The emerging roles of YAP/TAZ in melanoma: A scoping review of the currently available evidence
- Authors:
- Published online on: May 28, 2025 https://doi.org/10.3892/wasj.2025.356
- Article Number: 68
-
Copyright : © Wardhani et al. This is an open access article distributed under the terms of Creative Commons Attribution License [CC BY 4.0].
Abstract
Introduction
Melanoma is an aggressive form of skin cancer that arises from melanocytes, the pigment-producing cells in the basal layer of the epidermis. These cells produce melanin, which protects the skin from ultraviolet (UV) radiation and provides the skin with its color (1). Melanin biosynthesis is itself a hormonally regulated metabolic pathway (2,3). However, active melanogenesis itself can generate reactive quinone intermediates that favor mutagenesis. These associations support a ‘Yin-Yang’ concept in which pigmentation may both suppress and, under certain conditions, promote melanoma progression and therapy resistance (1).
Over the past decade, the incidence of melanoma has markedly increased. In 2020, an estimated 325,000 new cases of malignant melanoma were reported worldwide, and by 2021, it was ranked as the fifth most commonly diagnosed type of cancer in the USA (4,5). Several challenges in the diagnosis and treatment of melanoma lead to the high incidence rate, such as its asymptomatic nature in the early stages of the disease, which often leads to delayed detection and metastatic progression before treatment administration (6). Additionally, melanoma exhibits a high mutation rate, allowing it to develop resistance to therapy through mutations in key oncogenic pathways (7). Modern melanoma management relies on small-molecule BRAF and MEK inhibitors for tumors harboring BRAF mutations and immune-checkpoint blockade for advanced disease (8,9). Although these regimens have markedly improved overall survival rates, the majority of cases of resistance emerge within 12 months (10). These factors contribute to melanoma being the leading cause of skin cancer-related mortality (11). Consequently, alternative molecular targets need to be explored in order to overcome resistance and extend clinical benefit, such as the Hippo pathway effectors, yes-associated protein (YAP)/transcriptional coactivator with PDZ-binding motif (TAZ).
The pathogenesis of melanoma is driven by multiple risk factors, with exposure to UV radiation being one of the most prominent. A recent, comprehensive synthesis on malignant melanoma emphasized that the incidence, molecular heterogeneity and outcomes of melanoma are strongly influenced by vitamin D status and vitamin D receptor genetics, linking cutaneous photobiology to systemic endocrine regulation (12). UV radiation is a complete carcinogen for cutaneous melanoma and also serves as a neuro-immuno-endocrine modulator that maintains systemic homeostasis (13-15). Skin exposure to UVA/UVB initiates the so-called photo-neuro-immuno-endocrine signaling. This signaling is defined by the interaction of keratinocyte-derived cytokines, neuropeptides and hormones, as they propagate through peripheral nerves and the bloodstream to the brain, endocrine organs and immune system, orchestrating stress adaptation and metabolic balance caused by UV radiation (14,15). Melanoma can also hijack neuroendocrine circuits at advanced stages, producing neurotransmitters, neurohormones and glucocorticoids that modify whole-body homeostasis to favor tumor survival (16). These systemic cues converge on intracellular oncogenic pathways that drive melanoma cell behavior. One such pathway is the mitogen-activated protein kinase (MAPK) pathway, which includes RAS, RAF, MEK and ERK. This pathway promotes melanoma proliferation, migration and survival (17). Another key pathway is the PI3K/PTEN/AKT pathway, which regulates cell survival, growth and apoptotic resistance (18). Although these pathways are well-established in melanoma biology, they represent only a portion of the complex regulatory network that promotes melanoma progression. Therefore, this highlights the need to investigate other regulators or signaling pathways that further play a role in melanoma progression.
The Hippo signaling pathway is an emerging regulatory pathway that plays a central role in controlling cell proliferation, differentiation and apoptosis. The core effectors of this pathway, YAP and TAZ, are increasingly recognized as crucial mediators of tumor progression in various types of cancer, including melanoma (19,20). In a cancerous setting, YAP and TAZ promote tumor growth through multiple mechanisms, including the activation of cell cycle regulators, such as cyclin E and interaction with transcription factors such as the AP-1 family, which drive S-phase progression in mitosis (21,22). Furthermore, YAP/TAZ has been implicated in epithelial-mesenchymal transition (EMT), a process associated with enhanced metastatic potential, as observed in breast cancer (21). The overexpression of these effectors has also been shown to be associated with a poor prognosis, therapeutic resistance, tumor dormancy and extracellular matrix remodeling, which collectively contribute to treatment failure and disease recurrence (23).
In melanoma, the role of YAP and TAZ has become the focus of recent research. Several studies have reported that the overexpression of YAP and TAZ promotes tumor proliferation, metastasis and resistance to apoptosis, leading to poor patient outcomes (24). Other studies suggest that YAP/TAZ inhibition can suppress melanoma progression, indicating their potential as therapeutic targets (25,26). Notably, their functions appear context-dependent, varying due to the complex signaling pathway involved in the specific type of cancer. Some findings suggest that YAP plays a more prominent role in tumor suppression than TAZ (25), while others have indicated the opposite (26). Functional redundancy between YAP and TAZ adds another layer of complexity, as TAZ has been observed to compensate for YAP loss in certain melanoma models (26). This context-dependent behavior is further influenced by mutation variability in melanomas, with some cases exhibiting constitutive YAP activation due to upstream mutations. By contrast, mutations occur directly on the Hippo pathway components in other cases and sometimes do not yield the same oncogenic effects (24).
However, several key gaps remain regarding the function of YAP/TAZ in melanoma. It remains unclear whether YAP/TAZ are essential drivers of therapeutic resistance or function as adaptive survival mechanisms that enable melanoma cells to persist under therapeutic pressure. Additionally, while various signaling pathways, including MAPK and PI3K, have been implicated in modulating YAP/TAZ activity, the precise upstream regulators sustaining their activation in melanoma remain incompletely understood. Furthermore, although preclinical studies have suggested that targeting YAP/TAZ can enhance therapeutic responses (27-29), whether such approaches can effectively overcome resistance to existing melanoma therapies and improve patient outcomes requires further investigation. The present scoping review aimed to systematically map the currently available literature on the role of YAP/TAZ in melanoma, identifying key mechanistic insights, therapeutic implications and knowledge gaps. By synthesizing findings from in vitro, in vivo and patient-derived studies, the present study provides a framework for future research to advance YAP/TAZ-targeted strategies in the treatment of melanoma.
Data and methods
The present scoping review followed the framework proposed by the Joanna Briggs Institute (JBI) methodology for scoping reviews (30) to ensure a systematic and transparent approach to collating existing literature on YAP/TAZ in melanoma. The results were reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Extension for Scoping Reviews (PRISMA-ScR) guidelines (31,32).
A literature search was conducted across multiple databases, including Medline through PubMed and Cochrane Central. The search strategy used medical subject headings (MeSH) terms to ensure sensitivity using the following terms: [‘YAP-Signaling Proteins’ (MeSH Terms) OR ‘Transcriptional Coactivator with PDZ-Binding Motif Proteins’ (MeSH Terms)] AND ‘melanoma’[MeSH Terms]. Additional relevant articles may be further identified by manually screening the reference list from included studies whenever appropriate. This search used up-to-date evidence published up to June, 2024.
All retrieved records were imported into Mendeley for reference management, and duplicates were removed. The studies were selected using a two-phase approach with an initial title and abstract screening followed by a full-text review by two independent reviewers. The articles were screened using the eligibility criteria in a table. Data were extracted using a standardized form with the study authors, methods and design, and key findings collected. Qualitative synthesis was then conducted to summarize trends and discoveries, identify research gaps and collate them into themes. No ethical approval was sought since the study only used publicly available peer-reviewed articles. The eligibility criteria used in the present study are listed in Table I.
Results
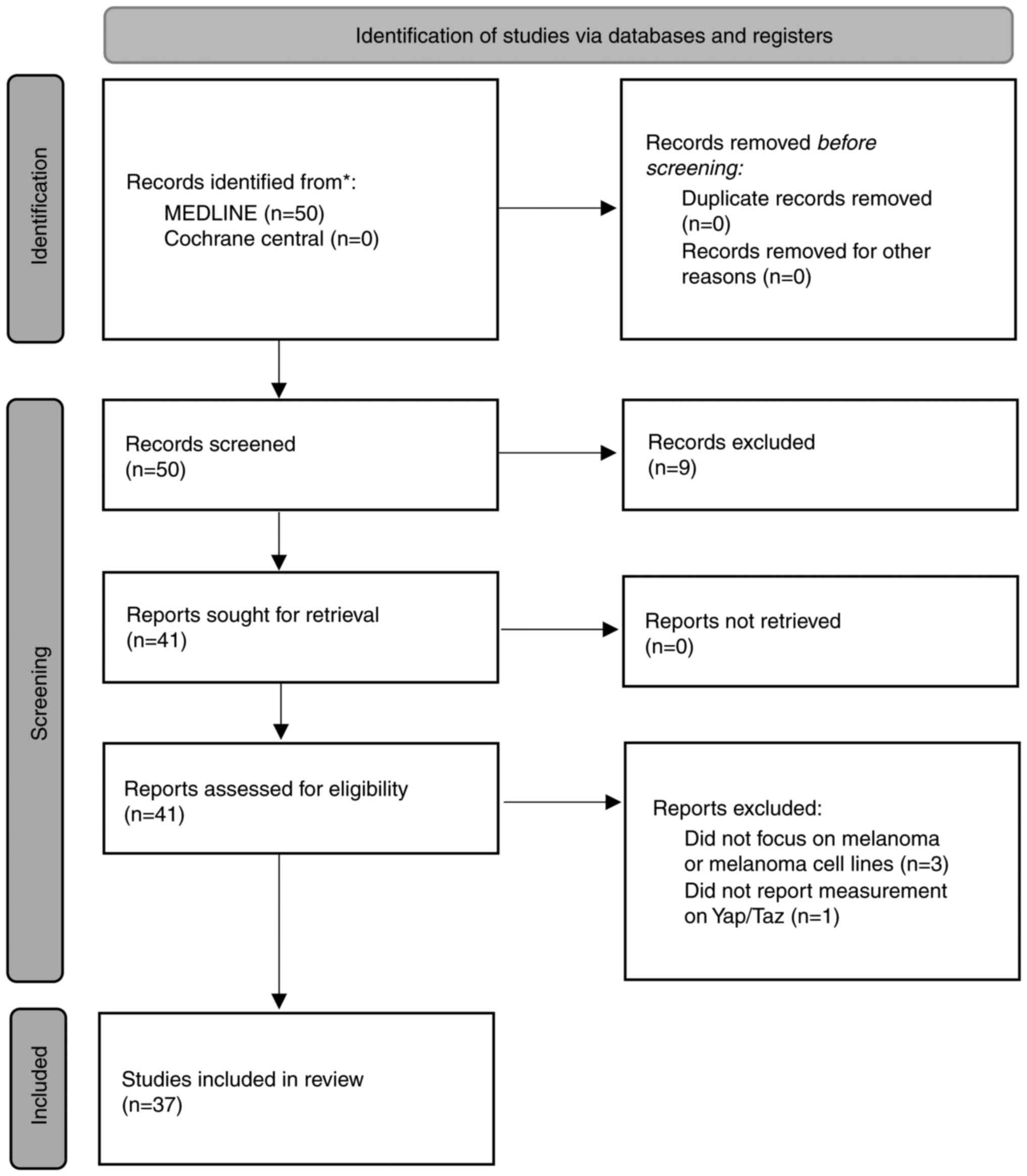
The present scoping review included 37 articles (Fig. 1). The present scoping review identified that YAP/TAZ plays a crucial role in the development of melanoma, influencing processes from proliferation to metastasis. Unlike in other types of cancer, where YAP/TAZ exhibits both pro-tumorigenic and protective roles, YAP/TAZ primarily functions as critical mediators of tumorigenesis in melanoma. Virtually all studies included in the scoping present review highlight their role as potential therapeutic targets. Multiple oncogenic pathways associated with YAP/TAZ activity were identified.
Hippo signaling and crosstalk between YAP/TAZ and other oncogenic signaling pathways
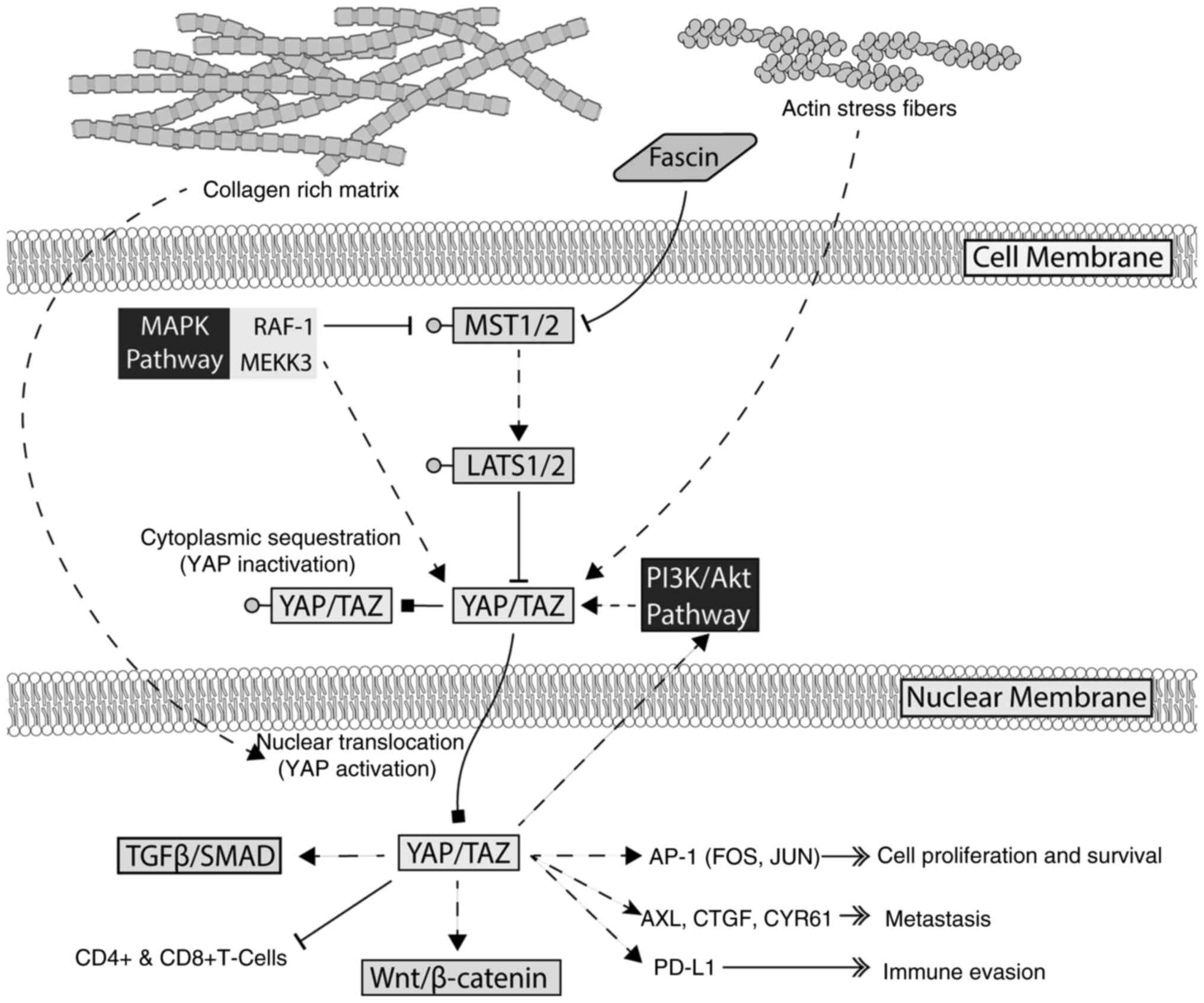
The main signaling regulator of YAP/TAZ activity is the Hippo pathway, which functions as a tumor suppressor by inactivating YAP/TAZ through phosphorylation. The knockdown of large tumor suppressor kinase (LATS)1, a core kinase of the Hippo pathway, enhances YAP nuclear localization, and increases melanoma cell proliferation and survival (33). However, YAP activation can also be sustained through Hippo-independent mechanisms, particularly MAPK signaling. The study by Park et al (28) identified MAP3K3 (MEKK3) as a crucial modulator of YAP stability. By phosphorylating YAP at serine 405, MAP3K3 prevents YAP degradation, enhancing its transcriptional activity (28). Additionally, RAF-1, another key MAPK signaling component, was previously found to regulate YAP/TAZ expression (34). The knockdown of RAF-1 reduced YAP and TAZ levels, inhibiting melanoma cell proliferation, migration and invasion while promoting apoptosis. RAF-1 was found to interact with MST2, linking the MAPK and Hippo pathways, though MST2 expression remains unchanged upon RAF-1 knockdown (34). This suggests that MAPK signaling enhances YAP stability by preventing its degradation and engages in crosstalk with Hippo signaling through RAF-1/MST2 interactions, further promoting the progression of melanoma.
The present study also identified several signaling pathways in cancer affecting the function of YAP/TAZ in melanoma. The study by Lüönd et al (35) identified the interplay between TGFβ/SMAD and β-catenin signaling, a well-established growth-promoting pathway in cancer. The findings of their study indicated that TGFβ signaling is essential for maintaining YAP/TAZ activity, while concurrent β-catenin activation further enhanced tumor growth. This finding confirmed by another study identifying Tankyrase inhibitors that both inhibit WNT/β-catenin and YAP signaling, re-sensitizing melanoma to checkpoint inhibitor therapy (36). Another key pathway influenced by YAP/TAZ is the AP-1 (FOS/JUN) transcription factor (37) YAP/TAZ directly regulates AP-1 activity, a key effector in tumorigenesis. The inhibition of AP-1 suppresses YAP-driven tumorigenesis, reinforcing its role as a crucial downstream mediator. Additionally, metabolic and epigenetic regulators contribute to YAP/TAZ-driven tumorigenesis. SIRT5, a critical energy homeostasis regulator, has been shown to mediate the acetylation of TAZ, enhancing its transcriptional activity (38). This finding establishes a direct link between metabolic signaling and melanoma progression, underscoring the central role of YAP/TAZ in oncogenesis.
Aside from metabolic regulation, the extracellular matrix also plays a crucial role in modulating YAP/TAZ activity. The study by Miskolczi et al (39) found that abundant collagen promoted YAP nuclear localization, facilitating a proliferative and differentiated melanoma phenotype. By contrast, TGFβ signaling promoted an invasive and dedifferentiated phenotype (39). Furthermore, actin cytoskeleton remodeling through stress fiber formations has been implicated in YAP/TAZ nuclear localization, contributing to resistance against BRAF inhibitors (40). Fascin, an actin-bundling protein, has been implicated in YAP activation by inhibiting MST2 kinase activity, a key upstream component of the Hippo pathway (41). This inhibition prevents the LATS-mediated phosphorylation of YAP, thereby sustaining its nuclear translocation and transcriptional activity. YAP, but not TAZ, has also been implicated explicitly in melanoma migration and metastasis through the direct regulation of actin-related protein 2/3 complex subunit 5(25). Additionally, interactions with cancer-associated fibroblasts via N-cadherin enhance tumor survival through PI3K/AKT signaling (42), further linking the tumor microenvironment with the progression of melanoma. These findings highlight the role of the extracellular matrix and cytoskeletal dynamics in modulating YAP/TAZ function and suggest that these functions may become therapeutic targets.
The present scoping review also identified several genetic and regulatory factors influencing YAP/TAZ activity. Three single nucleotide polymorphisms (YAP1 rs11225163, TEAD1 rs7944031 and TEAD4 rs1990330) have been found to be associated with melanoma-specific survival, highlighting potential genetic predispositions (43). RNA-based regulatory mechanisms were also evident, with multiple microRNAs (miRNAs/miRs) modulating YAP/TAZ expression. Notably, miR-590-5p is a tumor suppressor directly targeting YAP1, reducing melanoma proliferation (44). This miRNA is further regulated by lncRNA-ATB through endogenous competition (45). Another identified miRNA, miR-550a-3-5, suppresses YAP expression, decreasing cell proliferation and increasing sensitivity to BRAF inhibition, although the precise mechanisms involved remain unclear (46). The epigenetic regulation of the function of YAP/TAZ was also shown in the study by Luo et al (47). In their study, the transcriptional coactivator, PPARG coactivator 1α (PGC1α), a regulator of mitochondrial metabolism, was epigenetically silenced through EZH2-mediated histone (H3K27me3) modifications. This epigenetic silencing promoted the progression of melanoma by increasing the expression of WNT5A, stabilizing the YAP protein (47).
Therapeutic targeting of YAP/TAZ in melanoma
Given its involvement in multiple oncogenic pathways, tumor microenvironment interactions and resistance mechanisms, studies have shown that the expression of YAP/TAZ in melanoma is largely deleterious. TAZ nuclear localization amplifies TEAD transcriptional activity, accelerating melanoma proliferation and tumorigenesis (48). It has also been identified that a high expression of YAP/TAZ is strongly linked to increased tumor thickness, invasion depth and the number of lymph node metastases (27,49,50). YAP/TAZ knockdown can reduce melanoma invasiveness, colony formation and lung colonization, partially mediated by cellular communication network factor 2 (CCN2) expression (51,52). However, YAP-driven metastasis is primarily dependent on TEAD transcriptional activity (27,53), as mutations in other YAP domains (WW and PDZ-binding motif) had a minimal effect (53). YAP also influences the immune landscape of melanoma. Stampouloglou et al (54) demonstrated that YAP deletion heightened T-cell activation, leading to increased CD4+ and CD8+ T-cell infiltration into tumors, subsequently suppressing tumor growth (54). RNA sequencing further revealed the role of YAP role in regulating T-cell activation and migration-related genes (54). An increased YAP activity has also been shown to be associated with CD8+ T-cell exhaustion and MAPK inhibitor resistance (55). Notably, Kim et al (56) observed that YAP activation in BRAF-resistant melanoma cells increased programmed death-ligand 1 expression, enabling immune evasion, although anti-programmed cell death protein 1 therapy reversed this effect. Collectively, these findings reinforce YAP/TAZ as central drivers of melanoma pathogenesis, linking them to multiple oncogenic pathways, tumor microenvironment interactions and regulatory networks. Their influence on therapeutic resistance and immune evasion further underscores their potential as therapeutic targets, necessitating research into new inhibitors and combination therapies.
Several studies have demonstrated the beneficial effects of inhibiting YAP signaling in melanoma. Lüönd et al (35) investigated the hierarchical association between TGFβ/SMAD, Hippo/YAP/TAZ and Wnt/β-catenin pathways, revealing that inhibiting YAP/TAZ could serve as a therapeutic strategy in melanoma by disrupting the crosstalk with other oncogenic pathways. Ma et al (57) revealed that lactamase beta (LACTB), a mitochondrial protein, suppressed melanoma progression by inhibiting PP1A-mediated YAP dephosphorylation, thereby preventing YAP activation. In another study, in an in vivo metastasis model, targeting the HU177 collagen epitope inhibited YAP nuclear accumulation and CDK5 phosphorylation and was shown to decrease melanoma migration and metastasis (58). YAP activation is identified as a critical driver of anoikis resistance and melanoma metastasis. Anoikis-resistant melanoma cells exhibit higher YAP activation, increased migration and enhanced invasion, features which are crucial for metastatic dissemination. In another study, the pharmacological inhibition of YAP or genetic knockdown significantly reduced tumor invasion and metastatic potential (59). Sanchez and Aplin (60) demonstrated that YAP/TAZ knockdown reduced melanoma invasiveness, colony formation and lung colonization, revealing CCN2 as a downstream effector in promoting metastasis. Zhang et al (49) further established that YAP activity was elevated in invasive melanoma cell lines and associated with spontaneous metastasis in vivo, with AXL, THBS1 and CYR61 acting as key mediators of metastasis. Kazimierczak et al (33) studied LATS1, a key regulator of YAP/TAZ, and found that modulating its activity could impact melanogenesis, potentially influencing melanoma therapy. Kim et al highlighted the role of PIN1 in YAP/TAZ regulation, suggesting that targeting PIN1 could be a viable approach to suppress melanoma progression (48).
Several studies have explored the inhibition of YAP through previously identified drugs. Combination therapy using bromodomain and extraterminal domain (BET) and histone deacetylase (HDAC) inhibitors synergistically induces apoptosis in melanoma cells, including BRAF-resistant lines, by suppressing YAP and AKT signaling (29). These results are supported by another study which found an association between histone modifications and YAP modifications, suggesting that epigenetic regulators of YAP activity could serve as therapeutic targets (47). These findings suggest that targeting upstream modulators of YAP dephosphorylation may be a viable strategy to inhibit its oncogenic activity. The studies by Ryu et al (27) and Kim et al (48) investigated verteporfin, a known YAP inhibitor, and its effects on melanoma. The study found that nuclear YAP localization was associated with aggressive melanoma features, and verteporfin effectively disrupted YAP signaling, reducing melanoma cell proliferation and survival. This suggests that verteporfin may be a viable therapeutic strategy for targeting YAP-driven melanoma. Hajimoradi Javarsiani et al (61) explored metformin and its effects on the Hippo pathway in melanoma. They demonstrated that metformin treatment decreased melanoma cell viability and significantly suppressed melanoma proliferation when combined with dacarbazine. Their findings indicate that metformin may exert anti-melanoma effects by modulating YAP/TAZ activity, thus rendering it a potential adjuvant therapy.
YAP has emerged as a critical regulator of melanoma resistance to targeted therapies, particularly BRAF inhibitors. BRAF inhibitors selectively target the BRAF kinase and interfere with the MAPK signaling pathway, which controls cell proliferation and survival and was previously identified to interact with YAP/TAZ. Multiple studies have demonstrated that YAP activation and nuclear localization contribute to resistance. In BRAF-mutant melanoma, a genome-wide CRISPR/Cas9 screen identified solute carrier family 35 member B2 (SLC35B2), a sulfate transporter, as a novel modulator of YAP-mediated resistance (62). The loss of SLC35B2 led to reduced heparan sulfate expression, inhibited receptor tyrosine kinase activity, and sensitized melanoma cells to BRAF inhibitors. Another study by Misek et al (63) identified ibrutinib as a potential therapeutic strategy. Ibrutinib, a Bruton tyrosine kinase (BTK) inhibitor, reversed vemurafenib resistance by reducing YAP1 nuclear localization, acting through off-target inhibition of Src family kinases. In de-differentiated melanoma cells, Rho-mediated signaling, a signaling pathway involved in cytoskeletal signaling, was identified as a major driver of BRAF resistance (64). Inhibiting RhoA-ROCK-MRTF/YAP signaling restored sensitivity to vemurafenib, emphasizing the importance of YAP signaling in treatment resistance. These studies suggest that targeting YAP directly or through their upstream kinases may overcome treatment resistance in melanoma. Promising strategies include verteporfin, which disrupts YAP signaling, and combination therapies with BET and HDAC inhibitors that induce apoptosis in resistant melanoma cells.
Discussion
The findings of the present scoping review reinforce the critical role of YAP/TAZ as key regulators of melanoma progression, solidifying their position as central components of multiple oncogenic pathways, tumor microenvironment interactions and therapeutic resistance. Although YAP/TAZ have been extensively studied in various cancers, research indicates their function in melanoma is uniformly oncogenic. This is in contrast to other malignancies, where YAP/TAZ exhibit dual roles depending on the cellular context (21). The findings highlight the significance of YAP/TAZ in melanoma and their potential as therapeutic targets. A summary of YAP/TAZ activity in melanoma is presented in Fig. 2.
Studies have linked YAP/TAZ activation to EMT, (65) metastasis (53,66), and therapeutic resistance in several types of cancer (59,67,68). These findings further support the role of YAP/TAZ in these carcinogenic processes in melanoma. Studies have shown that the Hippo pathway is not the sole signaling route affecting YAP/TAZ in melanoma. Several alternative signaling pathways were identified, which are independent of the Hippo pathway, including MAPK, PI3K and TGFβ, which sustain YAP/TAZ activity (34,35). These findings suggest a potential loss of control by the Hippo pathway and highlight how multiple growth-promoting pathways converge on YAP/TAZ, presenting these proteins as potential therapeutic targets. Notably, these melanoma-specific effects are in contrast to the context-dependent behavior of YAP/TAZ, which has been reported in several other malignancies, such as breast cancer (69), renal cell carcinoma (70), esophageal (71) and lung cancer (72). A recent study demonstrated that YAP may function as a tumor suppressor in a specific context through interactions between TEAD and other proteins. For example, YAP disrupts the TEAD-Erα transcriptional complex in ER-positive breast cancer, hampering estrogen-driven proliferation (69). YAP also antagonizes HIF-2α signaling in renal clear-cell carcinoma and limits its growth (70). A large CRISPR-based pan-cancer screen also identified mutually exclusive pro- and anti-cancer YAP/TEAD transcriptional programs that are lineage-restricted (73). That study identified that YAP drives pro- and anti-cancer activity depending on the specific cancers. These differences underscore the need for a more in-depth understanding of the melanoma-specific regulatory and signaling mechanisms of YAP/TAZ.
Resistance to targeted therapies remains a significant challenge in cancer treatment, particularly with BRAF inhibitors in melanoma. A recent study demonstrated that BRAF-resistant melanoma cells maintain nuclear YAP through Hippo-independent, compensatory signaling pathways, most prominently MAP3K3-mediated phosphorylation that prevents lysosomal degradation of YAP (28), and a RhoA-ROCK-MRTF/YAP axis triggered by cell dedifferentiation (64). These studies identified that targeting YAP re-sensitizes tumor cells to therapy. Similar phenomena have been observed in other types of cancer, such as lung cancer, where YAP/TAZ mediates acquired resistance to targeted therapies. Several studies have demonstrated that YAP/TAZ inhibition can reverse tumor resistance. Notably, verteporfin, a YAP/TEAD interaction inhibitor, successfully reverses resistance to paclitaxel and gefitinib (71,72,74). Given these findings, investigating verteporfin in the context of BRAF-resistant melanoma is an intriguing avenue for future research. Additional adaptive routes include the loss of the heparan-sulfate transporter SLC35B2, which heightens receptor-tyrosine-kinase signaling and amplifies YAP-dependent resistance to MAPK inhibition (62), and actin-cytoskeleton remodeling that strengthens the YAP/TAZ nuclear accumulation (40). Targeting these upstream nodes, alone or combined with verteporfin, may further enhance re-sensitization strategies.
Beyond therapeutic resistance, YAP activation also drives metabolic adaptations supporting tumor survival. In melanoma, silencing PGC-1α, a key regulator of mitochondrial metabolism, increases YAP activity, whereas the expression of LACTB, a mitochondrial protein, inhibits YAP activity (47,57). These findings demonstrate the ability of YAP to influence metabolic adaptation, allowing tumor cells to survive in hostile conditions through glycolytic metabolism. Previous studies have highlighted the role of YAP/TAZ in metabolic regulation by increasing the expression of almost all glycolytic enzymes and glucose transporters (75,76). These findings suggest that combining metabolic inhibitors with YAP/TAZ-targeting agents could help overcome treatment resistance in melanoma. YAP/TAZ has also been shown to mediate treatment resistance through immune evasion. A previous study has linked YAP activity with PD-L1 expression and the effectiveness of PD-L1 inhibition in reversing YAP-induced immune evasion (56). However, that study did not explore the specific effects of YAP activation on immune cell functions in melanoma.
Despite strong preclinical evidence supporting the tumor-promoting role of YAP/TAZ in melanoma, translating these findings into clinical applications remains challenging. To the best of our knowledge, no studies to date have specifically explored verteporfin, a YAP/TEAD interaction inhibitor, in melanoma. However, several studies have investigated other drugs with off-target effects on YAP/TAZ. For instance, ibrutinib, a BTK inhibitor, has been shown to re-sensitize melanoma to chemotherapy through its off-target effects on YAP (63). However, concerns about toxicity persist, as ibrutinib has been shown to be associated with an uncertain risk of developing skin cancers (77). Other research has explored the combination of BET and HDAC inhibitors for epigenetic modulation, demonstrating synergy and enhanced effectiveness through YAP signaling inhibition (29). These studies highlight the promising potential of YAP/TAZ as therapeutic targets in melanoma. While pan-TEAD inhibitors and YAP-disrupting drugs, such as verteporfin exhibit antitumor activity in pre-clinical melanoma models, the systemic suppression of Hippo signaling can provoke hepatotoxicity, nephrotoxicity, immunosuppression, and impaired tissue regeneration (78). Several strategies to mitigate these effects in future studies include delivery systems, such as nanoparticles or antibody conjugates to confine inhibitor exposure to tumors and combination regimens that allow dose reduction. Given the emerging significance of YAP/TAZ, further research is warranted to evaluate the effects of YAP/TAZ-targeting therapies in clinical trials. Additionally, further research is required focus on selective targeting strategies to minimize off-target effects and reduce toxicity.
The present scoping review established the central role of YAP/TAZ in melanoma pathogenesis and therapeutic resistance. While their oncogenic role in melanoma is well-documented, their context-dependent regulation within the tumor microenvironment warrants further investigation. These findings emphasize that therapeutic strategies need to be carefully designed, with the preferential inhibition of YAP/TAZ in melanoma cells being a more viable treatment approach. Future research is required to focus on developing selective inhibitors and a more comprehensive characterization of YAP/TAZ signaling pathways in melanoma through functional screens and gene manipulation studies. To refine therapeutic strategies, a more in-depth understanding of tumor-specific YAP/TAZ dependencies is warranted. For example, comprehensive CRISPR functional screens and in vivo models are required to map melanoma-specific YAP/TAZ dependencies. These data can help guide selective YAP/TAZ-targeted therapies and combination strategies capable of circumventing drug resistance, while minimizing off-target toxicity. Emerging evidence also links YAP/TAZ to immune modulation (54) and metabolic adaptations (47,75,76) in cancer, although research in these areas remains limited. Further exploration of these pathways could lead to the development of novel therapeutic strategies to overcome drug resistance and improve patient survival outcomes.
Acknowledgements
The authors would like to thank the Maranatha Christian University for providing the necessary facilities.
Funding
Funding: The present study was funded through the Associate Professor Internal Research Grant from Maranatha Christian University.
Availability of data and materials
The data generated in the present study may be requested from the corresponding author.
Authors' contributions
SRW and AS conceived the study. AS designed and conducted the search strategy. SRW, RSS and ALB screened abstracts and full texts of the articles for the scoping review. RSS and AS performed the thematic analysis. SRW and RSS drafted the initial manuscript, and all authors contributed to the final version of the manuscript. SRW, AS and RSS confirm the authenticity of all the raw data. All authors have read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
|
Slominski RM, Sarna T, Płonka PM, Raman C, Brożyna AA and Slominski AT: Melanoma, melanin, and melanogenesis: The Yin and Yang relationship. Front Oncol. 12(842496)2022.PubMed/NCBI View Article : Google Scholar | |
|
Slominski A, Zmijewski MA and Pawelek J: L-tyrosine and L-dihydroxyphenylalanine as hormone-like regulators of melanocyte functions. Pigment Cell Melanoma Res. 25:14–27. 2012.PubMed/NCBI View Article : Google Scholar | |
|
Slominski A, Tobin DJ, Shibahara S and Wortsman J: Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol Rev. 84:1155–1228. 2004.PubMed/NCBI View Article : Google Scholar | |
|
Arnold M, Singh D, Laversanne M, Vignat J, Vaccarella S, Meheus F, Cust AE, de Vries E, Whiteman DC and Bray F: Global burden of cutaneous melanoma in 2020 and projections to 2040. JAMA Dermatol. 158:495–503. 2022.PubMed/NCBI View Article : Google Scholar | |
|
Roky AH, Islam MM, Ahasan AMF, Mostaq MS, Mahmud MZ, Amin MN and Mahmud MA: Overview of skin cancer types and prevalence rates across continents. Cancer Pathog Ther. 3:89–100. 2024.PubMed/NCBI View Article : Google Scholar | |
|
Ward WH, Lambreton F, Goel N, Yu JQ and Farma JM: Clinical presentation and staging of melanoma. In: Cutaneous Melanoma: Etiology and Therapy. Codon Publications, pp79-89, 2017. | |
|
Kolathur KK, Nag R, Shenoy PV, Malik Y, Varanasi SM, Angom RS and Mukhopadhyay D: Molecular susceptibility and treatment challenges in melanoma. Cells. 13(1383)2024.PubMed/NCBI View Article : Google Scholar | |
|
Rizos H, Menzies AM, Pupo GM, Carlino MS, Fung C, Hyman J, Haydu LE, Mijatov B, Becker TM, Boyd SC, et al: BRAF inhibitor resistance mechanisms in metastatic melanoma: Spectrum and clinical impact. Clin Cancer Res. 20:1965–1977. 2014.PubMed/NCBI View Article : Google Scholar | |
|
Nebhan CA and Johnson DB: Pembrolizumab in the adjuvant treatment of melanoma: Efficacy and safety. Expert Rev Anticancer Ther. 21:583–590. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Luebker SA and Koepsell SA: Diverse mechanisms of BRAF inhibitor resistance in melanoma identified in clinical and preclinical studies. Front Oncol. 9(268)2019.PubMed/NCBI View Article : Google Scholar | |
|
Saginala K and Barsouk A, Aluru JS, Rawla P and Barsouk A: Epidemiology of melanoma. Med Sci (Basel). 9(63)2021. | |
|
Slominski RM, Kim TK, Janjetovic Z, Brożyna AA, Podgorska E, Dixon KM, Mason RS, Tuckey RC, Sharma R, Crossman DK, et al: Malignant melanoma: An overview, new perspectives, and vitamin D signaling. Cancers (Basel). 16(2262)2024.PubMed/NCBI View Article : Google Scholar | |
|
Zamudio Díaz DF, Busch L, Kröger M, Klein AL, Lohan SB, Mewes KR, Vierkotten L, Witzel C, Rohn S and Meinke MC: Significance of melanin distribution in the epidermis for the protective effect against UV light. Sci Rep. 14(3488)2024.PubMed/NCBI View Article : Google Scholar | |
|
Slominski RM, Chen JY, Raman C and Slominski AT: Photo-neuro-immuno-endocrinology: How the ultraviolet radiation regulates the body, brain, and immune system. Proc Natl Acad Sci USA. 121(e2308374121)2024.PubMed/NCBI View Article : Google Scholar | |
|
Slominski AT, Zmijewski MA, Plonka PM, Szaflarski JP and Paus R: How UV light touches the brain and endocrine system through skin, and why. Endocrinology. 159:1992–2007. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Slominski RM, Raman C, Chen JY and Slominski AT: How cancer hijacks the body's homeostasis through the neuroendocrine system. Trends Neurosci. 46:263–275. 2023.PubMed/NCBI View Article : Google Scholar | |
|
Kim HJ and Kim YH: Molecular frontiers in melanoma: Pathogenesis, diagnosis, and therapeutic advances. Int J Mol Sci. 25(2984)2024.PubMed/NCBI View Article : Google Scholar | |
|
Strashilov S and Yordanov A: Aetiology and pathogenesis of cutaneous melanoma: Current concepts and advances. Int J Mol Sci. 22(6395)2021.PubMed/NCBI View Article : Google Scholar | |
|
Fu M, Hu Y, Lan T, Guan KL, Luo T and Luo M: The Hippo signalling pathway and its implications in human health and diseases. Signal Transduct Target Ther. 7(376)2022.PubMed/NCBI View Article : Google Scholar | |
|
Zhong Z, Jiao Z and Yu FX: The Hippo signaling pathway in development and regeneration. Cell Rep. 43(113926)2024.PubMed/NCBI View Article : Google Scholar | |
|
Luo J, Deng L, Zou H, Guo Y, Tong T, Huang M, Ling G and Li P: New insights into the ambivalent role of YAP/TAZ in human cancers. J Exp Clin Cancer Res. 42(130)2023.PubMed/NCBI View Article : Google Scholar | |
|
Guo Y, Luo J, Zou H, Liu C, Deng L and Li P: Context-dependent transcriptional regulations of YAP/TAZ in cancer. Cancer Lett. 527:164–173. 2022.PubMed/NCBI View Article : Google Scholar | |
|
Piccolo S, Panciera T, Contessotto P and Cordenonsi M: YAP/TAZ as master regulators in cancer: Modulation, function and therapeutic approaches. Nat Cancer. 4:9–26. 2023.PubMed/NCBI View Article : Google Scholar | |
|
Leask A, Nguyen J, Naik A, Chitturi P and Riser BL: The role of yes activated protein (YAP) in melanoma metastasis. iScience. 27(109864)2024.PubMed/NCBI View Article : Google Scholar | |
|
Lui JW, Moore SPG, Huang L, Ogomori K, Li Y and Lang D: YAP facilitates melanoma migration through regulation of actin-related protein 2/3 complex subunit 5 (ARPC5). Pigment Cell Melanoma Res. 35:52–65. 2022.PubMed/NCBI View Article : Google Scholar | |
|
Kazimierczak U, Przybyla A, Smielowska M, Kolenda T and Mackiewicz A: Targeting the Hippo pathway in cutaneous melanoma. Cells. 13(1062)2024.PubMed/NCBI View Article : Google Scholar | |
|
Ryu HJ, Kim C, Jang H, Kim SI, Shin SJ, Chung KY, Torres-Cabala C and Kim SK: Nuclear localization of yes-associated protein is associated with tumor progression in cutaneous melanoma. Lab Invest. 104(102048)2024.PubMed/NCBI View Article : Google Scholar | |
|
Park S, Ryu WJ, Kim TY, Hwang Y, Han HJ, Lee JD, Kim GM, Sohn J, Kim SK, Kim MH and Kim J: Overcoming BRAF and CDK4/6 inhibitor resistance by inhibiting MAP3K3-dependent protection against YAP lysosomal degradation. Exp Mol Med. 56:987–1000. 2024.PubMed/NCBI View Article : Google Scholar | |
|
Heinemann A, Cullinane C, De Paoli-Iseppi R, Wilmott JS, Gunatilake D, Madore J, Strbenac D, Yang JY, Gowrishankar K, Tiffen JC, et al: Combining BET and HDAC inhibitors synergistically induces apoptosis of melanoma and suppresses AKT and YAP signaling. Oncotarget. 6:21507–21521. 2015.PubMed/NCBI View Article : Google Scholar | |
|
Peters MDJ, Godfrey C, McInerney P, Munn Z, Tricco AC and Khalil H: Scoping reviews. In: JBI Manual for Evidence Synthesis. Aromataris E, Lockwood C, Porritt K, Pilla B and Jordan Z (eds). JBI, 2024. Available from: https://synthesismanual.jbi.global. https://doi.org/10.46658/JBIMES-24-09. | |
|
Tricco AC, Lillie E, Zarin W, O'Brien KK, Colquhoun H, Levac D, Moher D, Peters MDJ, Horsley T, Weeks L, et al: PRISMA extension for scoping reviews (PRISMA-ScR): Checklist and explanation. Ann Intern Med. 169:467–473. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, et al: The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ. 372(n71)2021.PubMed/NCBI View Article : Google Scholar | |
|
Kazimierczak U, Dondajewska E, Zajaczkowska M, Karwacka M, Kolenda T and Mackiewicz A: LATS1 is a mediator of melanogenesis in response to oxidative stress and regulator of melanoma growth. Int J Mol Sci. 22(3108)2021.PubMed/NCBI View Article : Google Scholar | |
|
Feng R, Gong J, Wu L, Wang L, Zhang B, Liang G, Zheng H and Xiao H: MAPK and Hippo signaling pathways crosstalk via the RAF-1/MST-2 interaction in malignant melanoma. Oncol Rep. 38:1199–1205. 2017.PubMed/NCBI View Article : Google Scholar | |
|
Lüönd F, Pirkl M, Hisano M, Prestigiacomo V, Kalathur RK, Beerenwinkel N and Christofori G: Hierarchy of TGFβ/SMAD, Hippo/YAP/TAZ, and Wnt/β-catenin signaling in melanoma phenotype switching. Life Sci Alliance. 5(e202101010)2021.PubMed/NCBI View Article : Google Scholar | |
|
Waaler J, Mygland L, Tveita A, Strand MF, Solberg NT, Olsen PA, Aizenshtadt A, Fauskanger M, Lund K, Brinch SA, et al: Tankyrase inhibition sensitizes melanoma to PD-1 immune checkpoint blockade in syngeneic mouse models. Commun Biol. 3(196)2020.PubMed/NCBI View Article : Google Scholar | |
|
Koo JH, Plouffe SW, Meng Z, Lee DH, Yang D, Lim DS, Wang CY and Guan KL: Induction of AP-1 by YAP/TAZ contributes to cell proliferation and organ growth. Genes Dev. 34:72–86. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Kim G, Bhattarai PY, Lim SC, Lee KY and Choi HS: Sirtuin 5-mediated deacetylation of TAZ at K54 promotes melanoma development. Cell Oncol (Dordr). 47:967–985. 2024.PubMed/NCBI View Article : Google Scholar | |
|
Miskolczi Z, Smith MP, Rowling EJ, Ferguson J, Barriuso J and Wellbrock C: Collagen abundance controls melanoma phenotypes through lineage-specific microenvironment sensing. Oncogene. 37:3166–3182. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Kim MH and Kim J, Hong H, Lee S, Lee J, Jung E and Kim J: Actin remodeling confers BRAF inhibitor resistance to melanoma cells through YAP/TAZ activation. EMBO J. 35:462–478. 2016.PubMed/NCBI View Article : Google Scholar | |
|
Kang J, Wang J, Yao Z, Hu Y, Ma S, Fan Q, Gao F, Sun Y and Sun J: Fascin induces melanoma tumorigenesis and stemness through regulating the Hippo pathway. Cell Commun Signal. 16(37)2018.PubMed/NCBI View Article : Google Scholar | |
|
Xiao Y, Zhou L, Andl T and Zhang Y: YAP1 controls the N-cadherin-mediated tumor-stroma interaction in melanoma progression. Oncogene. 43:884–898. 2024.PubMed/NCBI View Article : Google Scholar | |
|
Yuan H, Liu H, Liu Z, Zhu D, Amos CI, Fang S, Lee JE and Wei Q: Genetic variants in Hippo pathway genes YAP1, TEAD1 and TEAD4 are associated with melanoma-specific survival. Int J Cancer. 137:638–645. 2015.PubMed/NCBI View Article : Google Scholar | |
|
Mou K, Ding M, Han D, Zhou Y, Mu X, Liu W and Wang L: miR-590-5p inhibits tumor growth in malignant melanoma by suppressing YAP1 expression. Oncol Rep. 40:2056–2066. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Mou K, Liu B, Ding M, Mu X, Han D, Zhou Y and Wang LJ: lncRNA-ATB functions as a competing endogenous RNA to promote YAP1 by sponging miR-590-5p in malignant melanoma. Int J Oncol. 53:1094–1104. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Choe MH, Yoon Y, Kim J, Hwang SG, Han YH and Kim JS: miR-550a-3-5p acts as a tumor suppressor and reverses BRAF inhibitor resistance through the direct targeting of YAP. Cell Death Dis. 9(640)2018.PubMed/NCBI View Article : Google Scholar | |
|
Luo C, Balsa E, Perry EA, Liang J, Tavares CD, Vazquez F, Widlund HR and Puigserver P: H3K27me3-mediated PGC1α gene silencing promotes melanoma invasion through WNT5A and YAP. J Clin Invest. 130:853–862. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Kim G, Bhattarai PY, Lim SC, Kim JY and Choi HS: PIN1 facilitates ubiquitin-mediated degradation of serine/threonine kinase 3 and promotes melanoma development via TAZ activation. Cancer Lett. 499:164–174. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Zhang X, Yang L, Szeto P, Abali GK, Zhang Y, Kulkarni A, Amarasinghe K, Li J, Vergara IA, Molania R, et al: The Hippo pathway oncoprotein YAP promotes melanoma cell invasion and spontaneous metastasis. Oncogene. 39:5267–5281. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Feng Q, Guo P, Kang S and Zhao F: High expression of TAZ/YAP promotes the progression of malignant melanoma and affects the postoperative survival of patients. Pharmazie. 73:662–665. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Nallet-Staub F, Marsaud V, Li L, Gilbert C, Dodier S, Bataille V, Sudol M, Herlyn M and Mauviel A: Pro-invasive activity of the Hippo pathway effectors YAP and TAZ in cutaneous melanoma. J Invest Dermatol. 134:123–132. 2014.PubMed/NCBI View Article : Google Scholar | |
|
Xiong H, Yu Q, Gong Y, Chen W, Tong Y, Wang Y, Xu H and Shi Y: Yes-associated protein (YAP) promotes tumorigenesis in melanoma cells through stimulation of low-density lipoprotein receptor-related protein 1 (LRP1). Sci Rep. 7(15528)2017.PubMed/NCBI View Article : Google Scholar | |
|
Lamar JM, Stern P, Liu H, Schindler JW, Jiang ZG and Hynes RO: The Hippo pathway target, YAP, promotes metastasis through its TEAD-interaction domain. Proc Natl Acad Sci USA. 109:E2441–E2450. 2012.PubMed/NCBI View Article : Google Scholar | |
|
Stampouloglou E, Cheng N, Federico A, Slaby E, Monti S, Szeto GL and Varelas X: Yap suppresses T-cell function and infiltration in the tumor microenvironment. PLoS Biol. 18(e3000591)2020.PubMed/NCBI View Article : Google Scholar | |
|
Hugo W, Shi H, Sun L, Piva M, Song C, Kong X, Moriceau G, Hong A, Dahlman KB, Johnson DB, et al: Non-genomic and immune evolution of melanoma acquiring MAPKi resistance. Cell. 162:1271–1285. 2015.PubMed/NCBI View Article : Google Scholar | |
|
Kim MH, Kim CG, Kim SK, Shin SJ, Choe EA, Park SH, Shin EC and Kim J: YAP-induced PD-L1 expression drives immune evasion in BRAFi-resistant melanoma. Cancer Immunol Res. 6:255–266. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Ma Y, Wang L, He F, Yang J, Ding Y, Ge S, Fan X, Zhou Y, Xu X and Jia R: LACTB suppresses melanoma progression by attenuating PP1A and YAP interaction. Cancer Lett. 506:67–82. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Caron JM, Han X, Contois L, Vary CPH and Brooks PC: The HU177 collagen epitope controls melanoma cell migration and experimental metastasis by a CDK5/YAP-dependent mechanism. Am J Pathol. 188:2356–2368. 2018.PubMed/NCBI View Article : Google Scholar | |
|
Zhao B, Xie J, Zhou X, Zhang L, Cheng X and Liang C: YAP activation in melanoma contributes to anoikis resistance and metastasis. Exp Biol Med (Maywood). 246:888–896. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Sanchez IM and Aplin AE: Hippo: Hungry, hungry for melanoma invasion. J Invest Dermatol. 134:14–16. 2014.PubMed/NCBI View Article : Google Scholar | |
|
Hajimoradi Javarsiani M, Sajedianfard J and Haghjooy Javanmard S: The effects of metformin on the hippo pathway in the proliferation of melanoma cancer cells: A preclinical study. Arch Physiol Biochem. 128:1150–1155. 2022.PubMed/NCBI View Article : Google Scholar | |
|
Dieter SM, Lovecchio D, Pataskar A, Zowada MK, Körner PR, Khalizieva A, van Tellingen O, Jäger D, Glimm H and Agami R: Suppression of heparan sulfation re-sensitizes YAP1-driven melanoma to MAPK pathway inhibitors. Oncogene. 41:3953–3968. 2022.PubMed/NCBI View Article : Google Scholar | |
|
Misek SA, Newbury PA, Chekalin E, Paithankar S, Doseff AI, Chen B, Gallo KA and Neubig RR: Ibrutinib blocks YAP1 activation and reverses BRAF inhibitor resistance in melanoma cells. Mol Pharmacol. 101:1–12. 2022.PubMed/NCBI View Article : Google Scholar | |
|
Misek SA, Appleton KM, Dexheimer TS, Lisabeth EM, Lo RS, Larsen SD, Gallo KA and Neubig RR: Rho-mediated signaling promotes BRAF inhibitor resistance in de-differentiated melanoma cells. Oncogene. 39:1466–1483. 2020.PubMed/NCBI View Article : Google Scholar | |
|
Feldker N, Ferrazzi F, Schuhwerk H, Widholz SA, Guenther K, Frisch I, Jakob K, Kleemann J, Riegel D, Bönisch U, et al: Genome-wide cooperation of EMT transcription factor ZEB1 with YAP and AP-1 in breast cancer. EMBO J. 39(e103209)2020.PubMed/NCBI View Article : Google Scholar | |
|
Lee C, Jeong S, Jang C, Bae H, Kim YH, Park I, Kim SK and Koh GY: Tumor metastasis to lymph nodes requires YAP-dependent metabolic adaptation. Science. 363:644–649. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Lin L, Sabnis AJ, Chan E, Olivas V, Cade L, Pazarentzos E, Asthana S, Neel D, Yan JJ, Lu X, et al: The Hippo effector YAP promotes resistance to RAF- and MEK-targeted cancer therapies. Nat Genet. 47:250–256. 2015.PubMed/NCBI View Article : Google Scholar | |
|
Yang CE, Lee WY, Cheng HW, Chung CH, Mi FL and Lin CW: The antipsychotic chlorpromazine suppresses YAP signaling, stemness properties, and drug resistance in breast cancer cells. Chem Biol Interact. 302:28–35. 2019.PubMed/NCBI View Article : Google Scholar | |
|
Li X, Zhuo S, Zhuang T, Cho YS, Wu G, Liu Y, Mu K, Zhang K, Su P, Yang Y, et al: YAP inhibits ERα and ER+ breast cancer growth by disrupting a TEAD-ERα signaling axis. Nat Commun. 13(3075)2022.PubMed/NCBI View Article : Google Scholar | |
|
Li X, Cho YS, Zhu J, Zhuo S and Jiang J: The Hippo pathway effector YAP inhibits HIF2 signaling and ccRCC tumor growth. Cell Discov. 8(103)2022.PubMed/NCBI View Article : Google Scholar | |
|
Wang XW, Yang ZY, Li T, Zhao XR, Li XZ and Wang XX: Verteporfin exerts anticancer effects and reverses resistance to paclitaxel via inducing ferroptosis in esophageal squamous cell cancer cells. Mol Biotechnol. 66:2558–2568. 2024.PubMed/NCBI View Article : Google Scholar | |
|
Jeong SB, Das R, Kim DH, Lee S, Oh HI, Jo S, Lee Y, Kim J, Park S, Choi DK, et al: Anticancer effect of verteporfin on non-small cell lung cancer via downregulation of ANO1. Biomed Pharmacother. 153(113373)2022.PubMed/NCBI View Article : Google Scholar | |
|
Pearson JD, Huang K, Pacal M, McCurdy SR, Lu S, Aubry A, Yu T, Wadosky KM, Zhang L, Wang T, et al: Binary pan-cancer classes with distinct vulnerabilities defined by pro- or anti-cancer YAP/TEAD activity. Cancer Cell. 39:1115–1134.e12. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Saini H, Sharma H, Mukherjee S, Chowdhury S and Chowdhury R: Verteporfin disrupts multiple steps of autophagy and regulates p53 to sensitize osteosarcoma cells. Cancer Cell Int. 21(52)2021.PubMed/NCBI View Article : Google Scholar | |
|
Zhang X, Zhao H, Li Y, Xia D, Yang L, Ma Y and Li H: The role of YAP/TAZ activity in cancer metabolic reprogramming. Mol Cancer. 17(134)2018.PubMed/NCBI View Article : Google Scholar | |
|
Sanjaya A, Goenawan H, Setiawan I, Gunadi JW, Limyati Y and Lesmana R: Elaborating the physiological role of YAP as a glucose metabolism regulator: A systematic review. Cell Physiol Biochem. 55:193–205. 2021.PubMed/NCBI View Article : Google Scholar | |
|
Sun X, Ma Z, Guo Q, Zhao Z and Liu L: Ibrutinib-related skin cancer: A pharmacovigilance study from the food and drug administration adverse event reporting system. Eur J Cancer. 160:277–278. 2022.PubMed/NCBI View Article : Google Scholar | |
|
Baroja I, Kyriakidis NC, Halder G and Moya IM: Expected and unexpected effects after systemic inhibition of Hippo transcriptional output in cancer. Nat Commun. 15(2700)2024.PubMed/NCBI View Article : Google Scholar |