were analyzed. The samples were classified as MSI-High (MSI-H) if two or more markers showed altered allelic size, MSI-Low (MSI-L) if one marker showed allelic shift and MSS if none showed allelic shift (20).
For the independent MDACC test dataset, the paired neoplastic and non-neoplastic tissue controls were analyzed for MSI according to the standard-of-care immunohistochemistry test method (21). Immunoperoxidase stains were performed on formalin-fixed paraffin embedded sections with antibodies for the DNA mismatch repair enzymes MLH1, MSH2, MSH6 and PMS2. The samples were classified as MSI-H if two or more markers were altered, and MSS if none of the markers were altered. The samples unevaluated for MSI were filtered out for the analysis.
Genotyping for MDACC dataset
DNA was extracted from frozen tissue blocks by QIAamp Mini columns according to manufacturer's directions (cat. no. 56304; Qiagen) and was analyzed by the Sequenom MALDI TOF MassArray system and software (Sequenom Inc, San) for the following mutations [PIK3CA (E542A, E542K, E545A, E545K, H1047R/L), AKT1 (E17K), BRAF (V600E), KRAS (284 G12X, 285 G12C, G13D)].
Microarray data analysis
The Agilent Feature Extraction software was used to identify chromosomal gains and losses, with significance being considered at P-value less than 0.01 (22). The ploidy information on the cell lines obtained from spectral karyotyping (SKY) was used to correct for the software's assumption of overall diploidy in both test and reference channels.
For expression array analysis, the Axon generated fluorescence intensity for test and reference channels were treated as follows: median background was subtracted from median signal for each probe and the 75th percentile over probes in each channel was standardized to 1,000 with a multiplicative constant. The resulting expressions were then truncated at a lower minimum value of 25. These values were then base 2 log transformed. A linear model including factors for experimental and biological variation was fit to each probe to account for differential expression by cell line model (Patient), primary vs. metastatic strains nested within patient and date blocks as a random factor: y g,i,m(i),j,k=µg + Patientg,i + Metastasisg,m(i) + Dateg,j + εg,i,m(i),j,k (1); Dateg,j ~ N(0, σ2D,g) (2); εg,i,m(i),j,k(i,j) ~ N(0, σ2e,g) (3); g=1 to 41,675 i=1, 2 m (1)=1 to 3 and m (2)=1, 2 (4); j=1,2 replicates k=1 to n k(i,j) (5).
Expression analysis was performed based on the above models generated with variable factors, such as, biological variables of the samples, date variables when experiments were performed, g refers to signal differentials, i refers to dye swaps, j refers to replicates.
TCGA and MDACC tumor set validation studies
For the analysis using TCGA sample cohort, a total of 195 patients with complete tumor profiles and clinical data were used as the data set (23). Copy Number Variation and Expression array data (RNAseq) for these patients were downloaded from the website cBioPortal.org (14). All patients who showed an expression z-score >=1.96 for the candidate genes were considered to have a gain of function altered gene status.
The second dataset from MDACC has been profiled using Agilent Microarray, which were all hybridized against a common normal colon reference pool, according to manufacturer's protocol. Lowess based normalization was performed using the Agilent Feature extraction software with background subtraction. Samples that had both expression and mutation profiling data (n=182) were only considered for the subsequent analysis. Expression data corresponding to the four candidate genes of interest was extracted for further analysis. The gene BMP7 has three probes in the dataset. The probe A_24_P91566 was filtered due to poor correlation with the other two probes-A_23_P154643 and A_23_P68487 (Pearson correlation ~0.09). The Mean expression of the remaining two probes is taken as the representative expression of BMP7 for the downstream analysis.
Chi-square or Fisher's exact test were computed to test the differences in the distribution of categorical variables between patients with genetic alteration in any of the four genes in the signature and those without any alteration as the reference group. The categorical variables included Expression Subtypes, aggressiveness (defined as having lymph node spread and/or distant metastasis), MSI status and the BRAF mutation status. To assess the association between clinical outcomes and genetic alteration status, the adjusted odds ratios (ORs) with the 95% confidence intervals (95% CI) were estimated using unconditional multivariable logistic regression analyses, with adjustment for the covariates. All statistical tests were two-sided with a statistical significance level of P<0.05. Data were analyzed using Statistical Analysis System/Genetics software program (SAS/STAT version 9.1.3; SAS Institute, Inc.).
SKY
Mitotic shake-off preparations for each of the cell lines were fixed at room temperature in Carnoy's fixative (3:1 methanol: acetic acid) for 30 min and slides were prepared by dropping cell suspensions onto wet slides. SKY was carried out on aged slides using the Human SkyPaint Probes kit according to manufacturer's protocol (Applied Spectral Imaging Inc.). Visualization and analysis were carried out with an Olympus AX70 microscope using Image Analysis (Applied Spectral Imaging Inc.) and karyotype images were generated using SKYVIEW software (Applied Spectral Imaging Inc.). Modality was determined based on an average of 5 metaphases captured for each cell line.
Fluorescence in situ hybridization (FISH)
Genomic bacterial artificial chromosome (BAC) and P1-derived artificial chromosome (PAC) clones with inserts ranging in size of 150–200 kb representing the genomic intervals of minimum common region of amplification (MCR) on chromosome 20q and residing near or containing the candidate genes were obtained from the Sanger Institute or generously provided by Dr. Craig Chinault at Baylor College of Medicine. These BAC and PAC clones were cultured and their DNA isolated using the Eppendorf miniprep kit. The DNA was then labeled in a polymerase reaction with digoxigenin-dUTP (cat. no. 11573152910; Millipore Sigma). Human Cot-1 DNA (cat. no. 15-279-011; Invitrogen; Thermo Fisher Scientific, Inc.) was added, and the DNA was precipitated in ethanol, followed by 70% ethanol wash. The DNA pellet was briefly dried in a speed-vac then re-suspended in Hybrisol (cat. no. S4040; EMD Millipore). Probe was added to the slides prepared as stated above and hybridized under coverslip in a humidified chamber for 24 h. The coverslip was then removed, the slides were washed and the probe was detected with anti-digoxigenin-rhodamine FAB (cat. no. 11207750910; Millipore Sigma) and counterstained with DAPI (1 µg/ml). Slides were examined and signals counted in 5–10 interphase cells.
Results
SKY
To validate accurate copy number estimates from the intensity normalized aCGH data, the modal number for each cell line was determined by SKY (Fig. S1). In addition, the status of chromosome 20 in the karyotypes of the cells utilized was compared with previously published karyotypes. Notable genomic changes in all the cell lines are listed in Table SII. Most rearranged chromosomes were observed previously (16). Gain of chromosome 13 was identified in KM12C, which had not been previously reported. KM12C (near diploid), KM12L4A (pseudo-triploid) as well as KM12SM (near tetraploid) karyotypes contained the translocation of chromosome 20 to 22. In the primary tumor-derived SW480 cells (pseudo-diploid) as well as the metastasis-derived SW620 (near-diploid), translocation between chromosomes 5 and 20 were consistent with the previous observations (24). Ploidy correction was employed to aid in the normalization of the fluorescence intensity ratios obtained from the array GCH experiments analyzed by the Feature Extraction software from Agilent Technologies (Version 7.5.1).
aCGH and expression analysis
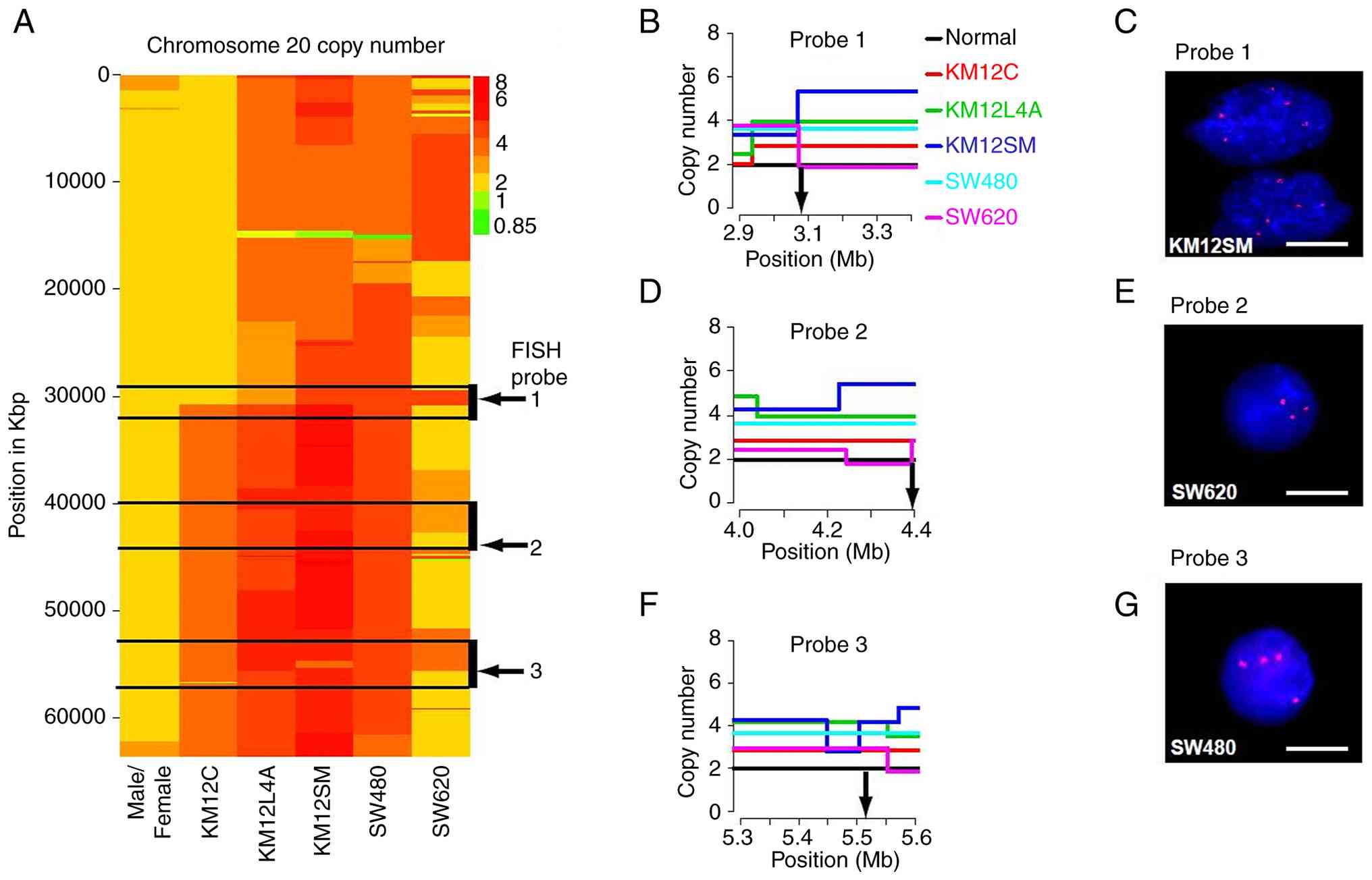
CGH to the Agilent Human Genome CGH 44A Microarray (resolution of ~75 kb) was carried out on the five cell lines, with dye swaps. Human female genomic DNA was used as the reference. The genome-wide profile was generated after circular binary segmentation analysis for each cell line (data not shown). Overall, KM12C, KM12L4A, and SW480 showed a lower level of noise in the data than KM12SM and SW620. Several genomic regions showed amplifications and deletions in more than one cell line, but since our primary aim was to identify candidate genes on the 20q amplicon, our analysis was focused on chromosome arm 20q. The high-resolution aCGH profile of chromosome 20 for each of the five cell lines is represented in a heat map (Fig. 1A), demonstrating copy gains along the q arm in all the five cell lines. After considering the copy gain profile of all the cell lines, three MCRs of amplification across the 20q arm were identified spanning 29–34 megabases (Mb), 40–45 and 54–57 Mb. Copy number verification for these intervals was carried out through interphase FISH using BAC and PAC probes located within these intervals (Fig. 1B-G, Table I). Based on FISH analysis of each probe, the genomic copy number data estimated from the aCGH analysis was confirmed.
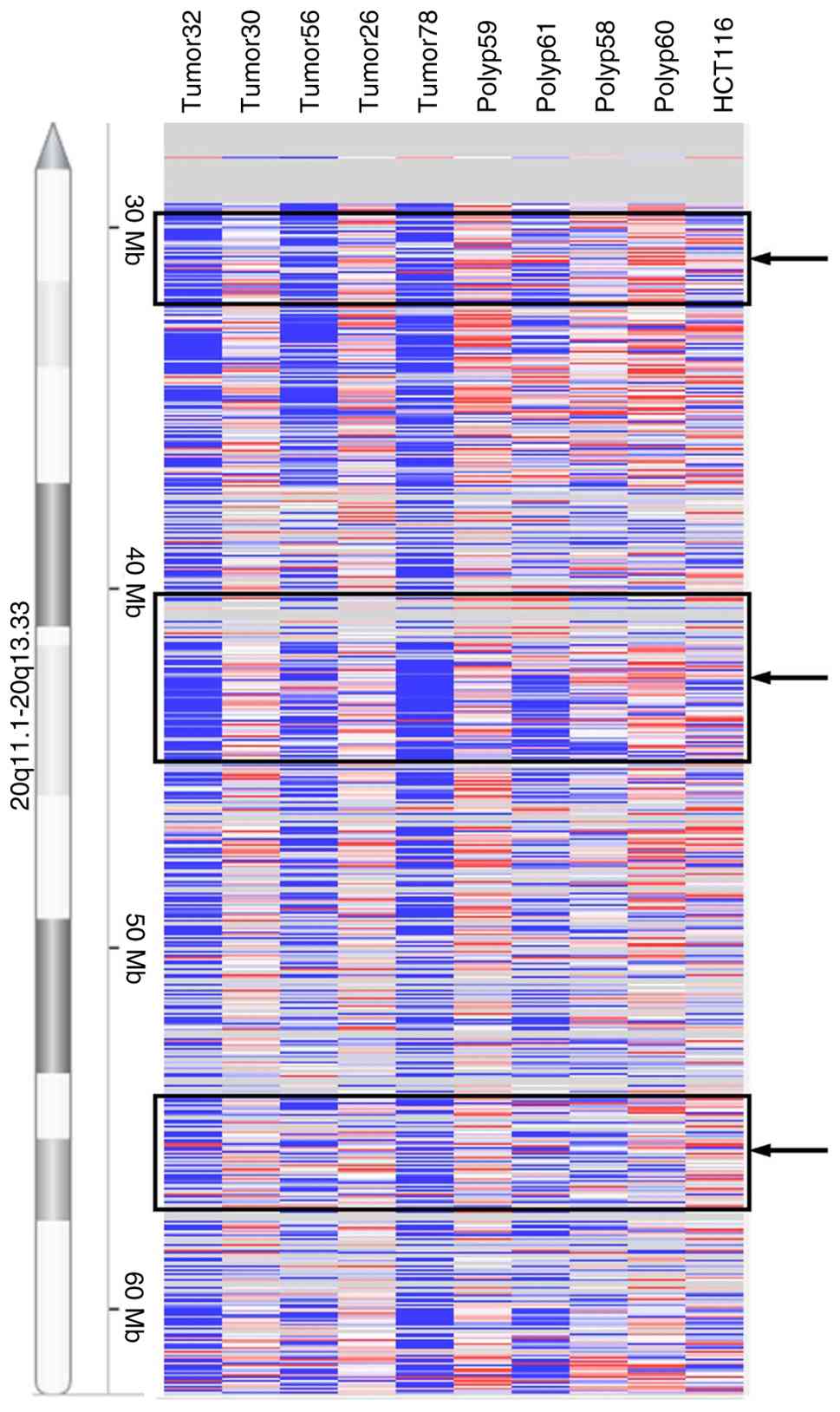
The present study was extended to primary tumor samples by performing aCGH analyses on a set of five colorectal tumor and four polyp samples. Differential amplification of varying genomic intervals within the three MCRs was detected in the tumor and the polyp samples (Fig. 2). The overall significant gains observed in polyps and tumors are included in Table SIII. The HCT116 CRC cell line, with no known chromosome 20q gain (25,26), was also investigated as a reference cell line for amplification profile and as expected, did not show any significant gains or losses on chromosome 20q.
To identify the genes with gain of expression localized in the MCRs identified above, genome-wide microarray expression analysis of the five cell lines compared with commercially available normal colon total RNA was carried out, and the expression patterns of the 1061 oligo probes for chromosome 20 were examined. It was found that there was a variety of expression patterns within these intervals, including significantly downregulated genes as well as upregulated and unchanged gene expression profiles. To focus on the genes residing within the MCRs, the data was filtered based on the chromosomal position of the genes represented in the list of 86 probes that displayed overexpression on chromosome 20q in the five cell lines. This reduced the list to 18 probes representing 14 known genes and 2 open reading frames. Further filtering of the list to include the probes showing elevated expression in the metastasis-derived cell lines led to the identification of four genes (BMP7, DNMT3B, UBE2C and YWHAB) as the candidate gene signature for further investigation. The RPL13A transcript was selected as the endogenous control for its consistent expression detected across the cell lines and reference normal colon total RNA.
RT-qPCR
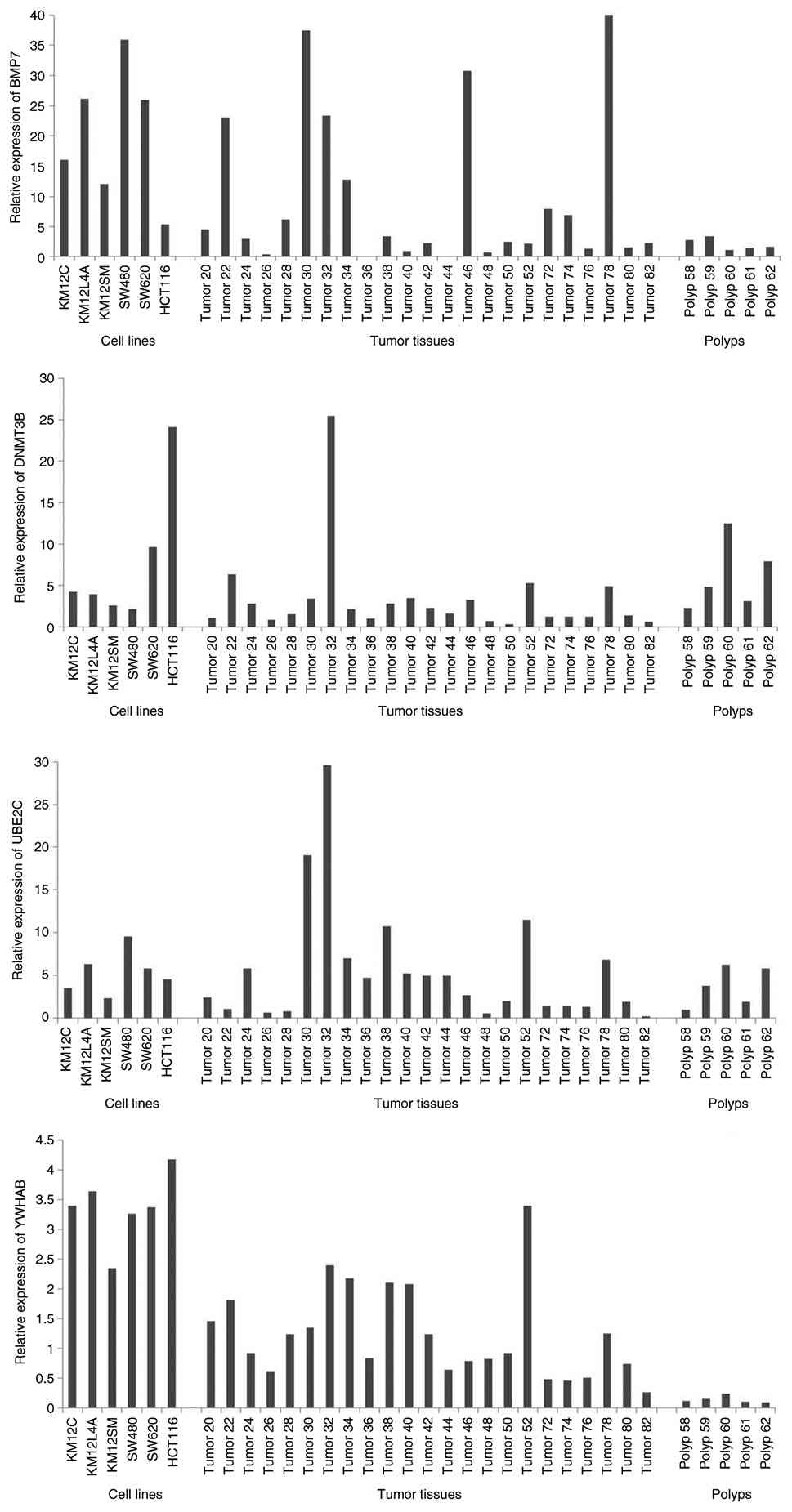
To validate the expression array data, RT-qPCR for the gene signature consisting of BMP7, DNMT3B, UBE2C and YWHAB was carried out in the cell lines and the training set of twenty-three tumors and five polyps. The expression patterns detected with both methods (expression array and RT-qPCR) were consistently altered in the same direction, with varying amplitude in the cell lines, tumors and polyps. The tumor samples showed elevated expression of the candidate genes compared with the matched normal, though the degree of increase was relatively less compared with the cell lines. A reference cell line HCT116 also revealed increased expression of the candidate genes following RT-qPCR analyses (Fig. 3) suggesting that alteration in the expression levels of these genes do not always result from gain of gene dosage. Furthermore, the tumor samples were analyzed for their MSI status. Of the 20 tumors analyzed, 16 tumors had altered status of the four gene panel and of these, eleven revealed MSS or MSI-L phenotypes.
TCGA and MDACC dataset analysis
To validate the statistical significance of the data obtained with the training set in a larger cohort of test set patient samples, copy number and mRNA expression data were analyzed in patients with CRC included in TCGA database (23), accessible through the cBioPortal for Cancer Genomics (14), as well as an independent set of colorectal adenocarcinoma samples that were annotated in-house at MD Anderson Cancer Center. Our primary aim was to investigate whether increased expression of the gene signature was associated with aggressive tumor behavior such as lymph node spread and/or presence of distant metastasis, as well as their degree of association with MSS or MSI. Table II shows the total number of patients from the TCGA dataset with an increased expression of the four candidate genes. Of the 182 patients that had complete tumor profiles, ~56% of the patients showed alteration in one or more of the four genes. A total of 74 patients showed lymph node spread and/or distant metastasis, with a significant number of these patients overexpressing the gene signature (P<0.05, TCGA data as of August 2013) (Table III). Specifically, a 2-fold increased risk for lymph-node spread/distant metastasis was observed in patients with at least one gene showing alteration (defined as ‘altered’ group; adjusted OR=1.97, 95% CI=1.05–3.67) after adjustment for sex, cancer site, family history of cancer and prior diagnosis of cancer. Furthermore, a significant association between the gene signature and absence of MSI in these patients was also observed (Table III), with majority of these patients showing the MSS phenotype, as observed in the 20 tumor samples of the training set analyzed in this study. Overall, the results of the TCGA data analyses corroborated the results obtained from the training set samples. A similar trend of overexpression associating with MSS status was also observed in the MDACC dataset, although statistical significance for this association was evident at lower stringency of z>1.28, 80% CI (P=0.03) (Fig. S2).
Table II.Incidence of 20q candidate genes alteration and driver gene mutations in patients with colorectal cancer (The Cancer Genome Atlas dataset containing 182 patients, data as of August 2013). |
Table III.Association of 20q gene signature with CRC characteristics of lymph node spread and/or distant metastasis, MSI status, Expression Subtype, Methylation Subtype and Stage of cancer (The Cancer Genome Atlas data as of August 2013). |
The four gene signature showed statistically significant association with the outcome ‘Expression subtypes’ in the TCGA data defined by the gene expression profile correlated with genomic instability. Furthermore, the gene signature showed association with additional CRC characteristics, such as, methylation status (P<0.0001) and stage of cancer (P=0.048). These findings were not surprising since expression subtype, MSI status, lymph node spread/distant metastasis and stage of cancer outcomes were themselves associated with each other (P<0.01, data not shown). Additionally, the percentage of patients with overexpression of gene signature was examined in relation to the status of known CRC ‘driver gene’ mutations. The gene signature expression tended to correlate with mutations in the APC gene (P=0.065; Table II). It was noteworthy that the frequency of patients with overexpression in the gene signature was markedly higher compared with the frequency of patients with alterations in other well-characterized genes associated with CRC such as BRAF, PIK3CA, SMAD4, FBXW7 (23) (data not shown). Interestingly, association of gene signature with absence of BRAF mutation was also observed in both the TCGA (P=0.06, z>1.96, 95% CI) and MDACC (P=0.07, z>1.28, 80% CI) datasets (Fig. S3).
Taken together, these observations suggest that expression of the chromosome 20q gene signature, identified in the present study, represents functionally significant genetic alterations cooperating with the driver gene alterations in the progression of CRC.
Discussion
The presence of 20q copy gain at the cytogenetic level has been previously reported in the CRC metastatic model cell lines of the KM and SW series (16,24). While expression analyses of these cell line models by cDNA array to identify metastasis-specific expression patterns was previously reported (27), integrated genomic and transcriptomic profiling of the 20q amplicons in these cells were not characterized in detail. In the present study, the commonly gained genomic intervals (between 29 and 34, 40 and 45, and 54 and 57 Mbs) corresponding to the 20q11.2, proximal and distal 20q13 regions, which have previously been associated with liver metastasis (28) and tumor aggressiveness (23), were identified. Chromosome 20q amplification characterizes distinct subtype of MSS-CRC unlike 20q non-amplified MSS subtype harboring mutant KRAS and BRAF oncogenes (29). Copy number gain of 20q13 has been reported to correlate with faster tumor progression and worse patient survival (8). Since inflammatory and immune response have also been implicated in the pathogenesis and prognosis of patients with CRC (30), possible involvement of the 20q amplicon genes associated with disease progression in inflammation and immune response pathways was explored.
Amplification and overexpression of the four genes in polyps suggested that elevated expression of this gene signature may be an early predisposing event in the progression of colorectal adenoma to carcinomas. This finding is reminiscent of the observations made in a previous study (28), in which allelic status of metastatic lesions was found similar to that of primary lesions. The association of the gene signature expression profile with the data from TCGA on lymph node spread and/or distant metastasis indicates the role of these genes in driving CRC progression and with worse patient prognosis, as previously described for chromosomal instability+/MSS subtype (31,32).
BMP7, UBE2C and YWHAB are directly or indirectly involved in critical cellular pathways, while DNMT3B epigenetically regulates several of these pathways through de novo DNA methylation at CpG islands. The secreted signaling molecule BMP7 (residing in the MCR between 54–57 Mbs) has been reported to mediate multiple critical cancer relevant processes, such as, activation of TAK1 downstream of KRAS, subsequently augmenting Wnt-dependent transcriptional program (33) as well as inhibition of transforming growth factor beta (TGFβ) antiproliferative signaling (34,35). Furthermore, BMP7 was found to modulate expressions of E-cadherin and MMP-9 to regulate cell migration and metastasis (36). More recently, secreted BMP7 was reported to act on macrophages and CD4+ T cells in the TME impairing pro-inflammatory and immune response (37).
Increased levels of the ubiquitin conjugating enzyme UBE2C (localized in the proximal region of 20q13 between 40–45 MB) correlates with chromosome instability accompanying tumor formation in mouse models (38) and with poor prognosis-overall survival in patients with lung adenocarcinoma and CRC (39,40). UBE2C amplification and protein overexpression has been detected during adenocarcinoma progression (39). Integrated gene expression profiling of multiple datasets derived from patients with CRC with liver metastasis has identified UBE2C as an amplified core gene for live metastasis of CRC (41,42). Additionally, UBE2C has been implicated in promoting immunotolerant proangiogenic TME (43) and inflammation-induced cell motility (44).
The 40–45 Mb MCR copy gain harbors YWHAB (14-3-3β), which mediates signal transduction through binding to phosphoserine-residues of proteins. The reported YWHAB interaction with cell division cycle 25 (CDC25) and v-raf-1 homolog 1 (RAF1) suggests a role in linking mitogenic signaling and cell cycle regulation (45,46). Increased expression of YWHAB is known to promote cell spreading and migration through its interaction with Integrin beta (47) and predictive of metastasis with worse survival in hepatocellular carcinoma and CRC (48,49). Interestingly, altered expression of YWHAB observed in peripheral blood mononuclear cells from patients with chronic inflammatory autoimmune disease suggests that the gene is involved in inflammatory response pathways (50).
Finally, increased levels of the de novo DNA methyltransferase DNMT3B (residing in the MCR between 29–34Mbs), has been associated with poor prognosis in pancreatic ductal adenocarcinoma (51), tumorigenicity in prostate cancer cells (52) as well as progression of colon microadenoma to macroadenoma (53), indicating functional role of DNMT3B in both early stages of colorectal tumor progression and also in predisposition to undergo metastasis. It is plausible that DNMT3B upregulation occurs because of chronic inflammation induced accumulation of myeloid derived suppressor cells leading to its IL-10 mediated activation and silencing of tumor suppressor IRF8 in colonic epithelial cells linking chronic inflammation to initiation and progression of colon cancer (54).
In summary, four genes were identified on chromosome 20q constituting a CRC progression-associated gene signature, which could help identify adenomas and carcinomas predisposed to undergo metastasis with worse prognosis. Association of the gene signature with aggressive tumors and poorer outcome may be a consequence of not only their role in genetic and epigenetic mechanisms driving proliferation and migration of tumor cells but also due to involvement in altered inflammation and immune responses underlying pathogenesis of CRC (30) in a subset of patients with significant clinical implications in determining appropriate therapeutic interventions. The present study has the limitation of not analyzing inflammatory and immune response pathways in the colon cancer cell line models and tissue samples investigated. Future studies to address this limitation with pre-clinical mouse models and human tumor samples is warranted to validate the proposed functional significance of the gene signature in the development of aggressive CRC.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
The authors acknowledge the technical assistance of Ms Aimee LeBlanc, Ms. Mary Brandt, Ms. Trupti Mehta (Department of Molecular Pathology, The University of Texas M.D. Anderson Cancer Center), and help from Drs David Gold, David Stivers (Department of Biostatistics, The University of Texas, M.D. Anderson Cancer Center), and Kaori Sasai (Department of Molecular Pathology, The University of Texas M.D. Anderson Cancer Center) in the present study.
Funding
The present study was supported in part by funding from the National Cancer Institute (grant no. RO1CA089716), the MDACC University Cancer Foundation, the American Legion Auxiliary Fellowship and the Frederick F. Becker Cancer Research Endowment.
Availability of data and materials
The data generated in the present study may be found in the Gene Expression Omnibus under accession numbers GSE131274 and GSE131275 or at the following URL: https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE131274; https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE131275.
Authors' contributions
JCJ, AMH, SRH and SS conceptualized the study. JCJ and AMH developed methodology, conducted investigation and validated data. JCJ and AMH confirm the authenticity of all the raw data. JCJ, AMH, YH, GM, VS, JHS, YC, RK, WT, SJM, SK and HK conducted data analysis. SK provided access to MDACC datasets. JCJ, AMH, YH and GM curated data. JCJ, AMH, SRH and SS wrote the original draft. JCJ, HK and SS wrote, reviewed and edited the manuscript. SRH and SS supervised the study. SS acquired funding. All authors read and approved the final version of the manuscript.
Ethics approval and consent to participate
All primary colorectal tumor and polyp samples including adjacent normal tissues and WBC samples were obtained under IRB approved protocols with ethics approval and participant consent approved (approval no. LAB90-018) by the Institutional Review Boards of UTMD Anderson Cancer Center and Johns Hopkins University School of Medicine.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Glossary
Abbreviations
Abbreviations:
|
aCGH |
array comparative genomic hybridization |
|
ATCC |
American Type Culture Collection |
|
CI |
confidence interval |
|
CIMP |
CpG island methylator phenotype |
|
CN gain |
copy number gain |
|
CRC |
colorectal cancer |
|
FISH |
fluorescence in situ hybridization |
|
Mb |
mega base |
|
MCR |
minimal common region |
|
MSI |
microsatellite instability |
|
MSI-H |
MSI-High |
|
MSI-L |
MSI-Low |
|
MSS |
microsatellite stable |
|
OR |
odds ratio |
|
RT-qPCR |
reverse transcription-quantitative PCR |
|
SKY |
spectral karyotyping |
|
TCGA |
The Cancer Genome Atlas |
|
TGFb |
transforming growth factor beta |
References
|
Siegel RL, Giaquinto AN and Jemal A: Cancer statistics, 2024. CA Cancer J Clin. 74:12–49. 2024.PubMed/NCBI | |
|
De Smedt L, Lemahieu J, Palmans S, Govaere O, Tousseyn T, Van Cutsem E, Prenen H, Tejpar S, Spaepen M, Matthijs G, et al: Microsatellite instable vs stable colon carcinomas: Analysis of tumour heterogeneity, inflammation and angiogenesis. Br J Cancer. 113:500–509. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
O'Connell JB, Maggard MA and Ko CY: Colon cancer survival rates with the new American joint committee on cancer sixth edition staging. J Natl Cancer Inst. 96:1420–1425. 2004. View Article : Google Scholar : PubMed/NCBI | |
|
Aragane H, Sakakura C, Nakanishi M, Yasuoka R, Fujita Y, Taniguchi H, Hagiwara A, Yamaguchi T, Abe T, Inazawa J and Yamagishi H: Chromosomal aberrations in colorectal cancers and liver metastases analyzed by comparative genomic hybridization. Int J Cancer. 94:623–629. 2001. View Article : Google Scholar : PubMed/NCBI | |
|
Hidaka S, Yasutake T, Takeshita H, Kondo M, Tsuji T, Nanashima A, Sawai T, Yamaguchi H, Nakagoe T, Ayabe H and Tagawa Y: Differences in 20q13.2 copy number between colorectal cancers with and without liver metastasis. Clin Cancer Res. 6:2712–2717. 2000.PubMed/NCBI | |
|
Mamlouk S, Simon T, Tomás L, Wedge DC, Arnold A, Menne A, Horst D, Capper D, Morkel M, Posada D, et al: Malignant transformation and genetic alterations are uncoupled in early colorectal cancer progression. BMC Biol. 18:1162020. View Article : Google Scholar : PubMed/NCBI | |
|
De Angelis PM, Stokke T, Beigi M, Mjåland O and Clausen OP: Prognostic significance of recurrent chromosomal aberrations detected by comparative genomic hybridization in sporadic colorectal cancer. Int J Colorectal Dis. 16:38–45. 2001. View Article : Google Scholar : PubMed/NCBI | |
|
Diep CB, Thorstensen L, Meling GI, Skovlund E, Rognum TO and Lothe RA: Genetic tumor markers with prognostic impact in Dukes' stages B and C colorectal cancer patients. J Clin Oncol. 21:820–829. 2003. View Article : Google Scholar : PubMed/NCBI | |
|
Aust DE, Muders M, Köhler A, Schmidt M, Diebold J, Müller C, Löhrs U, Waldman FM and Baretton GB: Prognostic relevance of 20q13 gains in sporadic colorectal cancers: A FISH analysis. Scand J Gastroenterol. 39:766–772. 2004. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang B, Wang J, Wang X, Zhu J, Liu Q, Shi Z, Chambers MC, Zimmerman LJ, Shaddox KF, Kim S, et al: Proteogenomic characterization of human colon and rectal cancer. Nature. 513:382–387. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Ptashkin RN, Pagan C, Yaeger R, Middha S, Shia J, O'Rourke KP, Berger MF, Wang L, Cimera R, Wang J, et al: Chromosome 20q amplification defines a subtype of microsatellite stable, left-sided colon cancers with wild-type RAS/RAF and better overall survival. Mol Cancer Res. 15:708–713. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Mosse YP, Greshock J, Margolin A, Naylor T, Cole K, Khazi D, Hii G, Winter C, Shahzad S, Asziz MU, et al: High-resolution detection and mapping of genomic DNA alterations in neuroblastoma. Genes Chromosomes Cancer. 43:390–403. 2005. View Article : Google Scholar : PubMed/NCBI | |
|
Lo KC, Stein LC, Panzarella JA, Cowell JK and Hawthorn L: Identification of genes involved in squamous cell carcinoma of the lung using synchronized data from DNA copy number and transcript expression profiling analysis. Lung Cancer. 59:315–331. 2008. View Article : Google Scholar : PubMed/NCBI | |
|
Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, Jacobsen A, Byrne CJ, Heuer ML, Larsson E, et al: The cBio cancer genomics portal: An open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2:401–404. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Morikawa K, Walker SM, Nakajima M, Pathak S, Jessup JM and Fidler IJ: Influence of organ environment on the growth, selection, and metastasis of human colon carcinoma cells in nude mice. Cancer Res. 48:6863–6871. 1988.PubMed/NCBI | |
|
Camps J, Morales C, Prat E, Ribas M, Capellà G, Egozcue J, Peinado MA and Miró R: Genetic evolution in colon cancer KM12 cells and metastatic derivates. Int J Cancer. 110:869–874. 2004. View Article : Google Scholar : PubMed/NCBI | |
|
Leibovitz A, Stinson JC, McCombs WB III, McCoy CE, Mazur KC and Mabry ND: Classification of human colorectal adenocarcinoma cell lines. Cancer Res. 36:4562–4569. 1976.PubMed/NCBI | |
|
Brattain MG, Fine WD, Khaled FM, Thompson J and Brattain DE: Heterogeneity of malignant cells from a human colonic carcinoma. Cancer Res. 41:1751–1756. 1981.PubMed/NCBI | |
|
Livak KJ and Schmittgen TD: Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI | |
|
Goel A, Nagasaka T, Hamelin R and Boland CR: An optimized pentaplex PCR for detecting DNA mismatch repair-deficient colorectal cancers. PLoS One. 5:e93932010. View Article : Google Scholar : PubMed/NCBI | |
|
Gibson J, Lacy J, Matloff E and Robert M: Microsatellite instability testing in colorectal carcinoma: A practical guide. Clin Gastroenterol Hepatol. 12:171–176.e1. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Olshen AB, Venkatraman ES, Lucito R and Wigler M: Circular binary segmentation for the analysis of array-based DNA copy number data. Biostatistics. 5:557–572. 2004. View Article : Google Scholar : PubMed/NCBI | |
|
Cancer Genome Atlas Network, . Comprehensive molecular characterization of human colon and rectal cancer. Nature. 487:330–337. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Melcher R, Steinlein C, Feichtinger W, Müller CR, Menzel T, Lührs H, Scheppach W and Schmid M: Spectral karyotyping of the human colon cancer cell lines SW480 and SW620. Cytogenet Cell Genet. 88:145–152. 2000. View Article : Google Scholar : PubMed/NCBI | |
|
Masramon L, Ribas M, Cifuentes P, Arribas R, García F, Egozcue J, Peinado MA and Miró R: Cytogenetic characterization of two colon cell lines by using conventional G-banding, comparative genomic hybridization, and whole chromosome painting. Cancer Genet Cytogenet. 121:17–21. 2000. View Article : Google Scholar : PubMed/NCBI | |
|
Roschke AV, Stover K, Tonon G, Schäffer AA and Kirsch IR: Stable karyotypes in epithelial cancer cell lines despite high rates of ongoing structural and numerical chromosomal instability. Neoplasia. 4:19–31. 2002. View Article : Google Scholar : PubMed/NCBI | |
|
Hegde P, Qi R, Gaspard R, Abernathy K, Dharap S, Earle-Hughes J, Gay C, Nwokekeh NU, Chen T, Saeed AI, et al: Identification of tumor markers in models of human colorectal cancer using a 19,200-element complementary DNA microarray. Cancer Res. 61:7792–7797. 2001.PubMed/NCBI | |
|
Yamamoto S, Midorikawa Y, Morikawa T, Nishimura Y, Sakamoto H, Ishikawa S, Akagi K and Aburatani H: Identification of chromosomal aberrations of metastatic potential in colorectal carcinoma. Genes Chromosomes Cancer. 49:487–496. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang B, Yao K, Zhou E, Zhang L and Cheng C: Chr20q amplification defines a distinct molecular subtype of microsatellite stable colorectal cancer. Cancer Res. 81:1977–1987. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Schmitt M and Greten FR: The inflammatory pathogenesis of colorectal cancer. Nat Rev Immunol. 21:653–667. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Sinicrope FA, Rego RL, Halling KC, Foster N, Sargent DJ, La Plant B, French AJ, Laurie JA, Goldberg RM, Thibodeau SN and Witzig TE: Prognostic impact of microsatellite instability and DNA ploidy in human colon carcinoma patients. Gastroenterology. 131:729–737. 2006. View Article : Google Scholar : PubMed/NCBI | |
|
Walther A, Houlston R and Tomlinson I: Association between chromosomal instability and prognosis in colorectal cancer: A meta-analysis. Gut. 57:941–950. 2008. View Article : Google Scholar : PubMed/NCBI | |
|
Singh A, Sweeney MF, Yu M, Burger A, Greninger P, Benes C, Haber DA and Settleman J: TAK1 inhibition promotes apoptosis in KRAS-dependent colon cancers. Cell. 148:639–650. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Alarmo EL, Rauta J, Kauraniemi P, Karhu R, Kuukasjärvi T and Kallioniemi A: Bone morphogenetic protein 7 is widely overexpressed in primary breast cancer. Genes Chromosomes Cancer. 45:411–419. 2006. View Article : Google Scholar : PubMed/NCBI | |
|
Sunde JS, Donninger H, Wu K, Johnson ME, Pestell RG, Rose GS, Mok SC, Brady J, Bonome T and Birrer MJ: Expression profiling identifies altered expression of genes that contribute to the inhibition of transforming growth factor-beta signaling in ovarian cancer. Cancer Res. 66:8404–8412. 2006. View Article : Google Scholar : PubMed/NCBI | |
|
Xu G, Tang S, Yang J, Chen K, Kang J, Zhao G, Feng F, Yang X, Zhao L, Lu Q, et al: BMP7 expression in esophageal squamous cell carcinoma and its potential role in modulating metastasis. Dig Dis Sci. 58:1871–1879. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Cortez MA, Masrorpour F, Ivan C, Zhang J, Younes AI, Lu Y, Estecio MR, Barsoumian HB, Menon H, Caetano MDS, et al: Bone morphogenetic protein 7 promotes resistance to immunotherapy. Nat Commun. 11:48402020. View Article : Google Scholar : PubMed/NCBI | |
|
van Ree JH, Jeganathan KB, Malureanu L and van Deursen JM: Overexpression of the E2 ubiquitin-conjugating enzyme UbcH10 causes chromosome missegregation and tumor formation. J Cell Biol. 188:83–100. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Bavi P, Uddin S, Ahmed M, Jehan Z, Bu R, Abubaker J, Sultana M, Al-Sanea N, Abduljabbar A, Ashari LH, et al: Bortezomib stabilizes mitotic cyclins and prevents cell cycle progression via inhibition of UBE2C in colorectal carcinoma. Am J Pathol. 178:2109–2120. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
Kadara H, Behrens C, Yuan P, Solis L, Liu D, Gu X, Minna JD, Lee JJ, Kim E, Hong WK, et al: A five-gene and corresponding protein signature for stage-I lung adenocarcinoma prognosis. Clin Cancer Res. 17:1490–1501. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
Niu L, Gao C and Li Y: Identification of potential core genes in colorectal carcinoma and key genes in colorectal cancer liver metastasis using bioinformatics analysis. Sci Rep. 11:239382021. View Article : Google Scholar : PubMed/NCBI | |
|
Takahashi Y, Ishii Y, Nishida Y, Ikarashi M, Nagata T, Nakamura T, Yamamori S and Asai S: Detection of aberrations of ubiquitin-conjugating enzyme E2C gene (UBE2C) in advanced colon cancer with liver metastases by DNA microarray and two-color FISH. Cancer Genet Cytogenet. 168:30–35. 2006. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang Q, Liu S, Zhang Q, Xiong Z, Wang AR, Myers L, Melamed J, Tang WW and You Z: Interleukin-17 promotes development of castration-resistant prostate cancer potentially through creating an immunotolerant and pro-angiogenic tumor microenvironment. Prostate. 74:869–879. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Zhou H, Wang L, Huang J, Jiang M, Zhang X, Zhang L, Wang Y, Jiang Z and Zhang Z: High EGFR_1 inside-out activated inflammation-induced motility through SLC2A1-CCNB2-HMMR-KIF11-NUSAP1-PRC1-UBE2C. J Cancer. 6:519–524. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Uchida S, Kuma A, Ohtsubo M, Shimura M, Hirata M, Nakagama H, Matsunaga T, Ishizaka Y and Yamashita K: Binding of 14-3-3beta but not 14-3-3sigma controls the cytoplasmic localization of CDC25B: binding site preferences of 14-3-3 subtypes and the subcellular localization of CDC25B. J Cell Sci. 117:3011–3020. 2004. View Article : Google Scholar : PubMed/NCBI | |
|
Van Der Hoeven PC, Van Der Wal JC, Ruurs P, Van Dijk MC and Van Blitterswijk J: 14-3-3 Isotypes facilitate coupling of protein kinase C-zeta to Raf-1: Negative regulation by 14-3-3 phosphorylation. Biochem J. 345:297–306. 2000. View Article : Google Scholar : PubMed/NCBI | |
|
Rodriguez LG and Guan JL: 14-3-3 Regulation of cell spreading and migration requires a functional amphipathic groove. J Cell Physiol. 202:285–294. 2005. View Article : Google Scholar : PubMed/NCBI | |
|
Liu TA, Jan YJ, Ko BS, Chen SC, Liang SM, Hung YL, Hsu C, Shen TL, Lee YM, Chen PF, et al: Increased expression of 14-3-3β promotes tumor progression and predicts extrahepatic metastasis and worse survival in hepatocellular carcinoma. Am J Pathol. 179:2698–2708. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
Ahluwalia P, Mondal AK, Bloomer C, Fulzele S, Jones K, Ananth S, Gahlay GK, Heneidi S, Rojiani AM, Kota V and Kolhe R: Identification and clinical validation of a novel 4 gene-signature with prognostic utility in colorectal cancer. Int J Mol Sci. 20:38182019. View Article : Google Scholar : PubMed/NCBI | |
|
Song X, Zhang Y, Zhao L, Fan J, Peng T, Ma Y, Guo N, Wang X, Liu X, Liu Z and Wang L: Analyzation of the peripheral blood mononuclear cells atlas and cell communication of rheumatoid arthritis patients based on single-cell RNA-Seq. J Immunol Res. 2023:63006332023. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang JJ, Zhu Y, Zhu Y, Wu JL, Liang WB, Zhu R, Xu ZK, Du Q and Miao Y: Association of increased DNA methyltransferase expression with carcinogenesis and poor prognosis in pancreatic ductal adenocarcinoma. Clin Transl Oncol. 14:116–124. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Gravina GL, Ranieri G, Muzi P, Marampon F, Mancini A, Di Pasquale B, Di Clemente L, Dolo V, D'Alessandro AM and Festuccia C: Increased levels of DNA methyltransferases are associated with the tumorigenic capacity of prostate cancer cells. Oncol Rep. 29:1189–1195. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Lin H, Yamada Y, Nguyen S, Linhart H, Jackson-Grusby L, Meissner A, Meletis K, Lo G and Jaenisch R: Suppression of intestinal neoplasia by deletion of Dnmt3b. Mol Cell Biol. 26:2976–2983. 2006. View Article : Google Scholar : PubMed/NCBI | |
|
Ibrahim ML, Klement JD, Lu C, Redd PS, Xiao W, Yang D, Browning DD, Savage NM, Buckhaults PJ, Morse HC III and Liu K: Myeloid-derived suppressor cells produce IL-10 to elicit DNMT3b-dependent irf8 silencing to promote colitis-associated colon tumorigenesis. Cell Rep. 25:3036–3046.e6. 2018. View Article : Google Scholar : PubMed/NCBI |